Accumulation of polyunsaturated fatty acid-derived metabolites in the sarcopenic muscle of aging mice
Abstract
Aim
Although it is known that advanced age alters skeletal muscle lipid metabolism, the role(s) of polyunsaturated fatty acid-derived metabolites (mostly eicosanoids and docosanoids) in sarcopenia are not clear. We therefore examined the changes in the metabolites of arachidonic acid, eicosapentaenoic acid and docosahexaenoic acid in the sarcopenic muscle of aged mice.
Methods
We used 6- and 24-month-old male C57BL/6J mice as healthy and sarcopenic muscle models, respectively. Skeletal muscles were removed from the lower limb and subjected to a liquid chromatography–tandem mass spectrometry analysis.
Results
The liquid chromatography–tandem mass spectrometry analysis detected distinct changes of metabolites in the muscles of the aged mice. Of the 63 metabolites identified, nine were significantly higher in the sarcopenic muscle of aged mice compared with the healthy muscle of young mice. In particular, prostaglandin E2, prostaglandin F2a, thromboxane B2, 5-hydroxyeicosatetraenoic acid, and 15-oxo-eicosatetraenoic acid (arachidonic acid-derived metabolites), 12-hydroxy-eicosapentaenoic acid and 14,15-epoxy-eicosatetraenoic acid (eicosapentaenoic acid-derived metabolites) and 10-hydroxydocosa-hexaenoic acid and 14-hydroxyoctadeca-pentaenoic acid (docosahexaenoic acid-derived metabolites) were significantly higher in aged tissue compared with young tissue (all P < 0.05).
Conclusions
We observed the accumulation of metabolites in the sarcopenic muscle of aged mice. Our results may provide new insights into the pathogenesis and progression of aging- or disease-related sarcopenia. Geriatr Gerontol Int 2023; 23: 297–303.
Introduction
Muscle atrophy during aging, known as sarcopenia, is characterized by progressive reductions in muscle mass and muscle strength that result in disability, poor quality of life and eventual mortality.1 Sarcopenia in humans and other mammals is known to be associated with a decrease in the number of muscle fibers and a simultaneous decrease in the size of the individual fibers,2 via the contribution of several factors including the production of reactive oxygen species (ROS) and/or dysregulation of protein synthesis and degradation pathways.3 Mechanisms by which the decline of muscle mass and function during the aging process could be slowed are of great interest; however, the precise mechanisms of sarcopenia in aging are still far from understood.
Eicosanoids and docosanoids are locally acting bioactive signaling lipids derived from arachidonic acid (AA) and related polyunsaturated fatty acids (PUFAs) that regulate a diverse set of homeostatic and inflammatory processes linked to numerous diseases.4, 5 Mass spectroscopy-based lipidomics profiling is now being used to identify, monitor and quantify many eicosanoids and docosanoids that are known to be involved in diseases.6 Lipidomics is also currently being used to screen more effectively for potential disease biomarkers.7, 8 There is growing evidence to supporting roles of fatty acids (FAs) and their metabolites in the regulation of skeletal muscle mass and function.9 However, the changing profiles of eicosanoids and docosanoids during aging are not fully known. Therefore, we conducted the present study to examine these metabolites in the sarcopenic muscle of aged mice and the muscle of healthy young controls.
Materials and methods
Animals
Twelve-week-old male C57BL/6J mice were randomly assigned to the young (6-month-old) and aged (24-month-old) groups. The mice were housed in an animal room under controlled conditions with a 12 h/12 h light/dark cycle. The animals were all individually identified by a numeric code. After the mice reached the respective ages of 6 and 24 months, they were killed by cervical dislocation under deep anesthesia with isoflurane; the gastrocnemius muscle from the lower limb of each mouse was then excised and weighed, frozen in liquid nitrogen and stored at −80°C until analysis.
The 24-month-old mice were used because we and other researchers have observed and documented sarcopenia in mice at that age.10, 11 In cases of death, sickness or cannibalism, the mouse was excluded from the analyses.
All experimental and animal care procedures were approved by our institution's Animal Research Committee and conformed with its Guidelines for the Care and Use of Laboratory Animals.
