Efficacy, safety and bioequivalence of the human-derived B-domain-deleted recombinant factor VIII TQG202 for prophylaxis in severe haemophilia A patients
Trial registration: ClinicalTrials.gov NCT04061109.
Abstract
Introduction
Current treatment of severe haemophilia A includes prophylaxis with factor VIII (FVIII) replacement. The supply of plasma-derived FVIII is short in China.
Purpose
To evaluate the efficacy and safety of a new B-domain deleted (BDD) recombinant FVIII (TQG202) produced by human-derived cells for prophylaxis in severe haemophilia A patients and compare the bioequivalence with Xyntha.
Methods
This multicentre, clinical trial consisted of an open-label, randomized, two-period cross-over trial assessing single-dose pharmacokinetics (PK), and a single-arm clinical trial evaluating the efficacy and safety of 24 weeks of TQG202 prophylaxis, and repeated PK were assessed after prophylaxis phase. The single-dose was 50 IU/kg in PK assessment, and the initial dose was 30 ± 5 IU/kg for prophylaxis. The primary endpoints of prophylaxis were the annualized bleeding rate (ABR) and the incremental recovery rate of the first administration. Adverse events (AEs) were recorded.
Results
Twenty-six participants were enrolled in the PK assessment and 81 participants in the prophylaxis phase. Mean age was 25.9 ± 10.8 years and all participants were male. The results of PK assessment showed TQG202 is bioequivalent to Xyntha. The total ABR was 2.0 (95% CI: 1.2–2.9) in prophylaxis phase. The mean incremental recovery rate of the first administration was .027 (95% CI: .026–.028) (IU/ml)/(IU/kg). AEs occurred in 42 participants, with an incidence of 51.9%. One severe AE not related to TQG202 occurred. No participants developed FVIII inhibitors.
Conclusion
TQG202 shows bioequivalence with Xyntha. The promising efficacy and tolerability in the severe haemophilia A prophylaxis support the use of TQG202in clinical practice.
1 INTRODUCTION
Haemophilia A is an X-linked recessive bleeding disorder caused by a chromosomal mutation resulting in a deficiency or absence of coagulation factor VIII (FVIII).1-3 Spontaneous bleeding or bleeding following trauma or surgery primarily occurred in patients with severe hemophilia.1-4 The prevalence of haemophilia A is about 25 cases per 100,000 live male births of all severities.1, 2 From 2007 to 2019, the Hemophilia Treatment Center Cooperation Network of China (HTCCNC) discovered and registered 17,779 patients with haemophilia A, of which 86.2% were moderate to severe.5 Complications include synovitis and arthropathy secondary to recurrent hemarthrosis, delayed post-traumatic bleeding, and inhibitor formation.1-3 Although the most common causes of death in Haemophilia A patients were major or intracranial haemorrhage, chronic joint disease due to joint bleeding was the main cause of disability.1-3
At present, the treatment for severe haemophilia A is prophylactic exogenous factor VIII injection, and it can reduce the annual bleeding rate by 95.9%.4, 6-8 Still, the proportion of Chinese severe haemophilia A patients receiving prophylaxis only reaches 16.2%, which is much lower than in developed countries.5, 9, 10 The main reasons are poor patient education and heavy economic burden, as well that the FVIII used in clinical practice in China is mainly plasma-derived. Due to the serious shortage of plasma and the risk of viral infection, it is necessary to develop recombinant human FVIII to alleviate the shortage of FVIII products in China and improve the prognosis of patients with haemophilia A.
Recombinant FVIII products (Advate, Xyntha, and Kovaltry), and the third-generation B-domain deleted (BDD) recombinant FVIII product SCT800 are marketed in China.11, 12 Advate and Kovaltry are similar to plasma-derived FVIII with varying degrees of truncation in the B domain, while Xyntha and SCT800 are recombinant FVIII products with BDD,11, 12 leading to better stability and higher purity of preparations and binding with von Willebrand Factor.13 Still, Advate, Kovaltry, and SCT800 are all produced by Chinese hamster ovary (CHO) cells, while Xyntha is produced by albumin-free CHO cells.14 Factor VIII produced by murine-derived cells such as CHO cells displayed strong immunogenicity and introduced a high risk of FVIII inhibitors development in the patients. At present, the marketed humanized recombinant FVIII products with BDD in China still cannot meet the needs.
