Self-recognition through Dectin-1 exacerbates liver inflammation
Communicated by: Yusaku Nakabeppu
Abstract
Dectin-1 is a well-characterized C-type lectin receptor involved in anti-fungal immunity through the recognition of polysaccharides; however, molecular mechanisms and outcomes initiated through self-recognition have not been fully understood. Here, we purified a water-soluble fraction from mouse liver that acts as a Dectin-1 agonist. To address the physiological relevance of this recognition, we utilized sterile liver inflammation models. The CCl4-induced hepatitis model showed that Dectin-1 deficiency led to reduced inflammation through decreased inflammatory cell infiltration and lower pro-inflammatory cytokine levels. Moreover, in a NASH model induced by streptozotocin and a high-fat diet, hepatic inflammation and fibrosis were ameliorated in Dectin-1-deficient mice. The Dectin-1 agonist activity was increased in the water-soluble fraction from NASH mice, suggesting a potential pathogenic cycle between Dectin-1 activation and hepatitis progression. In vivo administration of the fraction into mice induced hepatic inflammation. These results highlight a role of self-recognition through Dectin-1 that triggers hepatic innate immune responses and contributes to the exacerbation of inflammation in pathogenic settings. Thus, the blockade of this axis may provide a therapeutic option for liver inflammatory diseases.
1 INTRODUCTION
Innate immune receptors were typically associated with pathogen recognition (Takeuchi & Akira, 2010); however, recent reports showed that the receptors also recognize self-components and are thought to detect perturbation of internal and external stress (Guenther et al., 2022). Intracellular components are typically not released into the extracellular milieu, but in response to injury or disease intracellular constituents can be released and they can act as damage-associated molecular patterns (DAMPs) in the host (Roh & Sohn, 2018). Self-recognition of DAMPs can be beneficial in the maintenance of homeostasis or detrimental when associated with autoimmune and metabolic disorders (Gong et al., 2020; Marshak-Rothstein, 2006).
Chronic hepatitis is a dangerous condition that damages hepatocytes, leading to fibrosis and even liver cancer. Although viral hepatitis has been well-studied (Shin et al., 2016), sterile hepatitis is being studied, especially a molecular mechanism of the progression of non-alcoholic fatty liver disease (NAFLD) to non-alcoholic steatohepatitis (NASH), which is serious sterile hepatitis, remains largely unknown (Huby & Gautier, 2022). NAFLD is a disease caused by lifestyle-related diseases and the number of patients has been reported to be increasing worldwide in recent years (Pouwels et al., 2022). Fat accumulation in hepatocytes induces cell death, which releases DAMPs, activates Kupffer cells and infiltrated inflammatory myeloid cells such as monocytes and neutrophils (Arrese et al., 2016; Cai et al., 2019). These cells play detrimental roles by inducing inflammatory mediators like TNF-α and IL-1β (Krenkel & Tacke, 2017; Weston et al., 2019) as well as injuring hepatocytes and inducing fibrosis (Arrese et al., 2016; Huby & Gautier, 2022). However, the triggering receptors and endogenous ligand(s) that are responsible for liver inflammation have not been identified. In addition, there is no effective treatment for patients with NASH.
Adaptor molecules like Myd88 and Syk, which are involved in innate immune responses, also contribute to NASH progression (Huang et al., 2023; Kurniawan et al., 2018). Upstream molecules, such as TLR-2, 9, and C-type lectin receptor Mincle, are known to act as DAMP sensors for proteins, nucleic acids, and/or lipids (Dambuza & Brown, 2015; Drouin et al., 2020; Guenther et al., 2022; Huang et al., 2023). Dectin-1 (gene symbol Clec7a), a C-type lectin receptor, is widely expressed on myeloid cells and induces the production of inflammatory cytokines such as TNF and IL-1β through recognition of β-1,3 glucan and plays an essential role in anti-fungal immunity (Mata-Martínez et al., 2022). On the other hand, Dectin-1 is reported to be involved in progression of hepatitis model using thioacetamide (Seifert et al., 2015). However, innate immune responses in other sterile hepatitis models through Dectin-1 and a hepatic endogenous agonist for Dectin-1 have not been investigated.