Liquid chromatography–tandem mass spectrometry analysis of eicosanoid and docosanoid profiling in the skeletal muscle
A lipidome analysis was performed with the Lipidome Lab Eicosanoid/Docosanoid Scan package (LIPIDOME LAB Co., Ltd., Akita, Japan), as described.12, 13 Briefly, 40-mg frozen muscle samples were homogenized with 1 mL of methanol containing internal standards, 2 ng each of 5S-hydroxy-eicosatetraenoic acid deuterated (15-HETE-d8), leukotriene B4-d4 and prostaglandin E2 (PGE2)-d4 and 4 ng of AA-d8 (Cayman Chemicals, Ann Arbor, MI, USA), and diluted with low-concentration hydrochloric acid. The samples were extracted by solid-phase extraction with an Oasis HLB columns (Waters Corporation, Milford, MA, USA). They were separated using a high-performance liquid chromatography system (Nexera LC-30AD; Shimadzu Corporation, Kyoto, Japan) equipped with an XBridge C18 column (particle size 3.5 μm, length 150 mm, inner diameter 1.0 mm; Waters Corporation).
The column oven temperature was set at 30°C. A gradient solvent system used with mobile phase A (acetonitrile/methanol [4:1, v/v]) and mobile phase B (water with 0.1% acetic acid [v/v]). The samples were analyzed on a triple quadrupole mass spectrometer (LCMS-8040; Shimadzu Corporation) using with argon gas for collision-induced dissociation. The desolvation line and heat block temperatures were set at 250 and 400°C, respectively. The quantification of FA metabolites was performed by multiple reaction monitoring, as described.12 For quantification, calibration curves were prepared for each compound, and recoveries were monitored using deuterated internal standards.
The data analysis was performed using LabSolutions software (Shimadzu Corporation). A hierarchical cluster analysis (HCA) and a principal components analysis (PCA)14 were performed by the Human Metabolome Technologies proprietary MATLAB and R programs, respectively. Although there are various analytical approaches to metabolomic research, a PCA is routinely used to visualize high-dimensional metabolomic data in a two- or three-dimensional space, and a scatter plot of PC score vectors can be used to detect outliers and identify biologically significant patterns; we thus used this approach.
Immunoblotting
Immunoblotting was carried out as described.10, 15 The antibody was used 4-hydroxy-2-nonenal (4-HNE) (JaICa; Nikken SEIL, Shizuoka, Japan). Equal loading of the protein was normalized by immunoblotting with α-tubulin (Abcam, Cambridge, MA, USA). The proteins were quantified (band × volume) using an LAS-3000 Imaging System (FujiFilm, Kanagawa, Japan).
Statistical analysis
The results are expressed as the means ± SE. Welch's t-test was used for the analyses. P < 0.05 was considered significant. All computations were performed using R software (R Developmental Core Team: R, 2005).
Results
Characteristics of eicosanoids and docosanoids in the skeletal muscle
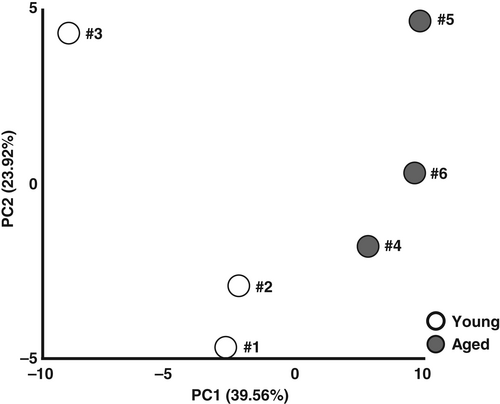
The body weights of the aged mice were significantly higher than those of the young mice (young vs. aged; 31.7 ± 0.8 vs. 47.1 ± 2.5 g; P < 0.05), and the gastrocnemius muscle mass in the aged mice was significantly lower than that of the young mice (young vs. aged: 176.6 ± 1.9 vs. 142.6 ± 2.2 mg; P < 0.05). We compared the metabolomic data from skeletal muscle samples obtained from young and aged mice to explore the effects of aging. Skeletal muscle samples from three mice from each age group were used for the metabolomic analysis, and 63 metabolites were identified and quantified. The score plot of the PCA is shown in Figure 1. As a clear separation between the groups was observed on the principal component 1 (PC1) axis (x-axis), we focused on that component. The PC1 scores of the young and aged mice showed positive and negative values, respectively, suggesting that the PC1 score is positively associated with sarcopenia.
