Loss of a single Zn finger, but not that of two Zn fingers, of GATA3 drives skin inflammation
Communicated by: Sho Yamasaki
Abstract
Transcription factor GATA3 is essential for the developmental processes of T cells. Recently, the silencer of a cytokine IFNγ gene was identified, the inhibitory activity of which requires GATA3. GATA3 has 2 Zn fingers and the commonly used GATA3 deficient mice lack both fingers (D2). We have established a mouse line that lacks only one Zn finger close to the C terminus (D1). The D1 mice line developed dermatitis, which was not observed in D2 mice. The expression of S100a8/S100a9 was elevated in D1 to a level higher than in D2, suggesting their roles in dermatitis development. CD8 T cells of both D1 and D2 lines expressed inhibitory receptors associated with the exhausted state. In the absence of MHC class II, the skin inflammation was exacerbated in both lines. The gene expression pattern of CD8 T cells became similar to that of effector T cells. Blocking Ab against LAG3 upregulated the expression of the effector molecules of T cells. These results suggest that the disfunction of GATA3 can lead to the spontaneous activation of CD8 T cells that causes skin inflammation, and that suppressive activity of MHC class II - LAG3 interaction ameliorates dermatitis development.
1 INTRODUCTION
The transcription factor GATA3 is involved in multiple steps of T cell development. In the thymus, GATA3 is one of the transcription factors required for establishing the T cell lineage commitment (Petrie & Zuniga-Pflucker, 2007; Rothenberg, 2014). In addition, GATA3 is required for the first phase of the gene rearrangement called β-selection in which either the gene rearrangement of TCRβ chain or that of both TCRγ and TCRδ chains takes place (Hosoya et al., 2010). In the CD4 CD8 double positive stage, GATA3 is required for the development of CD4 single positive cells. In the periphery, GATA3 is a critical positive regulator for Th2 subset induction (Zhu et al., 2004). For CD8 T cells, functional deficiency of GATA3 results in a hyporesponsiveness for Ag-dependent activation and proliferation (Tai et al., 2013; Wang et al., 2013). Recently, a negative regulatory element, a silencer of IFNγ gene transcription, was identified (Cui et al., 2023). The interaction of GATA3 with the silencer causes transcriptional suppression of IFNγ gene through the alteration of chromatin configuration.
Persistent antigen exposure of CD8 T cells takes place when pathogens are not eliminated by the effector T cells (Jenkins et al., 2023; Philip & Schietinger, 2022; Wherry et al., 2007). In tumor microenvironment, tumor cells become resistant to the cytotoxic T cells partly due to the expression of the ligands of inhibitory receptors such as PD-L1, which are expressed on tumor cells and deliver suppressive signal to T cells expressing inhibitory receptors such as PD-1 (Larkin et al., 2015; Tawbi et al., 2022; Waldman et al., 2020). Blocking this inhibitory signal by Ab shows therapeutic efficacy, called immune checkpoint blockade therapy (Jenkins et al., 2023; Waldman et al., 2020). Under immune checkpoint operation, CD8 T cells differentiate into a state called exhaustion. Exhausted T cells are hyporesponsive to Ag stimulation and exhibit a unique transcriptional profile. Exhausted cells are characterized by their expression of multiple inhibitory receptors. Inhibitory receptors mediate suppressive signals to cells upon the binding of their ligand molecules. Lag3 is one of the constitutively expressed inhibitory receptors of exhausted T cells. Various ligand molecules for Lag3 have been discovered, while the canonical ligand is MHC class II (Aggarwal et al., 2023; Baixeras et al., 1992; Kouo et al., 2015; Nguyen & Ohashi, 2015; Wang et al., 2019).
GATA3 contains 2 Zn finger motifs. One of the Zn fingers close to the C terminus and its adjacent conserved basic region are essential for the binding to its recognition motif (Pai et al., 2003; Shinnakasu et al., 2006; Tai et al., 2013; Wang et al., 2013; Zhu et al., 2004). The conventional GATA3 mutant mice lack both Zn fingers, and it has not been known whether the loss of a single Zn finger causes similar phenotypes. Thus, we established the mouse line (D1GATA3) that has lost only one of the two Zn fingers close to the C terminus. Similar to D2GATA3 mice lacking both of the Zn fingers, D1GATA3 mice showed impaired CD4 T cell development. However, D1GATA3 mice developed skin inflammation, which was not observed in the D2GATA3 mice.
2 RESULTS
2.1 Deletion of the Zn finger close to the C terminus of GATA3 leads to the suppression of type II immune response
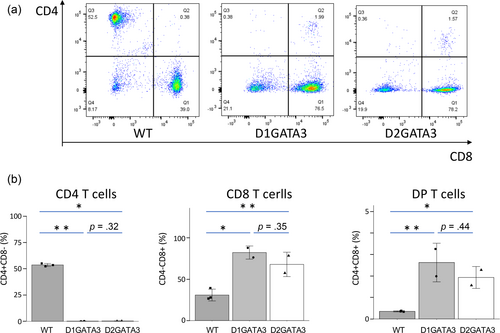
Disruption of GATA3 activity in T cells has been induced by the CD4Cre-mediated deletion of 2 Zn fingers (D2GATA3). The Zn finger close to C terminus and adjacent basic region are essential for the DNA binding of GATA3, suggesting an essential role of this Zn finger for various functions of GATA3 (Ho et al., 2009; Nakayama et al., 2017). We have established the mouse line (D1GATA3) carrying C-terminal truncation resulting in the loss of only the C-terminal Zn finger. Both D2GATA3 and D1DATA3 mice showed impairment of CD4SP T cell development in the thymus leading to a significantly reduced number of CD4 T cells in the periphery (Figure 1). In addition, CD4+ CD8+ double positive population was detected in the periphery of both lines which was not reported by other groups.