In this study, we investigated the nature of hepatic agonist for Dectin-1 and the contribution of Dectin-1 in sterile hepatitis. An endogenous agonist for Dectin-1 was enriched in a hydrophilic fraction derived from murine liver using various purification steps and enzymatic analysis revealed that Dectin-1 agonist possesses α-1,4 glucosyl linkage using GFP reporter cells. In a rodent CCl4 hepatitis model, the degree of inflammation was reduced in the absence of Dectin-1. Furthermore, in a murine NASH model, combining STZ and HFD, Dectin-1 deficiency resulted in reduced inflammation, hepatitis biomarkers, and liver fibrosis. Finally, administration of a hepatic agonist into mice induced inflammatory cytokines in serum and increased infiltration of inflammatory cells into the liver, indicating that hepatic hydrophilic agonist possesses a potent proinflammatory stimulus. These results indicate that Dectin-1 recognizes an endogenous agonist in liver and exacerbates inflammation during sterile hepatitis.
2 RESULTS
2.1 Murine liver possesses an endogenous agonist for Dectin-1
To search for an endogenous agonist in liver, we examined the presence of Dectin-1 ligand activity in the liver of mice. When the liver-derived hydrophilic agonist for Dectin-1 was purified using ethanol precipitate, ultrafiltation, anion exchange column chromatography, and gel exchange column chromatography, a fraction with a sufficiently increased specific activity was obtained (Figure 1a). The purified fraction was not recognized through CLRs other than Dectin-1 (Figure S1a), although TLR2 reporter cells were slightly stimulated with purified fraction, but not TLR4 (Figure S1b). This purified fraction included the alpha glucan, and the purity was enriched following purification steps (Figure 1b). To check whether the agonist is endogenous or exogenous component, both the hydrophilic component from SPF and germ-free mouse liver showed bioactivity, indicating that the active component is an endogenous ligand (Figure 1c). Although it has been reported that Dectin-1 recognizes β-1,3 glucan, treatment of endogenous ligand with cellulase or westase did not inhibit the activity. Importantly, the activity was completely abolished by amylase, indicating that the endogenous Dectin-1 agonist was composed of α-1,4 glucose linkage (Figure 1d). Consistent with this result, TLC analysis of enzyme-treated Dectin-1 agonist showed that they were degraded to monosaccharides by amylase (Figure 1e). The recognition of this ligand required the same recognition site of Dectin-1 as that of β-glucan (Figure 1f). These results suggest that murine liver-derived hydrophilic component possesses Dectin-1 agonist activity.
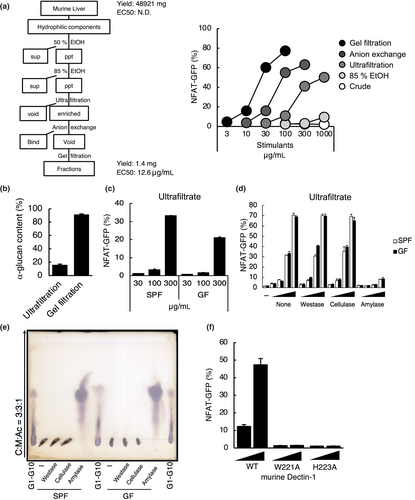
2.2 Dectin-1 deficiency attenuates the inflammatory response to CCl4-induced hepatitis
To determine whether Dectin-1 contributes to the exacerbation of sterile inflammation in liver, which possesses Dectin-1 agonist as shown in Figure 1, we analyzed Dectin-1 function using a model of CCl4-induced hepatitis (Figure 2a) (Scholten et al., 2015). The hepatitis was induced in wild-type and Dectin-1-deficient mice using CCl4 for 4 weeks. Liver/body weight ratio, a measure of liver inflammation, showed a decreasing trend in Dectin-1-deficient mice, but there was no significant difference (Figure 2b). FACS analysis of liver showed infiltration of inflammatory cells such as neutrophils and monocytes were reduced in Dectin-1-deficient mice (Figure 2c). The production of inflammatory cytokines such as TNF and IL-1β in the serum was decreased in Dectin-1-deficient mice (Figure 2d). However, there was no difference in the degree of fibrosis and the hepatitis markers such as alanine amino transferase (ALT) and aspartate amino transferase AST (Figure 2e,f). These findings indicate that Dectin-1 induces sterile inflammation in liver.