Recently, Chia Tai Tianqing Pharmaceutical Group Co., Ltd. (China) developed a BDD-type recombinant FVIII (named TQG202) produced by human-derived HEK293cells, achieving a post-translational modifications (PTMs) pattern similar to that of humans, making it similar to endogenous FVIII molecules, without carrying non-human epitopes. Therefore, TQG202 has certain advantages in reducing the immunogenicity of drugs and reducing the formation of inhibitors.15, 16
The purpose of this study was to evaluate the efficacy and safety of TQG202 produced by human-derived cells for prophylaxis of Chinese patients with severe haemophilia A, and compare the bioequivalence with Xyntha.
2 MATERIALS AND METHODS
2.1 Study design and participants
This multicentre, clinical trial consisted of two phases. The first phase was an open-label, randomized; two-period cross-over trial aiming to assess the single-dose pharmacokinetics (PK) characteristics of TQG202, and the second phase was a single-arm clinical trial to evaluate the efficacy and safety of TQG202 prophylaxis. The participants who completed the first phase continued to complete the second phase. The participants were enrolled from 20 February 2019 to 15 July 2019. The trial was approved by the institutional review boards of the Blood Diseases Hospital, Chinese Academy of Medical Sciences (XT2018049-EC-1). Written informed consent was obtained from all participants at enrolment. The trial was registered at ClinicalTrials.gov (#NCT04061109).
The eligibility criteria included 12–65 years of age and severe haemophilia A with a baseline coagulation FVIII activity (FVIII:C) <1%; previous treatment with FVIII concentrates for ≥100 exposure days (EDs); the record of at least 3 months of bleeding and treatment before enrolment could be obtained; absence of FVIII inhibitor. In addition, the participants recruited for the first phase needed to meet additional requirements: no other drugs for the treatment of haemophilia within 96 h before TQG202 administration, including recombinant or plasma-derived FVIII, cryoprecipitate, fresh plasma, and whole blood; and no obvious bleeding symptoms or active bleeding. The participants recruited for the second phase had to meet additional requirements: no other drugs for treating haemophilia A, including recombinant or plasma-derived FVIII, cryoprecipitate, fresh plasma, and whole blood, within 72 h before administration.
The exclusion criteria included: personal or family history of FVIII inhibitors; any other bleeding disease or coagulation dysfunction, or under anticoagulation therapy; human immunodeficiency virus infection; planning to perform elective surgery during the trial; treated with immunosuppressive agents within 1 week before TQG202 administration, and the wash-out period was less than seven half-lives; known to be allergic to the test drug or any excipients; severe anaemia and need for blood transfusion, or obvious liver or kidney or platelet dysfunction, uncontrollable hypertension, alcohol abuse, drug abuse, mental disorders, or other severe comorbidities.
2.2 Pharmacokinetics assessment
In the first phase of the trial, the participants were randomized stratified by age (<17, ≥18 years of age). A random seed was generated using the SAS 9.4 statistical software package PROC PLAN (SAS Institute, Cary, NY, USA), and the participants were randomized to the treatment-reference (TR) group or the reference-treatment (RT) group.
The PK assessment consisted of two stages (PK1 stage: days 1 to 4; PK2 stage: days 5 to 8). The wash-out period between the two stages was 96 h. The TR group received TQG202 (Chia Tai Tianqing Pharmaceutical Group Co., Ltd., 250 IU/bottle) in the PK1 stage and the reference Xyntha (Pfizer, New York, NY, USA; 500 IU/bottle) in the PK2 stage; the RT group received the opposite sequence. Factor VIII was administered by intravenous injection. The duration of each administration was 5 ± .5 min, at a single-dose of 50 IU/kg.
The dosage of each participant remained consistent, and participants who completed the first phase entered the second phase. After the second phase was completed, a repeat PK assessment was carried out, and the dose of TQG202 in the repeated PK was the same as in the first phase.