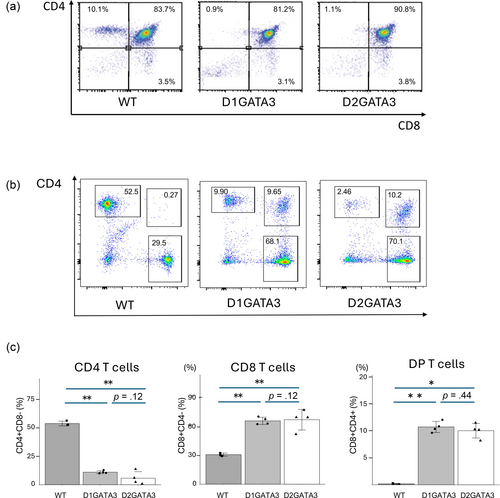
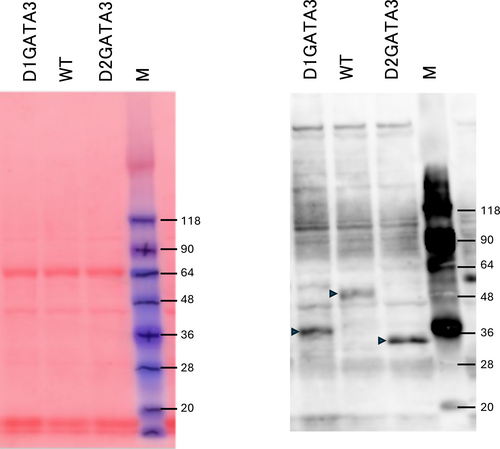
Western blot of CD8 T cell extracts was performed to elucidate whether the mutated proteins were expressed. As shown in Figure 2, a truncated protein with a single Zn finger deletion (D1GATA3) and that with both Zn fingers deleted (D2GATA3) were detected. The expression levels of those mutated proteins were comparable to or slightly elevated to that of wild-type GATA3 protein. Those truncated proteins might have partial activity or aberrant activity. Using D2GATA3 mice, other groups reported that CD8 T cells of D2GATA3 mice are impaired in immune responses including responsiveness to Ag stimulation and the peripheral maintenance of CD8 T cells (Tai et al., 2013; Wang et al., 2013).
Topical application of vitamin D analog MC903 induces TSLP production from keratinocytes in mice. TSLP affects various immune cells in the skin to drive type II immune responses such as elevated production of IgE and infiltration of eosinophiles; therefore, it is considered a mouse atopic dermatitis (AD) model. TSLP is highly expressed in the human skin of AD patients (Ebina-Shibuya & Leonard, 2023). MC903 application to D1GATA3 mice induced skin inflammation. The serum IgE level was upregulated in MC903-administered control mice, as expected, while IgE was significantly suppressed in MC903-administered D1GATA3 mice. The amount of IgE of D1GATA3 was not affected by the administration of MC903 and was close to the basal level in the control mice (Figure 3a). Some of the p-values exceeded 0.05 (0.12) due to variability of the data in WT mice. Therefore, we cannot use the term “significant difference.” However, we believe that the data generally support our conclusions.
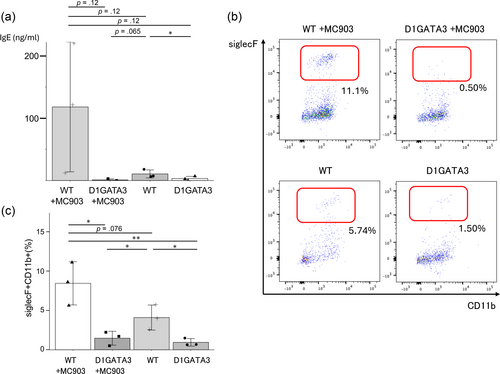
Eosinophil infiltration into the skin was induced in MC903-administered control mice. The eosinophil infiltration was significantly suppressed in D1GATA3 mice treated with MC903 (Figure 3b, c). Therefore, type 2 inflammatory response was inhibited in D1GATA3 mice. The p-value of the data between MC903 treated and control mice exceeded 0.05 (0.076) due to variability of the data. Therefore, we cannot use the term “significant difference.” However, we believe that the data generally support our conclusions.
2.2 D1GATA3 mice developed dermatitis
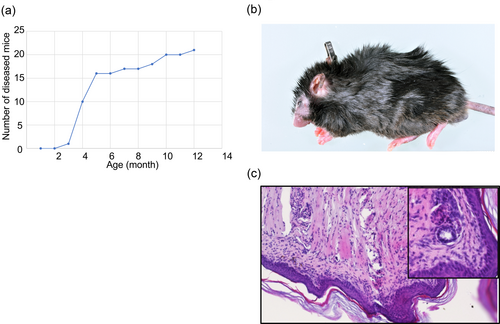
It has been reported that D2GATA3 mice develop age-dependent transient lymphadenopathy due to the expansion of CD8 T cells. Besides that, D2GATA3 mice do not develop any inflammatory diseases (Tai et al., 2013). D1GATA3 mice developed dermatitis. The onset of the dermatitis is mainly 3–6 months after birth (Figure 4a). Some mice develop the disease much later. As shown in Figure 4b, edema, erosion, and alopecia appear in the face, ears, and paws. H&E-stained section from the limb of D1GATA3 mice showed hypertrophic stratum corneum with lymphocytic infiltration in the dermis and subcutis (Figure 4c).