We conducted an HCA to classify the metabolites into clusters of different trends (Fig. 2). The significantly changed metabolites were increased in the muscle of aged mice compared with that of the young mice. In the HCA analysis, there were trends in the clustering of the skeletal muscle sample in the aged and young groups, indicating that aging has effects on the eicosanoids and docosanoids profiles of the skeletal muscle.
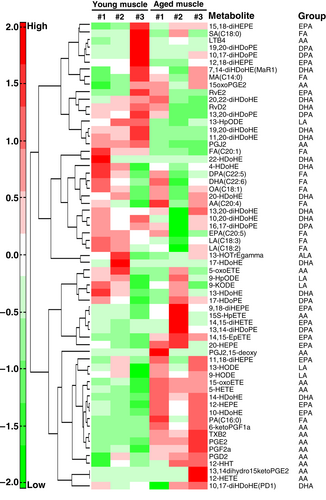
Compared with the young mice, some AA-, docosahexaenoic acid (DHA)- and eicosapentaenoic acid (EPA)-derived metabolites in the aged mice were increased by varying degrees. The results of the PCA and HCA analyses demonstrated that aging had a significant influence on the metabolite profiles of skeletal muscle. All metabolites are listed in Tables S1 and S2.
Tissue levels of eicosanoids and docosanoids and oxidative stress in the skeletal muscle
We identified a significant increase in metabolites derived from AA, EPA, and DHA in the sarcopenic muscle of aged mice compared with the healthy muscle tissue of young mice (Fig. 3). Among the AA-derived metabolites, PGE2, prostaglandin F2a (PGF2a), thromboxane B2 (TXB2, the stable metabolite of TXA2), 5-HETE and 15-oxo-eicosatetraenoic acid (15-oxoETE) were significantly increased in the sarcopenic muscle of the aged mice compared with the muscle of the young mice (P < 0.05) (Fig. 3a). Among the EPA-derived metabolites, 12-hydroxy-eicosapentaenoic acid (12-HEPE) and 14(15)-epoxy-eicosatetraenoic acid (14,15-EpETE) were significantly increased in the aged mice versus the young mice (P < 0.05). Among the DHA-derived metabolites, 10-hydroxy-docosahexaenoic acid (10-HDoHE) and 14-hydroxy-docosahexaenoic acid (14-HDoHE) were significantly increased in the aged mice versus the young mice (P < 0.05) (Fig. 3b).
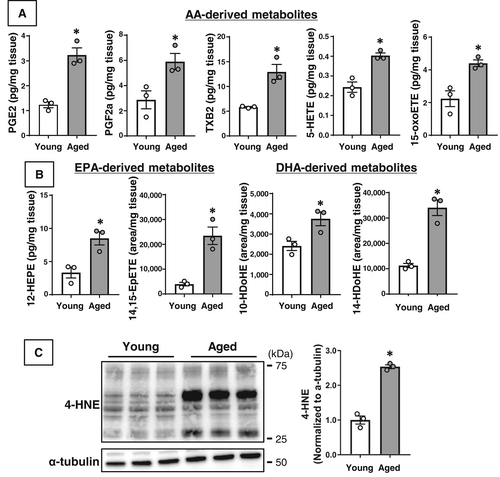
In addition, the 4-HNE protein expression level was significantly increased in the sarcopenic muscle of the aged mice compared with the level in the young mice (Fig. 3c).
Discussion
We investigated the differences in the profiles of eicosanoids and docosanoids for aged mice with sarcopenia compared with the healthy muscle of young control mice. We observed increases in nine metabolites in the skeletal muscle of the aged mice and decreases in none of the metabolites. The accumulation of metabolites in sarcopenic muscle may thus be associated, at least in part, with the progression of sarcopenia during aging.
