Clinical evaluation of bleeds and response to haemostatic treatment in patients with acquired haemophilia: A global expert consensus statement
Abstract
Background
Acquired haemophilia (AH) is a rare bleeding disorder with significant morbidity and mortality. Most patients initially present to physicians without experience of the disease, delaying diagnosis and potentially worsening outcomes. Existing guidance in AH is limited to clinical opinion of few experts and does not address monitoring bleeds in specific anatomical locations.
Aim
Derive consensus from a large sample of experts around the world in monitoring bleeding patients with AH.
Methods
Using the Delphi methodology, a structured survey, designed to derive consensus on how to monitor bleeding patients with AH, was developed by a steering committee for completion by a group of haematologists with an interest in AH. Consensus was defined as ≥75% agreement with a given survey statement. After three rounds of survey refinement, a final list of consensus statements was compiled.
Results
Thirty-six global specialists in AH participated. The participants spanned 20 countries and had treated a median of 12.0 (range, 1-50) patients with AH within the preceding 5 years. Consensus was achieved in all items after three survey rounds. In addition to statements on general management of bleeding patients, consensus statements in the following areas were presented: urinary tract, gastrointestinal tract, muscles, skin, joints, nose, pharynx, mouth, intracranial and postpartum.
Conclusions
Here, we present consensus statements derived from a broad sample of global specialists to address monitoring of location-specific bleeds and evaluating efficacy of bleeding treatment in patients with AH. These statements could be applied in practice by treating physicians and validated by individual population surveys.
1 INTRODUCTION
Acquired haemophilia (AH) is a bleeding disorder caused by the development of anticoagulation factor VIII (FVIII) autoantibodies.1 It is very rare with an incidence of approximately 1.3-1.5 per million people per year, and most commonly occurs in older adults with a median age of 70-78 years.2-6 AH has a high mortality, ranging from 20% to 33% in the literature with deaths specifically related to bleeds ranging from 3.5% to 9%.2, 3, 5, 7 Most often, mortality is not directly linked to the inhibitor itself, but to the underlying disease or secondary causes, such as infectious complications in patients receiving immunosuppression.3, 5, 7 However, eradication of the inhibitor is linked to a significantly better clinical outcome.7 Treatment of the underlying condition may also affect treatment outcome of AH, as demonstrated in a recent systematic review of patients with AH and cancer that responded better to AH treatment with simultaneous eradication of the malignancy.8
The diagnosis of AH is complex, requiring informed clinical assessment and specialist laboratory facilities. In around 50% of cases, it is idiopathic, with the remaining patients having an identified predisposing condition, most commonly autoimmune disease, malignancy, pregnancy and certain drugs.3-6 Patients usually present with spontaneous bleeds, which may occur anywhere in the body, but most commonly in the subcutaneous, intramuscular or mucosal regions, and less commonly intracranially or in joints (the latter type of bleeds is reported in <5% of patients)4; postoperative bleeds are also observed.2-6 The bleeding phenotype can be devastating, with severe, life-threatening bleeds occurring in over 80% of patients.5, 7 Laboratory confirmation of AH is made based on a prolonged activated partial thromboplastin time (aPTT) without correction after mixing tests following incubation, an isolated low FVIII and a quantified inhibitor titre.1 Due to the potential high morbidity and mortality, it is important that diagnosis and management of AH is undertaken quickly. It has been demonstrated in a recent European Acquired Hemophilia Registry (EACH2) analysis that delayed diagnosis has a significant impact on timing of treatment initiation in 33.5% of 501 enrolled patients.4 While it did not affect patient outcome in this study, it was acknowledged that patients were still at increased risk of fatal bleeds until eradication of the autoantibody.3, 4
Diagnostic delay in AH is almost certainly due to a lack of timely recognition. Due to its rarity, many physicians (including haematologists) never encounter AH.9 There are only a small number of specialists experienced in diagnosis and treatment of this condition, and they are based almost exclusively in academic tertiary centres. Thus, most patients with AH initially present to non-specialists with no prior experience of AH, which can lead to delayed diagnosis and inappropriate treatment.10 It has been demonstrated that a knowledge and clinical management gap exists among physicians when presented with a bleeding patient with an increased aPTT. In these instances, it has been demonstrated that physicians may not consider a rare bleeding disorder or consult a haematologist, particularly in emergency and critical care, leading to adverse patient outcomes.9 Furthermore, the bleeding pattern is different from congenital haemophilia as the severity does not correlate with level of FVIII or inhibitor titre, further complicating diagnosis.1
Due to its rarity, diagnostic and treatment guidelines rely on registry data and expert advice, with current guidance in the form of limited narrative literature reviews, expert panel recommendations based on literature searches, and ‘how I treat' articles.10-19 While providing an excellent resource on diagnosis and treatment, existing guidance is limited in its methodology, being based largely on clinical opinion of few experts with a relatively narrow geographical spread and derived from relatively unstructured methods. Furthermore, the existing guidance lacks focus on clinical assessment of bleeding in specified anatomical locations and the evaluation of the treatment approach utilized in relation to these location-specific bleeds. This is especially important as many physicians will never have encountered these types of clinical scenarios.
