A Review of Analytical Techniques to Characterise Nanomaterial Associations with Minerals, Organic Matter and Organisms
Abstract
Nanomaterials (NMs) have unique properties and control processes relevant to the fate of contaminants in soils, air, and aquatic systems and within the carbon cycle. Many NMs often occur in association with larger mineral grains, organic matter, or living organisms such as microbes, plants and fungi. The preservation of the spatial, textural, chemical, and mineralogical relations between NMs and minerals, organic matter, and organism (NM-associations) is of fundamental importance as it provides information about the origin and formation mechanisms of NMs. Here we review analytical approaches and techniques to study NM-associations at the bulk-, micro-, nano- and atomic-scale. We will focus on (a) X-ray diffraction and mass-spectroscopy techniques; (2) automatisms within software packages that permit the search of features without operators; (3) preparation and analytical techniques such as the focused-ion beam technology, transmission electron microscopy and atom probe tomography; (4) nano-spectroscopic techniques such as tip-enhanced Raman spectroscopy, synchrotron infrared nanospectroscopy, and nano-X-ray fluorescence spectroscopy; (5) ptychographic X-ray computer tomography. This review paper concludes with selected new perspectives such as (a) the characterisation of NM-precursors, (b) the role of NM-associations in the stabilisation of soil organic matter and (c) the interaction of NM-associations in wildfire smoke with contaminants from other sources.
Nanomaterials (NMs) composed of minerals make up by far the bulk of this planet and are a major part of the critical zone of the Earth. Nanomaterials have been considered one of the principal catalytic components of Earth throughout its history (Hochella et al. 2019), which include for example their roles in the polymerisation and self-assembly of the molecular building blocks of life during early evolution (Ferris and Ertem 1992, Oleson et al. 2010, Xu et al. 2012). Despite their importance through time, NMs are not considered in studies of biogeochemical processes throughout Earth's history, and overall are rarely considered major components of the Earth. Reasons for this may have been a lack of analytical tools and procedure to quantify the presence, size, shape and chemical and mineralogical composition of NMs. Only in the past 25 years, the properties of NMs have been rigorously studied and have been addressed in many review papers (Table 1). The majority of NMs form during weathering and alteration processes of their parent minerals or through heterogeneous nucleation processes involving bacteria, fungi and plants. Hence, associations of NMs with these environmental constituents are of great importance to understand their origin, properties and chemical and mineralogical composition. This review paper addresses the current state of analytical techniques that are used to characterise NMs in associations with minerals, organic matter and organisms.
| Review paper on preparation and analytical techniques to characterise nanoparticles | References |
|---|---|
| Nanoparticles/ultra-fine particles in air | (Kittelson 1998, Buseck and Adachi 2008, Karjalainen et al. 2014, Rabajczyk et al. 2020) |
| Nanoparticles/colloids in aquatic systems | (Lead and Wilkinson 2006, Baalousha et al. 2009, Delay and Frimmel 2012, Santschi 2019, Singh et al. 2023) |
| Nanoparticles in soils | (Wilson et al. 2008, Dinesh et al. 2012, Cornelis et al. 2014, Ahmed et al. 2021, Ameen et al. 2021, Campos et al. 2022) |
| Nanoparticle size characterisation with non-microscope-based analytical techniques | (Xie et al. 2022) |
| Nanoparticles from coal combustions | (Saikia et al. 2018, Silva et al. 2022) |
| Carbon nanoparticles/PM in air | (Islam and Saikia 2022) |
| Analytical chemistry of metallic nanoparticles in natural environments | (Silva et al. 2011a) |
| Combustion-derived nanoparticles | (Donaldson et al. 2005) |
|
Soil organic matter with examples for characterising organic matter-mineral associations |
(Weng et al. 2022) |
| Review papers on preparation and analytical techniques | |
| Analytical techniques for features at the bulk- and micrometre-scale | |
| Identification and quantification of minerals with X-ray diffraction and the Rietveld method | (Santini 2015, Zhou et al. 2018, Ali et al. 2022) |
| Laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) | (Maloof et al. 2020, Balaram et al. 2022) |
| Spectroscopy techniques in Earth Sciences (Fourier transform infra-red (FTIR), Raman, Mössbauer, X-ray absorption fluorescence spectroscopy (XAFS) | (Berthomieu and Hienerwadel 2009, Newville 2014, Rostron and Gerber 2016, Jones et al. 2019, Terzano et al. 2019) |
| Surface analytical techniques in Earth Sciences | (Qian et al. 2015, Greczynski and Hultman 2020, Krishna and Philip 2022) |
|
Time-of-flight secondary ion mass spectrometry (ToF-SIMS) |
(Fearn 2015) |
| Micro-proton-excited X-ray emission analysis (micro-PIXE) | (Pallon et al. 2017) |
| Micro-X-ray fluorescence spectroscopy (micro-XRF) | (Fittschen and Falkenberg 2011, Kaskes et al. 2021, Vanhoof et al. 2021, Guilherme Buzanich 2022) |
| Scanning electron microscopy (SEM)/electron microprobe analysis (EPMA) | (Reed 2005, Rinaldi and Llovet 2015, Llovet et al. 2021) |
| Secondary ion mass spectrometry (SIMS) | (van der Heide 2014, Huang et al. 2017, Walker 2017) |
| Spectroscopic techniques for features at the micro- and nanometre-scale | |
| Nano-X-ray absorption spectroscopy and nano-X-ray fluorescence spectroscopy | |
| Surface enhanced Raman spectroscopy (SERS), tip-enhanced Raman spectroscopy (TERS), synchrotron infrared nanospectroscopy (SINS) | (Stiles et al. 2008, Dierolf et al. 2010, Langelüddecke et al. 2015, Bechtel et al. 2020) |
| Preparation techniques for subsequent characterisation at the nanometre-scale | |
| Focused ion beam technique | (Heaney et al. 2001, Wirth 2004, Giannuzzi and Stevie 2005, Wirth 2005, Kim et al. 2017), Gu et al. (2020) |
| Ultramicrotomy and ion milling | (Heaney et al. 2001, Wirth 2004, Ayache et al. 2010, Lee 2010, Li 2012, Wen et al. 2015, Tizro et al. 2019)) |
| Analytical techniques for features at the nanometre- and atomic-scale | |
| Transmission electron microscopy (TEM) | (Lee 2010) |
| Atom probe tomography (APT) | (Gault et al. 2012, Lefebvre et al. 2016, Saxey et al. 2018, Reddy et al. 2020) |
| Electron energy-loss spectroscopy (EELS) | (Avouris and Demuth 1984, Hofer et al. 2016) |
| Ptychographic X-ray computed tomography (PXCT) | (Dierolf et al. 2010, Pfeiffer 2018) |
Nanomaterials are organic, inorganic, or organometallic materials with size- and shape-dependent chemical, physical, and/or electrical properties (Hochella et al. 2019). They may be atomically ordered (crystalline), disordered, or amorphous, and range in size from less than a nanometre up to several tens of nanometres in at least one direction. Their properties depend on their chemical and mineralogical composition and small compositional or structural changes can result in very different chemical and/or physical properties. Contrary to their macroscopic counterparts (with the same mineralogical composition but with sizes larger than tens of nanometres in each direction), NMs have dramatically different chemical and physical properties that affect Earth systems in many important ways. For example, nanometre-size iron oxyhydroxides affect in contrast to their macroscopic counterparts the productivity of phytoplankton in oceans, which control the sequestration of carbon and consequently the carbon cycle and global temperatures (Coale et al. 1996, Blain et al. 2007).
The decrease in size from macroscopic minerals to NMs is associated with a decrease in the total number of atoms and with an increasing fraction of surface atoms (Banfield and Zhang 2001). An increasing fraction of surface atoms leads to a higher degree of surface hydration and protonation, which often results in the weakening of bonds in the surface structure and thus in an increasing destabilisation of NMs (Banfield and Zhang 2001). Depending on the nature of the surrounding environment, this destabilisation leads often but not always to a higher solubility of NMs relative to their larger counterparts (Tang et al. 2004, Dutta et al. 2017, Ramos et al. 2017, Saikia et al. 2018, Sánchez-Peña et al. 2018, Oliveira et al. 2019, Lütke et al. 2020), an important aspect in terms of the bioavailability of toxic elements incorporated into NMs.