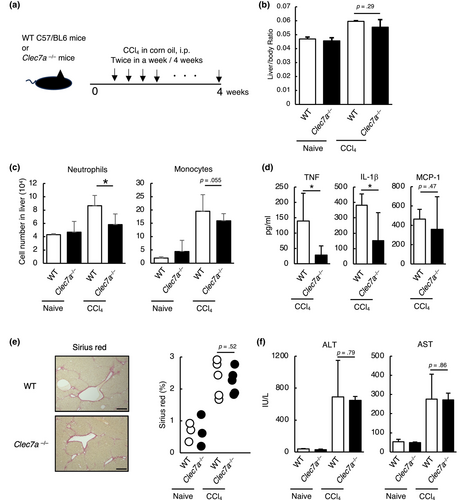
2.3 Dectin-1-deficient mice improved hepatitis exacerbation in a NASH model
Using the STAM mouse model of human NASH (Fujii et al., 2013), we attempted to analyze the function of Dectin-1 in actual liver disease. We induced NASH in wild-type and Dectin-1-deficient mice using streptozotocin and high-fat diet (Figure 3a). Eight weeks after induction of NASH, liver/body weight ratio showed a decreasing trend in Dectin-1-deficient mice, but there was no significant difference (Figure 3b). Although scoring of NASH in liver tissue sections showed no significant difference between WT and Dectin-1-deficient mice (Figure 3c), detailed examination of the cell population by FACS revealed a decreased infiltration of neutrophils and monocytes into the liver (Figure 3d). Furthermore, liver fibrosis, a characteristic pathology in the NASH model, was reduced in Dectin-1-deficient mice (Figure 3e). The production of IL-1β in serum was decreased in Dectin-1-deficient mice, although that of TNF and MCP-1 tended to decrease (Figure 3f). Moreover, AST tended to decrease in Dectin-1-deficient mice, while ALT was decreased in Dectin-1-deficient mice (Figure 3g). Interestingly, the agonist activity was increased in the NASH-induced liver (Figure 3h). These findings indicate that hepatitis pathology is improved in Dectin-1-deficient mice.
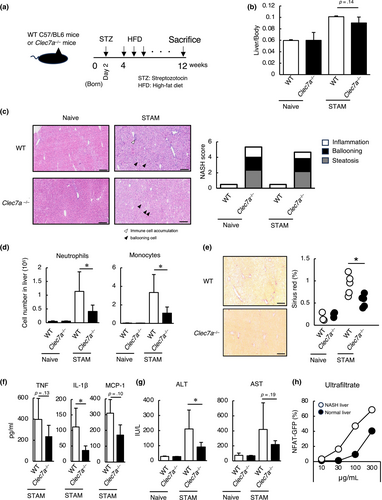
2.4 Hepatic agonist induces inflammation in liver
To investigate whether a hepatic agonist itself cause inflammatory responses in liver, it was intravenously administered to mice (Figure 4a). An accumulation of immune cells was observed in the liver following hepatic agonist administration (Figure 4b). Detailed examination revealed infiltration of neutrophils and monocytes, and increased production of TNF and IL-1β in the serum (Figure 4c,d). To examine whether this immune activation is mediated by Dectin-1, bone marrow-derived macrophages (BMDMs) were stimulated with a hepatic agonist. Wild-type BMDMs produced TNF and IL-1β, but the production was attenuated when Dectin-1 was deficient (Figure 4e). Partial decrease of the cytokine production may be attributed to recognition of the ligand through TLR2 (Figure S1b). These findings indicate that a hepatic agonist activates innate immune responses via Dectin-1. This study demonstrates that Dectin-1 recognizes an endogenous agonist in liver and triggers an innate immune response, and that Dectin-1 contributes to the exacerbation of NASH.
