Screening of and Mechanistic Insights Into the Enteric Methane Mitigation Potential of European Native and Non-Native Forage Trees, Shrubs, and Herbs Using In Vitro Batch Culture
Funding: This work was supported by Flemish Government, Department of Agriculture and Fisheries; Fonds Wetenschappelijk Onderzoek, 1SC6324N.
ABSTRACT
Enteric methane (CH4) emissions from ruminant livestock must be mitigated to reduce their climate impact. Trees, shrubs, and herbs have gained attention for their nutritional value, climate resilience, and CH4 reduction potential. This in vitro study evaluated 45 forage species harvested in Flanders, Belgium (July 2022), for their effects on enteric CH4 production (μmol/g DM), total volatile fatty acid (VFA) production (μmol/g DM), and relative CH4 production (CH4:total VFA, mol/mol). Leaf traits from the TRY database were included. Twelve promising species were selected for a second experiment (July 2023) using fresh and ensiled substrate. Polyethylene glycol (PEG) was used to assess the activity of tannins in fresh substrates. Different headspace conditions (100% CO2 vs. 50% CO2/50% H2) were used to assess the impact on methanogens. Reduced CH4 production in the first experiment was mainly linked to reduced fermentability, and leaf traits are more closely related to fermentability than direct CH4 mitigation. Alnus glutinosa, Castanea sativa, Catalpa bignonioides, Populus nigra, and Hedera helix emerged as the most effective CH4 mitigators. Ensiling reduced the concentration of phenolic compounds. PEG assays confirmed the role of tannins in some species; however, PEG was ineffective against the hydrolysable tannins in C. sativa. C. sativa, A. glutinosa, H. helix, and C. bignonioides exhibited anti-methanogenic effects, likely due to plant secondary metabolites, some of which were only indirectly evaluated (tannins and total phenolic compounds). Further phytochemical and microbiological analyses, along with in vivo trials, are needed to confirm these forages' practical application in livestock diets.
1 Introduction
Enteric methane (CH4) is a major contributor to climate change, accounting for approximately 30% of global anthropogenic CH4 emissions (FAO 2017). To limit the climate impact of ruminant livestock, feeding strategies can be implemented (Beauchemin et al. 2022). Forages play a central role in dairy cattle diets (Hassanat et al. 2013), providing essential nutrients such as carbohydrates and proteins (Vuolo et al. 2019). However, these forages also contribute to enteric CH4 production, with their impact influenced by factors such as chemical composition and fermentability (Russell and Hespell 1981). In response to the environmental challenges posed by dairy production, there is a growing interest in partially replacing traditional forages with alternative plant species such as herbs, shrubs, and trees. This approach is particularly relevant to organic farming, where many existing solutions (e.g., 3-NOP, nitrate) do not align with the principles or legal requirements. Fodder trees and shrubs are more resilient to climate change than grasses, maintaining foliage production in tough conditions (Balehegn 2017) and providing ecosystem services (Vandermeulen et al. 2018). Beyond providing essential nutrients, these alternative forages may also help to reduce enteric CH4 production. Several studies performed in vitro screenings to identify the potential of alternative forage plants for their CH4 mitigating potential (Jayanegara et al. 2011; Macheboeuf et al. 2014; Terranova et al. 2018). However, there is a high variability in their effects on ruminal fermentation variables between different plant species but also within a single species. The mitigating potential of these plants is often attributed to their chemical composition, and more specifically the presence of secondary plant metabolites (PSM) (Ku-Vera et al. 2020; Huang et al. 2023; Lambo et al. 2024). However, plants rich in PSM may also be less palatable or even toxic. Nevertheless, species with promising CH4-mitigating properties may still be valuable as sources of bioactive compounds for extract-based applications. Additionally, some species may currently have limited availability, although this could potentially be overcome through cultivation.
Within the plant kingdom, leaf traits can be used to identify the resource use strategy. This concept is captured by the Leaf Economic Spectrum (LES), a universal gradient ranging from acquisitive (fast-growing) to conservative (slow-growing) strategies (Wright et al. 2004). Furthermore, several of these leaf traits (e.g., leaf N content, leaf dry matter content) are correlated with forage digestibility (Pontes et al. 2007; Gardarin et al. 2014). Additionally, plant defence strategies, including structural defences (e.g., lignin and cellulose) and chemical defences (e.g., phenolic content) are likely to depend on LES traits (Mohanbabu et al. 2023). If such correlations exist, leaf traits may serve as reliable indicators to predict the CH4 mitigation potential of a plant species. PSM-like tannins and saponins are already studied extensively (Ku-Vera et al. 2020); however, understanding in the mode of action and potential to reduce enteric CH4 production is rather limited. In vitro batch incubations can be a useful tool to evaluate the mitigating potential of different herbs, shrubs, and trees. Despite its limitations (Yáñez-Ruiz et al. 2016), small adjustments to this system can create artificial conditions that provide more insights into the CH4 mitigation mechanism. For instance, polyethylene glycol (PEG) can be added to the in vitro system to assess the effect of tannins on fermentation variables (Makkar et al. 1995). The addition of PSM can inhibit H2-producing ruminal microorganisms, indirectly reducing CH4 production by limiting the availability of H2 or directly by inhibiting methanogens (Ku-Vera et al. 2020; Lambo et al. 2024). Since H2 uptake by methanogens is directly proportional to its concentration in the headspace, higher H2 levels result in increased CH4 production (Czerkawski et al. 1972). Thus, artificially increasing the H2 concentration in the headspace may serve as an indicator of methanogen inhibition. While in vitro systems have limitations, they provide a controlled, simplified environment to examine impacts on ruminal fermentation.