The fewer atoms in NMs result in fewer electron energy levels (as observed in micro- to bulk-size materials), and electron energy bands become discrete electron energy states in NMs, thus affecting their physical properties such as conductivity and light absorption (i.e., colour) (Hochella 2008). Higher surface reactivities and different physical properties also alter growth, dissolution, melting, evaporation, and aggregation state of NMs and thus play key roles in their environmental fate. The larger surface area of NMs relative to their larger counterparts also allows them to sorb and transport more effectively solutes, controlling often the fate of hazardous organic compounds and toxic elements in contaminated areas of the critical zone (Hochella and Madden 2005).
The stability of NMs towards dissolution, aggregation and transformation depends among many factors, on their size (which controls their surface energy), surface charge (controlled by pH), the presence of counterions and the presence of polymerised species on the surfaces. All these factors control for example, the stability of Fe-(hydr)oxides (Fe-Ox) (Navrotsky et al. 2008, Guo and Barnard 2013), with adsorbed silica species being the most prominent species that can stabilise FeOx NMs. It should be noted, and this is important for the later discussion, that (a) NMs can be also products of the aggregation of even smaller constituents such as clusters in aqueous solution (considered precursors of NMs); (b) the interaction of NMs with organic matter leads to the stabilisation of the latter and (c) dissolution, aggregation and transformation as well as adsorption processes involving NMs do not only occur in the critical zone but also in the atmosphere, which can result in modified particulate matter (PM) released by for example wildfires. One commonly distinguishes three groups of NMs: (1) Natural NMs which are part of the element cycling on Earth and which form through abiotic and biotic-controlled processes; (2) Incidental NMs which are unintentionally by-products of human activity and have steadily increased since the beginning of the Industrial Revolution; (3) engineered NMs used in many applications due to their designed physical and chemical properties. As the latter group of NMs is less common in the environment, we will focus in this review on natural and incidental NMs (Hochella et al. 2019).
Objectives
Nanomaterials form during abiotic and biotic alterations of minerals or organic matter or nucleate on their surfaces or within their pore spaces. Consequently, NMs are often associated with larger constituents such as mineral grains, organic matter, and organisms such as microbes, fungi and plants. In many cases, structure, stability, chemistry, charge, electronic and magnetic properties and reactivity of NMs are products or controlled by the interaction of NMs and their host materials (Becker et al. 2010). Hence, a thorough understanding of the formation and long-term environmental fate of NMs require the characterisation of associations between NMs and minerals, organic matter and organisms (from here on referred to as NM-associations) as individual entities rather than focusing exclusively on NMs.
The study of NM-associations should include the chemical and mineralogical characterisation of NMs as well as of their hosts, their spatial and textural relation and in the case of their biotic-controlled formation, the identification of the microbial community composition. The analytical characterisation of NM-associations is however challenging as the chemical and physical properties of NMs and associated larger materials and organisms can greatly differ from each other. For example, the chemical and mineralogical characterisation of NMs at the bulk scale is often hindered due to their smaller volume and lower degree of crystallinity relative to their hosts. Furthermore, the textural characterisation of the interface between NMs and their micrometre-size host is not straight forward as this interface is fragile due to differences in hardness and porosity and thus cannot often be preserved during preparation for electron microscopy studies. Here, the range of small versus large volumes of NMs and soft NMs versus hard micrometre-size hosts varies for example between NM-bearing mineral surface coatings in sand- or sandstone-based aquifers to NMs in clay-rich soils to apatite-precursors in association with bioapatite. In sand or sandstone aquifers, NMs are commonly undetected with bulk analytical techniques due to the high proportion of quartz and the heterogeneous and nanocrystalline character of the coatings (Coston et al. 1995, Penn et al. 2001). The phases in the latter coatings are often hydrous and soft in comparison to the hard micrometre-size quartz grains. In clay-rich soils, NMs occur in large volumes as detectable clay minerals, whereas only a small fraction of NM-precursor phases to clay minerals such as amorphous Al-silicates, Fe-Ox and silica remain still undetected with bulk analytical techniques (Tazaki and Fyfe 1987). The latter phases occur commonly on the surface of weathered silicate minerals such as minerals of the feldspar group and are much softer and hydrous relative to their hard micrometre-size silicate minerals. In the case of nano-size precursors to bioapatite such as amorphous calcium phosphate (ACP) and/or octacalcium phosphate (OCP) (Brown and Chow 1976), the difference in their hardness with respect to bioapatite is smaller than between clay minerals and quartz/feldspar and their associations are less difficult to prepare for studies at the micro- and nanometre scale (Witzke et al. 2022). Considering possible differences in volume and physical properties of NMs relative to their macroscopic counterparts, the objectives of this review are: (1) to provide a short overview on different types of NM-associations identified in sediments, soils, ore deposits, tailings and PM, (2) to describe common analytical techniques used for their characterisation, (3) to recommend analytical approaches and to point out advantages and disadvantages of preparation techniques, (4) to suggest new analytical approaches for the study of NM-associations in soils and PM.
There have been numerous review papers on analytical and preparation techniques for the characterisation of NMs in the environment (Table 1), but these reviews focused mainly on NMs rather than on NM-associations. Similarly, reviews exist for each of the below-listed analytical- and preparation techniques (Table 1) and readers interested in detail in one of the techniques should refer to those. This review paper will also not address analytical or computational techniques used to characterise NM-associations in experimental studies (e.g., atomic force and scanning tunnelling microscopy studies of mineral surfaces modified in laboratory experiments). Readers should refer here to the review articles by Becker et al. (2010) and Putnis and Ruiz-Agudo (2021).
Occurrence of nanomaterials in association with minerals, organic matter and microbes
This section addresses the formation of natural and incidental NMs and the environments in which they are associated with minerals, organic matter and microbes. Silicates, oxides, phosphates, and other minerals that are unstable under Earth's surface conditions dissolve or react to form other phases. Intermediate products of this equilibration process are often natural NMs such as amorphous gel-like precursors (Combes et al. 1989, Schindler et al. 2009a, Mantha et al. 2019), which transform into NMs such as amorphous or opaline silica (Jones et al. 1966, Guthrie et al. 1995, Banfield and Barker 1998), hydrous aluminosilicates such as allophane (Henmi and Wada 1976, Wada and Wada 1977), the tubular aluminosilicate imogolite (Gustafsson 2001, Schindler et al. 2019), clay minerals such as kaolinite (Schindler et al. 2019), oxides (e.g., magnetite and haematite; (Banfield and Eggleton 1990, Schindler et al. 2019) and oxyhydroxides (e.g., ferrihydrite and goethite, Schindler et al. (2019), Figure 1a). Note that the minerals allophane and ferrihydrite only form particles in the nano-size range and are termed nanominerals. Other minerals such as 1:1 or 2:1 sheet silicates can undergo further “solid-state conversion” (which is essentially a nano-scale dissolution-reprecipitation process within the sheet structure) to form either interstratified clay minerals (mixtures between different types of sheet silicates) or sheet silicates with a different structure-type such as chlorite (Środoń 1999). During these types of weathering processes, minor elements in the parent material such as Ti, Fe, Cr and Au may be expelled and re-precipitate as anatase (TiO2), Fe-Ox, chromite and gold NMs (Schindler et al. 2017). Hence, a first generation of NMs can further transform via dissolution-reprecipitation processes leading to the formation of numerous types and generations of NMs.