During the PK assessment, any drugs were forbidden except for drugs used to treat adverse events (AEs) and comorbidities. Venous blood was collected at 12 time points such as the 5 min (complete/immediately after drug infusion), 20 min, 35 min, 60 min, 1.5 h, 2 h, 3 h, 6 h, 9 h, 24 h, 36 h and 48 h after the drug infusion started; all blood samples were collected The time points were based on the starting point of medication, and about 2.7 ml of whole blood was collected at each time point. The deviation within the PK blood collection time window is calculated according to the planned blood collection time. If it exceeds the time window, it is calculated according to the actual blood collection time and administration time. Missing individual plasma drug concentration data were reported as “Missing” and were excluded from descriptive statistics and PK parameter calculations for plasma drug concentrations at each time point. For data below the lower limit of quantification (BLQ), the following principles are adopted in the descriptive statistical analysis of concentration and the calculation of PK parameters: (1) The BLQ appearing before the first measurable concentration point participates in the calculation with a zero value; (2) Other BLQs are recorded as “ND” and do not participate in the calculation; (3) Only when at least two-thirds of the individual data at a specific sampling time point meet or exceed the LLOQ, the concentration descriptive statistical analysis can be performed.
2.3 Prophylaxis procedure
TQG202 was injected at an initial dose of 30 ± 5 IU/kg. If two spontaneous bleeding events occurred in same major joint (such as hip, shoulder, knee, and elbow) or over three spontaneous bleeding events in any body part within 28 days, the dosage was adjusted to 45 ± 5 IU/kg. Prophylaxis was performed three times a week or once every other day for 24 weeks.
In the case of a bleeding event during the prophylaxis period, on-demand treatment could be carried out. The dosage was calculated according to the formula: dose (IU) = weight (kg) × factor VIII expected increase value (IU/dl or % normal value) × .5(IU/kg)/(IU/dl). The use of on-demand treatment was decided by the investigator according to the clinical situation.
Unless medically necessary, other medications should be avoided.
2.4 Endpoints
The primary PK parameters were the area under the plasma concentration curve from 0 to treatment (AUC0-t) and the area under the plasma concentration curve from 0 to infinity (AUC0-∞). Secondary PK parameters included peak concentration (Cmax), peak time (Tmax), in vivo elimination half-life (t1/2), apparent terminal elimination rate constant (λz), and incremental recovery rate. The incremental recovery rate is defined as the ratio of Cmax measured within 1 h after the end of the injection and the injection dose.
The primary endpoints of the prophylaxis phase were the annualized bleeding rate (ABR) and the incremental recovery rate of the first administration. The ABR included spontaneous and traumatic bleeding rate. Incremental recovery rate was the ratio of Cmax measured within 1 h after the end of the injection and the injection dose. The secondary endpoints were the annualized joint bleeding rate (AJBR), the treatment effect of bleeding events (none, fair, good, and excellent), the monthly average number of bleedings, dose and number of injections for each bleeding event, incremental recovery rate after repeated single-dose, joint function, and health status. The function of the knee, elbow, and ankle joints was assessed by the Hemophilia Joint Health Score (HJHS) Chinese version.17 The health status was evaluated by Chinese version of the EQ-5D scale.18
The safety endpoint included inhibitor development and all AEs. Inhibitory antibodies were determined using the Nijmegen Bethesda assay. Inhibitor development was confirmed when two consecutive samples were tested positive (defined as ≥.6 BU/ml). The threshold of low-titer inhibitor was determined to ≥.6 BU/ml, and the high-titer inhibitor was set to >5 BU/ml. AEs were assessed through physical examination, vital signs, electrocardiogram, and laboratory examinations according to World Health Organization (WHO) handbook for reporting results of cancer treatment.19
2.5 Statistical analysis
It was planned to enrol 20–24 participants in the pharmacokinetic assessment and 40–44 in the prophylaxis phase, for a total number of 64.
In this trial, the PK parameters were calculated by the non-compartmental analysis module of WinNonlin V6.4 (Pharsight Certara Co., Princeton, NJ, USA). The analysis of the efficacy and safety of the prophylaxis treatment was performed in the full analysis set (FAS) and safety analysis set (SS), which included all participants who received at least one administration of the study drug.
The statistical analysis was performed using SAS 9.4 (SAS Institute, Cary, NY, USA). Continuous data were presented as mean ± standard deviation or median (range). Categorical data were presented as n (%). ABRs, AJBRs, average monthly bleeding, and incremental recovery rate were calculated as median (Q1, Q3) and a fitted mean according to a negative binomial regression model [95% confidence intervals (CIs)] corrected for overdispersion. The safety endpoint of inhibitor development was evaluated using the Bayesian analysis model, and the β incidence distribution was used to simulate the incidence of inhibitor development. The joint function and health status at baseline and 24 weeks after treatment were compared using the paired t-test, with p < .05 indicating a statistically significant difference. Other efficacy and safety endpoints and repeat PK parameters were analysed using descriptive statistics.
