Adherence to prophylaxis and its association with activation of self-management and treatment satisfaction
Abstract
Introduction
Prophylactic replacement therapy (prophylaxis) in patients with haemophilia (PWH) requires lifelong, frequent (self)infusions. Prophylaxis effectiveness depends on adherence, and the drivers of treatment adherence among PWH are unclear.
Aim
To quantify prophylaxis adherence and associations between adherence and patients’ treatment attitudes and satisfaction in a large cohort of children and adults with haemophilia.
Methods
In a nationwide, cross-sectional, questionnaire-based study, PWH with complete information currently using prophylaxis were selected. Validated Hemophilia Regimen Treatment Adherence Scale-Prophylaxis (VERITAS-Pro; normalised score range: 0–100, optimum 0) measured treatment adherence; the Patient Activation Measure (PAM-13; total score range 0–100, optimum 100) measured activation of self-management; Hemophilia Patient Satisfaction Scale (Hemo-Sat; range 0–100, optimum 0) measured treatment satisfaction. Groups were compared according to age (children: <12 years; adolescents: 12–18 years; adults >18 years) and adherence levels using non-parametric tests, and correlations were assessed using Spearman's rho.
Results
Among 321 participants (median age 33 years, interquartile range [IQR]:15–54 years), adherence was high (median VERITAS-Pro total score 17, 89% adherent) but worsened with age, with median scores of 5, 14 and 20 in children, adolescents, adults, respectively (p < .001). Attitudes towards treatment (median 66 vs. 68) participants and treatment satisfaction (12 vs. 10) were similar between adherent and non-adherent patients. The VERITAS-Pro total score was moderately correlated with PAM-13 (r = .41) but not with Hemo-Sat (r = −.11).
Discussion
Prophylaxis adherence was high (89%) but decreased significantly with age and was not correlated with treatment attitude or treatment satisfaction.
1 INTRODUCTION
Repeated bleeding events in the joints and soft tissues are hallmarks of haemophilia. Especially, patients with severe haemophilia (clotting factor VIII or IX activity ≤1%) are at high risk for these spontaneous bleeds.1 Prophylactic treatment is recommended for this specific group, consisting of intravenous self-infusions approximately 2–3 times/week.1 This demanding treatment typically starts before the age of 3 years.2 In young children, parents administer these injections at home, and by 12 years, children begin learning to administer the injections themselves (known as self-infusion).3 Adherence to prescribed treatment regimens is necessary to prevent bleeds in all age groups.1
In a previous study, 57% of Dutch patients with severe haemophilia (N = 241 patients) were reported to be adherent to their prescribed treatment.4 Other studies have reported adherence levels between 53%5 and 76% among haemophilia patients using other evaluation methods.6, 7 For example, adherence was determined by evaluating infusion logs, using the adherence definition described by Schrijvers et al.8 or performing short interviews. Most studies have been performed with limited datasets, varying from 317 to 785 patients. Some have reported decreased adherence levels starting at puberty,9, 10 whereas others have not.5
Reasons for (non-)adherence in patients with haemophilia (PWH) were explored in two qualitative studies.11, 12 Among adults, the perception of adherence and the ability to perform prophylaxis were the primary contributors to (non-)adherence.11 Among adolescents and young adults, the level of treatment responsibility and the estimated risk per activity had strong influences on adherence.12 Reasons for (non-)adherence in PWH were explored in t quantitative European study (N = 180) demonstrated that longer visits to the treatment centre and good relationships with the treatment staff were associated with better treatment adherence.13 To date, whether adherence and satisfaction are related remains unknown. While several studies in cystic fibrosis and in general chronically ill patients have suggested that patients’ level of activation of self-management [measured using the patient activation measure (PAM-13)] might impact health conditions, including adherence.14, 15 Based on these reports, we hypothesised that (1) adherent patients would show a higher level of activation of self-management, and (2) adherent patients would be more satisfied with their treatment.
The aim of this study was to quantify adherence to prophylaxis and assess its association with activation of self-management and treatment satisfaction in a large cohort of children and adults with haemophilia.