2.3 Innate-like T cells and Tregs in D1GATA3 and D2GATA3 mice
The percentage of the NKT cells of D1GATA3 mice was comparable to that of wild-type mice (Figure 5a). It has been reported that the NKT cell development is impaired in GATA3 deficient mice equivalent to D2GATA3 mice (Kim et al., 2006). It may suggest that D1GATA3 mutant may have some compensatory pathways for the NKT cell development.
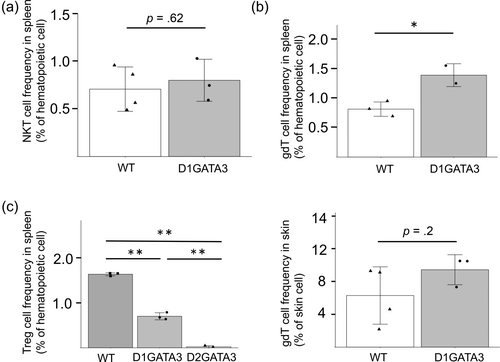
The number of γδT cells was elevated in the spleen while there was no difference in the skin (Figure 5b). The branching point of αβ and γδT cell lineage development is in the DN stage in the thymus, therefore, the GATA3 disruption by the CD4-Cre allele does not affect the γδT cell development directly. The increase of γδT cells in the spleen of D1GATA3 mice might be caused by the inflammatory state of D1GATA3 mice.
The numbers of CD4+ T cells and those of Tregs in D1GATA3 and D2GATA3 mice were reduced compared to those of WT mice (Figure 5c). It has been reported that the Treg-specific disruption of GATA3 by the deletion of both Zn fingers impairs the Treg function leading to the inflammation of lung, pancreas, lacrimal glands, and salivary glands (Wang et al., 2011). Those organs were not affected in either D1GATA3 or D2GATA3 mice (data not shown). We performed CD4 T cell depletion by injecting anti-CD4 Ab into D1GATA3 mice. The skin inflammation was not induced by CD4 T cell depletion. Although the involvement of CD4 helper T cells for the induction of dermatitis cannot be eliminated, depletion of Tregs may not cause the inflammation of skin tissue.
2.4 Transcriptional profiling of CD8 T cells and CD4 CD8 double-positive T cells in spleen of D1GATA3 mice shows features of T cell exhaustion
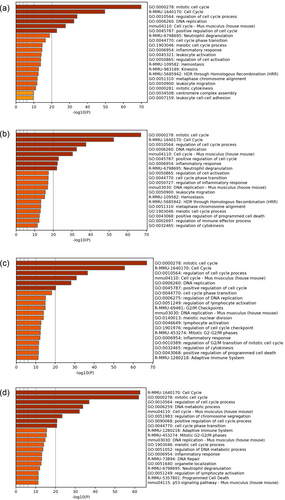
To elucidate the difference in gene expression profiles of D1GATA3 and D2GATA3 mice, spleen T cells were isolated from the mice of 8–9 weeks of age with no dermatitis observed, and the transcriptional profiling was performed by RNA-seq. RNA was extracted from CD4, CD8, and DP T cells of D1GATA3, D2GATA3, and control mice. To figure out the differentially expressed genes (DEGs), the CD8 and DP T cells of the GATA3 mutated mice and the control mice were compared. Expression of effector genes of cytotoxic T cells such as various granzymes, perforin, and IFNγ was elevated in DP T cells of D1GATA3 and D2GATA3 mice compared to that in WT CD8 T cells, while genes expressed highly in CD4 T cells such as CD40L and ThPOK were downregulated. Thus, we consider that the gene expression profile of DP T cells is close to that of CD8 T cells and the DEGs between DP T cells and the control CD8 T cells was determined. The expression profiles of CD8 T cells and CD4 CD8 DP T cells of the D1GATA3 mice were compared with those of the CD8 T cells of WT mice. A total of 319 upregulated and 74 downregulated DEGs from CD8 T cells and 2111 upregulated and 465 downregulated DEGs from DP T cells were identified (Supplementary Table S1). Gene ontology (GO) biological process analysis of upregulated genes revealed that CD8 T cells of D1GATA3 mice include pathways related to immune response such as “inflammatory response” (p-value −13.1), “regulation of inflammatory response”(p-value −9.68) and “leukocyte migration” (p-value −8.67). GO biological process analysis of DP cells of D1GATA3 mice includes pathways related to cell cycle regulation such as “mitotic cell cycle process” (p-value −19.7) and pathways related to immune response such as “cytokine-cytokine receptor interaction” (p-value −10.1).
Unsupervised hierarchical clustering of the DEGs revealed the close relatedness of the transcriptional profiles of CD8 T cells of D1GATA3 and D2GATA3 mice as well as DP T cells of D1GATA3 and D2GATA3 mice (Figure 6a, b). However, PCA analysis indicated that the transcriptional profiles of those populations of D1GATA3 and D2GATA3 mice are related but distinct (Figure 6c).