3 DISCUSSION
We found that Dectin-1 recognizes an endogenous agonist found in the liver and that Dectin-1 can contribute to the exacerbation of NASH. Myeloid cells expressing Dectin-1 produce proinflammatory cytokines such as TNF and IL-1β (Figure 4e), which are known to pathogenic factors (Weston et al., 2019). Thus, it is reasonable that Dectin-1 contributes to NASH. Dectin-1 is widely expressed in myeloid cells, and it is possible that tissue macrophages respond to a hepatic agonist released by hepatocellular injury and attract neutrophils and monocytes into liver.
We showed the possibility that endogenous α-glucan is recognized by Dectin-1, while the innate receptor recognizes exogenous β-glucan typically. β-Glucan is not present in mammal; thus, this glucan is exploited for a pathogen associated molecular pattern (PAMP). Germ-free mice-derived liver possess an agonist activity that supports the endogenous ligand is not a PAMP (Figure 1c) and β-glucanase did not abolish the agonist activity (Figure 1d), suggesting that an endogenous agonist is not β-glucan. Importantly, amylase dampened the activity of agonist, strongly suggesting that the active component is glycogen, which is an endogenous α-glucan. Indeed, it has been reported that α-glucan from fungi can activate innate immune response through Dectin-1 (Xisto et al., 2023), thus these results support the possibility that glycogen is a Dectin-1 agonist. While the amino acids at W221 and H223 in Dectin-1 were already shown to be important for the β-glucan recognition (Adachi et al., 2004), the mechanism of α-glucan recognition was unknown, thus we analyzed the recognition mechanism of a hepatic agonist through Dectin-1 using reporter assays with its mutants (Figure 1f). This result suggests that amino acids W221 and H223 in Dectin-1 were also essential for the recognition of an endogenous agonist. On the other hand, the site in the ligand that is recognized by Dectin-1 is uncovered. It is also noteworthy that hepatic agonist is present at very low abundance, that is, 0.003% of total liver in terms of yield (Figure 2a), while around 5% glycogen of total liver is collected from liver in general (Deng et al., 2016). Thus, it is possible that hepatic agonist has unique modifications and linkages not found in the most glycogens, suggesting that a hepatic agonist may be an abnormal glycogen possessing potent agonist activity, though further structural analysis of agonist is required.
We demonstrated the presence of murine liver-derived endogenous Dectin-1 agonist, which may be an abnormal glycogen, emphasizing the physiological relevance of this finding (Figures 2 and 3). Abnormalities in glycogen-metabolizing enzymes cause a condition called glycogen storage diseases (Kanungo et al., 2018). In addition, it has been recently reported that intracellular metabolite accumulation induces inflammation and contributes to the exacerbation of the pathology through an activating C-type lectin receptor (Shimizu et al., 2023). Although a hepatic agonist is not considered to be a major component based on the low yield, the agonist may act as a DAMP in NASH when the agonist is abnormally accumulated. The effect of Dectin-1 function was more prominent in the NASH model than in CCl4-induced hepatitis. The STAM model is based on type 2 diabetes and western diet, thus normal glycogen is thought to be degraded into glucose (Petersen et al., 2017), suggesting the possibility that the ratio of abnormal hepatic agonist is greater, and Dectin-1-endogenous agonist axis may contribute to the exacerbate NASH. Indeed, comparison of activity of murine hepatic hydrophilic fraction from normal and NASH livers showed that the agonist was enriched in the NASH liver (Figure 3g). Thus, murine hepatic agonist is enriched in the liver under NASH conditions, which may suggest that disruption of homeostasis of glycogen metabolism is a risk factor for NASH. NASH has been reported to be exacerbated after rapid weight loss (Tsai et al., 2017), which may also be due to the host receptor sensing of increased murine Dectin-1 agonist levels in liver caused by starvation and damage of hepatocytes.