2 METHODS
A cross-sectional, web-based survey was conducted among all Dutch PWH. The ‘Haemophilia in the Netherland's (HIN-6) nationwide survey, an initiative of the Leiden University Medical Centre, the Netherlands. This is the sixth version of this survey which started 50 years ago.16 Medical ethical approval was obtained from the Medical Ethical Committee Leiden, Den Haag, Delft under number NL59114.058.17.
2.1 Population
Male PWH from all six Dutch treatment centres were invited to participate in a national, multi-centre, cross-sectional study. In 2019, patients received an e-mail (and reminder e-mails) containing a link to the HIN-6 survey (paper version available on request). This e-mail was sent on behalf of the physicians of their own treatment centre. It was described that the survey was part of a study, all data would be pseudonymised and therefore not usable for daily care, and participation was voluntary. Participants who did not sign informed consent for blood and urine collection but did complete the questionnaire were considered as having consented to participate (opt-in inclusion). For this particular study, patients who were prescribed prophylactic treatment who completed at least one domain of the VERITAS-Pro questionnaire were included from the HIN-6 database.
2.2 Data collection
The HIN-6 study questionnaire included several validated questionnaires (eg adherence, quality of life, sport, work, satisfaction). The questions were adapted to the respondents’ age: (1) the parents of children with haemophilia (0–11 years), adolescents (12–18 years) and adults (>18 years). The parent version included the Hemo-Sat questionnaire, and the adult version included both the PAM-13 and Hemo-Sat questionnaires. Completing all questionnaires took approximately 30–60 min, and intermediate pausing or stopping was allowed. Data were pseudonymised and coded. Additionally, research nurses extracted treatment characteristics from medical files from those who signed informed consent for blood and urine (eg age, diagnoses, treatment details, concomitant infections). All data were collected using an online case report form (via Castor EDC); paper version available on request. This system kept track of percentage of questions completed and was programmed to send reminders. For this study, the following variables were analysed: demographic characteristics, the adherence17 questionnaire, level of activation of self-management questionnaire14 and the treatment satisfaction questionnaire.18
2.2.1 Adherence
The Dutch version of the validated VERITAS-Pro was used to assess prophylaxis adherence over the past 2 weeks.17 This haemophilia-specific questionnaire consists of 24-items, generating a total score and six domain scores: Time (ie scheduled days and times per week), Dose (ie increase or decrease in dose), Plan (ie supplies at home), Remember (ie forget or remember infusions), Skip (ie skip or postpone infusions) and Communicate (ie contacting the treatment centre in case of bleeds or medical interventions). The VERITAS-Pro can be used as a continuous variable (normalised to 0–100, optimum 0) or as a categorical variable (adherent/non-adherent, cut-off normalised score ≥3419). Cronbach's alpha for the VERITAS-Pro was α = .70.
2.2.2 Adherence, according to the consensus definition
In addition to the VERITAS-Pro score, adherence was assessed according to the consensus definition reported by Schrijvers et al.8 Normally, the three components of this definition (ie missed infusions, dose changes and changes in timing of infusions) are assessed in person during a clinic visit. In this case, this was done by selecting and separately analysing three VERITAS-Pro questions. VERITAS-Pro question 2 represents the domain of missed infusions, question 5 represents dose changes, and question 3 represents time changes.
2.2.3 Patients’ activation of self-management
The Dutch version of the validated generic PAM-13 was used to assess motivators, attitudes, behaviours towards the patients’ illness, referred to activation of self-management.14 The questionnaire consists of 13-items resulting in a 0–100 (optimum 100) score. In addition, patients can be categorised into four levels: (1) disengaged and overwhelmed; (2) becoming aware, but still struggling; (3) taking action; and (4) maintaining behaviours and pushing further.17, 20 Cronbach's alpha for the PAM-13 was 0.87.14 Only adult participants completed the PAM-13.
2.2.4 Patient satisfaction
The Dutch version of the Hemo-Sat15, 16 was designed to assess patient satisfaction with treatment. The Hemo-Sat is a haemophilia-specific instrument consisting of a total score and six domains: Ease and Convenience, Efficacy, Burden, Specialist/Nurse, Centre Hospital and General Satisfaction. The questionnaire consists of 32 questions, measured on a scale of 0–100 (optimum 0). Cronbach's alpha for the Hemo-Sat was 0.85.15 Only parents of patients and adult patients completed the Hemo-Sat.