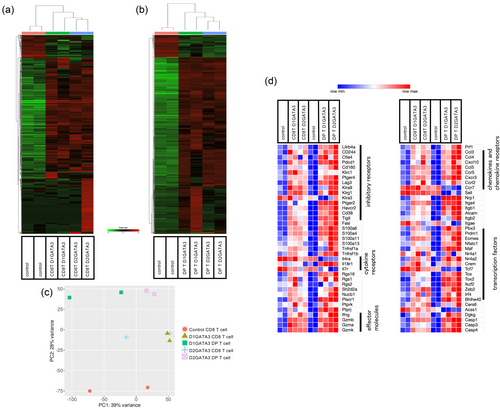
In D2GATA3 mice, 310 upregulated and 69 downregulated DEGs from CD8 T cells, and 1359 upregulated and 805 downregulated DEGs from DP T cells were identified (Supplementary Table S1). Upregulated genes of CD8T cells of D2GATA3 mice include pathways related to immune response such as “inflammatory response” (p-value −12.1), “regulation of leukocyte migration”(p-value −8.8), and “innate immune response” (p-value −8.3). In DP T cells of D2GATA3 mice, pathways related to cell cycle regulation such as “mitotic cell cycle” (p-value −54.3) and immune response regulation such as “inflammatory response” (p-value −14.1) and “cytokine-cytokine receptor interaction” (p-value −12.4) were identified as being upregulated.
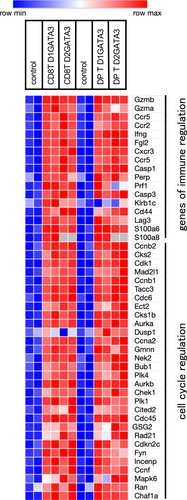
Figure 6d shows the heat map illustrating the relative expression of genes that are associated with the terminally exhausted T cells. Those include inhibitory receptors Lilrb4a, CD244, Ctla4, Pdcd1, Cd160, Lag3, Havcr2, Cd38, and Tigit as well as NK receptors, Klrc1 and Klrg1. Transcription factors upregulated in exhausted T cells include Prdm1, Eomes, Maf, Nr4a2, Crem, Tox, Tox2, Ikzf2, Egr2 Zeb2, Irf4, and Bhlhe40.
Those genes associated with the exhausted state are upregulated in CD8 T cells and DP T cells of D1GATA3 as well as D2GATA3 mice. Tox, Tox2, and Nr4a transcription factors have been reported to form a transcriptional network and to facilitate differentiation of exhausted T cells. Moreover, the exhausted cell population contains the exhausted progenitor population capable of self-renewal and required for the maintenance of the terminally exhausted cell population. The progenitor population expresses Tcf7 (Tcf1) and IL7 receptors. The expression of Tcf7 as well as IL7 receptor was downregulated in CD8 T cells and DP T cells, supporting the idea that most of the CD8 T cells and DP T cells are in the terminally exhausted state.
The upregulated genes such as inhibitory receptors of exhausted state showed more elevated expression in the DP population than the expression in the CD8 T cell population. The expression of the downregulated genes such as Il4r, Il7r, Ccr7, Sell, and Tcf7 of the exhausted state is clearly suppressed in the DP population, while in CD8 T cells, the extent of suppression was small. This might indicate that the CD8 T cell population contains cells other than the exhausted cells.
2.5 Expression of the antimicrobial peptides S100a8/S100a9 is significantly higher in CD8 T cells with D1GATA3 than D2GATA3
Antimicrobial proteins S100a8 and S100a9 are expressed abundantly in myeloid cells. In neutrophils, more than 50% of cytoplasmic proteins consist of S100a8/S100a9 complex (Striz & Trebichavsky, 2004). Monocytes, macrophages, and non-hematopoietic cells such as keratinocytes also express S100a8/S100a9. S100a8/a9 complex functions as one of danger-associated molecular patterns to promote inflammation. Toll-like receptor 4 (TLR4) and receptor for advanced glycation end products (RAGE) have been shown to act as the receptors for S100a8/S100a9 (Narumi et al., 2015; Vogl et al., 2007). Elevated expression of S100a8/a9 is associated with various inflammatory conditions such as sepsis, asthma, psoriasis, and inflammatory bowel diseases (Aoki et al., 2009; Benoit et al., 2006; Gray et al., 2008; Palmer et al., 2019; Wang et al., 2023; Wang & Zhang, 2023). S100a8/S100a9 exerts both anti-inflammatory effects as well as pro-inflammatory effects, especially in sepsis, uncontrolled systemic inflammatory response to pathogens (Wang et al., 2023).
The transcriptional profiles of the CD8 T cells and DP T cells of D1GATA3 mice were compared directly to those of D2GATA3 mice (Supplementary Table S2). The expression of S100a8/S100a9 was significantly higher in the CD8 T cells of D1GATA3 mice than that of D2GATA3 mice. S100a9, but not S100a8 was also detected as a DEG in DP T cells. The expression of S100a8/S100a9 is enhanced in psoriasis patients as well as in the mouse model of psoriasis (Benoit et al., 2006; Defrene et al., 2021; Xie et al., 2023). Therefore, the elevated expression of S100a8/S100a9 in D1GATA3 mice might be the cause of dermatitis development.