Accumulation of arachidonic acid-derived metabolites in the sarcopenic muscle of aged mice
Liquid chromatography–tandem mass spectroscopy analysis revealed significant changes in AA-, EPA- and DHA-derived metabolites in the sarcopenic muscle of aged mice compared with the healthy muscle of young mice by liquid chromatography–tandem mass spectroscopy analysis. AA is metabolized by cyclooxygenase (COX) enzymes to prostaglandins (PGE2 or PGF2a); these mediators are closely involved in inflammatory processes in aging and disease states, acting through specific G-protein coupled receptors to produce cytokines including interleukins.16 PGE2, in particular, is a potent mediator of skeletal muscle wasting, and several animal models of disease have linked an excessive production of prostaglandins to muscle wasting.17 In an animal model of arthritis, muscle wasting was also related to the induction of E3 ubiquitin ligases including muscle RING finger-1 (MuRF-1) and muscle atrophy F-box (MAFbx), which are markers of protein degradation; these changes were restored by COX inhibitor treatment.18 As mentioned above, the activation of prostaglandins via COX enzymes might be an important factor in the progression of disease-related muscle wasting.
5-HETE is an endogenous eicosanoid, an intermediate in AA metabolism via the 5-lipoxygenase (5-LOX) pathway. 5-LOX is a member of the LOX family pathway that converts AA into lipid mediators including leukotriene B4 and other HETEs.19 Activation of this pathway has been linked to increases in the intracellular production of ROS, in particular 4-HNE.20, 21 Our earlier investigation as well as other studies10, 22 have detected an association between increased 4-HNE protein expression in skeletal muscle and sarcopenia in aged mice. Consistent with these results, the 4-HNE protein expression level in the sarcopenic muscle of the aged mice markedly increased compared with the level in the young mice (Fig. 3c).
15-oxoETE is the final metabolite of the 15-LOX pathway. In a previous study, 15(S)-HETE, an upstream metabolite of 15-oxoETE, was upregulated in skeletal muscle of denervation-induced muscle wasting model mice.23 Consistently, NADPH oxidases (NOXs), a major source of ROS, were markedly increased in the atrophic muscle of that model, and those changes were reversed by 15-LOX inhibition using genetic and pharmacological approaches.23 Protein kinase B (Akt)—mammalian target of rapamycin (mTOR)—p70 ribosomal S6 protein kinase (p70S6K) is a major signaling pathway of protein synthesis in mammals. Protein kinase C (PKC), in particular, the activation of the PKCθ isoform via excessive FAs, acts as a potent repressor of this signaling pathway. Indeed, the FAs exposure can induce PKCθ activation, which directly inhibits this signaling in mouse-derived skeletal myocyte cells.9, 24 Intriguingly, it has been shown that total PKC activity including PKCθ was increased in 15-oxoETE-treated endothelial cells.25 We also demonstrated that excessive NOX-derived ROS production in skeletal muscle contributes to the progression of sarcopenia via a downregulation of the Akt–mTOR-p70S6K pathway in aging and cardiovascular diseases model mice.10, 15
Similar to other AA-derived metabolites, TXB2 is also produced by a COX-dependent pathway in the AA metabolism process. Interestingly, a blockade of TX production by using gene deletion or pharmacological approaches attenuated the myocardial damage with a concomitant inhibition of 4-HNE and NOX-derived ROS production in ischemic reperfusion injury model mice.26 These findings suggest an association between TXB2 and ROS production in sarcopenic muscle and it is supported in our present results (Fig. 3c). Taken together, our results and the research focused on AA-derived metabolites and skeletal muscle still hold unanswered questions; however, we speculate that activation of the COX and LOX pathways and an acceleration of ROS production in skeletal muscle are involved in the dysregulation of protein synthesis and degradation that may cause metabolites to accumulate and thus induce sarcopenia in aged mice.
Accumulation of eicosapentaenoic acid- and docosahexaenoic acid-derived metabolites in the sarcopenic muscle of aged mice
EPA and DHA, which are major components of omega-3 PUFAs, have a range of beneficial effects on aspects of health such as improved blood lipid regulation, immune function, cognitive function and neuromuscular function.27 The beneficial effects of EPA and DHA on health markers are often associated with increases in the FA content of phospholipids in cell membranes. An imbalance in the phospholipid FAs in cell membranes has been shown to induce changes in a multitude of biological processes including inflammation and signaling pathway alterations.9, 27 In addition, these FA metabolites have complex effects on skeletal muscle and the cardiovascular system.9, 28 Therefore, dysregulation of EPA and DHA metabolism or its metabolites is an essential factor in sarcopenia during aging.