3 RESULTS
3.1 Baseline characteristics
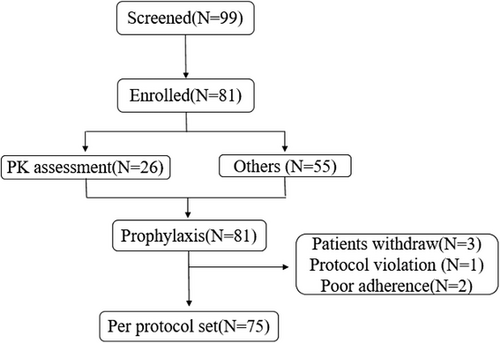
A total of 26 participants were enrolled in the PK assessment (13 in each group), with a mean age of 30.0 ± 9.9 years. All participants were male. A total of 81 participants (including the 26 participants who completed the PK assessment) were enrolled in the prophylaxis phase (Figure 1), with a mean age of 25.9 ± 10.8 years. All participants were male. The median baseline FVIII activity level was .50%. Seventy-one participants had bleeding events in the past 3 months. The median of the total number of bleeding and the total number of joint bleedings within 3 months was two respectively. There were 36 participants (44.4%) with family history of haemophilia. Table 1 presents the baseline characteristics of the participants.
| Characteristics | Total (N = 81) |
|---|---|
| Age, years, mean ± SD | 25.9 ± 10.8 |
| 12–17 years | 19 (23.5) |
| 18–65 years | 62 (76.5) |
| Male, n (%) | 81 (100) |
| Height, cm, mean ± SD | 171.1 ± 7.5 |
| Han nationality, n (%) | 75 (92.6) |
| KPS, mean ± SD | 88.2 ± 10.7 |
| Age at diagnosis, years, median (range) | 19.4 (4.0–44.4) |
| Family history of haemophilia, n (%) | 36 (44.4) |
| History of bleeding in the past 3 months, n (%) | 71 (87.7) |
| Bleedings events in the past 3 months, times, median (range) | 2 (0–29) |
| Joint bleedings, times, median (range) | 2 (0–28) |
| History of surgery, n (%) | 24 (29.6) |
| aHJHS score, median (range) | 18 (0–63) |
| EQ-5D scale, median (range) | 8 5(30–100) |
| Baseline factor VIII activity, %, median (range) | .5 (.0–1.0) |
| Previous factor VIII treatment, n (%) | |
| Plasma-derived human factor VIII | 15 (18.5) |
| Recombinant human factor VIII | 8 (9.9) |
| Plasma-derived + recombinant human factor VIII | 58 (71.6) |
| Factor VIII treatment in the past 3 months, n (%) | |
| On-demand | 33 (40.7) |
| Prophylaxis | 10 (12.4) |
| Both | 37 (45.7) |
| Factor VIII treatment exposure days, n (%) | |
| 100–150 days | 61 (75.3) |
| >150 days | 20 (24.7) |
- Abbreviations: KPS: Karnofsky performance status; SD: standard deviation. HJHS: Hemophilia Joint Health Score.
- a Assessment of HJHS: total: 0(best) to 124(worst).
3.2 Pharmacokinetics
The detailed pharmacokinetic parameters of TQG202 and Xyntha are shown in Table S1. The geometric mean ratios (GMRs) of AUC0-t and AUC0-∞ were 104.5% (90% CI: 97.9%, 111.6%) and 103.6% (90% CI: 96.6%, 111.1%), respectively. The GMRs of Cmax and incremental recovery rate were 125.7% (90% CI: 118.2%, 133.6%) and 124.5% (90% CI: 117.0%, 132.5%), respectively. The results of the time-dependent analysis showed that the GMRs of AUC0-t, AUC0-∞, Cmax, and incremental recovery rate of repeated PK assessment were 114.8% (90% CI: 100.7%, 131.0%), 115.3% (90% CI: 99.5%, 133.5%), 108.6% (90% CI: 95.7%, 123.3%), and 109.6% (90% CI: 96.4%, 124.6%), respectively. The results of the PK assessments are shown in Table 2.