This study aimed (1) to evaluate the CH4-mitigating potential of a range of native and non-native European plant species, (2) to identify common leaf traits among those with effective mitigation properties, (3) to explore the effect on CH4-mitigating properties under artificial in vitro conditions to provide insights into the underlying mechanisms of CH4 reduction. To the best of our knowledge, our study is the first to combine screening with the investigation of potential mechanisms of action by making simple setup modifications to the in vitro system, such as adjusting the headspace composition.
2 Materials and Methods
2.1 Overview of the Experimental Setup
A schematic overview of the experimental setup is shown in Figure 1. Here an overview of the experimental setup is given, while details on collection, transport, storage and handling of the plant material as well as in vitro incubations, chemical analysis and statistical treatment are given below. In the first experiment, 45 plant species commonly found in Western Europe, including both native and non-native species, were selected for an initial screening of their CH4 mitigation potential. Plant species were primarily selected based on their potential relevance for ruminant diets or their widespread occurrence in the landscape. However, not all selected plant species are intended for direct inclusion in ruminant feed. Data from the TRY Plant Trait Database (Kattge et al. 2020) was used to categorise the plant species into two groups: woody and herbaceous plant species. All plant species were collected within a single harvest window of 10 days in early July 2022. To introduce variation in plant material, each species was harvested from three different geographical locations in Flanders (Belgium). The specific locations, however, varied between plant species and were not fixed. Fresh plant material was incubated for 24 h with rumen inoculum, a bicarbonate/phosphate buffer under 100% CO2 headspace. To assess their potential to reduce both absolute CH4 production (mmol/g dry matter (DM)) and relative CH4 production (expressed as mmol CH4 per mmol volatile fatty acids (VFA)), a control mixture was included in the incubation. The control mixture was composed of 65% perennial ryegrass (Lolium perenne) and 35% white clover (Trifolium repens), harvested at the same time and from the three different locations, and represents the standard basal roughage used for organic ruminants in Flanders.
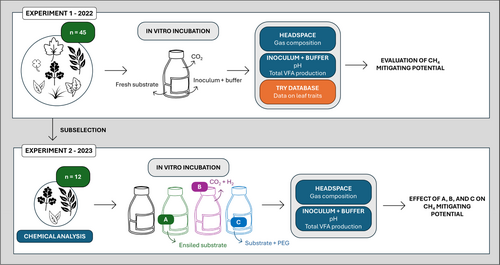
Based on the potential to reduce relative CH4 production of the first experiment, 12 plant species were selected for a second experiment to explore the working mechanism underlying the CH4 reduction (Tables 1–3). For this selection procedure, besides mitigating potential, practical relevance, effect on overall ruminal fermentation, and mitigation potential in literature were considered. The selected species were harvested within a 10-day window in July 2023, across the same three geographical locations sampled in 2022. Plant material was pooled over different locations and used as substrate in this experiment. Again, fresh material of the 12 selected plant species and the control mixture was incubated under the same conditions as in the first experiment. Furthermore, all selected plant species and the control mixture were also subjected to three additional treatments, including (A) ensiled substrate, (B) 50% CO2 + 50% H2 headspace, and (C) fresh substrate + PEG. Throughout both experiments, in vitro batch incubation techniques were used.
| No. | Group | CH4 (μmmol/g DM) | Total VFA (mmol/g DM) | Relative CH4 (mol/mol) | ||||
|---|---|---|---|---|---|---|---|---|
| F | S | F | S | F | S | |||
| Control mixture | 1 | A | 1.58 | 1.47 | 5.43b | 5.09a | 0.29 | 0.29 |
| Alnus glutinosa | 3 | B | 0.30* | 0.36* | 2.91*a | 3.17*b | 0.10* | 0.11* |
| Castanea sativa | 6 | C | 0.09*a | 0.93*b | 1.37*a | 3.63*b | 0.06*a | 0.26b |
| Catalpa bignonioides | 7 | C | 0.44*a | 1.16*b | 3.11*a | 4.15*b | 0.14*a | 0.28b |
| Corylus avellana | 9 | E | 0.62* | 0.67* | 2.59* | 2.66* | 0.24 | 0.25 |
| Cydonia oblonga | 10 | E | 0.55*a | 0.74*b | 2.61* | 2.74* | 0.21*a | 0.27b |
| Hedera helix | 13 | D | 0.93*a | 1.12*b | 5.22 | 5.06 | 0.18* | 0.22 |
| Myrica gale | 15 | E | 0.53*a | 0.73*b | 2.16* | 2.11* | 0.25a | 0.35b |
| Populus nigra | 17 | D | 0.82*a | 1.49b | 4.14*a | 4.49*b | 0.20*a | 0.34b |
| Ribes × nidigrolaria | 25 | E | 0.84*a | 1.03*b | 3.57*a | 3.84*b | 0.24 | 0.27 |
| Sambucus nigra | 29 | D | 1.06* | 1.17* | 4.89* | 4.99 | 0.22* | 0.24 |
| Hypericum perforatum | 36 | D | 0.85* | 0.94* | 3.96*b | 3.47*a | 0.21*a | 0.27b |
| Rumex obtusifolius | 41 | E | 0.65*a | 1.00*b | 2.65*a | 3.39*b | 0.24a | 0.30b |
- Note: Different letters indicate a significant difference within a species between fresh and ensiled plant material based on Tukey post hoc test. Group (A, B, C, D, and E) indicate the plant species that cluster together in the PCA in Figure 8 and can be considered as one group.