2.3 Data analysis
Only patients who completed one domain of the VERITAS-pro were selected for this study. All data analyses for each questionnaire were performed according to the following age groups: children (0–11 years), adolescents (12–18 years) and adults (>18 years). Missing data were excluded from the analysis. The VERITAS-Pro total and domain scores were normalised [(total score −24)/96 × 100% and (domain score −4)/16 × 100%]. In the HIN6 study questionnaire for children, one question for the domain ‘Communicate’ was erroneously omitted. Normalised total scores for children were calculated for the remaining questions (23 questions instead of 24). Patients were divided into adherent or non-adherent groups based on the VERITAS-Pro total score (normalised cut-off value for non-adherence: ≥34).10 The data were not normally distributed; therefore, the differences between 3 groups were tested using the Kruskal-Wallis test.
To assess adherence according to the consensus definition of Schrijvers et al., three questions were selected from the VERITAS-Pro: (1) ‘I infuse the recommended number of times per week’; (2) ‘I use the doctor-recommended dose for infusions’; and (3) ‘I perform prophylaxis infusions in the morning, as recommended’. Patients were categorised as adherent (always), suboptimal (often), or non-adherent (Sometimes, Rarely, and Never) based on the response to each question. For this specific analysis, only those who completed this specific question were selected for this analysis. To analyse patient activation, adult patients who completed the entire PAM-13 questionnaire were selected. The PAM-13 total scores and corresponding levels were calculated by ‘Insignia Health’, the developers of the PAM-13.20 Patient characteristics were analysed using descriptive statistics and presented as the median and interquartile range (IQR, 25th percentile–75th percentile). The relationship between adherence and the PAM-13 or Hemo-Sat scores was analysed using the Mann-Whitney U test, and the relationship between the PAM-13 levels (1–4) and adherence was analysed using Pearson's correlation. Additionally, the correlations between the VERITAS-Pro and PAM-13 or Hemo-Sat scores were analysed using Spearman's rho. Significant correlation coefficients above 0.4 were considered clinically relevant.20
3 RESULTS
The study included 61 children, 29 adolescents and 321 adults who had completed the Veritas-Pro questionnaire (278 complete all domains and 2 at least one domain). Patient characteristics are shown in Table 1. The median overall age was 33 years (IQR: 15–54 years), for children was 6 years (IQR: 4–9 years), for adolescents was 14 years (IQR: 13–16 years), and for adults was 47 years (IQR: 31–59 years). Most patients were diagnosed with haemophilia A (87%) and were distributed equally across all age groups. The majority had a severe phenotype (90%). Overall, patients with haemophilia B were prescribed higher doses per injection (median 25 IU/kg vs. 16 IU/kg in haemophilia A) with lower frequencies per week (median 2×/week vs. 3×/week in haemophilia A).
|
All N = 321 |
Children (0–11 year) N = 61 |
Adolescents (12–18 year) N = 29 |
Adults (>18 year) N = 231 |
|
|---|---|---|---|---|
| Median (IQR) or N (%) | ||||
| Age (years) | 33 (15–54) | 6 (4–9) | 14 (13–16) | 47 (31–59) |
| Weight (kg) | 74 (56–87) | 23 (17–32) | 56 (48–64) | 82 (72–90) |
| Haemophilia A | 279 (87%) | 52 (84%) | 23 (80%) | 204 (88%) |
| Severe (A and B) | 290 (90%) | 54 (87%) | 28 (97%) | 208 (90%) |
| Moderate (A and B) | 20 (6%) | 6 (10%) | 1 (3%) | 13 (6%) |
| Dose per infusion (IU/kg) | ||||
| Haemophilia A | 16 (13–24) | 28 (22–40) | 17 (13–22) | 14 (12–20) |
| Haemophilia B | 25 (16–38) | 43 (29–56) | 28 (23–41) | 23 (14–30) |
| Infusion frequency per week | ||||
| Haemophilia A | 3 (2–3) | 3 (2–3) | 3 (3–3) | 3 (2–3) |
| Haemophilia B | 2 (1–3) | 2 (1–2) | 2 (1–2) | 3 (1–4) |
| Self-infusion | 222 (69%) | 3 (5%) | 22 (76%) | 197 (85%) |
| Positive inhibitor (Both history of inhibitor or current) | 49 (15%) | 12 (19%) | 2 (7%) | 35 (15%) |
| HIV history | 21 (7%) | a | a | 21 (9%) |
| HCV history | 125 (39%) | a | a | 125 (54%) |
- Abbreviations: HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range.