| Pharmacokinetic parameters | TQG202 mean | Xyntha mean | GMR (TQG202 /Xyntha) % | 90% CI | Intersubject CV | Power (%) |
|---|---|---|---|---|---|---|
| PK assessment (N = 26) | ||||||
| AUC0-t (h × IU/ml) | 15.18 | 14.52 | 104.54 | 97.94–111.58 | 13.81 | 99.8 |
| AUC0-∞ (h × IU/ml) | 16.10 | 15.53 | 103.64 | 96.64–111.14 | 14.82 | 99.8 |
| Cmax (IU/ml) | 1.337 | 1.064 | 125.65 | 118.19–133.57 | 12.95 | 3.70 |
| Incremental recovery rate (IU/ml)/(IU/kg) | .0265 | .0213 | 124.50 | 117.03–132.46 | 13.11 | 6.20 |
| T1/2 | 9.92 | 11.15 | 88.98 | 81.59–97.05 | 18.4 | 65.3 |
| Pharmacokinetic parameters | Mean of single-dose | Mean of repeated single-dose PK | GMR (single/repeated) % | 90% CI |
|---|---|---|---|---|
| Repeat PK assessment (N = 25) | ||||
| AUC0-t (h × IU/ml) | 15.17 | 17.43 | 114.83 | 100.68–130.98 |
| AUC0-∞ (h × IU/ml) | 16.10 | 18.55 | 115.26 | 99.50–133.52 |
| Cmax (IU/ml) | 1.337 | 1.452 | 108.59 | 95.65–123.28 |
| Incremental recovery rate, (IU/ml)/(IU/kg) | .0265 | .0290 | 109.59 | 96.36–124.63 |
- Abbreviations: GMR: geometric mean ratios; CI: confidence interval; CV: coefficient of variation; AUC0-t: area under the plasma concentration curve from 0 to treatment; AUC0-∞: the area under the plasma concentration curve from 0 to infinity; Cmax: peak concentration; Tmax: peak time; t1/2: in vivo elimination half-life.
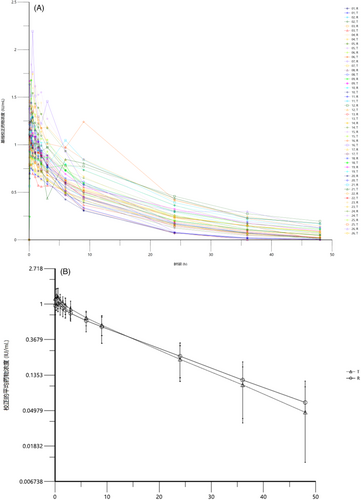
The individual plasma concentration-time curve (Figure 2A) and the average plasma concentration-time curve (Figure 2B) showed that TQG202 exhibited first-level linear elimination characteristics after reaching the peak quickly, and the plasma concentration curve is concentrated. The curve of TQG202 was close to Xyntha's curve.
3.3 Prophylaxis efficacy
The therapeutic effect during the prophylaxis phase is shown in Table 3. There were 69 bleeding events, for a mean ABR of 2.0 (95% CI: 1.2–2.9), including a mean of .8 (95% CI: .4–1.2) for spontaneous ABR and 1.2 (95% CI: .6–1.9) for traumatic ABR. The mean incremental recovery rate after the first administration was .027 (95% CI: .026–.028) (IU/ml)/(IU/kg). The AJBR was 1.7 (95% CI: .9–2.4), including a spontaneous AJBR of .7 (.4–1.1) and traumatic AJBR of .9 (.4–1.5). When used to manage bleeding events, 13 (18.8%) participants rated the treatment effect as excellent, 45 (65.2%) as good, with 84.1% rating of excellent or good.