- * A significant difference with control mixture (65% Lolium perenne, 35% Trifolium repens) based on a Dunnet's post hoc test.
| No. | Group | Absolute CH4 production (mmol CH4/g DM) | ||
|---|---|---|---|---|
| CO2 | H2 + CO2 | |||
| Control mixture | 1 | A | 1.58a | 3.01b |
| Alnus glutinosa | 3 | B | 0.30* | 0.50* |
| Castanea sativa | 6 | C | 0.11* | 0.10* |
| Catalpa bignonioides | 7 | C | 0.44* | 0.51* |
| Corylus avellana | 9 | E | 0.62*a | 1.81*b |
| Cydonia oblonga | 10 | E | 0.55*a | 1.32*b |
| Hedera helix | 13 | D | 0.90* | 0.96* |
| Myrica gale | 15 | E | 0.53*a | 1.25*b |
| Populus nigra | 17 | D | 0.82*a | 2.30*b |
| Ribes × nidigrolaria | 25 | E | 0.84*a | 1.88*b |
| Sambucus nigra | 29 | D | 1.06*a | 2.16*b |
| Hypericum perforatum | 36 | D | 0.85*a | 1.45*b |
| Rumex obtusifolius | 41 | E | 0.65*a | 1.22*b |
- Note: Different letters indicate a significant difference within a species between different conservation methods based on Tukey post hoc test. Group (A, B, C, D, and E) indicate the plant species that cluster together in the PCA in Figure 8 and can be considered as one group.
- * A significant difference with the control mixture (65% Lolium perenne, 35% Trifolium repens) based on a Dunnet's post hoc test.
| No | Group | Absolute CH4 (mmol/g DM) | Total VFA production (mmol/g DM) | Relative CH4 (mmol/mmol) | ||||
|---|---|---|---|---|---|---|---|---|
| CTRL | PEG | CTRL | PEG | CTRL | PEG | |||
| Control mixture | 1 | A | 1.58 | 1.53 | 5.43 | 5.23 | 0.29 | 0.29 |
| Alnus glutinosa | 3 | B | 0.30a* | 0.44b* | 2.91a* | 3.46b* | 0.10* | 0.13* |
| Castanea sativa | 6 | C | 0.11* | 0.10* | 1.37a** | 2.25b* | 0.06* | 0.05* |
| Catalpa bignonioides | 7 | C | 0.44* | 0.46* | 3.11* | 3.18* | 0.14* | 0.14* |
| Corylus avellana | 9 | E | 0.62a* | 1.16b | 2.59a* | 3.98b* | 0.24a | 0.30b |
| Cydonia oblonga | 10 | E | 0.55a* | 1.18b | 2.61a* | 4.47b* | 0.21a | 0.26b |
| Hedera helix | 13 | D | 0.91* | 0.93* | 5.27 | 5.26 | 0.17 | 0.18 |
| Myrica gale | 15 | E | 0.53a* | 0.95b* | 2.16a* | 3.33b* | 0.25a | 0.29b |
| Populus nigra | 17 | D | 0.82* | 0.83* | 4.14* | 4.22* | 0.20 | 0.20 |
| Ribes × nidigrolaria | 25 | E | 0.84a* | 1.37b | 3.57a* | 4.78b* | 0.24a | 0.29b |
| Sambucus nigra | 29 | D | 1.06* | 1.08 | 4.89* | 5.08 | 0.22 | 0.21 |
| Hypericum perforatum | 36 | D | 0.85a* | 1.03b | 3.96a* | 4.67b* | 0.21 | 0.22 |
| Rumex obtusifolius | 41 | E | 0.65a* | 1.26b | 2.65a* | 4.21b* | 0.24a | 0.30b |
- Note: Different letters indicate a significant difference within a species between with and without PEG based on Tukey post hoc test. Group (A, B, C, D, and E) indicate the plant species that cluster together in the PCA in Figure 8 and can be considered as one group.
- * A significant difference with the control mixture (65% Lolium perenne, 35% Trifolium repens) based on a Dunnett's post hoc test.
2.2 Harvesting and Storing Substrates
For the trees and shrubs, 300–400 g of fresh material was collected in a plastic bag that was closed airtight and stored in a cooling box. The aerial vegetative components were collected from multiple plants at the same site for each plant species. Only non-woody twigs with attached leaves or leaflets that were grown during the growing season of 2022 or 2023 were collected at a plant height between 1 and 2.5 m as this represents the browsing height of cows. For the herbaceous plant species, 100 g of fresh material including leaves and stems from different plants was harvested and placed in an airtight plastic bag. The longest interval between harvesting and arrival at the laboratory was overnight, with all samples received the following morning. During transport, samples were stored in closed containers with ice packs, ensuring dark conditions and maintaining temperatures below 4°C. The fresh plant material (tree, shrubs, and herbs) was frozen at −20°C and subsequently freeze-dried, as this method best preserves the characteristics of fresh material (Fievez et al. 2004). Before using it as an incubation substrate, this plant material was ground and sieved (0.212 mm < substrate particles > 1 mm) and stored in a desiccator in the dark. Throughout the text, the freeze-dried substrate is referred to as “fresh” substrate, since freeze-drying. Lab-scale silages were made as previously described by Gadeyne et al. (2016) with small adaptations. Fresh material (100 g) of the harvest in July 2023 was cut (pieces of 1–2 cm), sprayed with formic acid solution (5%) and placed in a vacuum bag (250 × 300 mm, 90 μm, FPS-multiproducts NV). These bags were stored in a dark place at room temperature for up to 30 days. After the ensiling process was finished, substrates were frozen (−20°C), freeze-dried, ground, and sieved in the same way as the fresh samples.