IL17A and IL17F have been reported as critical inducer cytokines for S100a8 and S100a9 in keratinocytes. RNA-seq analyses showed that the expression of IL17A in D1GATA3 CD8 T cells is higher than that in D2GATA3 CD8 T cells, although its expression level was low (Supplementary Table S3). Furthermore, the expression of IL17A in DP T cells of D1GATA3 mice was much higher than that of D2GATA3 mice in the same experiment (Supplementary Table S3). qPCR analysis of splenic CD8 T cells stimulated with anti-CD3ε Ab and anti-CD28 Ab indicated that D1GATA3 CD8 T cells produced more IL-17A mRNA than D2GATA3 CD8T cells did (Figure 7a). The p-value between the WT and D1GATA was 0.09. However, the p-value between D1GATA and D2GATA is less than 0.05, and our results are in support of the conclusion that IL-17a is induced in D1GATA3 mice. These suggest a possibility that the elevated production of IL17a in D1GATA3 mice may lead to increased A100a8/9 in keratinocytes and to dermatitis development.
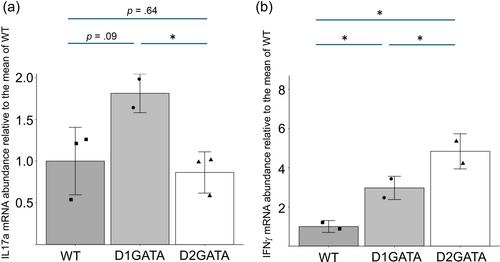
In addition, the expression of granzyme A, one of the effector molecules, was higher in D1GATA3 mice than that of D2GATA3 mice. Granzyme A has been shown to play important roles in inflammation and cell death induction (Lieberman, 2010; Rawle et al., 2022). Granzyme A is another candidate gene that might drive dermatitis in D1GATA3 mice.
IFNγ was detected as DEGs in DP T cells of D1GATA3 and D2GATA3 mice (Supplementary Tables S1 and S3). The expression level was higher in D2GATA3 mice than in D1GATA3 mice. qPCR analysis of the isolated CD8 T cells was performed (Figure 7b). The expression of IFNγ of stimulated CD8 T cells was much higher in D2GATA3 mice than in D1GATA3 mice. This suggests that IFNγ may not be the critical cytokine to drive the dermatitis in D1GATA3 mice. It may also indicate that D1GATA3 mutation that retains one Zn finger has some residual activity to suppress the transcription of the IFNγ gene.
2.6 MHC class II deficiency of GATA3 mutated mice results in the exacerbation of skin inflammation
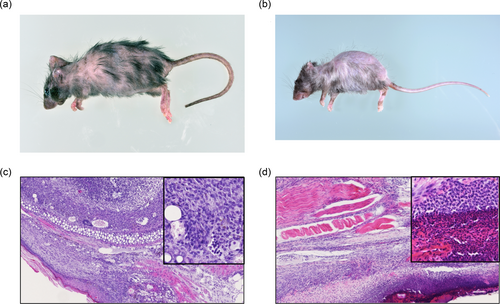
As shown in Figure 8a, D1GATA3 mice with the MHC class II deficient background (MHC class IIdef) show severe alopecia of the back skin, graying hair, and erosion of ears. The onset of the inflammation is about 3 weeks with 100% penetrance. Although D2GATA3 mice do not develop dermatitis, D2GATA3 MHC class IIdef mice suffered from similar severe dermatitis (Figure 8b). Histological analysis of the ear of the D1GATA3 MHC class IIdef mice showed the structural breakdown of the ear tissue, thickening of the dermis, and infiltration of lymphocytes (Figure 8c). A cluster of lymphocytes was found in the sebaceous and adipose tissues around the elastic cartilage. Lymphocytic infiltrates were found in collagenous tissue, nerve tissue, and rhabdomyocytes in the underlying layer of thickened squamous epithelium of the tail (Figure 8d). CD4 T cells were almost undetectable in the periphery of both D1GATA3 and D2GATA3 MHC class IIdef mice, while the number of CD8 T cells was comparable to those of D1GATA3 and D2GATA3 MHC class II sufficient mice (Figure 9). Therefore, it is inconceivable that the GATA3 mutated CD4 T cells cause the dermatitis.
RNA-seq analyses of CD8 and DP T cells of D1GATA3 MHC class IIdef mice were performed. In the absence of MHC class II molecule, the numbers of DEGs of CD8 and DP T cells from D1GATA3 and D2GATA3 mice were comparable (Supplementary Table S4). GO analyses of those T cells are shown in Figure 10a–d. In all those T cell populations, pathways related to cell cycle regulation and cell proliferation are significantly enriched. Pathways related to immune regulation such as “inflammatory response” and “lymphocyte activation” appeared next to the pathways of cell cycle regulation.
Resting naive CD8 T cells are activated by Ag stimulation to become effector CD8 T cells that proliferate extensively and produce various effector molecules such as various cytokines, chemokines, chemokine receptors, caspases, perforin, and granzymes. Gene signature of effector T cells includes genes involved in cell cycle regulation such as Cdc45, Cdk1, Cdc6, Ccna2, and Chk1. The other enriched group of effector pathways includes effector molecules of activated T cells such as Gzmb, Gzma, Ccr5, Ccr2, IFNγ, Cxcr3, and Casp1.