| Total (n = 81) | ||
|---|---|---|
| Median (Q1, Q3) | Mean (95% CI) | |
| ABR | 0 (.00, 2.14) | 2.03 (1.17, 2.89) |
| Spontaneous ABR | 0 (.00, .00) | .80 (.43, 1.18) |
| Traumatic ABR | 0 (.00, 2.11) | 1.23 (.56, 1.89) |
| Incremental recovery rate after the first administration, (IU/ml)/(IU/kg) | .026 (.023, .032) | .027 (.026, .028) |
| AJBR | 0 (.00, 2.12) | 1.66 (.93, 2.40) |
| Spontaneous AJBR | 0 (.00, .00) | .73 (.35, 1.10) |
| Traumatic AJBR | 0 (.00, .00) | .94 (.38, 1.50) |
| Average monthly bleeding, times | 0 (.00, .18) | .17 (.10, .24) |
| Incremental recovery rate after repeated administration, (IU/ml)/(IU/kg) | .030 (.026, .036) | .057 (.020, .093) |
- Abbreviations: ABR: annualized bleeding rate; AJBR: annualized joint bleeding rate; CI: confidence interval.
Out of 72 new bleeding treatments, 33 (45.8%) participants received one injection to stop bleeding, 23 (31.9%) received two injections to stop bleeding, eight (11.1%) received three injections to stop bleeding, five (6.9%) received four injections to stop bleeding, and three (4.2%) received more than four injections to stop bleeding. These three bleeding treatments came from two patients (Table 4). Subject 06005 had a total of three bleeding treatments during the trial, of which one bleeding was slightly relieved after the first treatment, and after five injections for consolidation therapy, the efficacy evaluation was no relief according to the four-level scoring standard. One of the two other bleeding treatments for this subject was assessed as moderate and one was not assessed for efficacy (the subject was given as-needed medication for suspected bleeding due to pain, but no bleeding was assessed by the investigator). Subject 06006 had received a total of four bleeding treatments during the trial, two of which had no remission after the first treatment, and were slightly relieved after five and four injections respectively, according to the four-level scoring standard, the efficacy was evaluated as no remission. The other two additional bleeding treatments were evaluated as good and moderate. During the trial, the inhibitor detection of the two subjects was negative, and there was no related symptoms such as decreased efficacy. The incremental recovery rate is shown in Table 4, and the coagulation FVIII activity can reach the expected level without obvious abnormality. During the trial, seven subjects received nine dose adjustments, the reasons for dose adjustment were: bleeding (four times), ankle swelling or pain (two times), medication status in the past 4 weeks (two times), and weight change (one time). The maximum adjusted dose is 40 IU/kg. Subject 06006 received two dose adjustments due to two bleeding events. The average injection dose was 1907.47 IU, and the mean consumption per patient of TQG202 for the treatment of bleeding was 3819.44 IU.
| No. | First injection | 12 weeks | 24 weeks | |||
|---|---|---|---|---|---|---|
| Dosage IU/kg | Incremental- recovery (IU/ml)/(IU/kg) | Dosage IU/kg | Incremental- recovery (IU/ml)/(IU/kg) | Dosage IU/kg | Incremental- recovery (IU/ml)/(IU/kg) | |
| 06005 | 25 | .0305 | 30 | .0313 | 30 | .0408 |
| 06006 | 25 | .0216 | 30 | .0238 | 35 | .0260 |
Regarding the joint function assessment, the 24-week HJHS score was lower than at baseline (mean: from 22.5 ± 15.4 to 19.2 ± 13.2; p < .0001; the difference was -3.3 ± 6.8). Regarding the health status, the 24-week EQ-5D score was higher than at baseline (mean: from 83.4 ± 13.2 to 86.2 ± 12.3; p = .025; the difference was 3.3 ± 12.7) (Table 5).