2.3 In Vitro Incubation
The in vitro incubation was performed as described in Vlaeminck et al. (2014) with small adaptations. In short, substrates were incubated for 24 h in an in vitro batch system using rumen fluid from Holstein Friesian rumen fistulated lactating dairy cows. The rumen fluid was collected just before morning feeding (8:00 a.m.) through the rumen cannula using a vacuum suction pump and a perforated sampling probe. Approximately 1 L of rumen fluid was collected from at least three different cows that were fed the same diet. The basal diet was composed of 50% maize silage and 50% wilted grass silage (on a DM basis) supplemented with some straw and urea. Additionally, soybean meal and balanced (based on net energy and protein digestible at the level of the small intestine, using the Belgian/Dutch VEM and DVE evaluation system) compound feed was administered to fulfil the animals' requirements for milk production. The collected rumen fluid was transferred into a pre-heated insulated flask and transported to the laboratory. After filtration through a sieve with a pore size of 1 mm and under continuous CO2 flushing at 39°C, the rumen fluid of the different cows was combined and then mixed with a bicarbonate/phosphate buffer (containing 3.58 g Na2HPO4·12H2O, 1.55 g KH2PO4, 0.124 g MgCl2·6H2O, 8.74 g NaHCO3, and 1 g NH4HCO3 per litre of distilled water). Incubation was performed in triplicate in 120-mL flasks. The flasks were sealed and flushed with CO2. Afterwards, CO2-saturated bicarbonate/phosphate-buffered rumen fluid was added (buffer + water:rumen fluid 4:1, v:v) to reach a total liquid volume of 25 mL. The flasks were incubated at 39°C in a shaking incubator (Edmund Bühler GmbH, Hechingen, Germany) for 24 h. The amount of substrate used per flask was 250 mg. Blanks were not included as plant secondary metabolites can influence fermentation not only of the added substrate but also of organic matter present in the inoculum (Araujo et al. 2011). Although additive-specific blanks as suggested by Araujo et al. (2011) could help correct for this, such an approach was not possible in our study, as the substrate itself contained the secondary metabolites. For the second experiment on the working mechanism, the headspace was either filled with 100% CO2 or 50% H2 and 50% CO2. To determine if tannins contribute to the reduction in CH4 production, additional flasks containing 50 mg of Polyethylene glycol (PEG 6000) were included, as PEG is known to bind tannins and reduce their impact on ruminal fermentation variables (Makkar et al. 1995). After 24 h, the fermentation process was stopped by placing the incubation bottles in an ice bath to stop microbial activity.
After each incubation, gas composition of the headspace was analysed by connecting the incubation flasks directly to the micro gas chromatograph (990 Micro GC System, Agilent). The system automatically withdrew a subsample of the headspace gas for injection. Ethane was used as the internal standard. After opening the bottle, pH of incubation contents was measured. A 1 mL aliquot of the incubation content was acidified with 100 μL formic acid, containing the internal standard (10 mg 2-ethyl butyric acid/mL formic acid) for VFA analysis using a gas chromatograph (HP 7890A, Agilent Technologies) (Gadeyne et al. 2016). The net production of VFA was calculated by subtracting the amounts in rumen fluid before the incubation from the amounts measured after incubation.
2.4 Chemical Analysis
Upon arrival in the laboratory, fresh plant material was weighed before and after freeze-drying to calculate the dry matter (DM) content. Crude fibre was analysed with the Ankom fibre analyser (Ankom Technology, USA) after boiling the freeze-dried samples in sulphuric acid and sodium hydroxide (EC 1992). Crude protein (N × 6.25) was analysed using the Kjeldahl method (ISO:5983-2 2005). Carbon and nitrogen concentrations were measured by high temperature combustion at 1150°C using an elemental analyser (Vario MACRO cube CNS, Elementar, Germany) and the C:N ratio was calculated. For the analysis of minerals and trace elements, approximately 1 g (to the nearest 0.1 mg) of ground sample was weighed into a crucible. This crucible was placed in a muffle furnace at 500°C for 4 h. The resulting ash was dissolved in 5 mL of 6N HCl (Supelco) and heated at 100°C for 30 min. Subsequently, 5 mL of 3N HCl (Supelco) was added, and the solution was reheated at 100°C for another 30 min. After cooling, the solution was quantitatively transferred to a 50 mL volumetric flask with ultrapure water. Before ICP analysis, the solution was filtered using a Whatman 5 filter. All elements were analysed using an ICP-OES (iCAP 7000, Thermo Scientific) except for selenium, which was determined using ICP-MS (iCAP Q, Thermo Scientific). Total phenolic concentration was quantified using the Folin–Ciocalteu colorimetric method as described by Marinova et al. (2005) with small adaptations. Briefly, 0.125 g of the freeze-dried substrate was mixed with 10 mL of 70% ethanol to extract. The mixture was shaken in an orbital shaker for 2 h at 509 × g and then centrifuged at 7633 × g at 21°C. Then, 25 μL of the substrate extract or a gallic acid standard solution (ranging from 0.10 to 1.25 mg gallic acid/mL) was added to 250 μL Folin–Ciocalteu reagent. After 6 min, the mixture was combined with 750 μL of 20% (w/v) Na2CO3 solution. The samples were then kept in the dark at room temperature for 2 h to develop colour. The absorbance was measured at 740 nm (Infinite M nano, Tecan) and the results were expressed as grams of gallic acid equivalent (GAE) per 100 g of freeze-dried substrate.
2.5 Leaf Traits
Data on leaf traits from the LES were obtained from the TRY plant trait database (www.TRY-db.org) (Kattge et al. 2020). Given the relevance of LES traits to resource use strategies and plant defence mechanisms, leaf traits were selected that are commonly associated with this spectrum. The following traits were chosen based on data availability for the plant species used in these experiments and their relevance to LES: leaf area (mm2), leaf C (mg/g DM), leaf N (mg/g DM), C:N ratio, leaf density (mg/cm3), leaf P (mg/g DM), leaf thickness (mm), leaf DM (mg/g FM). Due to varying availability, not all traits were recorded for every plant species. The number of available datapoints for each trait is as follows: leaf area: n = 26, leaf C: n = 37, leaf N: n = 36, C:N ratio: n = 32, leaf density: n = 27, leaf P: n = 31, leaf thickness: n = 31, leaf DM: n = 37.