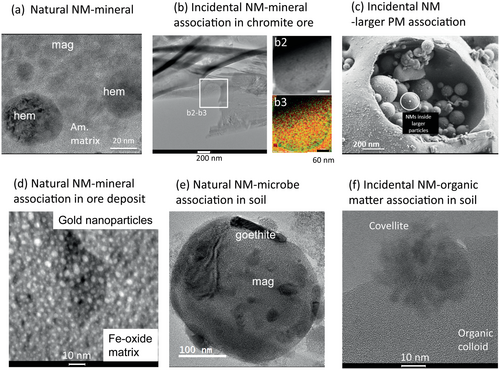
In the case of an incomplete equilibration process of the mineral (parent phase), newly formed NMs (daughter phases) are often associated with the parent phase, and the spatial and textural relations between the different daughters and parent provide insights into the underlying mechanisms of the equilibration process and in the sequence of formation of the daughters (Figure 1) (Schindler and Hochella 2015, 2016). Similarly, NM-mineral or NM-organic matter associations can form when fluids enter pore spaces of altered rocks, minerals and organic matter in which changes in the environmental conditions or number of nucleation or reactive surface sites leads to higher degrees of supersaturations with respect to the NM (Figure 1; Jadoon and Schindler 2022, Jadoon et al. 2022, Alavijeh et al. 2023). NM-organic matter associations can also form when organic matter sorbs to mineral surfaces in soils (Kleber et al. 2021). In this case, however, the minerals do not have to be nanometric and the formation of mineral-organic matter associations are controlled by the surface charges of the minerals, functional groups of the organic species and charge-neutralising constituents such as extracellular polymeric substances (Guhra et al. 2019).
Contrary to porous mineral- and organic matrices, the formation of NMs within denser high-T minerals (e.g., sulfides, oxides and silicates) occurs when (a) the concentration of an element exceeds the amount that can be structurally incorporated into their hosts (which is temperature dependent) (Reich et al. 2005, 2006); (b) the formation of NMs is energetically more favourable than the structural incorporation of its constituents into the mineral structure (Becker et al. 2010); (c) the growth of the host material is slow and thus provides enough time for the assembly of ions towards NMs (Fougerouse et al. 2016). Well-known examples are the formation and diffusion of Au nanoparticles in pyrite, arsenopyrite, amorphous silica, calcite, rutile and Fe-Ox within epithermal, porphyry and orogenic ore deposits (Reich et al. 2005, Reich et al. 2006, Hough et al. 2011, Fougerouse et al. 2016, Saunders and Burke 2017, Hastie et al. 2021, McLeish et al. 2021).
The formation of NMs such as Fe-Ox, aluminosilicates, Ca-carbonates, and Ca-phosphate can be also induced or controlled by biological processes (Banfield and Zhang 2001, Schindler and Hochella 2015, Sharma et al. 2015). During biologically induced mineralisation, NMs are formed indirectly through redox reactions in the microbial environment related to metabolic processes such as the oxidation of Fe2+ to Fe3+ or the reduction of sulfate to sulfide (Sharma et al. 2015). In contrast, the formation of NMs during biologically controlled mineralisation is entirely controlled by organisms. Here, NMs form in the microbial cells under certain conditions and are composed of well-defined crystals with narrow particle-size distributions (Sharma et al. 2015). Nanomaterials formed by biologically controlled mineralisation have various functions for the organisms such as the use of magnetite nanoparticles for navigation purposes in magnetotactic bacteria (Schüler and Frankel 1999). Similar to NM-mineral associations, preservation and examination of the spatial and textural associations between NMs and microbes, fungi and plants is of great importance for our understanding of the role of living organisms in alteration processes and thus in the re-cycling of elements within the Earth's crust (Finlay et al. 2019, Wild et al. 2022).
To conclude, natural NMs can form during abiotic- and biotic-induced and controlled processes with weathering processes providing the largest amount of NMs (Hochella et al. 2019). In comparison, incidental NMs are released as NMs during human activity or form during weathering or transformation of by-products of human-activities. Nanomaterials are directly released from human activities during for example combustion, smelting, metallurgical processes and transportation, whereas weathering and transformation of by-products can occur in mine tailings, aquatic bodies, water treatment plants, soils or even shooting ranges (Schindler et al. 2020). Incidental and natural NMs formed during weathering have similar chemical and physical properties whereas incidental NMs directly emitted as NMs can have unique chemical and physical properties rarely observed in the environment. For example, high-T combustion and smelting processes can result in the release of highly reactive NMs such as O-deficient Ti-oxide phases (so-called Magnelli phases) and metallic Pb (Batonneau et al. 2004, Yang et al. 2017), which have not been identified as products of wildfires, ore-forming processes or volcanic activity.
NM-associations involving incidental NMs occur for example in mine tailings. Here, NMs such as ferrihydrite, schwertmannite and jarosite are common constituents and form on the surfaces of primary Fe-sulfides and silicates during their alteration (Bigham and Nordstrom 2000, Blowes et al. 2003). The stability of these NM-associations is of great environmental concern as the corresponding NMs can sequester and mobilise metal(loids) such as As, Pb, Cd, Cu and even transport those for many kilometres within a hydrographic basin (Rodriguez-Iruretagoiena et al. 2016). Incidental NM-associations also occur in mining- and smelter-affected soils. For example, elementary Cu and Ag nanoparticles as well as Cu- and Ag-sulfides and -oxide nanoparticles form in pore spaces of organic matter within the surface layers of organic-rich soils (Figure 1f) (Mantha et al. 2019, Jadoon and Schindler 2022, Jadoon et al. 2022, Alavijeh et al. 2023).
Similar to mine tailings, coal combustion and oil refineries are major anthropogenic activities that generate NM-associations. For example, NMs emitted by oil refineries include fullerenes, graphenes, C nanotubes, and several amorphous phases (Sanchís et al. 2013), whereas those emitted by coal power plants and coal combustion processes are chemically and mineralogically more complex and are composed of fullerenes, C-nanotubes, soot and tar particles as well as amorphous phases, mainly sulfate- and silicate-minerals and complex mixtures of amorphous phases and minerals (Kronbauer et al. 2013, Sanchís et al. 2013, Wilcox et al. 2015, Ward 2016, León-Mejía et al. 2018, Silva et al. 2022, Figure 1c). Carbon-based nanoparticles such as soot are also emitted from wildfires and can interact with other atmospheric components such as sea spray and mineral dust forming micrometre-size NM-associations (Donado et al. 2023). These examples show that incidental NM can also occur in NM-associations, and that those play an important role in the biogeochemical cycling of elements and their impact on environment and health.
As indicated above, characterisations of NM-associations rather than solely NMs are necessary to understand their origin and long-term fate but are challenging to their differences in volume and degree of crystallinity relative to their macrometre-size counterparts. We will start this review paper with analytical techniques that characterise NM-associations at the bulk scale before addressing their characterisation at the micro- and nanometre scale.
Analytical techniques to study NM-associations at the bulk- and micrometre-scale
Formations of NM-associations in rocks, soils and tailings are a direct result of the bulk properties of their hosts namely their mineralogical and chemical composition, physical properties such as porosity and environmental conditions such as water-rock ratio, Eh, pH, T and P. Mineralogical and chemical compositions of environmental samples (e.g., soil, suspended and/or precipitated sediments, organic matter, ashes, rocks and PM) are extremely complex and their characterisation requires often multiple preparation methods, analytical techniques and sometimes even thermodynamic calculations on the potential occurrence of NM-associations in environmental samples that have been affected by pressure and temperature gradients (Silva et al. 2022).
In addition, as a number of structural and electronic properties of NMs and their interfaces towards their host are difficult to determine experimentally, molecular simulations can provide a better understanding of nanoscale phenomena at the molecular- and atomic-scale. These molecular simulations commonly include quantum-mechanical calculations, force-field calculations and molecular dynamics (MD) (Becker et al. 2010).
In terms of the analytical characterisation of NM-associations, one can distinguish between techniques that analyse bulk properties versus those that characterise properties as well as textural and spatial information at the micro- to nanometre scale (Figure 2).
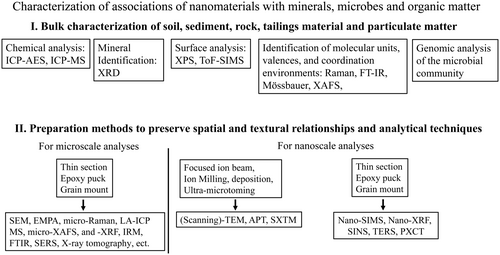
Chemical analysis and powder X-ray diffraction of the bulk material
Inductively coupled plasma-atomic emission spectrometry (ICP-AES) and X-ray fluorescence spectroscopy (XRF) can be used to quantify major, minor and trace elements of a sample.