| Baseline (N = 81) | 24-week (N = 76) | Changes (N = 76) | |
|---|---|---|---|
| HJHS score | |||
| Mean ± Std | 22.5 ± 15.4 | 19.3 ± 13.2 | -3.3 ± 6.8 |
| Min–Max | .0, 63.0 | .0, 50.0 | -30.0, 14.0 |
| EQ-5D score | |||
| Mean ± Std | 83.4 ± 13.2 | 86.2 ± 12.3 | 3.33 ± 12.68 |
| Min–Max | 30.0, 100.0 | 45.0, 100.0 | -40.0, 55.0 |
3.4 Safety
The mean consumption per patient of TQG202 for prophylaxis was 134,327.2 IU, and the average days of exposure reached 73.8. AEs occurred in 42 participants, with an incidence rate of 51.9% (42/81) (Table 6). The most common AE was upper respiratory tract infection (10 cases, 12.4%). Most AEs were grade I or II, and there was only one case of AEs ≥grade 3. According to the investigator's judgement, there were 16 cases of study drug-related AEs, with an incidence rate of 19.8% (16/81). The severity of study drug-related AEs was grade I or II, and there was no adverse event ≥grade 3. The most common AE related to the study drug was an abnormality of the QRS waves (3.7%). This adverse reaction occurred in three patients, numbered 10,002, 10,006, and 10,007, respectively. The adverse reactions in the three patients were all RV5 high voltage, which were classified as abnormal of the QRS waves in the statistical analysis of the data. Both of the three patients had ECG abnormalities during the screening period, including sinus bradycardia, sinus rhythm V1 lead R/S >1, and sinus tachycardia with QT interval prolongation. None of the three patients underwent symptomatic treatment or medication dose adjustment, symptoms resolved in two patients, and persisted in one patient at the last visit. It is not yet clear how this adverse reaction is deeply related to the drug or disease. Other adverse reactions according to the incidence rate were: increased alanine aminotransferase (2.47%), increased aspartate aminotransferase (2.47%), first-degree atrioventricular block (2.47%), sinus rhythm tachycardia (2.47%), upper respiratory tract infection (2.47%), dizziness (2.47%), arthralgia (2.47%), decreased lymphocyte count (1.23%), prolonged ECG QT interval (1.23%), ECG ST segment abnormal (1.23%), ventricular extrasystoles (1.23%), palpitations (1.23%), left bundle branch block (1.23%), sinus arrhythmia (1.23%), herpes simplex (1.23%), oral Sore throat (1.23%). In this trial, one serious AE (SAE) occurred, which was not related to TQG202. The participant was hospitalized due to pneumonia requiring treatment. None of the 81 participants developed FVIII inhibitors during the trial.
| Adverse events | n (%) |
|---|---|
| Any adverse events | 42 (51.9) |
| Grade 3–4 adverse events | 1 (1.2) |
| Adverse events led to withdrawal from the trial | 0 |
| Severe adverse events | 1 (1.2) |
| AEs related to TQG202 | 16 (19.8) |
| Grade 3–4 AEs related to TQG202 | 0 |
| AEs related to TQG202 that led to the withdrawal from the trial | 0 |
| SAE related to TQG202 | 0 |
- Abbreviations: AE: adverse event; SAE: serious adverse event.
4 DISCUSSION
This multicentre, clinical trial aimed to evaluate the efficacy and safety of the BDD recombinant FVIII (TQG202) produced by human-derived cells for prophylaxis of severe haemophilia A patients in China, and compare the bioequivalence with Xyntha. The results suggest that TQG202 is bioequivalent to Xyntha. The application of TQG202 effectively reduce the ABR and AJBR. The AE profile suggests that TQG202is tolerable. The promising efficacy and tolerability in the prophylactic treatment of severe haemophilia A patients support the use of TQG202 in clinical practice.
This is the first study reporting PK data about TQG202. Compared with a single-dose in PK assessment, the estimates GMR of AUC0-t and AUC0-∞ in repeated single-dose PK after 24 weeks of prophylaxis (114.8% and 115.3%, respectively) all fall within the specified range of 80.00%–125.00%. Therefore, the PK assessment showed that the PK parameters after 24 weeks of prophylaxis with TQG202 were similar to those of the first administration, with no time dependence. Moreover, the PK parameters of this study are similar to those of other recombinant factor VIII formulations.11, 20-23
Previous studies reported relatively high ABR and AJBR, with an ABR of 2.82 (95% CI: 2.01–3.96) for SCT800,11 1.9 (range, 0–4.2) for BAY94-9027,20 1.9 (range, 0–5.8) for BAX855,21 median of 1 (individualized prophylaxis) or 2 (weekly prophylaxis) for rFVIIIFc #13,22 and 1.14 (range, 0–4.2) for rVIII-SingleChain.23 The ABR of severe haemophilia A participants under prophylaxis was 0 (.00, 2.14), and the AJBR was 0 (.00, 2.12) in this trial, superior to or similar to the other recombinant FVIII products. In addition, the incremental recovery rates of the first and repeated medications were stable, which were .026 (.023, .032) (IU/ml)/(IU/kg) and .030 (.026, .036) (IU/ml)/(IU/kg), respectively. The low ABR and AJBR observed in the present study strongly support the prophylactic efficacy of TQG202, which might be related to weaker immunogenicity of TQG202 produced by human-derived cells other than CHO cells.24 In addition, when treating bleeding events during the trial, TQG202 displayed a promising efficacy with 84.1% rating of excellent or good, which is similar to other studies, at 92.6% for Xue et al.11 and 72.4% for Reding et al.20 Furthermore, most bleeding episodes were controlled using one or two injections of TQG202.