2.6 Statistical Analysis
3 Results
3.1 Screening of Plant Species for Their Methane-Mitigating Potential
Absolute CH4 production (mmol/g DM), total VFA production (mmol/g DM), and relative CH4 production (mol CH4/mol VFA) differed (p < 0.05, Table S1) between plant species. Initially, K-means clustering was used as an explorative method to cluster plant species based on absolute CH4 and total VFA production. The clustering resulted in three clusters (Figure 2): (1) no significant or partial effect; (2) moderate effect; and (3) strong effect. In the ‘no significant or partial effect’ cluster, 7 of the 17 plant species had no significant effect on either CH4 or VFA production, 2 species significantly affected CH4 production only, and 6 species significantly influenced VFA production only. Melilotus albus (no. 39) and Saponaria officinalis (no. 42) even showed an increased total VFA production of 15% and 22% respectively. Hedera helix (no. 13) was allocated to the ‘moderate effect’ cluster, although it uniquely showed a 59% reduction (p < 0.001) in absolute CH4 production without affecting total VFA production. Castanea sativa (no. 6) exhibited the lowest absolute CH4 production and total VFA production, placing it at the edge of the ‘strong effect’ cluster. Figure 3 illustrates the absolute CH4 production, total VFA production and relative CH4 production of the different clusters. The correlation matrix of parameters of fermentation and leaf traits of the LES is shown in Figure 4. Absolute CH4 and total VFA production were negatively correlated with leaf C (r = −0.39 and r = −0.45) and leaf DM (r = −0.44 and r = −0.52). However, these Pearson correlations disappeared in relative CH4 production. The best multiple linear regression to predict absolute CH4 production from leaf traits (R2 = 0.19, p < 0.01) is: with y = absolute CH4 production (mmol/g DM), and x1 = leaf DM (g/kg FM). To predict total VFA production, the best multiple linear regression (R2 = 0.33, p < 0.01) is: with y = total VFA production (mmol/g DM), x1 = leaf DM (g/kg FM), and x2 = leaf C (g/kg DM). From all screened traits of the LES, leaf DM (mg/g FM) was the only leaf trait that showed a trend (p = 0.061) for a difference between the ‘no effect’ cluster (0.261 mg/g FM) and the ‘moderate effect’ cluster (0.329 mg/g FM) (Figure 5). The ‘no effect’ cluster is mainly composed of herbaceous plant species, with lower DM content. A PCA was performed to generate linear combinations of these leaf traits. The first two PCs accounted for 43.8% and 21.2% of the variance, respectively (Figure 6). Leaf N and Leaf P were correlated with PC1, while Leaf C, Leaf DM, and Leaf thickness were correlated with PC2. However, no clear relation between the leaf traits and the predefined clusters could be observed. An overview of the chemical composition of the plant species selected for the second experiment is shown in Table S2.
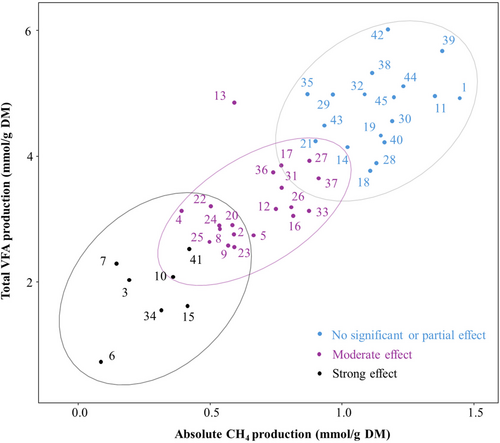
 ) no significant or partial effect (n = 18); (
) no significant or partial effect (n = 18); ( ) moderate effect (n = 20); and (
) moderate effect (n = 20); and ( ) strong effect (n = 7). Each point represents an individual plant species, identified by its corresponding number (Table S1). Ellipses indicate the 95% confidence interval for each cluster.
) strong effect (n = 7). Each point represents an individual plant species, identified by its corresponding number (Table S1). Ellipses indicate the 95% confidence interval for each cluster.
 ) no significant or partial effect (n = 18); (
) no significant or partial effect (n = 18); ( ) moderate effect (n = 20); and (
) moderate effect (n = 20); and ( ) strong effect (n = 7) cluster. The box spans the interquartile range (IQR) from the first quartile (Q1) to the third quartile (Q3), with the horizontal line indicating the median (Q2). Whiskers extend to the smallest and largest values within 1.5 times the IQR. The mean is displayed as a red dot, and any dot beyond the whiskers is considered an outlier.
) strong effect (n = 7) cluster. The box spans the interquartile range (IQR) from the first quartile (Q1) to the third quartile (Q3), with the horizontal line indicating the median (Q2). Whiskers extend to the smallest and largest values within 1.5 times the IQR. The mean is displayed as a red dot, and any dot beyond the whiskers is considered an outlier.