Interestingly, the pathologies of CCl4-induced hepatitis between WT and Dectin-1-deficient mice were not changed, while inflammatory cytokines were decreased in Dectin-1-deficient mice. There was no difference in infiltration of monocytes in WT and Dectin-1-deficient liver in CCl4-induced hepatitis, which is thought to contribute to hepatitis exacerbation (Cai et al., 2019). MCP-1 tended to be decreased in NASH model of Dectin-1-deficient mice (Figure 3f), which may improve pathophysiology in the NASH model due to decrease of monocytes, although the production of MCP-1 of WT mice was comparable to that of Dectin-1-deficient mice in CCl4-induced hepatitis. These results suggest that the level of chemokines via the Dectin-1 may related to be pathology of hepatitis.
Administration of a hepatic agonist to mice induces infiltration of innate immune cells such as monocytes and neutrophils into the liver and increased production of inflammatory cytokines, such as TNF and IL-1β, that may contribute to hepatitis exacerbation (Figure 4c,d) (Krenkel & Tacke, 2017). This innate immune activating capacity of hepatic agonist may be mediated by Dectin-1, as evidenced by reduced cytokine production in Dectin-1-deficient macrophages (Figure 4e). This emphasizes the direct recognition of Dectin-1 in the innate immune response induced by hepatic agonist in vivo. It was reported that an inhibitor of Syk, a downstream mediator of Dectin-1, suppressed the pathogenesis of NASH (Kurniawan et al., 2018). On the other hand, deficiency of Card9, a major downstream molecule of Syk, exacerbated NASH (Liu et al., 2024). This may be due to the suppression of the anti-inflammatory response in Card9-deficient mice (Liu et al., 2024). Indeed, several C-type lectin receptors can produce IL-10, an anti-inflammatory cytokine, via Card9 (LeibundGut-Landmann et al., 2007; Robinson et al., 2009) as well as inflammatory cytokines. Although Card9 is downstream of C-type lectin receptors, Dectin-1 differs from Fc receptor gamma chain-coupled receptors and exploits Raf-1-mTOR pathway (Mata-Martínez et al., 2022) and inhibition of mTOR ameliorates NASH (Feng et al., 2022; Gosis et al., 2022), suggesting the possibility that Dectin-1-Raf-1-mTOR pathway may be important in the pathogenesis of NASH exacerbated by Dectin-1.
In this study, we show that Dectin-1 partially contributes to NASH-induced inflammation. This is reasonable since various innate immune receptors have been reported to contribute to NASH-induced inflammation (Ganz & Szabo, 2013; Huang et al., 2023). Combining drugs that collectively inhibit innate immune receptors may provide a synergistic inhibition of inflammation and a new therapeutic approach to NASH.
4 EXPERIMENTAL PROCEDURES
4.1 Animals
Clec7a−/− mice on a C57BL6/J background was established through CRISPR-Cas9 (Sander & Joung, 2014) (Figure S2). Target sequence was produced by CHOPCHOP (https://chopchop.cbu.uib.no/results/1703662351633.6182/details/22). Forward typing primer is TTTCAGGGTTTGGGTTAGTGAG, and reverse typing primer is CTACTTCTGTTATTTCAGTTAATGAGAACAAGG. Aged and sex matched wild type control animals were obtained from CLEA Japan (C57BL6/J). All mice were bred and maintained under specific pathogen-free conditions and provided with standard laboratory food and water ad libitum. All experiments were conducted according to the guidelines for animal care approved by the Research Institute for Microbial Diseases, Osaka University.