Powder X-ray diffraction (XRD) identifies minerals with a modal abundance larger than 5% (Santini 2015, Zhou et al. 2018, Ali et al. 2022). The powder diffraction file can be further analysed with the Rietveld method using programs such as SIROQUANT system (Ward et al. 2001, Permana et al. 2013). The Rietveld method allows the quantification of minerals and the amorphous fraction but cannot be used to quantify various types of amorphous NMs within a mixture of amorphous materials.
The identification and quantification of NMs in NM-associations depends on their modal abundances and their degree of crystallinity. For example, magnetite NMs formed during the alteration of volcanic glass can be easily identified with XRD as amorphous glass produces only a broad peak in the diffraction pattern (Schindler et al. 2019). On the contrary, Fe-Ox NMs in mineral surface coatings in soils remain commonly undetected in XRD pattern with diffraction peaks exclusively associated to the underlying soil grains (Schindler et al. 2016).
Organic matter often hosts NMs in organic-rich soils, which remain undetected in an XRD pattern due to their low abundance. The relative proportion of NMs can be, however, enriched through removal of the organic matter during low-temperature ash, a method conducted at low temperature (< 200 °C) in an electronically excited oxygen plasma (Ward et al. 2001, Silva et al. 2011b, Oliveira et al. 2012). Removal of organic matter at a temperature of 370 °C in a conventional oven should be avoided as it results in phase transformations or oxidations within the mineral matrix such as the decomposition of carbonates, sulfides and hydroxides (Ward 2016).
Surface analytical techniques: XPS and ToF-SIMS
Information on the chemical compositions and valences of cations and anions on the surfaces of samples containing NM-associations can be gained with time-of-flight secondary ion mass spectrometry (ToF-SIMS) and X-ray photoelectron spectroscopy (XPS) (van der Heide 2014, Qian et al. 2015, Greczynski and Hultman 2020, Krishna and Philip 2022).
The development of new generations of XPS spectrometers with detectors of higher sensitivities, smaller apertures and charge-compensation systems allow measurements of well-resolved high-resolution XPS spectra from non-conductors samples. Consequently, XPS became one of the most effective techniques for characterising surfaces of NMs (Silva et al. 2013). Chemical and structural information gained with XPS include: (1) the chemical composition of the surface structure (Mantha et al. 2012); (2) structural information on the coordination environment of cations/anions (Schindler et al. 2009b, c); (3) valences of cations and anions on the surfaces (Schindler et al. 2009b, c) and (4) changes in composition with depth using depth profiling (Cerqueira et al. 2011, Mantha et al. 2012). The depth to which this information can be extracted is called information depth and depends on the inelastic mean free path of the respective photoelectrons. It lies for most of the elements in the upper 10 nm (Hochella 1988).
The determination of the chemical composition of a sample with XPS includes low atomic number elements such as N, O, and C (Leung et al. 1999, Haerle et al. 2002, Brydson et al. 2008) as well as the proportion of O, OH and H2O groups (Schindler et al. 2009c). Structural information gained with XPS include for example an estimation of the polymerisation degree of silicate tetrahedra (important in the case of NM-amorphous silica association; Schindler et al. 2010) or the proportion of strongly versus weakly covalent bonds in NM containing for example hexavalent uranium (i.e., uranyl bonds versus the more ionic U-O equatorial bonds; Schindler et al. 2009b).
ToF-SIMS measures the mass of secondary ions emitted from a surface, which is bombarded by a pulsed primary ion beam of typically 25 keV (van der Heide 2014). The primary beam consists of a low flux ion (Bi, Cs or Ga), which results in the excitation of secondary ions from only the uppermost layers of the surface. The secondary ion beam consists of ionised molecular fragments, which are analysed with respect to their mass to charge ratio. In a time-of-flight (ToF) mass spectrometer, the separation is based on the fact that ions of different mass have different velocities (but the same kinetic energy) and thus different flight times towards the detector (Fearn 2015).
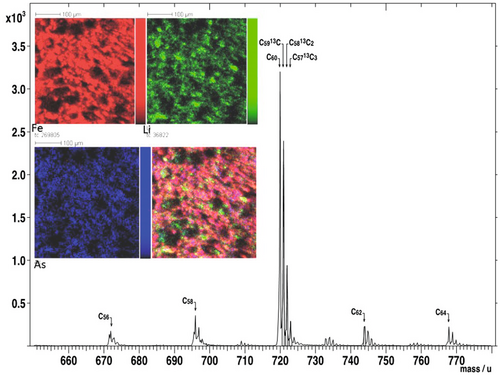
The ToF-SIMS is an important analytical tool for the quantification of chemical elements as well as their isotopes. It creates a complimentary dataset to XPS with respect to the occurrence of major and minor elements on the surface of NM-associations. As both analytical techniques can detect low atomic number elements such as C and as many carbon-bearing NMs have dimensions in the range similar to the depth of information for both instruments, ToF-SIMS and XPS provide valuable information on (a) the occurrence and composition of different carbon-allotropes (e.g., fullerenes) (Cerqueira et al. 2011, 2012, Oliveira et al. 2012) (Figure 3); (b) the chemical composition of nanometre-thick coatings on minerals (Scheidegger et al. 1993); (c) the valence of elements which are exclusively associated with NMs and are absence in their host materials (silica on Fe-hydroxides, (Vempati et al. 1990)).
Fourier Transform Infra-Red (FTIR) and Raman spectroscopy at the bulk scale
The spectroscopy techniques FTIR and Raman are used to identify molecular-size units or functional groups in organic, inorganic, and organometallics phases (McMillan and Hofmeister et al. 1988, Berthomieu and Hienerwadel 2009, Rostron and Gerber 2016, Jones et al. 2019). The strengths of both techniques are to characterise NMs or functional groups of NMs (which cannot be detected with X-ray diffraction) in carbonaceous materials such as glassy carbon, graphitic and amorphous carbon films, pitch and coal fibres, fullerene and activated carbon. For example, Raman spectroscopy can identify structural variations of graphite (crystalline versus highly disordered), their molecular structural order/disorder (short-range order), and their nanostructures (Wang et al. 1990). From the environmental viewpoint, Raman spectroscopy can be used to identify (a) graphite-like carbon in diesel engine soot and (b) different types of soot PM emitted from various sources on the basis of their degree of graphitisation (Nikiel and Jagodzinski 1993). Similarly, FTIR spectroscopy can be used to qualitatively and semi-quantitatively determine aromatic and aliphatic functional groups in soot PM and associated by-products formed during combustion processes. (Paul and Datta 2014, Ulusoy 2020, Samoudi et al. 2022).
Mössbauer spectroscopy
Mössbauer spectroscopy is predominantly used to identify and characterise Fe-bearing NMs such as ferrihydrite (Dyar et al. 2006). The technique is thus a complimentary study to XRD, Raman, and XPS. Structural information gained with Mössbauer spectroscopy includes valence, coordination number and distortion of the Fe-polyhedron due to the interaction of the Fe-bearing NMs with organic and inorganic ligands. An example of a useful application of Mössbauer spectroscopy would be the monitoring of the valence of Fe in NM-associations with depth in soils, sediments, and tailings.
X-ray absorption spectroscopy techniques
X-ray absorption spectroscopy (XAS) has become one of the most popular techniques to study the valence and crystal chemical environment of an element of interest in complex environmental samples (Newville 2014, Terzano et al. 2019). Its application in Earth and Environmental Sciences has been reviewed numerous times (Table 1). Information in an XAS spectrum can be gained in the structure of the spectrum near the edge and at the extended part of the edge. The X-ray absorption near edge structure (XANES spectrum) can be used to determine the valence and coordination geometry, whereas the extended part of the edge (EXAFS) can be used to determine the local molecular structure of a particular element within a sample (Newville 2014). The crystal chemical information gained with these XAS techniques represents an average over the entire bulk samples and is thus more representative than data collected at the micro- and nanometre-scale. Many beamlines also allow measurements of XAS spectra from micrometre-size features (micro-XAS) and 4th generation synchrotron beamlines even permit the recording of XAS spectra from features at the nanometre scale (nano-XAS) (see below).