Figure 11 shows the heat map illustrating the relative expression of genes that are associated with the effector function of T cells. CD8T cells and DP T cells of both D1GATA3 MHC class IIdef and D2GATA3 MHC class IIdef mice express genes induced in effector T cells including cell cycle regulatory molecules and effector molecules. Interestingly S100a8/S100a9 are extremely highly expressed in D1GATA3 MHC class IIdef mice, while S100 proteins of other members such as S100a4 and S100a6 were highly expressed in D2GATA3 MHC class IIdef mice (Supplementary Table S4). Severe dermatitis might be developed by those S100 proteins. In addition, many members of granzymes were also upregulated in those cells suggesting that those molecules might be the cause of the severe dermatitis.
Since the onset of the dermatitis of the mice with the MHC class II deficiency was very early, the T cells were isolated from the mice that had shown the symptoms of dermatitis. There is a possibility that the transcriptional profile of T cells might be affected by the inflammation of the skin. Indeed, the increase of cDC and granulocytes in the spleen was observed (data not shown). Therefore, the transcriptional profile of T cells might represent the combined effect of GATA3 mutation and those myeloid lineage cells.
2.7 MHC class II negatively regulates CD8 T cells through MHC class II-Lag3 axis
An important role of MHC class II molecule in the immune system is to present Ag to CD4 positive helper and regulatory T cells. Another role is to suppress Lag3 expressing cells through MHC class II—Lag3 interaction. To elucidate the role of Lag3 of GATA3 mutated T cells, administration of the blocking Ab against Lag3 into D1GATA3 mice was performed (Figure 12). To evaluate the state of activation, the expression of granzyme B was analyzed by flow cytometry. D1GATA3 mice were administered either 200 μg of anti-LAG3 Ab or the control Ab every 3 days for 2 weeks. At 4 h before the isolation of T cells, 10 μg of LPS was injected to stimulate T cells in vivo to induce granzyme B expression on the cell surface.
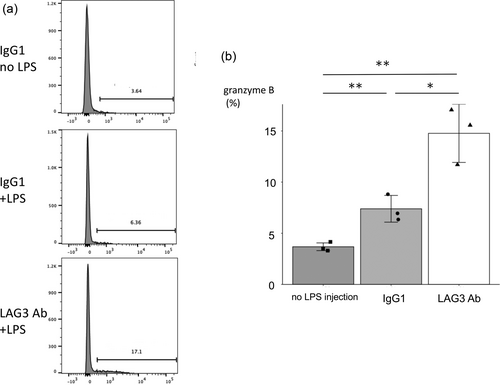
The blocking of the MHC class II—Lag3 axis by anti-LAG3 Ab revealed that for D1GATA3 CD8 T cells, the negative signal through Lag3 is effective in inducing granzyme B expression. This indicates that the Lag3 signal induced exhausted state to avoid extensive activation and the induction of inflammation. However, we did not observe the induction of skin inflammation in D1GATA3 mice upon Lag3 Ab injection. This could be because the period of Ab injection might not be long enough to induce inflammation. Alternatively, the inhibitory activity of the Ab may not be effective enough to induce dermatitis.
3 DISCUSSION
Various regulatory elements for the expression of IFNγ gene have been identified. Recently, silencer CNS28 was discovered (Cui et al., 2023). GATA3 binds to this silencer and the chromatin complex (topological associated domain (TAD)) including CNS28 and CNS22, a nearby enhancer of IFNγ gene is formed to keep the enhancer away from the promoter of IFNγ gene. When CNS28 is deleted, the chromatin complex (TAD) allows the interaction of the CNS22 to the promoter of IFNγ gene, and the transcription is induced. GATA3 binding to this silencer is also required for its function. Therefore, the disruption of GATA3 leads to the transcriptional activation of IFNγ gene. Among the 2 Zn fingers, the one close to the C terminus is more important for DNA binding and this Zn finger is deleted in T cells of D1GATA3 mice (Shinnakasu et al., 2006). T cells of D2GATA3 mice lack both Zn fingers. Comparing the transcriptional profile of T cells from D1GATA3 and D2GATA3 mice, the expression of IFNγ is more elevated in CD8 T cells and DP T cells of D2GATA3 mice (Figure 6d). qPCR analysis of activated CD8 T cells showed higher expression of IFNγ of D2GATA3 mice than that of D1GATA3 mice (Figure 7b). This suggests that the interaction of GATA3 with CNS28 silencer to keep IFNγ gene completely quiescent requires both Zn fingers. The Zn finger close to the N terminus might retain GATA3 function to some extent. Further analysis of the association of these 2 mutant GATA3 proteins with CNS28 silencer and many other binding sites in chromatin is required.