4.2 In vitro analysis
2B4-NFAT-GFP reporter cells expressing murine Dectin-1, murine Dectin-1 W221A variant or murine Dectin-1 H223A were prepared as described previously (Takano et al., 2017). Reporter cells were suspended by 10% FCS supplemented RPMI1640 media (R8758, Sigma-Aldrich) at 300,000 cells/mL and 100 μL of the cell suspension was dispensed into 96-well plate (3596, Corning). The cells were stimulated at 37°C CO2 incubator for 16–20 h as indicated in the figure legends. After stimulation, the cells were harvested and GFP expression of the cells was analyzed using flow cytometry (Gallios, Beckman Coulter). For enzyme treatment, murine hepatic agonist was treated with none, amylase (A9857, Sigma-Aldrich) cellulase (C1184, Sigma-Aldrich), or westase (9005, TAKARA Bio) in citrate buffer (pH 4.5) for 16–20 h at 37°C. TLR2 or TLR4 reporter HEK cells were stimulated with purified fraction for 16–20 h at 37°C. The supernatants were incubated with QUANTI-Blue solution™ for 1 h at 37°C and the samples was measured at OD 630 nm. Bone marrow cells were differentiated into BMDMs by culturing in 10 mL of 10% MGM-5 culture supernatant and 10% FCS supplemented DMEM (D0822, Sigma-Aldrich) at a concentration of 500,000 cells/mL for 6 days. The macrophages were harvested by EDTA-PBS and washed by 10% FCS supplemented DMEM twice. For stimulation, the cells were suspended by 10% FCS supplemented DMEM at 500,000 cells/mL and 100 μL of the cell suspension was dispensed into 96-well plate (3596, Corning) and co-cultured with curdlan (tlrl-curd, Invivogen), zymosan (Z4250, Sigma-Aldrich), crude component from murine liver (G0885, Sigma-Aldrich), hepatic agonist (10, 100 μg/mL) or LPS (10 ng/mL) (L4516, Sigma-Aldrich) in total 200 μL of culture media for 24 h at 37°C with 5% CO2. TNF and IL-1β in supernatant was measured by ELISA (560478, BD OptEIA™, 432615, Biolegend).
4.3 Ligand isolation and treatment
For ligand purification, murine livers were homogenized with DDW and the supernatant was collected. The hydrophilic fraction was added up to 50% (v/v) ethanol and centrifuged (4°C, 10,000 rpm, 15 min) to precipitate low active component. The supernatant was adjusted at concentration of 85% (v/v) ethanol and centrifuged (4°C, 10,000 rpm, 15 min) to precipitate high active component. The precipitant was resuspended by DDW and ultrafiltered at 10 kDa filter (VS2001, Vivaspin 20, SARUTORIUS) and then the concentrated components were divided into 20 fractions using AEC (HiPrep™ DEAE Fast Flow 16/10, Cytiva). The flow-through fractions were combined and separated using gel filtration column chromatography (Superdex™ 200 10/300 column, Cytiva). For α-glucan measurements, a starch assay kit (Cell Biolabs, Inc.) was used.