Genomic analysis of the microbial community
An understanding of the biologically induced and controlled formation of NM-associations in soils, mine tailings and low-T ore deposits requires knowledge on the composition of the local microbial community (Parkes 1998, Konhauser 2009, Gadd 2010). For this purpose, genomic DNA from the total microbial community can be extracted and sequenced in laboratories specialised in nucleic acid sequencing (Banfield et al. 2005). The sequence data can be used to determine the taxonomic structure of the microbial community using microbial sequence databases by taxonomic rank: phylum, class, order, family, genus and species. Biomineralogical studies focus commonly on the order and/or genus rank to help distinguish, for example, Fe- and S-oxidisers from Fe- and S-reducers (Courchesne et al. 2021). However, a common caveat when comparing NM-associations with microbial community compositions is that the former associations may have formed under different environmental conditions than the conditions to which the microbial community has adapted. This discrepancy between mineralogy and microbiology can be however explained through (a) characterisation of multiple NM-associations; (b) considering the immediate environment of the sample location (i.e., whether the area is prone to occasional flooding or affected by fluctuations in the phreatic level); (c) examining whether the NM-associations occur within confinements that retard the equilibration with the bulk soil.
Analytical techniques to characterise samples at the micrometre-scale
- How frequent is a specific type of NM-association in a sample such as a specific type of mineral surface coating, biofilm, mineral inclusion or aggregate of PM?
- What are the variations in chemical composition of NMs?
- What are the chemical compositions of the underlying minerals or matrices hosting NMs?
- What are the textural and spatial relations between NMs and their hosts?
- Which sites in NM-associations are of interest and are suitable for the subsequent characterisation at the nanoscale?
There are many analytical techniques for the micro-scale characterisation of NM-associations such as scanning electron microscopy (SEM), electron probe microanalysis (EPMA), laser ablation inductively coupled plasma-mass spectrometry (LA-ICP-MS), micro-X-ray fluorescence spectroscopy (lab- or synchrotron-based μ-XRF), secondary ion mass spectrometry (SIMS), micro-proton-excited X-ray emission analysis (micro-PIXE), micro-Raman, surface enhanced Raman spectroscopy (SERS), micro-XAS, near edge X-ray absorption fluorescence spectroscopy (NEXAFS), micro-infrared and micro-XRD (Stiles et al. 2008, Weng et al. 2022) (Table 1). The latter six techniques follow similar principles as the corresponding bulk techniques except that all three methods can be used to record two-dimensional distribution maps of valences (micro-XAS) or abundances of phases and organic functional groups (micro-Raman, SERS, NEXAFS, micro-Infrared and micro-XRD).
The techniques micro-PIXE and SIMS use protons and ion beams (Cs, O) to excite atoms or generate secondary ion beams, respectively and are powerful techniques to measure and record the chemical distribution of low atomic number elements (Z > 9 for PIXE and Z ≥ 1 for SIMS) and to quantify isotope ratios (SIMS) (Huang et al. 2017, Pallon et al. 2017, Walker 2017).
Micro-XRF utilises X-rays to generate secondary X-rays (due to the fluorescence effect). In a laboratory-based setting, micro-XRF can provide quick answers on the chemical distribution of major and minor elements whereas synchrotron-based micro-XRF provides additional information on the distribution of trace elements (Fittschen and Falkenberg 2011, Kaskes et al. 2021, Vanhoof et al. 2021, Guilherme Buzanich 2022). The latter technique is often used in combination with micro-XAS as it allows the recording of chemical distribution and valence maps of an element of interest in the same NM-association (Masue-Slowey et al. 2011).
X-ray micro-computed tomography (μ-CT) can provide non-destructive analysis of micrometre-size features in soil aggregates containing NM-associations (Zhang et al. 2023). The method is based on differences in the attenuation coefficient of X-rays in materials of different electron density. Many 2D projections of a sample are first collected at different angles (typically between 500 and 3000 2D projections) and are then used to construct a 3D image.
The most common microbeam techniques in many Earth and environmental science departments are SEM, EPMA and LA-ICP-MS (Gu et al. 2020, Rinaldi and Llovet 2015, Llovet et al. 2021, Maloof et al. 2020, Balaram et al. 2022). Although the three techniques are collectively called microbeam techniques, there are important differences in interaction volumes that dictate their effective spatial resolution and thus their ability to characterise NM-associations. Typical penetration depth of secondary electrons, back-scattered electrons, and characteristic X-rays are 5–50 nm, 0.5–1 μm and 2–5 μm, respectively, depending on physicochemical properties of target materials and beam conditions (Gu et al. 2020). The spatial resolution of electron images is also influenced by the types of electron guns. For example, a field-emission electron gun (FE-SEM) allows the recording of high-resolution images of features down to the upper nanometre range (> 100 nm), which cannot be achieved with a conventional tungsten filament (Gu et al. 2020). In comparison, typical quantitative LA-ICP-MS measurements employ beam sizes of similar ablation depth in the range of tens of micrometres.
Preparation of NM-associations for their study at the atomic- and nanometre-scale
The most-often used preparation techniques for the study of NM-associations at the atomic- to nanometre-scale are the dispersion of fine particles on a holder/grid and the more site-specific techniques ultramicrotomy (UM), ion milling (IM) and focused ion beam technique (FIB) (Figure 2) (Heaney et al. 2001, Wirth 2004, Ayache et al. 2010, Tizro et al. 2019).
The simplest and most straightforward preparation technique for the study of NM-associations in colloids or PM is their deposition on grids/holders. Here, solvents such as methanol or mixtures of hexane and acetone can be used to deposit colloids/particles without dissolving water-soluble phases (Donado et al. 2023).
Fundamental principles and advantages and disadvantages of the site-specific preparation techniques UM, IM and FIB have been reviewed in numerous books and papers (Table 1). Here, we briefly describe their functionality and discuss their capabilities to prepare NM-associations as well as their cost efficiency.
In UM, thin slices (30–100 nm) are produced through propagating a fracture induced by the edge of a diamond knife. A pyramidal-shaped sample of either a solid material or a powder embedded into a mould is brought in close contact to the knife and follows a fixed path (so-called D-shaped trajectory) allowing the knife to shave a thin slice at very low speed (Ayache et al. 2010). Advantages of UM are that many slices can be collected in a short period of time (high-cost efficiency) and that a site-specific location of a NM-association can be mounted in the desired orientation within the mould. The major disadvantage is that tearing or crushing of components in mineralogical samples (Figure 1b) during shaving does not allow the preservation of textural and spatial relations in NM-associations, especially those where a soft and porous NM assemblage is associated with a physically hard and homogeneous mineral (e.g., a ferrihydrite coating on a pyrite grain or an amorphous silica coating on a feldspar). Less severe damage is commonly observed when shaving soft organic matter or microbes in association with NMs such as a bacteria-Fe-Ox assemblage in a mine tailings sample.
In the IM technique, a 5–30 μm-thick sample is thinned to electron transparency through abrading the sample with an Ar+ ion beam (ionic abrasion and ionic cleaning) (Ayache et al. 2010, Li 2012). The most common procedure for NM-associations includes the mechanically pre-thinning of a mineral fixed to a 3 mm diameter disk (grinding, polishing, dimpling) followed by its ion-mill thinning at various angles of incidence. Ion-mill thinning gradually proceeds from high beam energies at higher incident angle (~ 10°) to lower beam energies at lower incident angles (~ < 3°). The thinning procedure is monitored with an optical microscope or a CCD camera and lasts for several hours. The advantages of this method are that it allows the thinning of specific micrometre-size NM-associations that have been removed from an epoxy-embedded sample with a diamond knife. This micrometre-size sample can be fixated on a Cu-holder and can be subsequently thinned along a feathered loading edge extending laterally over 1000s of micrometres. The disadvantages of the technique are that I. the broad Ar+ beam (1–2 mm in diameter) does not allow the site-specific thinning of only a small, isolated area of interest within a NM-association; II. The thinning of mineralogical-heterogeneous samples containing materials of greatly different mill rates will result in a high surface roughness (e.g., nano- to micrometre-size uraninite particles embedded within organic matter). In comparison to the UM method, the IM method is less cost-efficient (normally one sample per day) and is less suitable for the preparation of NM-microbe associations. The advantage of this technique with respect to UM is the better preservation of spatial and textural relations in mineralogically heterogeneous NM-associations.
