Electronic diaries in the management of haemophilia gene therapy: Perspective of an expert group from the German, Austrian and Swiss Society on Thrombosis and Haemostasis (GTH)
Abstract
Introduction
Gene therapy (GT) is becoming a realistic treatment option for patients with haemophilia. Outside clinical trials, the complexity and potential complications of GT will pose unprecedented challenges to haemophilia care centres.
Aim
To explore the potential use of electronic tools to improve the delivery of GT under real-world conditions.
Methods
Considering the hub-and-spoke model, the GTH working group on GT considered the entire patient pathway and reached consensus on requirements for an integrative software tool to secure documenting and sharing information between treaters, pharmacies and patients.
Results
Six steps of the gene therapy process were identified, each requiring completion of the previous step as a prerequisite for entry. The responsibilities of GT dosing and follow-up treatment centres, read/write access rules, and the minimum data set were outlined. Data contributed by patients through mobile devices was also considered.
Conclusion
Important information needs to be shared between patients and treatment centres in a real-world GT hub-and-spoke model. Collecting and sharing this information in well-organised electronic applications will not only improve patient care but also enable national and international data collection in clinical registries.
1 INTRODUCTION
Liver-targeted AAV-based gene therapy for haemophilia (GT) promises to achieve potentially clinically significant coagulation factor levels that could last for years after a single intravenous administration of the gene therapy product. This would enable patients with haemophilia (PWH) to become independent of regular treatment with factor replacement or non-factor products, even in the event of trauma or surgery.
Gene therapy studies in both haemophilia A and haemophilia B show significant transgenic expression of FVIII and FIX levels, leading to almost absence of both the number of bleeds and the need for regular prophylaxic treatment. This is associated with tremendous clinical benefit and significantly improved quality of life. Recently, clinically sufficient factor expression has been confirmed in haemophilia A and haemophilia B for up to 5 years1, 2 and 8 years.3
Different results were reported in the various clinical trials with regard to the persistence of transgene expression and the number of bleedings. Recombinant AAV are produced in mammalian cell lines (HEK293) or insect cell lines (Sf9), with different dosages required to achieve similar therapeutic levels of FVIII or FIX.4, 5 In addition to some previously described unknowns of gene therapy, short- to medium-term adverse events were also noted in a considerable number of clinical and preclinical studies, mostly manifesting as a mild to moderate increase in alanine aminotransferase (ALT).6 This ALT elevation is asymptomatic and transient, but may result in a reduction or loss of expression of the transgene.7 Regular post-treatment protocols are essential to implement immunosuppressive treatment as early as possible, which has the potential to regulate this ALT pattern. In addition, long-term consequences of GT are being studied and long-term registries focusing on liver function, genotoxicity and malignancy are planned.8
In recent years, the complex treatment of haemophilia has been taken over by specialised multidisciplinary Haemophilia Comprehensive Care Centres (HCCCs) and Haemophilia Treatment Centres (HTCs), which are constantly adapting to the growing demands and offer a wide range of clinical and laboratory services to ensure safe and efficous treatment of haemophilia. For this, a multidisciplinary care model has been established with access to clinical specialities, emergency departments and appropriate laboratory facilities.9
Compared to existing treatment options for haemophilia, the gene therapy approach requires an additional number of different, sequential treatment steps to ensure the safe and effective application of gene therapy in haemophilia.
1.1 The hub-and-spoke model for gene therapy
Recently, a joint publication of the European Association for Haemophilia and Allied Disorders (EAHAD) and the European Hameophilia Consortium (EHC)10 proposed a networked hub-and-spoke model with a system for long-term monitoring of safety and efficacy. This model includes criteria for participating centres and their collaboration in gene therapy care, leading to a reassessment of the infrastructure of HTC's.