Comparison of the transcriptional profile of CD8 and DP T cells of D1GATA3 and D2GATA3 mice revealed that the expression of S100a8/S100a9 antimicrobial peptides was significantly upregulated in D1GATA3 compared to D2GAGTA3 mice. S100a8/S100a9 is not only sensitive biomarkers for inflammatory states but also critical for the pathogenesis of inflammatory diseases (Wang et al., 2013). Recently, S100a8/S100a9 levels in blood have been shown to discriminate severe acute respiratory symptoms of COVID-19 infection from the mild form (Shu et al., 2020). Myeloid cells such as neutrophils and monocytes express those peptides in a large amount while lymphocytes express a minute amount. S100a8/S100a9 binds to 2 types of innate immune receptors, TLR4 and RAGE inducing activation of neutrophils (Narumi et al., 2015; Vogl et al., 2007). In psoriasis patients' skin, S100a8 and S100a9 are highly expressed in keratinocytes and infiltrating neutrophils and monocytes. In several mouse models of psoriasis, the expression of S100a8/S100a9 correlates with the inflammatory state, and those molecules are involved in the pathogenesis (Christmann et al., 2020; Schonthaler et al., 2013; Xie et al., 2023). The development of dermatitis of D1GATA3 mice might be attributed to its elevated expression of S100a8/S100a9. In addition, CD8 T cells of D1GATA3 mice produced more IL17a than that of D2GATA3 mice (Figure 7a). IL17a induces S100a8/S100a9 proteins from keratinocytes, therefore, IL17a produced by CD8 T cells might be important to induce dermatitis.
Granzyme A was upregulated in D1GATA3 mice compared to D2GATA3 mice. Granzyme A is one of the granzymes that are required for the induction of the target cell death by CTL and NK cells. Granzyme A is secreted and activates inflammatory molecules such as IL1β by its proteolytic activity and induces inflammation (Hildebrand et al., 2014). Therefore, granzyme A is another candidate molecule to induce dermatitis in the D1GATA3 mouse.
Long-term maintenance of CD8 T cells in the periphery is impaired when CD8 T cells of the D2GATA3 mice are adoptively transferred into wild-type mice (Tai et al., 2013; Wang et al., 2013). Maintenance of T cells requires IL7 signal and compared to wild-type CD8 T cells, CD8 T cells of the D2GATA3 mice express reduced levels of IL7r. Reduced expression of IL7r is a characteristic of terminally exhausted T cells and it is detected in CD8 T cells of both D1GATA3 and D2GATA3 mice. It has been shown that the signal of Ag stimulation through TCR is impaired in CD8 T cells of D2GATA3 mice resulting in the poor proliferation and reduced production of cytokines in vitro. In addition, responses to cytokines such as IL2 and IL15 are reduced. In vivo, CD8 T cells of the D2GATA3 mice respond poorly to viral pathogens, tumors, and allogenic transplants. The dysfunction of T cells of the D2GATA3 mice might be interpreted by the exhausted state of those T cells. Terminally exhausted cells are not completely inactive, and they are able to produce effector molecules until they become fully exhausted state. Effector molecules such as IFNγ and granzymes are produced by exhausted cells and contribute to immune responses. Therefore, although most of the T cells of the D1GATA3 mice are in a state of terminal exhaustion, those T cells are able to contribute to skin inflammation.
In both D1GATA3 and D2GATA3 mice, deficiency of MHC class II molecules led to a significant exacerbation of dermatitis indicating that the suppressive signal through one of the inhibitory receptors LAG3 induces terminally exhausted state, and without this signal, GATA3 deficient T cells are activated to become effector cells. Blocking Ab against LAG3 enhanced the expression of one of the effector molecules granzyme B, indicating that MHC class II—LAG3 axis is important for the suppression of GATA3 deficient CD8 T cells. In MHC class II deficient mice, mild colitis is occasionally observed but not severe inflammation. This indicates that GATA3 functions to suppress the activation of T cells when the suppressive signal of the MHC class II—LAG3 axis is absent. This function of GATA3 may not be solely attributed to the silencing of IFNγ expression. MHC class II—LAG3 axis and GATA3 collaborate to keep the quiescent state of T cells.
D1GATA3 mice with MHC class I deficient background did not show the reduction of CD8 T cells and developed dermatitis. This is probably due to the redirection of MHC class II-restricted double-positive T cells towards CD8 T cells in the thymus. In addition, the development of dermatitis in the absence of MHC class I suggested that the inflammation was not induced by the self-Ag presented by MHC class I molecules. MHC class II deficiency exacerbated the dermatitis. Therefore, the dermatitis was not caused by the self-Ag presented by MHC molecules. However, we cannot eliminate the possibility that the cell populations other than CD8 T cells caused the dermatitis.
LPS can activate T cells of D1GATA3 mice in vivo, suggesting that T cells of the D1GATA3 mice can be activated by cytokines. To block the inhibitory signal through LAG3, antagonistic Ab was administered. T cells were stimulated in vivo by LPS before the analysis. Granzyme B expression was enhanced in the presence of the LAG3 blocking Ab. Therefore, the inhibitory signal through LAG3 suppressed T cell activation by cytokines. The mechanism of LAG3 suppression of the TCR signal is through its association to the TCR-CD3 complex and interferes with the interaction between lck and the CD4/CD8 coreceptors. However, the inhibitory signal of LAG3 may affect T cells activated by cytokines in the absence of GATA3 function.