The FIB technology is the most sophisticated preparation technique for site-specific TEM samples (Heaney et al. 2001, Wirth 2004). Here, a thin lamella of constant thickness is extracted at a precise area of the sample. In the first step, a metal- or carbon-layer is deposited using a gas injection system (GIS) in order to protect the surface area to be extracted (Figure 4a, b). A focused ion beam (commonly Ga+ ions) is used to machine two parallel trenches adjacent to the lamella and to cut oft the lamella from the sample prior to its extraction with an internal micromanipulator. Mounting of the lamella on a sample holder and its subsequent thinning completes the preparation of, for example, a TEM sample (Figure 4b). Each step of the preparation process can be monitored with a SEM using a so called a dual-beam system (i.e., focused ion beam and electron beam). In addition to the FIB, SEM and GIS system (Figure 4a), instruments such as the FEI DualBeam™ Helios 600 Nanolab™ are also equipped with a high-resolution field emission gun (FEG); multiple electron detectors for image acquisition, such as through-the-lens detector (TLD), an Everhart-Thornley (ETD), a backscattered electron (BSED) and a EDS detector (Oliveira et al. 2018). Artefacts such as the implantation of Ga+ ions, amorphisation, redeposition of material (metals such as native gold and silver are prone to redeposition) or heating of temperature-sensitive minerals can be minimised through tuning down the current of the focused ion beam from several nA during trenching and extraction to several pico-ampere during polishing. Additional cleaning of the extracted lamella with an advanced plasma system can further minimise the amount of implanted Ga+ ions or redeposited material (Ribeiro et al. 2010).
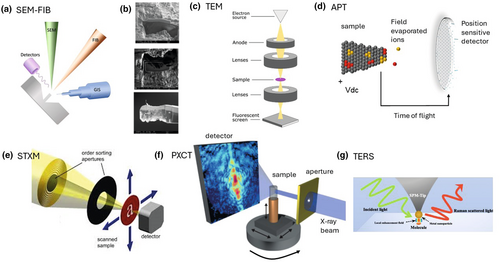
FIB lamellae for example TEM can be extracted from almost every interface observed in NM-associations such as NM−bacteria (Obst et al. 2005), NM−mineral−fungal biofilms (Bonneville et al. 2016, Gerrits et al. 2021), NM−mineral (Hellmann et al. 2012), and NM−organic matter (Mantha et al. 2019). However, the successful extraction of a lamella for TEM depends on the occurrence of fractures in the underlying material (not visible when selecting a site for FIB extraction), which often occur along interfaces between NMs and minerals or organic matter. The number of fractures may be minimised through embedding the material in a low viscous resin, but their occurrence cannot be completely avoided as fractures (a) are not always accessible by the resin and (b) form after the exposure of the sample to vacuum or the generation of heat during FIB-trenching.
The advantages of the FIB technology versus UM and IM are its high site-specificity and the preservation of almost all mineralogical, textural and spatial relations of the components in the selected NM-associations. The major disadvantages are (a) the very low cost-efficiency (> 800 $US per sample), (b) the technology is not available at every academic/research institute and (c) that the extraction of a FIB lamella requires highly skilled personal.
Analytical techniques to study nano-scale features
Analytical techniques to study nano-scale features commonly involves TEM but also recently developed techniques such as atom probe tomography (APT), scanning transmission X-ray microscopy (STXM), nano-spectroscopic techniques based on FTIR (synchrotron infrared nano spectroscopy; SINS), Raman (tip-enhanced Raman spectroscopy; TERS), X-ray fluorescence (nano-XRF) and X-ray absorption spectroscopy (nano-XAS) and ptychographic X-ray computer tomography (PXCT). The principles of some of these techniques are shown as schematics in Figure 4c–g.
Transmission electron microscopy
NM-associations prepared with UM, IM and FIB are commonly examined with TEM (Figure 4c). The microscope allows the characterisation of all phases present, the semi-quantification of the concentrations of the major and minor elements, morphological features such as particle attachments, particle sizes, surface area and structural features such as dislocations and twinning (Lee 2010). These features are commonly characterised with techniques available in the TEM such as convergent beam electron diffraction (CBED), microbeam diffraction (MBD), fast Fourier transformation (FFT) of high-resolution-TEM images, selected area electron diffraction (SAED), energy dispersive spectroscopy (EDS), high-angle annular dark field imaging (HAADF) and scanning TEM (STEM).
Additional information on the valences of cations and anions in NM-associations or the occurrence and proportion of organic functional groups in NM-organic matter associations (e.g., aromatic vs. carbonyl vs. carboxyl vs. ester groups) can be obtained with electron energy-loss spectroscopy (EELS), which is however not available in all TEM instruments (Avouris and Demuth 1984, Hofer et al. 2016).
Atom probe tomography
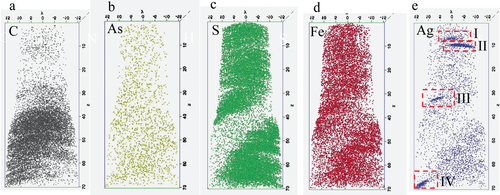
Atom probe tomography allows the three-dimensional compositional mapping of elements and isotopes in NM-associations (Gault et al. 2012, Lefebvre et al. 2016, Saxey et al. 2018, Reddy et al. 2020). In a process called field evaporation, either ultra-fast voltage or laser pulses are used to erode and convert the atoms on the tip of a needle-shaped specimen into charged ions (Figure 4d). An electric field accelerates the ions towards a position-sensitive detector that registers the time of flight and impact position of individual ion or species (Gault et al. 2012, Lefebvre et al. 2016, Saxey et al. 2018, Reddy et al. 2020). The energy applied to the surface of a specimen is used to calculate the mass-to-charge ratios of the ions based on their travel time, which is almost always sufficient for the identification of an individual atom. Impact positions on the position-sensitive-detector plot the locations of individual atoms (Figure 4d). Locations and the identity of individual atoms can be used to calculate a 3D plot which provide information on the location of NMs in minerals and organic matter (Alavijeh et al. 2023) (Figure 5). Samples for APT are commonly extracted with the FIB technology, where similar to the preparation of a TEM lamella, the selected site is marked and protected with deposited Pt, and then extracted and prepared with a Ga+ ions and a micromanipulator (Gu et al. 2020).
Synchrotron-based scanning transmission X-ray microscopy and ptychographic X-ray computer tomography
Scanning transmission x-ray microscopy is performed at synchrotron light sources, which produce intense and tuneable X-ray beams (Henderson et al. 2014). STXM images from X-ray transparent samples are generated with a highly focused X-ray beam with spot sizes as small as 15 nm in size (Figure 4e). The images are obtained at a given photon energy via a raster scan of the sample through a focal point. Here, a sequence of images is collected around the X-ray absorption near edge fine structure (XANES) of an element of interest as a function of photon energy. This sequence of images is commonly referred to as a “stack”. STXM provides data on the valence, chemical and mineralogical composition of NM-associations and similar to EELS, on the occurrence and distribution of organic functional groups across an X-ray image (Rothe et al. 2000, Plaschke et al. 2002). Consequently, STXM is a powerful tool to characterise NM-associations with organic matter and carbon-bearing PM as it allows to visualise (a) the occurrence of inorganic and organic carbon-components and (b) to chemically characterise the distribution of functional groups along the interface of a mineral surface and organic matter (see below). STXM measurements are ideally taken from FIB lamellae or PM and colloids deposited on TEM grids (Moffet et al. 2011, Gu et al. 2020).