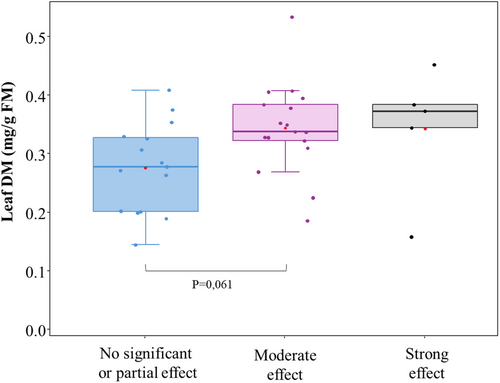
 no significant or partial effect (n = 18);
no significant or partial effect (n = 18);  moderate effect (n = 20); and
moderate effect (n = 20); and  strong effect (n = 7)). The box spans the interquartile range (IQR) from the first quartile (Q1) to the third quartile (Q3), with the horizontal line indicating the median (Q2). Whiskers extend to the smallest and largest values within 1.5 times the IQR. The mean is displayed as a red dot, and any dot beyond the whiskers is considered an outlier.
strong effect (n = 7)). The box spans the interquartile range (IQR) from the first quartile (Q1) to the third quartile (Q3), with the horizontal line indicating the median (Q2). Whiskers extend to the smallest and largest values within 1.5 times the IQR. The mean is displayed as a red dot, and any dot beyond the whiskers is considered an outlier.
 no significant or partial effect (n = 15);
no significant or partial effect (n = 15);  moderate effect (n = 16); and
moderate effect (n = 16); and  strong effect (n = 4) on absolute CH4 and total VFA production. Each point represents an individual plant species included in the PCA, identified by its corresponding number (Table S1), although some species are missing due to a lack of leaf trait data (i.e., Catalpa bignonioides, Cydonia oblonga, Phyllostachys spp., Ribes × nidigrolaria, Vitis spp., Lotus pendunculatus).
strong effect (n = 4) on absolute CH4 and total VFA production. Each point represents an individual plant species included in the PCA, identified by its corresponding number (Table S1), although some species are missing due to a lack of leaf trait data (i.e., Catalpa bignonioides, Cydonia oblonga, Phyllostachys spp., Ribes × nidigrolaria, Vitis spp., Lotus pendunculatus).3.2 Effect of Ensiling on the Methane Mitigating Potential
Overall, absolute and relative CH4 productions were not significantly affected by ensiling (Table 1). However, a significant interaction effect between plant species and substrate type was observed (p < 0.001), indicating that some plant species exhibited increased absolute and relative CH4 production after ensiling, whereas others did not. Total VFA production was affected by plant species (p < 0.001), substrate type (p < 0.01), and the interaction between both (p < 0.001). In most plant species where a difference in absolute CH4 production was observed between fresh and ensiled substrates, there was an associated increase in total VFA production. Only ensiled Hypericum perforatum (no. 36) led to a 12% reduction in total VFA production. Alnus glutinosa (no. 3) was the only plant species that consistently reduced relative CH4 production in both fresh and ensiled substrates. In contrast, all other species that reduced relative CH4 production in fresh substrates, that is, C. sativa (no. 6), Catalpa bignonioides (no. 7), Cydonia oblonga (no. 10), H. helix (no. 13), Populus nigra (no. 17), Sambucus nigra (no. 29), and H. perforatum (no. 36) did not differ from the control when ensiled. Total phenolic concentration was measured in the ensiled substrates (Figure 7) and was affected (p < 0.05) by substrate type, resulting in an overall reduced concentration in ensiled substrates compared to the fresh substrates.

3.3 Effect of Headspace Composition on Absolute Methane Production
The effect of different headspace gas compositions (CO2 or CO2 + H2) on absolute CH4 production is shown in Table 2. Absolute methane production was influenced (p < 0.001) by the gas composition of the headspace. However, this effect differed over the plant species (head space composition × plant species, p < 0.001). Based on the additional CH4 production under CO2 + H2 conditions compared to CO2 conditions, three groups can be distinguished: (1) substrate incubations where the additional CH4 production does not differ from that in the control medium under CO2 + H2 conditions; (2) substrate incubations where additional CH4 production is significantly higher under CO2 + H2 conditions but remains lower than the additional CH4 production in the control medium (e.g., H. perforatum (no. 36) and Rumex obtusifolius (no. 41)); and (3) substrate incubations where CH4 production does not differ between CO2 + H2 and CO2 conditions (e.g., A. glutinosa (no. 3), C. sativa (no. 6), C. bignonioides (no. 7), and H. helix (no. 13)).
3.4 Effect of PEG on Methane Production and Digestibility
The effect of the addition of PEG on the absolute CH4 production of individual plant species differed (PEG × Plant species p < 0.001, Table 3). In some of the plant species, the addition of PEG did not result in a significant effect on CH4 and total VFA production (C. bignonioides (no. 7), H. helix (no. 13), P. nigra (no. 17), and Sambucas nigra (no. 29)). C. sativa (no. 6) showed no significant effect on absolute CH4 production, but an effect on total VFA production. Additionally, the addition of PEG led to an effect on absolute CH4 production and total VFA production in A. glutinosa (no. 3), Corylus avellana (no. 9), C. oblonga (no. 10), H. perforatum (no. 39), Myrica gale (no. 15), Ribes × nidigrolaria (no. 25), and R. obtusifolius (no. 41).
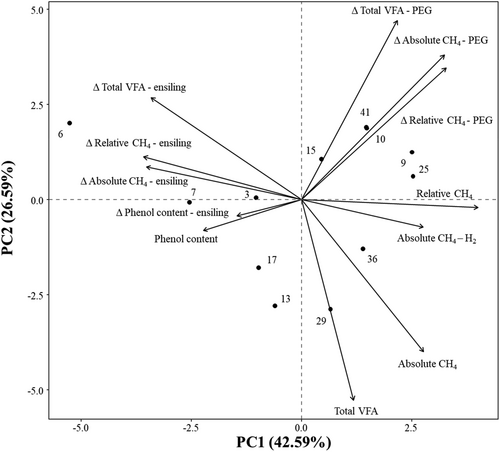
A PCA was performed to combine the effects of ensiling, addition of H2 in the headspace, and supplementation of PEG to the substrate on the fermentation variables. The PC1 and PC2 explained 42.59% and 26.59% of the variance (Figure 8). Relative CH4 production was positively correlated to PC1. Plant species on the negative side of the PC1 axis (A. glutinosa (no. 3), C. sativa (no. 6), C. bignonioides (no. 7), H. helix (no. 13), and P. nigra (no. 17)) showed the highest potential to reduce relative CH4 production. Absolute CH4 and total VFA production had a strong contribution to PC2, with plant species on the positive side of this axis showing lower absolute CH4 and total VFA production. The effect of ensiling on fermentation variables and on total phenolic concentration was negatively correlated with PC1. The addition of PEG influenced both absolute and relative CH4 production, contributing to both PC1 and PC2, while its effect on total VFA production primarily contributed to PC2. Additionally, the effect of H2 in the headspace on absolute CH4 production was positively correlated with PC1.