Here, we add to the existing literature by presenting the results from a study using Delphi methodology, utilizing a large group of specialists from around the world, to derive consensus in the location-specific management of bleeding in AH. The specific aims of the study were to: develop a deeper insight into expert opinion on clinical evaluation of bleeds and response to haemostatic treatment; determine areas of consensus, points for further discussion and important gaps in patient assessment/evaluation; and assess further steps required to develop clinical guidance on diagnosis and monitoring of patients with AH.
2 MATERIALS AND METHODS
2.1 The Delphi methodology
The Delphi methodology was selected as a framework to derive a consensus on haemostatic management of individuals with AH, among specialists in the field. The Delphi methodology uses a series of surveys distributed to a group of individuals to ascertain consensus on statements relating to a domain of expertise.20, 21 The process employs three survey rounds, and each participant is provided with the collated feedback of the entire participating group at the end of each round. After three successive survey rounds, a definitive list of statements that did and did not derive consensus is generated.21 The Delphi methodology has previously been used in the field of haemophilia to gain consensus in defining immune tolerance induction failure, standardizing definitions used in haemophilia care, assessing risk of FVIII immunogenicity associated with switching factor replacement products, management of acute coronary syndrome in patients with haemophilia and defining target plasma blood clotting factor levels for use in different clinical scenarios.22-26
2.2 Development of the initial survey
A steering committee composed of internationally renowned haematologists with an interest and experience in AH, recommended by the lead investigators, developed the initial survey based on current knowledge in the field. The initial survey was circulated via email for refinement and was formally corroborated at a face-to-face meeting. It was designed to ascertain consensus on who should treat bleeding patients with AH and to determine indicators of treatment effectiveness, worsening condition, intervals of assessment and defining response to treatment for location-specific bleeds. The questions in these areas were constructed to allow subsequent design of consensus statements for Round 2. Bleeds in the following sites were addressed in the survey: urinary tract, gastrointestinal tract, muscles, skin, joints, nose, pharynx, mouth, central nervous system (intracranial) and female genital tract (postpartum).
2.3 Defining consensus
Consensus was determined based on the proportion of received responses that ‘strongly agreed' or ‘agreed' with a given statement, or the percentage of participants selecting a given answer from a multiple-choice question. The strength of consensus was defined as follows21, 27: strong consensus (≥90% agreement), consensus (75% to <90% agreement), majority approval (50% to <75% agreement), and no consensus (<50% agreement). For questions in the Round 1 survey requiring selection of a single item, consensus was not specifically determined but responses gaining above one-third (33.3%) of votes were taken forward to form a subsequent statement for inclusion in the Round 2 survey, where consensus would be determined per the above. All items in Rounds 2 and 3 contained statements seeking strength of agreement.
2.4 Survey distribution and refinement
At each survey completion round, the survey was distributed to participants via a purpose-built online tool (literatureconsensusportal.eu, Forga Solutions) with an initial 2 weeks for participants to complete the survey. The review round was considered complete once ≥75% of the participant group had participated. Upon completion of the survey review round, the data were processed, collated and the strength of consensus determined. Statements achieving consensus were noted and excluded from the next refinement round. Statements that did not achieve consensus were further refined based on qualitative responses and then included in the subsequent survey round. After three survey rounds, all statements achieving consensus were consolidated.