Similar to micro-CT tomography, PXCT provides high penetrability and non-destructive 3D visualisation of constituents in NM-associations. A further development of STXM, PXCT is a coherent diffraction imaging technique with the highest spatial resolution currently available (Pfeiffer 2018) (Figure 4f). The technique combines STXM with diffraction imaging and subsequent reconstruction of the images (Dierolf et al. 2010, Pfeiffer 2018). It provides images of extended sample areas with single digit nanometre resolution (with respect to resolved features) and, thus, increases the resolution of a STXM by about one order of magnitude. Although the spatial resolution is ideally only limited by the diffraction of X-rays (i.e., wavelength-limited) rather than by the configuration of the beamline (i.e., X-ray optics), mechanical vibrations of the beamline system such as piezoelectric motors limit the resolution of PXCT to a couple of nanometres.
The basic principle of PXCT is to measure multiple diffraction patterns by scanning a finite X-ray beam over an extended specimen. Illumination of the object multiple times generates an overlap between adjacent illumination positions, which provides subsequently a sufficient over-determination of information on the areas of the object. This over-determination in combination with reconstruction algorithms leads to the generation of electron density plots in 3D space and thus in the visualisation of 3D features in biological and environmental samples (Pfeiffer 2018). Beamlines with PXCT abilities occur for example at the synchrotron facilities Sirius (Brazil; Caterete beamline (Meneau et al. 2021), and Carnauba beamline (Tolentino et al. 2023) Max IV (Lund, Sweden, (Johansson et al. 2021)), ESRF (Grenoble, France, (Martínez-Criado et al. 2016)), the Swiss Light Source at the Paul Scherrer Institute (cSAXS beamline, https://www.psi.ch/en/sls/csaxs) and the ALS at the Lawrence Berkeley National Laboratory (beamline 11.0.2, https://als.lbl.gov/beamlines/11-0-2/).
Advantages of PXCT versus the combination of FIB-TEM-APT are that no sample preparation is needed, and the samples can be characterised without any destruction (note that the volume of the area of interest is limited). Examples of NM-associations characterised with PXCT include for example swelling clay minerals in sandstone (De Boever et al. 2015), magnetite, kerogen and silica associations in micro-fossils (Figure 6, (Maldanis et al. 2020)), magnetosomes in bacteria (Zhu et al. 2016) or nano-size Mn-oxides clusters in rock varnish (Förster et al. 2021).

Nano-spectroscopic techniques
Nano-spectroscopic techniques include, for example TERS, SINS, nano-XRF and nano-XAS (see above and Table 1). Raman spectroscopy utilises vibrational spectroscopic techniques and measures the inelastic light scattering (Raman scattering) of the radiation of monochromatic light from a laser source with a vibrating molecule or functional group. The interaction of the light with the vibrating molecule yields a spectral shift, also called the "Raman" shift. The Raman scattering effect is often insufficient when characterising organic matter within mineral matrices and can be enhanced through the deposition of silver and gold nanoparticles on the corresponding sample surface (Silvestri et al. 2020). The recognition of the latter effect led to the development of SERS and consequently to TERS (Figure 4g, Table 1). The latter technique uses a combination of Raman spectroscopy with scanning probe microscopy, such as atomic force microscopy (AFM), scanning-tunnelling microscopy or shear force microscopy. The latter microscopy techniques are based on moving a sharp tip along the surface of a substrate recording changes in topography, conductivity, or chemical composition. In TERS, the sharp tip has an apex on ~ 10 to 20 nm, is coated commonly with gold and silver and moves at an equal distance (< 5 nm in depth) over the surface. The tip acts as antenna upon laser illumination and boosts the Raman scattering effect (Stiles et al. 2008, Langelüddecke et al. 2015). The small radius of the tip keeps the enhancement localised around the tip apex (Figure 4g). Overall resolution and enhancement of the Raman scattering depends on many factors, such as the size and shape of the tip, the tip material and substrate, as well as the distance between the tip and substrate. Hence, TERS can resolve changes in chemical composition and phase abundances in NM-associations at the single-nanometre range whereas SERS with a resolution of hundreds of nanometres can provide similar information for larger scale features such as NM-bacteria associations. Similar, SINS combines Infrared microscopy with AFM, where the metallic tip of the AFM acts as an antenna and bundles synchrotron-sourced mid- to far-IR radiation into the confinements between tip and surface (Bechtel et al. 2020). This so-called near-field microscopy technique collects information on IR absorption, IR reflectivity and morphology properties simultaneously and allows nanometre spatial resolution (~ 10 nm) that is determined by the apex radius of the tip. In terms NM-associations, SINS was used for example to spatially resolve the organic and mineral heterogeneity of shale at the nano- and micrometre- scale (Hao et al. 2018). Beamlines offering SINS are for example the IMBUIA-nano station at the infrared (IR) beamline of the Brazilian Synchrotron Light Laboratory (LNLS) or the beamline 2.4 at the Advanced Light Source (ALS) at the Lawrence Berkeley National Laboratory in California.
In addition to LNLS, ALS and other synchrotron facilities, new beamlines such as the ID16B at the European Synchrotron Radiation Facility, the Carnauba beam line at the Sirius synchrotron facility, Brazil or the NanoMAX beamline at the synchrotron facility MAX IV in Lund, Sweden have now the ability to generate brilliant high-energy synchrotron radiation with beam spot sizes down to a few tens of nanometres (Martínez-Criado et al. 2016, Johansson et al. 2021, Teixeira et al. 2023). Using nano-XRF, -XRD and -XAS techniques, nanometre beam sizes allow to resolve individual features within NM-associations. For example, lateral spatial resolutions of 120 nm and 30 nm are available at the Tarumã and Sapoti stations of the Carnauba beam line, respectively (Teixeira et al. 2023, Tolentino et al. 2023) and Nano-XRF imaging at the ID16B allowed the identification of Ag nanoparticles in plant vascular redion of sunflowers (Martínez-Criado et al. 2016, Teixeira et al. 2023).
Nano-XRF, -XRD and -XAS as well as TERS and SINS do not require the preparation of samples using the FIB or ion-milling techniques. In the case of TERS and SINS, challenges are the preparation of the gold and silver coatings on the scanning probe microscopy tips and the preparation of colloidal samples on flat substrates such as micas.
Challenges and perspectives
The most common challenge when studying NM-associations is that the data obtained at the nanoscale may not be representative for a given environment or medium such as soil, rock, tailings, particulate matter or colloids in a river, lake or sediment. The lack of representative data obtained at the nanoscale is due to the high costs involved in the preparation of FIB lamellae or hours of TEM or APT time to fully characterise a sample. This problem may be solved though creating representative data sets at the bulk and micrometre scale. The datasets obtained at these scales should show for example similar chemical and mineralogical compositions and textural features of NM-associations. A possible way to approach such a multi-scale approach would be a pyramidal analytical approach, where the number of analyses correlates with the width of a pyramid at a given height. In this approach, the analyses at the bulk scale would represent the base of the pyramid, those at the micrometre scale would be at half-height and those at the nanometre-scale at the tip of the pyramid. Considering the time effort to collect and analyse samples at each scale, multi-scale analyses are often conducted over several years and should be carefully planned with respect to a student thesis.
The data collection at the micro- and nanometre-scale can be accelerated using automatism software during SEM and TEM analyses. These are of great importance when examining unconsolidated environmental samples (sediments, soils and tailings material) at the micrometre scale with SEM and colloids and PM with TEM at the nanometre-scale. These analyses are often time consuming and even after many hours of analysis, the obtained data may still be not representative. In recent years, the QEMSCAN (quantitative evaluation of minerals by scanning electron microscopy) system has been used as a tool to search for features at the nanoscale. The QEMSCAN system was developed within the Commonwealth Scientific and Industrial Research Organization (CSIRO) of Australia as a counterpart to the QEM-SEM system. The latter system was based on an automated SEM fitted with four energy dispersive X-ray spectrometers (EDS), which was not capable of quantifying low atomic number elements such as carbon or oxygen in, for example, coal-bearing NM-associations (Ward 2016). Initial research in the characterisation of coal-bearing NM-associations with QEMSCAN commenced in the late 1980s and early 1990s when a proof-of-concept study was initiated, which experimented with various mounting media and automated SEM measurements. During 1997–98, CSIRO's first LEO QEMSCANTM became operational as a fully automated instrument, running a Windows-based PC operating system (Liu et al. 2005). This system has now enhanced low atomic number element capability (carbon and oxygen) due to the use of thin polymer windows and has opened new possibilities for characterising not only minerals in coal but the coal itself (i.e., the macerals), thus providing for the first time, information on the textural setting of minerals in coal-bearing NM-associations.