A modifiable hub-and-spoke model addresses all aspects related to gene therapy of haemophilia, including preparation and administration of the gene therapy product, determination of coagulation and immunological parameters, joint score and function, and liver health. According to this model, gene therapy is prescribed and administered by expert haemophilia comprehensive care centres (as the hubs) and longitudinally monitored by haemophilia treatment centres in close communication with the primary expert hub (as spokes linking into that hub) depending on the expertise already available in each centre. Several criteria were established for defining a centre, such as experience with previous gene therapy trials, facilities familiar with gene therapy and close collaboration with other disciplines.
Well-defined outcome criteria such as clotting factor concentration remain the appropriate endpoint in GT,11 but some variation has previously been reported between the one-stage assay and the chromogenic assay, with the implication that both assays should be used to obtain an accurate clinical picture of effectiveness.12, 13
In clinical trials, gene therapy requires non-routine measurement of cellular and humoral immunity against the AAV capsid as well as the determination of the release of vector particles through body fluids (vector shedding). As a result of gene transfer, coordinated action is required by all participants to ensure safe and effective use and to initiate immediate coordinated action in the event of unexpected safety issues to give each PWH an optimal chance of success and to provide clinicians and PWH with better information for decision-making.
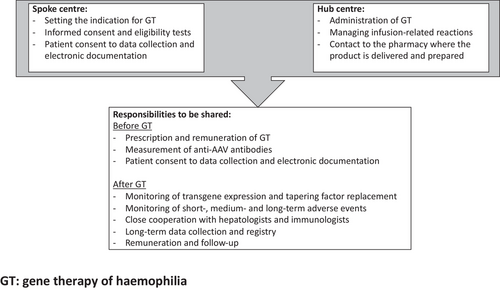
Even if these criteria are well defined, the elaboration between the centres has to be done individually. Figure 1 provides an overview of the different responsibilities that should be agreed and shared between centres. While some tasks can be clearly defined, such as the administration of gene therapy at the hub centre and the follow-up after gene therapy at a centre close to the patient's home, there are other tasks whose responsibilities need to be clarified between the centres involved. In the future, more experience with gene therapy may lead to an improvement in knowledge and a change in responsibilities so that the hub-and-spoke model can be adapted.
1.2 Benefit of electronic devices in gene therapy of haemophilia
The challenges of gene therapy for haemophilia can only be met through the combined efforts of haemophilia centres and require a re-evaluation of infrastructure and comprehensive care. In haemophilia care, several tools for accurate and reliable documentation of treatment have been developed using electronic diaries and mobile apps such as Haemoassist™ or SmartMedication™ in Germany. Based on such tools, a retrospective, multicentre study demonstrated improved treatment adherence in a large group of PWH with haemophilia A and haemophilia B from 10 centres in two countries.14 In addition, the quality of documentation and commitment to documentation could be improved. Adherence to treatment can be difficult to assess in clinical trials. In Germany, significant differences have been found between different age groups of patients.15
However, the use of electronic devices has been shown to improve adherence and compliance with data transmission compared to paper documentation.16 In addition, an observational study showed that quality of life improved and joint health scores stabilised.17
Given the complexity and potential complications of GT, it is the responsibility of healthcare professionals to ensure the prescribing, administration and monitoring of gene therapies. Following the hub-and-spoke model, HTCs will provide GT according to their technical and medical expertise and share responsibility for patients with haemophilia. The electronic devices developed recently have been gradually improved and would also help the HTC to manage the tasks involved after the introduction and delivery of gene therapy as safely and efficiently as possible.
Table 1 summarises the various needs in communication and cooperation that could be addressed by an electronic system available to each HTC and PWH. Each entry in the electronic system would be recorded there and lead to further tasks for the other parties involved. At the same time, an alert system would be implemented to provide mandatory reminders of required factor levels and liver enzyme tests at specific time intervals. This ensures that the various safety risks can be detected and dealt with in the best possible way and as transparently as possible for all those involved. The advantage of electronic platform would be the secure, comprehensible recording of data for all involved, combined with an overview of the resulting subsequent steps. Another advantage would be that the data can be viewed and entered by all parties at the same time, so that everyone is on the same level and possible actions are comprehensible for everyone. The data of the globally applicable electronic devices will only be shared between all those involved in the treatment of an individual patient.