Due to the relatively low numbers of specialists in AH, it was deemed necessary to maximize the number of participants included in each round of survey completion so that crucial expertise was not excluded from the process. Therefore, participants not able to complete the first survey were still eligible to complete the survey at its second iteration in Round 2. Members of the steering committee were also required to participate.
2.5 Recruitment
Participant recruitment was conducted based on international specialists known to, and nominated by, the steering committee. Each steering committee member provided a list of haematologists known in the AH field, and the face-to-face meeting provided a platform for evaluating and ratifying the final list of potential participants. This approach was chosen due to the relatively low numbers of haematologists with experience in AH globally, and it ensured a wide geographical spread of participants. As current guidance in AH is for referral of patients to an expert centre,10 it was assumed that expertise in managing AH would not be found outside of such facilities. All individuals that participated in the study did so on a voluntary basis.
2.6 Statistical analysis
For each survey round, the consensus for each statement was determined by calculating the percentage of participants who agreed with the statement. Descriptive statistical analysis was undertaken to derive means and standard deviations.
3 RESULTS
3.1 Participant characteristics
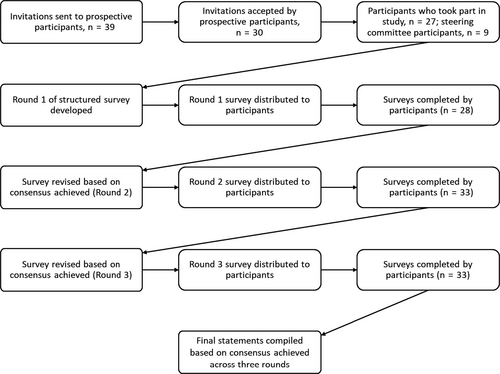
Thirty-nine haematologists were invited to take part in the survey, of whom 30 responded and 27 subsequently volunteered to participate (Figure 1). Including members of the steering committee, who were also required to take part in the survey, the total number of participants in the study was 36. The total number of specialists who participated across each survey round was 28 (78%), 33 (92%) and 32 (89%) in Rounds 1, 2 and 3, respectively. There was a wide geographical spread in participants with 20 countries represented (Austria, Brazil, Canada, China, Croatia, Egypt, France, Germany, Hungary, Italy, Japan, New Zealand, Poland, Saudi Arabia, Singapore, Slovak Republic, Spain, Thailand, United Kingdom and United States). Background information on the participants is shown in Table 1. The main area of practice within haematology was varied with most participants (62%) focusing on haemophilia/bleeding disorders. The median (range) number of patients diagnosed with and treated for AH over a 5-year period was 11.0 (1-49) and 12.0 (1-50), respectively. Access to laboratory and clinical tests varied, but all participants had access to aPTT, aPTT 1:1 mixing and FVIII level assays, and all participants had 24-hour access to a laboratory service at their institutions.
| Main area of practice | |
| General haematology | 14 (41) |
| Haemato-oncology | 4 (12) |
| General thrombosis and haemostasis | 18 (53) |
| Haemophilia/bleeding disorders | 21 (62) |
| Laboratory haematology | 8 (24) |
| Number of patients with AH diagnosed in past 5 y, median (range) | 11.0 (1-49) |
| Number of patients with AH treated in past 5 y, median (range) | 12.0 (1-50) |
| Number of patients managed by telephone only, median (range) | 0.0 (0-10) |
| Access to following laboratory assess/tests | |
| aPTT | 34 (100) |
| aPTT mixing test | 34 (100) |
| FVIII level assay | 34 (100) |
| Bethesda assay | 32 (94) |
| Anti-porcine FVIII antibody | 14 (41) |
| Ultrasound | 32 (94) |
| MRI | 30 (88) |
| CT | 32 (94) |
| Endoscopy | 31 (91) |
| Urinalysis | 33 (97) |
| Complete blood count | 33 (97) |
| Access to laboratory testing 24 h/day 7 d/wk | 34 (100) |
- Abbreviations: AH, acquired haemophilia; aPTT, activated partial thromboplastin time; CT, computed tomography; MRI, magnetic resonance imaging.