Similar to AA-derived metabolites, EPA- and DHA-derived metabolites were significantly increased in the sarcopenic muscle of aged mice in the present investigation. 12-HEPE is an oxidized EPA metabolite produced by the 12-LOX pathway; it can indirectly affect with endogenous AA for 5-LOX in stimulated human neutrophils,29 and it has inhibitory effects against platelet aggregation in humans.30 Similarly, 14,15-EpETE, a cytochrome P450-dependent EPA metabolite, has inhibitory effects on platelet aggregation,31 and it has been shown to induce dilatation of rat coronary microvessels potently by activation of large conductance Ca2+-activated K+ channels.32 Although these metabolites have various anti-inflammatory effects on tissues, it is unclear how these metabolites contribute to sarcopenia. However, interestingly, PGE2 is associated with platelet function and its regulatory factors including signal transducer and activator of transcription 3 protein in atherosclerotic33 or cancer cachexia model mice.34 Therefore, an accumulation of PGE2 or other AA-derived metabolites might counteract the anti-inflammatory effects of 12-HEPE and other EPA-derived metabolites in the sarcopenic muscle. We plan to elucidate this issue in a future study.
10-HDoHE and 14-HDoHE are oxidized lipids that can be formed non-enzymatically through the interaction of PUFAs with ROS.35 The roles of these metabolites in sarcopenia or disease are not yet clear, but we observed that the 4-HNE protein expression level in the sarcopenic muscle of the aged mice was markedly increased compared with the level in the healthy muscle of young mice (Fig. 3c), which is consistent with our previous work and other studies.10, 36 These results suggest that accumulation of 10-HDoHE and 14-HDoHE is related to both ROS production in skeletal muscle and sarcopenia more broadly.
This study has several limitations. The metabolite profiles in control (young) #3 was very different from those of controls #1 and #2, and this sample size is insufficient. Further studies with stable sample quality and a sufficient sample size in a lipidomics analysis are thus necessary. We could not assess the phospholipid contents or lipid species in the skeletal muscle. A decrease in PCs and altered profiles of lipid species were observed in aged muscle in the earlier study.37 In addition, sphingolipids such as ceramide also promote muscle wasting and contractile dysfunction via various mechanisms, including apoptosis, energy metabolism dysfunction, and dysregulation of protein synthesis and degradation,38, 39 and it is necessary to perform the above-mentioned experiment to gain a better understanding of the roles of the accumulations of eicosanoids and docosanoids and their metabolism in the sarcopenic muscle of aged mice in a future study.
Although the mechanisms of these processes and the specific involvement of eicosanoids and docosanoids in sarcopenia are unclear, a correction of their excessive accumulations by the inhibition of COX, LOX and ROS production might be useful for preventing sarcopenia. Our present results may thus provide the research community new insights into the mechanisms of aging- or disease-related sarcopenia.
Acknowledgements
This work was supported by a MEXT KAKENHI grant (no. 18K17935) and the Sportology Center, Juntendo University Graduate School of Medicine. We thank Reiko Mishima (Human Metabolome Technologies Inc., Yamagata, Japan), Hiroki Nakanishi, Takayo Ohto-Nakanishi (LIPIDOME LAB Co., Ltd., Akita, Japan), Emiko Nakamura (Department of Cardiovascular Biology and Medicine, Juntendo University Graduate School of Medicine), Hiroshi Koide, Tomomi Ikeda, and Takako Ikegami (Laboratory of Molecular and Biochemical Research [Kyodo-ken], Research Support Center, Juntendo University Graduate School of Medicine) for the technical assistance and biochemical measurements in the experiments.
Disclosure statement
The authors declare no conflict of interest.
Open Research
Data availability statement
The data that supports the findings of this study are available in the supplementary material of this article.