| Challenges | Significance for the outcome of GT | Participants involved |
|---|---|---|
| Documentation of inclusion/exclusion criteria | Requirement for GT | HTCs |
| Patient informed consent | Requirement for GT | HTCs and PWH |
| Documentation of factor levels (one-stage/chromogenic) | Relevant for tapering of previous treatment | HTCs |
| Tapering of factor replacement | Relevant for the determination of the transgene expression | HTCs and PWH |
| Documentation of ALT levels | Early detection of major safety issue | HTCs |
| Dosage and duration of immunosuppressive treatment | Possibility to limit reduction of factor expression after ALT elevation | HTCs |
| Direct communication | Important for the success of the therapy | HTCs and PWH |
| Alarm safety system | Immediate reaction on safety problems is important for the success of the therapy | HTCs and PWH |
| Documentation of GT | Possibility to transfer data to national and international registries | HTCs and PWH |
- Abbreviations: ALT, alanine aminotransferase; GT, gene therapy of haemophilia, HTCs, haemophilia treatment centres, PWH, patients with haemophilia.
Another advantage of using electronic devices in GT is electronically provided and regularly updated checklists that can be viewed, confirmed and reviewed by all participants. There are several steps in GT, starting with initial verification of inclusion and exclusion criteria. Six steps of gene therapy are formulated below, for each of which completion of the previous step is the entry requirement (Table 2). All steps can be viewed and interrogated by all participants with the opportunity to view and prepare the requirements for the next steps.
| Step 1: Prerequisites for treatment |
| Initial requirement: Interest of the PWH in GT |
|
| Next step requirement: Informed consent of the patient and fulfilment of the inclusion criteria |
| Step 2: Preparation of GT (not applicable if it is the dosing centre itself) |
|
| Next step requirement: Preparation of GT completed |
| Step 3: Administration of GT |
|
| Next step requirement: Administration of GT completed |
| Step 4: Follow-up during the first year after treatment |
|
| Next step requirement: One-year follow-up completed |
| Step 5: Follow-up phase in the following years |
|
| Next step requirement: Loss of transgene expression and restart with factor replacement or subcutaneous treatment |
| Step 6: Restarting factor replacement therapy or subcutaneous treatment |
|
At the same time, data privacy and protection must be secured according to established standards and informed consent must be obtained from patients before using health data in electronic devices.
An important early task for HTC would be to follow and monitor the selection criteria for gene therapy. Up to now, most patients could not be treated with gene therapy, as in many cases exclusion criteria for gene therapy exist, e.g. the presence of pre-existing antibodies against AAV, age younger than 18 years or comorbidities.7
Administration of AAV vectors will induce innate immune response, followed by adaptive immune responses leading to the development of neutralising antibodies in all individuals, that will be present long-term. In addition, cytotoxic T-cell responses against the vector capsid may occur in 30–60% of patients,7 which may be associated with ALT elevation and reduction or loss of transgene expression. Although the loss of transgene expression and restart with factor replacement or subcutaneous treatment has not been systematically studied so far, this will play an important role in the future, as in one study of GT of haemophilia A the levels seem to decrease successively per year.1 According to recent recommendations, the trough levels should not fall below a range of 3–5 %, so that prophylactic therapy should be restarted at least from this range.18
2 CONCLUSION
Gene therapy for haemophilia is the most innovative approach to treating haemophilia to date, reducing both the burden of haemophilia and the burden of treating haemophilia. It presents a challenge to the existing structure of haemophilia centres and requires an improved and adapted form of collaboration and information sharing with the aim of maximising benefits and minimising risks.
The strength of a hub-and-spoke model of gene therapy management structures would be to provide standardised gene therapy treatment regardless of which centre the patients are located in.
In order to make the cooperation of all participants effective and to keep all participants up to date, the introduction of an electronic administration of GT data would be useful.