- a Two participants were retired. Data are presented as n (%) unless otherwise stated.
3.2 Deriving a consensus across three survey rounds
Table 2 shows the final consensus statements derived from the three survey rounds. The first of these rounds resulted in consensus achieved for 2/4 statements on initial management (Item 1) with two statements revised for Round 2, based on qualitative responses. In the first round, three of 28 participants disagreed that ‘Emergency Department (ED) doctors should commence treatment for AH if specialist consultation is not immediately available' with responses highlighting the need for specialist consultation. Twenty-two of 28 participants disagreed that ‘only AH patients with bleed symptoms should be referred to expert centres as soon as possible' with respondents specifying that all patients suspected of having AH should be referred to expert centres.
| Statement | Level of agreement |
|---|---|
| Item 1. Initial management | |
| 1.1 Advice on management should be sought from an expert haematologist as soon as possible | 100% (strong consensus) |
| 1.2 The non-expert treating physician should consider commencing treatment for serious bleeds in acquired haemophilia if specialist consultation is not available | 93% (strong consensus) |
| 1.3 If specialist consultation is not immediately available, and the condition is life-threatening, Emergency Department doctors should commence treatment for AH in line with local or national recommendations until an expert haematologist becomes available. If there are no local or national recommendations available, published recommendations should be sought. | 97% (strong consensus) |
| 1.4 All AH patients should be referred to an expert centre as soon as possible. | 88% (consensus) |
| Item 2. Urological bleeds/haematuria | |
|
2.1 The following may be indicators of treatment effectiveness:
|
97% (strong consensus) |
|
2.2 The following may be indicators of worsening condition:
|
97% (strong consensus) |
| 2.3 The optimal interval for assessing whether haemostasis has been achieved is every 6-12 h. | 100% (strong consensus) |
| 2.4 A complete response to haemostatic treatment will be defined as cessation of bleeding from the site, with no evidence of recurrent bleeding from that site for 2 d after withdrawal of the haemostatic treatment. | 94% (strong consensus) |
| 2.5 If initial treatment for bleeding has not been effective, it is appropriate to consider a different/more intensive treatment every 6-12 h. | 94% (strong consensus) |
| Item 3. Gastrointestinal bleeds | |
|
3.1 The following may be indicators of treatment effectiveness:
|
97% (strong consensus) |
|
3.2 The following may be indicators of worsening condition:
|
100% (strong consensus) |
| 3.3 The optimal interval for assessing whether haemostasis has been achieved is every 6 h. | 79% (consensus) |
| 3.4 A complete response to haemostatic treatment will be defined as cessation of bleeding from the site, with no evidence of recurrent bleeding from that site for 2 d after withdrawal of the haemostatic treatment. | 94% (strong consensus) |
| 3.5 If initial treatment for bleeding has not been effective, it is appropriate to consider a different/more intensive treatment every 6-12 h. | 94% (strong consensus) |
| Item 4. Muscle bleeds | |
|
4.1 The following may be indicators of treatment effectiveness:
|
100% (strong consensus) |
|
4.2 The following may be indicators of worsening condition:
|
100% (strong consensus) |
| 4.3 The optimal interval for assessing whether haemostasis has been achieved is every 6-12 h. | 100% (strong consensus) |
| 4.4 A complete response to haemostatic treatment will be defined as cessation of bleeding from the site, with no evidence of recurrent bleeding from that site for 2 d after withdrawal of the haemostatic treatment. | 91% (strong consensus) |
| 4.5 If initial treatment for bleeding has not been effective, it is appropriate to consider a different/more intensive treatment every 12 h. | 91% (strong consensus) |
| Item 5. Skin bleeds | |
|
5.1 The following may be indicators of treatment effectiveness:
|
97% (strong consensus) |
|
5.2 The following may be indicators of worsening condition:
|
100% (strong consensus) |
| 5.3 The optimal interval for assessing whether haemostasis has been achieved is every 12-24 h. | 97% (strong consensus) |
| 5.4 A complete response to haemostatic treatment will be defined as cessation of bleeding from the site, with no evidence of recurrent bleeding from that site for 2 d after withdrawal of the haemostatic treatment. | 94% (strong consensus) |
| 5.5 If initial treatment for bleeding has not been effective, it is appropriate to consider a different/more intensive treatment every 24 h. | 88% (consensus) |