4 Discussion
Results indicated a strong relationship between absolute CH4 production and total VFA production. Leaf C content in this study (47.3% for woody plants, 44.5% for herbaceous plants) aligns closely with Ma et al. (2018), who reported 47.8% and 44.7%, respectively. The C-content in plants is positively correlated (r2 = 0.29) with lignin content in plants (Ma et al. 2018), known to have a negative influence on forage digestibility (Grabber et al. 2009). Furthermore, structural defences, such as lignin, were positively correlated with resource-conservative plant strategies (Mohanbabu et al. 2023). Wright et al. (2004) demonstrated that leaf traits associated with resource-use strategies can be represented along a single axis. Our results align with this framework, with Urtica dioica (no. 45) reflecting resource-acquisitive traits, and Pseudotsuga menziesii (no. 22), reflecting resource-conservative traits. These findings indicate that leaf traits are more related to fermentability than to a specific CH4 mitigating potential. However, within the current dataset, the relationship between DM and C with absolute CH4 and total VFA production is biased by the woodiness of the plant species. R. obtusifolius (no. 41) was the only herbaceous species in the ‘strong effect’ cluster, while other species in this cluster were woody plants. Most herbaceous species were in the ‘no significant or partial effect’ cluster. Among the tested plant species, H. helix (no. 13) is an outlier within the ‘moderate effect’ group as it reduced absolute CH4 production, without affecting total VFA production. This is in line with findings of Ramos-Morales et al. (2017), who found no influence of H. helix on total VFA concentration during an in vitro experiment.
Based on their reduction in relative CH4 production observed in the first experiment or, in cases where this was not observed, evidence of mitigation potential from the literature, 12 plant species were selected for further investigation. This second experiment indirectly explored the CH4 reduction mechanisms and involved lab-scale experiments with fresh versus ensiled material, variable in vitro headspace gas-phase H2 concentrations, and PEG addition. Ensiling resulted in an overall decrease in total phenolic content. This reduction may be related to cell rupture during chopping and ensiling (Huang et al. 2016). Besides decreased concentrations, PSM are known to undergo modifications and degradation during ensiling (Rodríguez et al. 2009; Muklada et al. 2021). For example, in willow, ensiling has been shown to result in complete metabolization of catechin, gallocatechin, and epicatechin (Muklada et al. 2021), a finding supported by De Bellis et al. (2022). This metabolization may be provoked by lactic acid bacteria involved in the ensiling process, which possess enzymatic activities such as tannase (Rodríguez et al. 2008) and glycosidase (Landete et al. 2014). As a result, deglycosylation of PSM is promoted during ensiling (Muklada et al. 2021). Based on these findings, it is plausible that the ensiled substrate in this study may exhibit reduced antimicrobial activity as glycosylated forms of these compounds have generally been reported to exhibit higher antibacterial and anti-virulence properties compared to their aglycones (Barros et al. 2024). The ensiled substrate of C. sativa (no. 6) strongly affected all fermentation variables, resulting in the loss of its reduction potential in relative CH4 production compared to the fresh control mixture. A reduction in the total phenolic concentration can be observed. Based on the tannase activity of lactic acid bacteria as mentioned above, hydrolysable tannins present in C. sativa are possibly degraded. On the other hand, P. nigra (no. 17), H. helix (no. 13), and S. nigra (no. 29) showed no or only minor loss of methane-mitigating potential after ensiling, which coincided with their total phenolic content remaining largely unaffected. This suggests that their effect may be linked to phenolic (or other) compounds that remain stable during ensiling, possibly due to their presence in the aglycone form. Quercetin, a stable aglycone flavonoid found in all three species, may be one such compound contributing to this effect (Napoli et al. 2018; Gavrila et al. 2023). C. avellana, C. oblonga, M. gale, and Ribes × nidigrolaria exhibited reduced total phenolic concentrations, yet ensiling had little to no effect on fermentation variables. These findings suggest that the compound(s) responsible for the effects on fermentation variables were not affected during ensiling. Notably, all these plant species showed tannin activity (Makkar et al. 1995), as demonstrated by the positive effect of PEG on fermentation variables. Although tannase activity from lactic acid bacteria has been suggested to reduce the effects of hydrolysable tannins during ensiling, tannases specifically target hydrolysable tannins (Belmares et al. 2003). Therefore, it is possible that the observed effects were, at least partly, due to condensed tannins, which may have remained active in both fresh and ensiled substrate. Although the phenolic content of C. sativa (92 and 131 g/kg DM in 2009 and 2010; Jayanegara et al. 2011) has been previously reported to be largely composed of (hydrolysable) tannins (78 and 119 g/kg DM in 2009 and 2010), and chestnut tannin extracts have been shown to reduce CH4 production in vitro (Hassanat et al. 2013; Battelli et al. 2023), the addition of PEG to C. sativa (no. 6) in the current study did not influence either absolute or relative CH4 production. However, total VFA production increased. It is suggested that PEG influences hydrolysable tannins less compared to condensed tannins (Bhatta et al. 2009; Pellikaan et al. 2011). The increase in total VFA production suggests some effect of tannins on substrate fermentability, likely driven by the presence of condensed tannins in C. sativa alongside hydrolysable tannins (Terranova et al. 2020).