4.4 Hepatitis models
For CCl4 hepatitis model, wild-type or Clec7a−/− male mice (8–10 weeks) were injected with 50 μL of CCl4 diluted corn oil (035-01273, Wako) or corn oil as vehicle (032-17016, Wako) intraperitoneally at a dose of 0.5 μL/g body weight twice a week for 4 weeks, that is, mouse that weighs 20 g is injected with 10 μL CCl4 in total 50 μL corn oil. Mice were euthanized and serum was collected, and livers were harvested. For NASH model, wild-type or Clec7a−/− male mice were injected with a single subcutaneous injection of 200 μg streptozotocin (STZ) (191-15151, Wako) at 2 days after birth. The mice were fed with HFD32 (CLEA Japan) from 4 weeks. After 8 weeks, mice were euthanized and serum was collected, and livers were harvested. ALT and AST in serum were measured by Oriental Yeast Co., Ltd (Tokyo, Japan). To evaluate agonist function in liver, wild-type or Clec7a−/− male mice (8–10 weeks) were injected intravenously with murine hepatic agonist (200 μg/head), curdlan (200 μg/head), or PBS. After 2 days, mice were euthanized and serum was collected, and livers were harvested. TNF, IL-1β, and MCP-1 in serum were measured by ELISA kit (560478, BD OptEIA™, 432615, Biolegend, 555260, BD OptEIA™). Liver cells were collected using Liver Dissociation Kit (130-105-807, Miltenyi) and analyzed by flow cytometry. All animal protocols were approved by the committee of Ethics on Research Institute for Microbial Diseases, Osaka University.
4.5 Flow cytometry
To obtain single cell suspensions for flow cytometry, whole livers were cut into pieces and digested by Liver Dissociation Kit (130-105-807, Miltenyi). Digested lung tissue was passed through a 70 μm cell strainer (352350, Falcon), and the cells recovered by centrifugation at 4°C. Following ACK buffer, cells were collected by centrifugation, resuspended in RPMI1640 media with 2% FBS twice and passed through a 40-μm cell strainer (352340, Falcon). Liver single cells were stained with the myeloid flow cytometry antibody cocktail: PE rat anti-mouse Ly6G (clone: 1A8, BD Biosciences), Alexa488 rat anti-mouse CD11b (clone M1/70, Biolegend), PE-Cy7™ rat anti-mouse Ly6C (clone: AL-21, BD Biosciences), BV421 rat anti-mouse CD45 (clone: 30-F11, Biolegnd). A blocking agent was added to the cells before staining for 30 min on ice to prevent non-specific binding of the antibodies: mouse BD Fc block™ rat anti-mouse CD16/CD32 antibody (clone:2.4G2, BD Biosciences). After centrifuging, pellet cells were suspended by cocktail. After staining, cells were washed, centrifuged, and resuspended in 2% RPMI1640 media. Data acquisition was performed using Gallios (Beckman Coulter). Flowjo software (v10.8.1) was used for post-acquisition analysis. For the current study, neutrophils were defined as CD11b+Ly6G+, inflammatory monocytes as CD11b+Ly6C+ in CD45+ cells.
4.6 Histological analysis
For the assessment of histopathology of liver, two H&E-stained sections per mouse were analyzed for score of steatosis, ballooning, and inflammation based on a previously described method (Kleiner et al., 2005). For the evaluation of fibrosis of livers from CCl4-induced hepatitis mice and NASH model mice, fibrosis positive aria was calculated by Image J in three randomly selected fields per sirius red-stained sections (Yamaguchi et al., 2007). Liver tissue sections were stained by staining kit (PSR-1, ScyTek Laboratories, Inc.) To count the inflammatory foci in liver following ligand administration, three sections were analyzed in the left lobe and five sites were observed per section in a 200× field of view.
4.7 Statistics
An unpaired two-tailed Student's t test was used for all the statistical analyses. Asterisks denote level of statistical significance (*p < .05).
ACKNOWLEDGMENTS
We thank M. Kurata, S. Iwai, and Y. Takeuchi for technical support; E. Ishikawa, K. Toyonaga, M. Watanabe, C. Kojima for discussion; M. Tanaka, Y. Baba, K. Kaseda, and M. Ikawa for embryonic engineering.
FUNDING INFORMATION
This work was supported by The Japan Agency for Medical Research and Development (JP21ak0101070) and the Japan Society for the Promotion of Science KAKENHI (JP22H05183). This work was also supported, in part, by the National Institutes of Health (NIH) Grants R01GM119197 and R01GM083016 to D.L.W.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.