| Item 6. Joint bleeds | |
|
6.1 The following may be indicators of treatment effectiveness:
|
100% (strong consensus) |
|
6.2 The following may be indicators of worsening condition:
|
100% (strong consensus) |
| 6.3 The optimal interval for assessing whether haemostasis has been achieved is every 6-12 h. | 100% (strong consensus) |
| 6.4 A complete response to haemostatic treatment will be defined as cessation of bleeding from the site, with no evidence of recurrent bleeding from that site for 2 d after withdrawal of the haemostatic treatment. | 94% (strong consensus) |
| 6.5 If initial treatment for bleeding has not been effective, it is appropriate to consider a different/more intensive treatment every 12 h. | 88% (consensus) |
| Item 7. Head and neck bleeds (nosebleed) | |
|
7.1 The following may be indicators of treatment effectiveness:
|
91% (strong consensus) |
|
7.2 The following may be indicators of worsening condition:
|
100% (strong consensus) |
| 7.3 The optimal interval for assessing whether haemostasis has been achieved is every 6 h. | 91% (strong consensus) |
| 7.4 A complete response to haemostatic treatment will be defined as cessation of bleeding from the site, with no evidence of recurrent bleeding from that site for 2 after withdrawal of the haemostatic treatment. | 91% (strong consensus) |
| 7.5 If initial treatment for bleeding has not been effective, it is appropriate to consider a different/more intensive treatment every 6-12 h. | 91% (strong consensus) |
| Item 8. Head and neck bleeds (pharyngeal bleed) | |
|
8.1 The following may be indicators of treatment effectiveness:
|
100% (strong consensus) |
|
8.2 The following may be indicators of worsening condition:
|
88% (consensus) |
| 8.3 The optimal interval for assessing whether haemostasis has been achieved is every 6-12 h. | 76% (consensus) |
| 8.4 A complete response to haemostatic treatment will be defined as cessation of bleeding from the site, with no evidence of recurrent bleeding from that site for 2 d after withdrawal of the haemostatic treatment. | 94% (strong consensus) |
| 8.5 If initial treatment for bleeding has not been effective, it is appropriate to consider a different/more intensive treatment every 6 h. | 82% (consensus) |
| Item 9. Head and neck bleeds (oral bleed–spontaneous or traumatic) | |
|
9.1 The following may be indicators of treatment effectiveness:
|
97% (strong consensus) |
|
9.2 The following may be indicators of worsening condition:
|
100% (strong consensus) |
| 9.3 The optimal interval for assessing whether haemostasis has been achieved is every 6 h. | 91% (strong consensus) |
| 9.4 A complete response to haemostatic treatment will be defined as cessation of bleeding from the site, with no evidence of recurrent bleeding from that site for 2 d after withdrawal of the haemostatic treatment. | 94% (strong consensus) |
| 9.5 If initial treatment for bleeding has not been effective, it is appropriate to consider a different/more intensive treatment every 6-12 h. | 88% (consensus) |
| Item 10. Intracranial haemorrhage | |
|
10.1 The following may be indicators of treatment effectiveness:
|
100% (strong consensus) |
|
10.2 The following may be indicators of worsening condition:
|
100% (strong consensus) |
| 10.3 Assessment intervals depend on the clinical situation and may vary from every 2 h (e.g. for clinical assessment) to every 24 hours (e.g. for imaging studies) | 91% (strong consensus) |
| 10.4 A complete response to haemostatic treatment will be defined as cessation of bleeding from the site, with no evidence of recurrent bleeding from that site for 2 d after withdrawal of the haemostatic treatment. | 94% (strong consensus) |
| 10.5 If initial treatment for bleeding has not been effective, a different/more intensive treatment should be considered every 6 h. | 76% (consensus) |
| Item 11. Postpartum haemorrhage–female genital tract bleeding | |
|
11.1 The following may be indicators of treatment effectiveness:
|
100% (strong consensus) |
|
11.2 The following may be indicators of worsening condition:
|
100% (strong consensus) |
| 11.3 The optimal interval for assessing whether haemostasis has been achieved is every 6 h. | 91% (strong consensus) |
| 11.4 A complete response to haemostatic treatment will be defined as cessation of bleeding from the site, with no evidence of recurrent bleeding from that site for 2 d after withdrawal of the haemostatic treatment. | 94% (strong consensus) |
| 11.5 If initial treatment for bleeding has not been effective, a different/more intensive treatment should be considered every 6 h. | 94% (strong consensus) |
- Abbreviations: AH, acquired haemophilia; GCS, Glasgow Coma Scale; NIH, National Institutes of Health.