- a Not applicable for this age group.
3.1 Adherence
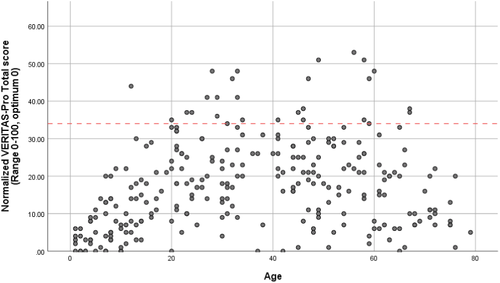
Adherence scores according to age are shown in Table 2. Overall, prophylaxis adherence was high (89% adherent, median total score of 17, IQR: 8–25), and only 11% were defined as non-adherent. Participants with haemophilia B (N = 42, 13%) showed a trend towards better adherence than participants with haemophilia A (score: 17 vs. 13, p = .34). Figure 1 shows a non-linear association between adherence and age group: adherence was best (lowest score) among the very young and deteriorated with increasing age (p < .01). In children, 0% were non-adherent, in adolescents 3% and in adults 15%. The top 3 domains with the highest non-adherence were Communicate (39% non-adherent), Plan (32%) and Dose (23%).
|
All N = 321 |
% Non-adherenta |
Children N = 61 |
% Non-adherenta |
Adolescents N = 29 |
% Non-adherenta |
Adults N = 231 |
% Non-adherenta | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) or (%) | |||||||||
| Total score | 17 (8–25) | 11%‡ | 5 (2–10) | 0% | 14 (8–19) | 3% | 20 (11–28) | 15% | ≤.00 |
| Time | 19 (0–31) | 12% | 6 (0–19) | 0% | 18 (0–25) | 7% | 25 (6–39) | 16% | ≤.00 |
| Dose | 6 (0–13) | 23% | 0 (0–6) | 7% | 0 (0–13) | 10% | 6 (0–19) | 29% | ≤.00 |
| Plan | 25(6–38) | 32% | 19 (0–25) | 23% | 25 (14–25) | 21% | 25 (13–38) | 36% | .02 |
| Remember | 19 (0–31) | 15% | 0 (0–6) | 3% | 19 (6–31) | 17% | 18 (6–38) | 18% | ≤.00 |
| Skip | 6 (0–25) | 9% | 0 (0–6) | 0% | 0 (0–13) | 7% | 13 (0–25) | 11% | ≤.00 |
| Communicate | 38 (19–50) | 39% | b | b | 25 (6–38) | 28% | 38 (19–56) | 52% | .02 |
Note
- Total score significantly different between all age groups, and significant differences between children and adults were observed for all domains.
- Abbreviations: IQR, interquartile range; VERITAS-Pro, Validated Hemophilia Regimen Treatment Adherence Scale-Prophylaxis.
- a Percentages are based on the total group. For example (‡), 11% of the N = 321 patients were non-adherent (above the cut-off score), based on total VERITAS-Pro score.
- b Not able to analyse because ≥25% missing data.
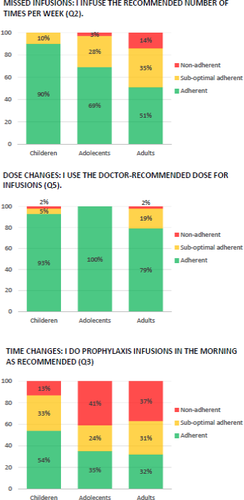
3.2 Adherence, according to the consensus definition
Adherence was evaluated based on three questions selected from VERITAS-Pro. In response to ‘I infuse the recommended number of times per week’, 0% of children, 3% of adolescents and 14% of adults were non-adherent, which was comparable to the non-adherence percentages determined using the VERITAS-Pro total score (0%, 3%, and 15%, respectively). In response to ‘I use the doctor-recommended dose for infusions’, 2% of children, 0% of adolescents and 2% of adults were non-adherent, which was lower for adolescents and adults than their respective VERITAS-Pro total scores. In response to ‘I perform prophylaxis infusions in the morning, as recommended’, 13% of children, 41% of adolescents and 37% of adults were non-adherent. Figure 2 shows adherence evaluated according to the consensus definition. For all subgroups, the percentages of non-adherence were higher than the percentages suggested by the VERITAS-Pro total score.