The assessments of joint function suggest that after 24 weeks of prophylaxis, joint function was better than that at baseline. The health index scores indicated that after 24 weeks of preventive treatment, the health status was better than that at baseline, similar to Xue et al.11 with SCT800 and Konkle et al.21 with BAX855. It demonstrated the use of TQG202 for prophylaxis not only reduce the risk of bleeding, but also further improve the joint function and health status of Chinese severe haemophilia A patients.
The safety of prophylactic TQG202, including the incidence rate of AEs and inhibitors in this study was analysed. AEs occurred in 51.9% (42/81) of the participants, including one SAE of pneumonia. No participants developed inhibitors. Xue et al.11 reported that SCT800, a BDD FVIII derived from CHO cells, had an AE incidence of 53.4%, all of mild or moderate severity, except for one SAE of moderate upper gastrointestinal bleeding. None of their participants developed inhibitors. Konkle et al.21 examined the safety of BAX855, a recombinant FVIII-rurioctacog Alfa pegol, and reported AEs in 53.3% of the participants without inhibitor development. A study of BAY94-9027 reported AEs in 75% of the patients, including two cases of withdrawals due to AEs.20 Using rFVIII Fc22 or rVIII-SingleChain,23 64%–66% of the patients reported AEs, though no inhibitor development was observed. Abnormality of the QRS waves was the most common AE related to TQG202, the deeply relationship between this adverse reaction and drug or disease is not clear yet. Zong et al.24 reported that patients with haemophilia A (PWHA) showed higher probability of developing left ventricular systolic dysfunction (LVSD), coronary artery disease or coronary microvascular disease (CAD/CMVD) and left ventricular hypertrophy defined as left ventricular electrical remodelling (LVH/LVER) than age-matched controls as evaluated by advanced electrocardiography (A-ECG). We believe that further studies and larger populations are needed to confirm this association and the underlying causes. Differences in AEs among the studies could be due to the source of the FVIII and different baseline characteristics of participants. Indeed, TQG202 is produced by human-derived cells (HEK293) and might be safer than FVIII produced by murine-derived cells.25
This study has limitations. Because haemophilia A is a rare disease, the sample size is relatively small, and it is difficult to observe rare AEs. Second, the follow-up time was short, and the long-term efficacy and safety were unclear. Third, this study only included treated participants, and further clinical trials are needed to provide efficacy and safety in newly treated severe haemophilia A patients. Further studies with larger sample size, longer follow-up time and wide population are warranted to support the clinical application of TQG202.
In conclusion, the recombinant Coagulation Factor VIII TQG202 is a BDD-type recombinant FVIII produced by human-derived cells and shows bioequivalence with Xyntha. The promising efficacy and tolerability in the prophylactic treatment of severe haemophilia A patients support the use of TQG202 in clinical practice. It might help add treatment options for Chinese patients with severe haemophilia A, alleviating the current shortage of FVIII in China.
ACKNOWLEDGEMENTS
The authors thank all of the clinical personnel and patients who participated in this study. This study was funded by Chia Tai Tianqing Pharmaceutical Group Co., Ltd.
CONFLICTS OF INTEREST
Haifei Jia is employed by Chia Tai Tianqing Pharmaceutical Group Co., Ltd. The remaining authors stated that they had no interests which might be perceived as posing a conflict or bias.
AUTHOR CONTRIBUTIONS
Research performed by Yaming Xi, Chenghao Jin, Wei Liu, Hu Zhou, Zhen Wang, Rongfu Zhou, Shifeng Lou, Xielan Zhao, Fangping Chen, Peng Cheng, and Zimin Sun. Study was designed by Lei Zhang. Data were analysed by Yaming Xi, Haifei Jia. Paper was written by Yaming Xi and reviewed by all authors.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request