For all items on location-specific bleeds (five statements for each of Items 2-11), responses were collated at the first survey round and used to construct the first statements posed to participants in the second round; statement 4 in each of Items 2-11 (‘complete response' to haemostatic treatment) was completely revised due to wide inconsistencies in responses. Respondents initially pointed out that assessment of bleeds depends on the site and that continued haemostatic treatment is often given to prevent re-bleeding although the initial bleed responded adequately. The final consensus was, however, that a complete response should be defined as ‘no signs of re-bleeding after haemostatic medication had been withdrawn for 2 days'. This statement achieved strong consensus (defined as ≥75% agreement).
Strong consensus was also achieved at the second survey round across all other statements except for one (statement 10.3, assessment of intracranial bleeds), for which there was only a majority approval for an assessment interval of ‘within 6 hours'. As this statement fell short of strong consensus, it was revised according to qualitative responses and 91% agreement was achieved at the third survey round.
3.3 Summary of final consensus statement, with key observations
The initial management statements (Item 1) specify that advice on management of AH should be sought as soon as possible from an expert haematologist (level of agreement: 100%; Table 2). If specialist consultation is not available, the non-expert treating physician should consider commencing treatment for serious bleeds (93%); if the condition is life-threatening, ED doctors should commence treatment in line with local or national recommendations or, in their absence, published recommendations (97%). All patients with AH should be referred to an expert centre as soon as possible (88%).
Overall, indicators of treatment effectiveness and worsening condition were varied according to the bleeding sites covered in Items 2-11. There were also differences in observation intervals for assessing whether haemostasis has been achieved, or if initial treatment for bleeding is ineffective; for example, the optimal observation interval for assessing achievement of haemostasis was much wider (2- or 12-24 hours) for skin or intracranial haemorrhage (the latter due to varying clinical situations and the need for different assessments, i.e. clinical or imaging) compared with gastrointestinal, head and neck bleeds and postpartum haemorrhage (all 6 hours). There was consensus to define a complete response to haemostatic treatment consistently, as ‘cessation of bleeding from the site, with no evidence of recurrent bleeding from that site for 2 days after withdrawal of the haemostatic treatment', regardless of bleeding site. The level of agreement was variable across the location-specific bleeding statements, although strong consensus was achieved throughout.
4 DISCUSSION
There is an unmet need for robust consensus on how bleeding patients with AH should be managed, and few haemophilia experts with extensive experience of AH are currently practicing; these experts are located in various geographical regions. Therefore, the Delphi methodology20, 21 was utilized in this study to derive a consensus, from a large group of global specialists, on how bleeding patients with AH should be managed and to provide additional information to that currently provided within the existing guidelines. One of the major purposes of this was to generate consensus endpoints which could be utilized for future clinical studies examining efficacy of traditional bypassing agents, disruptive therapies and gene therapy constructs which may be used in AH. Through three rounds of survey refinement, strong consensus was derived on all general and location-specific assessments of outcome. The high level of agreement between participants demonstrates that expert clinical practice in this area is comparable around the world and serves to reinforce the value of these consensus statements.