A. glutinosa (no. 3), C. sativa (no. 6), C. bignonioides (no. 7), H. helix (no. 13), and P. nigra (no. 17) showed little or no effect of H2 addition on absolute CH4 production, suggesting inhibitory effects on methanogens. C. sativa is known to mainly contain hydrolysable tannins, which have a more direct effect on the methanogens compared to condensed tannins (Patra and Saxena 2011). However, contrasting to what this study suggests, Battelli et al. (2023) found no effect on archaea as a result of the addition of hydrolysable tannins from C. sativa. Additionally, H2 + CO2- versus CO2-headspace incubations with A. glutinosa (no. 3) suggest a direct anti-methanogenic effect. Its bark is known to be rich in oligomeric proanthocyanidins (a type of condensed tannin; Skrypnik et al. 2019), while A. glutinosa acorns predominantly contain ellagitannins (a type of hydrolysable tannin) as their main phenolic compound (Nawirska-Olszańska et al. 2022). However, neither the bark nor acorns have been addressed in the current study and no literature data were found on oligomeric proanthocyanidins and ellagitannins in leaves and twigs of A. glutinosa, which were the subject of the current study. Nevertheless, the observed effect of PEG on fermentation variables suggests the presence of tannins in the A. glutinosa leaves and twigs. Furthermore, another group of phenolic compounds, the diarylheptanoids, predominantly oregonin, have been identified as major phenolic compounds in A. glutinosa (Smeriglio et al. 2022). Both oregonin and its glycosylated form have been associated with antimicrobial properties (Altınyay et al. 2015; Abedini et al. 2016). In the present study, H. helix (no. 13) and C. bignonioides (no. 7) showed anti-methanogenic effects. H. helix is known to contain saponins such as hederacoside C, α-hederin, and hederagenin, which have been reported to possess antimicrobial properties and may inhibit methanogens (Uddin et al. 2011; Tatia et al. 2023), in line with our results of the H2 + CO2- vs. CO2-headspace incubations. Similarly, C. bignonioides has shown antimicrobial effects in some studies (Torres et al. 2015), with saponins likely playing a role (Muñoz-Mingarro et al. 2003). Saponins are known to interact with the sterols present in the membrane of ciliate protozoa, resulting in reduced numbers or activity of protozoa (Patra and Saxena 2009). Methanogens are often found in endo- and ectosymbiotic associations with protozoa, contributing to approximately 37% of total methane production (Finlay et al. 1994). H. perforatum (no. 36) and R. obtusifolius (no. 41) both exhibit partial inhibition of methanogens, as CH4 production increased, but less than in the control mixture. H. perforatum has been reported to contain the aglycone quercetin (Napoli et al. 2018), a compound that has demonstrated a strong anti-methanogenic activity with both methanogens and protozoa being inhibited (Nørskov et al. 2023). Other species in this study, such as S. nigra, H. helix, and P. nigra, also contain quercetin (Senica et al. 2017; Tebbi and Debbache-Benaida 2022; Gavrila et al. 2023) which may contribute to the observed effects. On the other hand, R. obtusifolius is known to contain condensed tannins, mainly procyanidin polymers (Spencer et al. 2007; Rochfort et al. 2008). Different n-hexane, chloroform, and aqueous fractions of R. obtusifolius showed antimicrobial properties (Orbán-Gyapai et al. 2017), but methanol extracts showed particular efficacy (Harshaw et al. 2010). However, to fully understand the impact on ruminal microbiota, microbiological assessments are essential.
This study highlights the methane-mitigating potential of various plant species in modifying rumen metabolism. However, practical challenges must be considered when integrating these species into ruminant feed. Many investigated alternative forage species have lower competitiveness and limited tolerance to frequent cutting, making them less suitable as primary forage crops (Hamacher et al. 2021; Shackleton et al. 2024). Cultivating these species in monoculture on agricultural land may reduce the area available for high-yielding crops, potentially leading to production losses. However, incorporating trees, shrubs, and herbs into forage systems could enhance landscape diversification and biodiversity, contributing to more resilient agroecosystems with improved ecosystem services, such as erosion control, nutrient cycling, and weed suppression (Cappelli et al. 2022). Furthermore, although this study concentrated on ruminal fermentation, PSM may also affect feed palatability (Häring et al. 2008). Additionally, some species could present potential toxicity risks when consumed in large quantities (Acamovic and Brooker 2005).
5 Conclusion
This study highlights the mitigating potential of plant species and the important role of their PSM in modifying rumen metabolism. However, the observed mitigation was often associated with a reduced fermentability, as supported by an analysis of leaf traits and lower total VFA production. An in-depth analysis of a subselection of 12 plant species identified C. sativa, C. bignonioides, A. glutinosa, P. nigra, and H. helix as most promising CH4 mitigators. It is crucial to note that the effects of PSM on rumen metabolism are highly compound-specific, species-specific and influenced by abiotic and biotic factors with high impact on the CH4 mitigating potential of plant species. On the other hand, this study showed that controlled adjustments to the in vitro setup can aid in identifying potential underlying mechanisms. While this vitro setup provided valuable insights, identifying the specific phytochemical profiles responsible for CH4 mitigation and microbial analysis will help further elucidate the mechanisms behind these effects. To fully validate their CH4-mitigating potential, future research should explore synergistic effects and the inclusion of plants in mixed diets and conducting in vivo trials.
Acknowledgements
We would like to acknowledge the INSTALAB core facility at Ghent University for providing us with their analytical service and granting us access to their instrumental equipment. Special thanks to Charlotte Melis for the laboratory work.
Ethics Statement
The ethical commission of the Institute of Agriculture, Fisheries and Food, Belgium (EC2015-257), approved all experimental procedures, including the fistulation of dairy cows, required for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