All those involved could be kept up to date, which would simplify consultation in the event of new problems and make it comprehensible for everyone. At the same time, checklists are provided in the electronic platform, which can be used to initiate the various steps of gene therapy that build on each other.
Other questions remain open, such as the question of data ownership. In order for the data to be subsequently passed on to other registries, the data should belong to the centre that entered it. The rights to view and record data (right to write) should also be clarified. It would also be important here to clarify the source data inflow for registers and to go into detail about the structure and prerequisites.
With the establishment of the prospective, longitudinal Gene Therapy Registry (GTR) of the Word Federation of Haemophilia (WFH) in close collaboration with scientific organisations and other patient organisations, long-term follow-up of all patients with GT can be ensured. This GTR includes data on demographics, medical/clinical history, gene therapy infusion details, safety, efficacy, patient-reported outcomes and mortality. Data will initially be collected every three months and annually one year after treatment. In order to use established data repositories, WFH will collaborate with existing data registries and individual treatment centres if collaboration with existing national gene therapy registries is not possible.
If successfully implemented and used, this system can contribute to the further spread of gene therapy worldwide and be a guarantor for its safe and effective use. Especially after approval, it will be crucial for further success and safety that there are binding criteria according to which gene therapy can be applied. As gene therapy for haemophilia is a rapidly developing field, there is also a need for adapted, more advanced and effective systems for safe use and data transfer. Hopefully, other rare bleeding disorders could also benefit from this strategy of sharing data.
ACKNOWLEDGEMENTS
Open Access funding enabled and organized by Projekt DEAL.
CONFLICTS OF INTEREST
WM received fees from Bayer, Biomarin, Biotest, CSL Behring, Chugai, Freeline, LFB, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, sobi, Takeda/Shire, uniQure.
HE received research grants from Bayer, CSL Behring, and Pfizer, and honoraria for advisory boards or speaker fees from Bayer, Biotest, CSL Behring, Novo Nordisk, Pfizer, Roche, Sobi and Takeda.
KH received honoraria for advisory boards or speaker fees from Bayer, Biotest, Chugai, CSL Behring, Novo Nordisk, Pfizer, Roche, Sobi, Takeda and research grants from Bayer, CSL Behring, Sobi and Pfizer.
SH has acted as paid consultant to Biomarin, Roche and Pfizer.
RK5 received honoraria from Bayer, Biomarin, Biotest, CSL Behring, Chugai, Novo Nordisk, Octapharma, Pfizer, Roche, Sanofi, Sobi, Takeda.
RK6 has acted as a paid consultant of Biomarin Deutschland GmbH.
CM received personal honoraria (consultancy, speaker, chair) from Bayer, Biotest, CSL Behring, NovoNordisk, Roche, Pfizer; unrestricted grant to institution from Biotest, CSL Behring; fees to institution for study participation from Bayer, Baxalta/Shire/Takeda, Biotest, CSL Behring, NovoNordisk, SOBI.
MO reports grants and honoraria for speaking and/or consulting from Bayer, Biotest, Takeda, CSL Behring, Octapharma, Pfizer, Roche, Sobi, NovoNordisk, Biomarin.
JO reports grants and personal fees from Bayer, personal fees from Biogen Idec, personal fees from Biomarin, grants and personal fees from Biotest, personal fees from Chugai, grants and personal fees from CSL Behring, personal fees from Freeline, personal fees from Grifols, personal fees from Novo Nordisk, grants and personal fees from Octapharma, grants and personal fees from Pfizer, personal fees from Roche, personal fees from Sanofi, personal fees from Sparks, grants and personal fees from SOBI, grants and personal fees from Takeda/Shire, outside the submitted work.
AT received research support from Pfizer and honoraria for lectures or consultancy from Biomarin and Pfizer.
AUTHOR CONTRIBUTIONS
The authors are members of the gene therapy working group of the GTH. WM produced the first draft of this manuscript, which was subsequently revised and finalized with all authors. All authors approved the final manuscript.
Open Research
DATA AVAILABILITY STATEMENT
None.