This study was also conducted to supplement existing guidance on the assessment of the bleeding patient with AH, with specific regard to the location of the bleed. While it is acknowledged that the most common bleed types are subcutaneous, muscular and gastrointestinal,19 little attention has been given as to how these, and bleeds in other anatomical locations, such as genitourinary, intracranial and oral bleeds should be approached. Indeed, current recommendations comprising narrative reviews, expert panel recommendations and ‘how I treat' articles provide limited guidance on the diagnosis and treatment of location-specific bleeds,10-19 warranting a structured and methodologically robust approach to further the quality of the existing literature. This paper provides expert statements on initial consultation of bleeding patients, as well as on how treatment effectiveness can be assessed regarding various locations around the body. Furthermore, it considers assessment frequency for the monitoring of adequate haemostasis, definition of a ‘complete' haemostatic response, and frequency for considering a different treatment approach.
While strong consensus was achieved for all statements by the end of the three survey rounds, some areas proved challenging. For instance, the statement addressing optimal frequency for assessing haemostasis in intracranial bleeds (Table 2, Item 10.3) was the subject of much deliberation among the steering committee regarding timing of assessment. This statement did not achieve consensus until the third survey round, since there were differing opinions among the participants as to when patients with this life-threatening bleed should be assessed. Defining a ‘complete response' to haemostatic therapy (fourth statement in Table 2, Items 2-11) was similarly challenging. Due to wide inconsistencies in responses between each item after the first survey round, an encompassing statement defining a complete response to haemostatic treatment was considered the most appropriate resolution and was widely accepted during Round 2.
The major strength of our study is that 36 haematologists, spanning 20 countries across six continents, with a wealth of experience in AH, participated. Thus, we can conclude that our consensus statements came from a broad sample of haematologists with an interest in AH reflecting regional and economic differences. Furthermore, the study achieved a high retention rate with 28 (78%), 33 (92%) and 32 (89%) of the 36 participants taking part in survey Rounds 1, 2 and 3, respectively, which exceeded the 75% target participation rate, determined at the outset of the study. Finally, while the benefits of using Delphi methodology for generating consensus are well documented,22-26 this approach may have also avoided the potential bias that could have occurred using a face-to-face assessments, utilized in other consensus methodologies.27
Overall, we anticipate that our consensus statement will aid physicians treating patients with AH; however, it is acknowledged that patients and clinical scenarios vary, including when it is or is not appropriate to assess a patient or alter a treatment strategy. Also, geopolitical diversities and socioeconomic factors which may influence patient access to care and treatment options have not been accounted for in the current study. Therefore, excessively complex statements and use of the term ‘guidance' or ‘recommendation' have been avoided, as these are not the aims of this paper.
5 CONCLUSIONS
These consensus statements reflect the clinical opinion of specialists from around the world in the monitoring of bleeds and evaluating the efficacy of bleeding treatment, across specific anatomical locations in patients with AH. These findings could be applied in practice and validated by individual population surveys. In the future, we hope to again harness expert consensus in development of guidance in AH management.
ACKNOWLEDGEMENTS