3.3 Patients’ activation of self-management
In total, 181 (78%) adults completed the PAM-13 questionnaire. The median PAM-13 score among the total population was 66 (IQR: 53–75). Patients who were scored as adherent were primarily classified into PAM Levels 3 (34% taking action) and 4 (38% maintaining behaviours and pushing further). Unexpectedly, non-adherent patients were also primarily classified into PAM Levels 3 and 4 (level 3: 47%; level 4: 30%; p = .40). Patients’ activation of self-management according to adherence is shown in Table 3, and a correlation table can be found in the Appendix 1. The PAM-13 scores (0–100) were similar between adherent and non-adherent groups (66 vs. 68, p = .83) and were not significantly correlated (r = .41, p = .6) with the VERITAS-Pro total scores. None of the VERITAS-Pro domain scores correlated with the PAM scores (r values between −.12 and .11), all p > .05. The correlation table can be found in Appendix 1.
| Patients’ activation of self-management (PAM-13) | Total | Adherent | Non-adherent | Diff between score and crosstabs* (p-value) | |
|---|---|---|---|---|---|
| N = 181 (100%) | N = 151 (100%) | N = 30 (100%) | |||
| PAM−13 Scorea (median, IQR) | 66 (53–75) | 66 (53–78) | 68 (57–73) | .83 | |

|
Level 1: Disengaged and overwhelmed (N, %) | 12 (6%) | 2% | 10% | .40 |
| Level 2: Become aware, but still struggling (N, %) | 37 (20%) | 22% | 13% | ||
| Level 3: Taking action (N, %) | 66 (37%) | 34% | 47% | ||
| Level 4: Maintaining behaviours and pushing further (N, %) | 66 (37%) | 38% | 30% | ||
| Treatment satisfaction (median, IQR) | N = 208 | N = 180 | N = 28 | ||
| Total score | 12 (6–21) | 12 (5–20) | 10 (5–21) | .57 | |
| Ease and convenience | 15 (15–28) | 15 (5–27) | 10 (5–23) | .37 | |
| Efficacy | 21 (8–33) | 21 (8–33) | 17 (13–33) | .91 | |
| Burden | 13 (5–31) | 13 (6–31) | 6 (0–31) | .33 | |
| Specialist/Nurse | 0 (0–13) | 4 (0–14) | 0 (0–31) | .31 | |
| Centre hospital | 0 (0–13) | 0 (0–13) | 7 (0–13) | .39 | |
| General satisfaction | 0 (0–13) | 0 (0–13) | 0 (0–13) | .79 | |
- Abbreviations: IQR, Interquartile range; PAM-13, Patient Activation Measure.
- a Interpretation of PAM-13: Each point increase in the PAM-13 score correlates to a 2% increase in medication adherence.
3.4 Adherence and treatment satisfaction
Overall, patients reported high treatment satisfaction (median Hemo-Sat: 12, IQR: 6–21). In particular, the domains Specialist/Nurse, Centre Hospital and General Satisfaction had maximum scores (median: 0; IQR: 0–13). Treatment satisfaction was similar between adherent and non-adherent groups for both total score (median 12 vs. 10) and the domain scores. Treatment satisfaction according to adherence is shown in Table 3. Adults were significantly more satisfied with their treatment than parents of children (median 16 in children vs. 10 in adults, p = .01). No correlation between VERITAS-Pro total scores and Hemo-Sat total scores was observed (r = −.11), nor between any VERITAS-Pro domain scores and Hemo-Sat total scores (r between −.13 and .08). The correlation table can be found in Appendix 1.