Cytokine-dependent T-cell activation is referred to as bystander activation. Various inflammatory cytokines are able to activate various types of T cells. For CD8+ T cells, cytokines such as IL-15, IL-18, and type I IFN can drive activation and proliferation. It has been reported that the LAG3 signal suppresses STAT5 and Akt signaling in Treg cells infiltrated into the islet of NOD mice (Zhang et al., 2017). In addition, LAG3 signal inhibits IL2-dependent homeostatic proliferation which requires STAT5 as a signaling molecule (Durham et al., 2014). LAG3 limits the proliferation and activation of CD4 T cells by regulating the IL7-STAT5 signaling pathway (Previte et al., 2019). Therefore, cytokine-dependent pathways such as IL-15 signal of CD8 T cell activation and proliferation might be suppressed by LAG3 signaling through a similar mechanism. Taken together, MHC class II—LAG3 axis and GATA3 cooperate to keep the quiescent state of T cells to respond appropriately to Ag stimulation as well as cytokine stimulation.
4 EXPERIMENTAL PROCEDURES
4.1 Mice
The permission to use D2GATA3 mice (gata3 gene Exon4 is floxed) was obtained from Dr. Zhu at NIAID and the mouse was distributed from Dr. Kubo at Riken. The permission to use CD4Cre mouse was obtained from Dr. Willson at The University of Washington and the mice were distributed from Dr. Kubo at Riken. The permission to use I-Aβ deficient mouse (MHC class II deficient mouse) was obtained from Dr. Mathis and distributed from Dr. Ikuta at Kyoto University. D1GATA3 mouse (floxed mouse to delete one Zn finger close to C terminus) was produced at Tokyo Metropolitan Institute of Medical Science.
All mice were maintained in specific-pathogen-free barrier facilities and used according to protocols approved by the Tokyo Metropolitan Institute of Medical Science animal care and use committees.
4.2 Topical application of MC903 to induce skin inflammation
Mice were anesthetized using intraperitoneal injection of a combination of medetomidine, midazolam, and butorphanol. MC903 (calcipotriol; Tocris Bioscience) was dissolved in 100% ethanol and topically applied on mouse ears (1 nmol in 20 μL per ear). As vehicle control, the same volume of ethanol was applied. MC903 was applied to the ear every 2 days for 2 weeks.
4.3 Elisa
Mouse sera were collected, and IgE measurement was performed by Enzyme-linked immunosorbent assay according to the manufacturer's recommendations (Biolegend, San Diego, CA).
4.4 Cell staining for flow cytometry
The following antibodies were purchased from BioLegend.
CD3ε FITC (500A2), CD8a BV421 (53-6.7), CD4 PE (GK1.5), granzyme B APC (QA18A28), γδTCR PE(UC7-13D5), NK1.1 PerCP-Cy5.5 (S17016D), foxp3 PE (MF-14).
Dead cells were discriminated by staining with Zombie aqua dye (BioLegend, San Diego CA). Intracellular staining of granzyme B was performed using the Cyto-Fast™ Fix/Perm Buffer Set (Biolegend, San Diego, CA) in accordance with the manufacturer's protocol. Flow cytometry data were acquired on a BD LSR II instrument. Data were analyzed using FlowJo software (BD).
4.5 qPCR of IL-17a and IFNγ
RNA purification and quantitative PCR. Total RNAs were isolated using TRIzol (Invitrogen). cDNAs were prepared using ReverTra Ace qPCR RT Master Mix (Toyobo). Quantitative PCR was performed on ABI7500 Real-Time PCR System.
4.6 Ab injection
A total of 200 μg of anti-LAG3 Ab (C9B7W, BioXCell, Lebanon NH) or a control rat IgG1 (BioXCell, Lebanon NH) were injected ip every 3 days for 2 weeks. A total of 10 μg LPS was injected and 4 h later, mice were sacrificed, and spleen T cells were analyzed by flow cytometry.
4.7 Statistic
Student t-test was used for statistical analysis. *p <.05, **p <.01. In some cases, the calculated p-values were presented in the figures.
4.8 RNA-seq sample preparation
NEBNext® Ultra™II RNA Library Prep Kit for lllumina (NEB) was used for the library construction. Messenger RNA was purified from total RNA using poly-T oligo-attached magnetic beads. After fragmentation, the first strand cDNA was synthesized using random hexamer primers, followed by the second strand cDNA synthesis using dTTP for non-directional library. For the non-directional library, it was ready after end repair, A-tailing, adapter ligation, size selection, amplification, and purification. Quantified libraries will be pooled and sequenced on NovaSeq 6000.
4.9 RNA-seq data analysis
The sequencing reads were mapped to the mm10 genome using STAR (Dobin et al., 2013). The number of reads falling into each gene was quantified using RSEM (Li & Dewey, 2011). The iDEP.96 software was used to detect DEGs (padj<0.01) between any pair of biological conditions (Ge et al., 2018).
AUTHOR CONTRIBUTIONS
Tomohiro Iguchi: Investigation. Makiko Toma-Hirano: Investigation. Masakatsu Takanashi: Investigation. Hisao Masai: Funding acquisition; writing – review and editing. Shoichiro Miyatake: Conceptualization; funding acquisition; writing – original draft; writing – review and editing.
ACKNOWLEDGMENTS
We thank Dr. So Maezawa and Dr. Tatsuya Hattori for critical discussion. We thank our laboratory members including Hirokazu Sakamoto, Ayami Suzuki, Megumi Motokawa, Yumiko Miyagawa, and Asuka Ikeda for their technical assistance. This work was supported by JSPS KAKENHI (Grant Number JP18K07185, S.M.; Grant-in-Aid for Scientific Research [A], 6251004, H.M.; Grants-in-Aid for Scientific Research on Innovative Areas, 21H00264 and 22H04707, H.M.).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.