The authors would like to thank the following individuals who contributed their time and expertise in completing the surveys for this study, in alphabetical order: Pantep Angchaisuksiri (Ramathibodi Hospital, Mahidol University, Bangkok, Thailand), Angelika Batorova (University Hospital, Bratislava, Slovak Republic), Francesco Baudo (Ospedale Niguarda, Milan, Italy), Margareth Castro Ozelo (UNICAMP, São Paulo, Brazil), Ampaiwan Chuansumrit (Ramathibodi Hospital, Mahidol University, Bangkok, Thailand), Mohssen El Alfy (Ain Shams University, Cairo, Egypt), Magdy El-Ekiaby (Shabrawishi Hospital, Dokki, Egypt), Miguel Escobar (University of Texas Health Science Center, Texas, US), Satoshi Higasa (Hyogo College of Medicine, Nishinomiya, Japan), Masahiro Ieko (Health Sciences University of Hokkaido, Tōbetsu, Japan), Bettina Kemkes-Matthes (University Hospitals Giessen and Marburg GmbH, Giessen, Germany), Paul Knoebl (Medical University of Vienna, Vienna, Austria), Rebecca Kruse-Jarres (Bloodworks Northwest, Seattle, US), Alice Ma (UNC–Chapel Hill, Chapel Hill, US), Maria Eva Mingot Castellano (Hospital Regional Universitario de Málaga, Málaga, Spain), Yoshiyuki Ogawa (Gunma University Graduate School of Medicine, Maebashi, Japan), Ingrid Pabinger-Fasching (Medical University of Vienna, Vienna General Hospital, Vienna, Austria), Man-Chiu Poon (University of Calgary, Calgary, Canada), Elena Santagostino (Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy), Yoshinobu Seki (Niigata University Medical and Dental Hospital, Niigata, Japan), Mark Smith (Christchurch Hospital, Christchurch, New Zealand), Sim Leng Tien (Singapore General Hospital, Singapore), Paula Ribeiro Villaça (Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo, Brazil), Jerzy Windyga (Institute of Hematology and Transfusion Medicine, Warsaw, Poland), Renchi Yang (Chinese Academy of Medical Sciences, Peking Union Medical College, Tianjin, China), Galila Zaher (King Abdulaziz University Hospital, Jeddah, Saudi Arabia) and Silva Zupančić-Šalek (University Hospital Center Zagreb, Zagreb, Croatia). Support for the study design, survey development, and manuscript drafting was provided by Dr Ciaran Wright, PhD, of Bioscript Medical, Macclesfield, UK, and funded by Novo Nordisk AG. Methodological consultancy and management of the online Delphi survey was provided by Mr Torsten Karge and Dr Nadine Steubesand, PhD, of Forga Solutions GmbH, Berlin, Germany, and funded by Novo Nordisk AG.
DISCLOSURES
AT: grants and personal fees for lectures or consultancy from Alnylam, Bayer, Biogen Idec, Biotest, Boehringer Ingelheim, CSL Behring, Leo Pharma, Novo Nordisk, Octapharma, Pfizer, Portola, Roche, Shire and SOBI. PG: consultancy and/or lecture fees from Bayer, CSL Behring, NovoNordisk, Pfizer and Shire. JT: speaker fees and member of advisory boards for Novo Nordisk, Bayer, Takeda, Octapharma, Pfizer, Roche and Bioverativ. KA: speaker fees for lectures from Baxalta, Bayer, Biogen, Kaketsuken, Novo Nordisk and Pfizer; consultancy fees from Baxalta, Bayer, CSL Behring, Kaketsuken, Novo Nordisk and Pfizer; unrestricted grants supporting research from Pfizer.GB: grants and personal fees for lectures and consultancy from Novo Nordisk. LN: personal fees for lectures or advisory boards from Shire/Takeda, NovoNordisk, Bayer, Pfizer, Sobi, CSL Behring, Roche and Octapharma. VJ-Y: reimbursement for attending symposia/congresses and/or honoraria for speaking and/or consulting and/or funds for research from Shire, Bayer, CSL Behring, Grifols, Novo Nordisk, Octapharma and Pfizer. Rd'O: personal fees for lectures or advisory boards and received non-financial support for attending scientific meetings from Shire Pharmaceuticals/Baxalta, NovoNordisk, Bayer Pharma plc, Pfizer, Sobi, LFB, Roche and Octapharma. SBEF: employee and shareholder of Novo Nordisk. CMK: funding for clinical research from Bayer, Takeda, Bioverativ, Genentech, NovoNordisk, Sangamo and Octapharma. He has served on advisory boards for Bayer, Takeda, Bioverativ, Genentech, NovoNordisk, Octapharma, Pfizer, BioMarin and Sangamo. He is on DSMBs for NIH, Octapharma and Bayer. He is a paid consultant for LFB.
AUTHOR CONTRIBUTIONS
All authors discussed and agreed the content of the manuscript following completion of the study. The first draft of this manuscript was developed by the medical writer under guidance from AT, CMK and PG. JT, KA, GB, LN, VJ-Y, Rd'O and SBEF reviewed and provided comprehensive feedback of all manuscript drafts and approved the final version for journal submission. The sponsor provided methodological input and reviewed the manuscript draft for medical accuracy, but AT and CMK had final authority on presentation of content.