A new perspective: identifying precursors of nanomaterials
Hochella et al. (2019) proposed a cycle for NMs, in which the breakdown of larger minerals and grains results initially in the formation of NM-precursors. These precursors can be ions, clusters, complexes and small molecules, which may play a key role in the formation of nanoparticles during fluid-mediated mineral dissolution and precipitation processes. The NMs themselves either aggregate or attach to a growing mineral surface via processes such as crystallisation through particle attachment (De Yoreo et al. 2015), Ostwald ripening (Hastie et al. 2021) or flocculation (McLeish et al. 2021). Smaller NMs can be also precursors of larger NMs with the same (Au system (Hough et al. 2011)) or different structure type (Fe-sulfides (Morse et al. 1987) Fe-oxides (Banfield et al. 2000) and Ca-carbonates (Nielsen et al. 2014)) as well as of micro- to centimetre large minerals (De Yoreo et al. 2015).
Information on the formation of NMs from precursors (clusters, ions) comes from atmospheric studies (Merikanto et al. 2009) as well as experimental studies, especially with respect to the formation of engineered Au-, Ag- and Cu-nanoparticles (Dobbs et al. 2006, Zielińska et al. 2009, Baco-Carles et al. 2011). Contrary, structural information on the occurrence of NM-precursors in a natural setting is scarce. Potential precursors of Cu-, Fe- and Zn-sulfide nanoparticles may be higher order unprotonated clusters of the type M3S3, M4S6, M2S4 with M = Cu, Fe and Zn (Luther et al. 1999, Rozan et al. 2000, Luther et al. 2002, Lewis 2010). For example, (Luther et al. 1999) proposed that the formation of sphalerite (ZnS) occurs via the condensation of Zn2+(H2O)6 aqueous species towards the soluble precursors (Zn3S3)/(Zn4S6)4- and the subsequent aggregation of the neutral (Zn3S3) clusters.
Future studies on the identification of cluster-size precursors in NM-associations may be conducted with new generations of APT instruments, which have high detection efficiency (100%) and spatial resolution (Reddy et al. 2020). Targeted NM-associations may be mineral surface coatings in contaminated soils or ore deposits, in which Cu-, Pb-, As- Au-bearing precursors form and grow towards their corresponding NMs.
A new perspective: studying NM-organic matter associations in soils
The largest pool of C is soil organic matter (SOM), where even a small increase in the decomposition of SOM can cause large variations in the atmospheric concentrations of the greenhouse gases (GHG) CO2 and CH4 (Intergovernmental Panel on Climate 2014). Hence, soils are increasingly recognised as a key battleground in the fights against climate change as well as nutrient pollution, and other pressing global change challenges (Lehmann and Kleber 2015). Among many factors, mineral-organic matter associations control the C-budget in soils. These associations form through the adsorption of polar soluble organic constituents on mineral surfaces, slow down the decomposition of the latter organic matter, increase the stability of the corresponding minerals and have commonly a greater ability to sequester metallic cations than minerals or organic matter alone (Qu et al. 2019). Consequently, there has been an increasing interest in understanding the chemical composition and structure of interfaces between minerals and organic matter (Possinger et al. 2020, Kleber et al. 2021).
Recently, (Weng et al. 2022) reviewed analytical techniques that are commonly used to characterise soil organic matter including organic matter adsorbed to surfaces of minerals (see above). Here, combinations of cryo-UM-STXM, cryo-FIB-TEM-EELS, STXM and NEXAFS allow the identification of the molecular diversity of the organic matter and their two dimensional distribution at the 50 nm scale (Stuckey et al. 2017, Possinger et al. 2020). However, there is also a need to understand how organic matter affects the formation of NMs in soils (Kleber et al. 2021) and this knowledge can be achieved when studying NM-organic interfaces in particulate organic matter (POM). From the analytical viewpoint, the characterisation of the latter interfaces is challenging as they (a) cannot be stabilised with organic-based resins (as this would not allow the characterisation of the C-species) and (b) are sensitive towards ion- and electron-beams. Hence, future studies of NM-organic interfaces in POM may also include the use of inorganic resins such as indium and in addition to the analytical techniques listed above, APT, the nano spectroscopic techniques TERS and SINS as well as nano tomography (PXCT).
A new perspective: characterising the interaction of wildfire smoke and coal combustions with atmospheric pollutants
Processes within the C cycle have profound effects on climate and human health as the release the GHG CO2 and CH4 is a positive feedback to climate change increasing global temperatures and thus higher frequencies of wildfires and coal (Intergovernmental Panel on Climate 2014). A higher frequency of the latter fires results in an increasing atmospheric concentration of small PM (< 2.5 μm) with a high number of oxidative potential radicals (Aguilera et al. 2021). This leads to greater potentials of inflammation and oxidative stress in lungs and thus increases the risk of respiratory and cardiovascular diseases, and cardiopulmonary and lung cancer (Elliott and Copes 2011). The major component of PM released by wild- and coal fires are soot particles, which are composed of spherules with concentric curved graphene layers, like the layers of an onion. The physical, chemical and structural properties of the soot particles may change during their interactions with PM from different sources, which can affect their level of impact and toxicity toward the environment and humans (Li et al. 2011, Donado et al. 2023). The characterisation of the modified soot particles and associated nanoparticles and trace elements is challenging as these NM-associations can be highly complex in terms of their mineralogical and chemical composition (Donado et al. 2023a). However, combinations of TEM with EELS, SXTM, ToF-SIMS, LA-ICP-MS and perhaps single particle inductively coupled plasma time-of-flight mass spectrometry (sc-ICP-ToF-MS; a method to characterise the chemical composition of single nanoparticles) may be used to characterise changes in (a) the structure of the spherules; (b) the coordination environment of carbon (sp2 versus sp3 hybrid), (c) proportions of organic components such as polycyclic aromatic hydrocarbons and (d) concentrations of minor and trace elements within the NM-associations. These multi-analytical approaches should provide more information on atmospheric processes occurring during the mixing of wild- and coal-fire smoke with pollutants from other anthropogenic and natural sources and will thus help epidemiological studies to better understand the health impact of NM-associations within modified PM.
Conclusions
The study of NM-associations remains an analytical challenge due to their mineralogical and chemical complexities and differences in the physical properties of NMs and associated mineral grains, organic matter, microbes, fungi and plants. Analytical techniques such as the focused ion beam technology, new generations of TEM, automatism software for SEM and TEM analyses and combinations of APT with STXM and PXCT may provide new insights into the structure and properties of NM-associations. The use of the latter technologies offers also new perspectives such as the identification of precursors of NMs in ore deposits and contaminated soils, air and aquatic systems, a better understanding of the role of NM-associations in stabilising carbon in soils and in the transformation of carbon-bearing PM during its interaction with other atmospheric contaminants.
Acknowledgements
The authors thank Mark Cooper, Panseok Yang and Nadia Mykytczuk for discussions, two anonymous reviewers for their very valuable comments and Joint Editor in Chief Jacinta Enzweiler for handling the paper. MS was supported by a NSERC Discovery Grant RGPIN-2023-04726. Financial support for Mozhgan Akbari Alavijeh was provided by Natural Sciences and Engineering Research Council of Canada There are no conflicts or competing interests to declare. All authors provided their consent for publication. Authors' contributions: Funding acquisition, supervision, final draft of paper (Michael Schindler); data curation, wrote two sections of first draft (Mozhgan Akbari Alavijeh); wrote two sections of first draft (Marcos L.S. Oliveira); conceptualisation, wrote three sections of first draft (Luis F.O. Silva).
Scientific editing by Jacinta Enzweiler.
Open Research
Data availability statement
Availability of data and material is not applicable as none were generated for this review article.