4 DISCUSSION
The aim of this study was to quantify prophylaxis adherence and examine the association between adherence and Patients’ activation of self-management or satisfaction in a large cohort of children and adults with haemophilia. We hypothesised that (1) adherent patients would show a higher level of activation of self-management, and (2) adherent patients would be more satisfied with their treatment. Overall, Dutch PWH reported high adherence to prescribed treatment (89%). Adherence levels decreased significantly among older patients. The Patients’ activation of self-management (PAM-13 score) was similar between adherent and non-adherent patients. Both groups showed high levels of patients’ activation of self-management: with the majority categorised as level 3 (taking action) or 4 (maintaining behaviours and pushing further), which indicated that non-adherent patients make conscious choices regarding their treatment. Overall treatment satisfaction was high and showed no association with adherence. In summary, based on the hypothesis, it can be stated that (1) non-adherent patients showed similar patients’ activation of self-management as adherent patients and (2) treatment satisfaction was similar between adherent and non-adherent groups.
The HIN-6 is a repeated nationwide study associated with several limitations, first, the potential risk of selection bias exists, as the selection of patients on prophylaxis may potentially result in the selection of more adherent patients who took the time to complete the questionnaires. However, this potential bias is not expected to affect the association between adherence with treatment activation of self-management. Secondly, all outcomes were self-reported, which could be associated with a risk of under- or overreporting.6, 21 Underreporting by patients is a well-known phenomenon, particularly for adherence.6, 21 Therefore, actual adherence levels could be higher than those reported. Thirdly, it cannot be ruled out that some patients used extended half-life (EHL) time products (while participating in a clinical study) at the time of the data collection. At the moment, it is unknown if EHL products impact adherence. However, the VERITAs-Pro is expected to be useful regardless the type of concentrate used. Finally, three questions of the VERITAS-Pro were separate analysed. In daily care, the VERITAS-Pro is too long for a quick first impression as a guideline for a conversation. A selection of 3 questions addressing the 3 components of the definition of adherence is less onerous and can also be done as a first impression and guideline for a conversation.
Overall, the VERITAS-Pro scores reported in the present study were similar to those in earlier reports by Duncan et al.19 (mean score 18), who examined 67 patients with a mean age of 15 years (standard deviation [SD]: 12.7). The domain with the best adherence was Dose (mean: 9, range: 0–63) and the domain with the worst adherence was Time (mean: 22, range; 0–75).19 The VERITAS-Pro total and domain scores reported for the Dutch validation study were comparable to those reported in our sample for similar age groups (children and adolescents). Lock et al. reported a median total score of 13 (IQR: 5–18) among 60 children with a mean age of 10 years (SD: 4).17 Patients were reported to be most adherent for the domain Skip (median: 0, IQR: 0–13) but the least adherent for the domain Plan (median: 19, IQR: 0–31).17
This current study reported a significant difference in adherence between all age groups: children, adolescents and adults. Other studies have previously reported a significant difference between young children and adolescents,9, 22 which may be associated with the finding that patients begin learning how to infuse themselves around the age of 12 years.3 To the best of our knowledge, this study is the first to evaluate patients’ activation of self-management using the PAM-13 questionnaire in PWH. In the present study, both adherent and non-adherent patients were primarily categorised to PAM levels 3 and 4. In addition, this current study failed to identify any correlation between adherence and PAM-13 scores (r = .41; p = .6. These results are not comparable with those reported for several other studies that examined the association between PAM-13 scores and adherence in other chronic disorders.21, 23, 24 A possible explanation for this could be the difference in study design (RCT or Pre- to post-test) and use of an different method to assess adherence. Only one study used a comparable study design as our study. Jie Gao et al.24 studied adults with cystic fibrosis (N = 64) who were categorised into two levels of adherence. Non-adherent patients were typically categorised to Levels 3 (41%) and 2 (32%), whereas adherent patients were categorised to Levels 3 (64%) and 4 (27%).24 Other studies have reported low correlations between adherence questionnaire and PAM-13 scores (R = −.1823 and R = .1525 ).
Based on qualitative studies examining PWH (adults and adolescents),11, 12 we hypothesised that some patients are intentionally non-adherent, making the conscious choice not to administer prophylaxis. Based on the descriptions for the PAM-13 questionnaire levels, this hypothesis would be compatible with levels 3 (taking action) and 4 (maintaining behaviours and pushing further). Others are unintentionally or subconsciously non-adherent, which would be compatible with Levels 1 (disengaged and overwhelmed) and 2 (becoming aware but still struggling). However, this is not corroborated by the findings of the present study. Therefore, most haemophilia patients appear to be making a conscious choice to be non-adherent to their treatment regimen. Based on previous qualitative research,12 we suggest that this non-adherence might be caused by not experiencing bleeds after skipping or forgetting prophylaxis, which impacts patients’ estimations of the risks associated with skipping prophylaxis.
This study results showed that adherence among Dutch PWH is high and that both adherent and non-adherent patients have a high level of self-management and high satisfaction with their treatment. However, some patients were non-adherent to their prescribed treatment regimens, with high activation of self-management, which suggested that they were making the conscious choice to be non-adherent. Haemophilia nurses and treaters might overestimate their patients’ adherence levels. Therefore, haemophilia nurses and treaters should be aware that even knowledgeable patients with a high activation of self-management can be non-adherent and at risk for bleeds and joint damage. An open and non-judgmental conversation should enable assessment of patient's reasons for adherent or non-adherent behaviours. Motivational interviewing can be applied as a useful conversation technique, characterised by open questions, affirmation, reflective listening and summary reflections.26, 27 If non-adherence is associated with disease acceptance (in terms of prophylaxis or related complication), a cognitive behavioural intervention may be appropriate.28-30 Preliminary reports on haemophilia-specific acceptance and commitment training have shown promising results.31 The present study used quantitative research methods to test hypotheses based on the findings of qualitative research. Contrary to expectations, the hypotheses were not confirmed.
5 CONCLUSION
This paper describes high prophylaxis adherence levels among children, adolescents and adults with haemophilia. Overall, adherence decreased among older patients. Both adherent and non-adherent patients were generally categorised as PAM-13 scale levels 3 and 4, indicating that patients generally have high activation of self-management, and non-adherence likely represents a deliberate choice. No correlations between the adherence questionnaire (VERITAS-Pro) scores and the scores for the activation of self-management (PAM-13) or treatment satisfaction (Hemo-Sat) questionnaires were observed.
ACKNOWLEDGEMENTS
This letter was written on behalf of the Haemophilia in The Netherlands 6 Steering Group.
CONFLICT OF INTEREST
J.H.: has not declared any conflicts of interest. L.S.: has not declared any conflicts of interest. F.L. has received unrestricted research grants from CSL Behring, Takeda and uniQure, and is consultant for Takeda, uniQure, and Biomarin. He is DSMB member for a study sponsored by Roche. JE has received research support from CSL Behring and honorarium for educational activities of Roche and Celgene (all paid to the institute). SS has been paid for participation in an advisory board of NovoNordisk and she received a grant to participate in a masterclass of Takeda, both in 2020. CS has not declared any conflicts of interest. S.G. has received unrestricted research grants from Sobi. K.F. has received speaker's fees from Bayer, Baxter/Shire, Biotest, CSL Behring, Octapharma, Pfizer, NovoNordisk; performed consultancy for Baxter/Shire, Biogen, CSL Behring, Freeline, NovoNordisk, Pfizer, Roche and SOBI; and has received research support from Bayer, Pfizer, Baxter/Shire, and Novo Nordisk.
APPENDIX 1
Spearman's rho correlations between adherence (VERITAS-Pro) and activation of self-management (PAM-13) or treatment satisfaction (Hemo-Sat)
| Activation of self-management | Treatment satisfaction | ||
|---|---|---|---|
| Pam-13 score (0–100) | Pam-13 level (1–4) | Hemo-sat total score | |
| Adherence | |||
| Total score | 0.41 | −0.00 | −0.11 |
| Time | 0.03 | −0.00 | 0.01 |
| Dose | −0.12 | −0.14 | 0.03 |
| Plan | 0.04 | 0.02 | 0.08 |
| Remember | 0.11 | 0.05 | −0.13 |
| Skip | 0.09 | 0.06 | −0.6 |
| Communicate | −0.02 | −0.03 | −0.02 |
- None of the correlations reached significance at the 0.05 level (2-tailed).
- Abbreviations: VERITAS-Pro, Validated Hemophilia Regimen Treatment Adherence Scale-Prophylaxis; PAM-13, Patient Activation Measure; Hemo-Sat, Hemophelia Patient Satisfaction Scale.
Open Research
DATA AVAILABILITY STATEMENT
Data is available upon request.




