SquamBase—A database of squamate (Reptilia: Squamata) traits
Abstract
Motivation
I present a database that contains information on multiple key traits for all 11,744 recognised species of squamates worldwide. The database encompasses key traits and a reasonably comprehensive picture of available public knowledge. I present comprehensive description of the sources and rationale leading to the assignment of each particular trait state for each species. I hope the dataset can serve the scientific community, promote research and understanding of the group, comparisons with other taxa, and assessment of conservation needs. Furthermore, gaps in our knowledge of squamate traits become readily apparent and will hopefully lead to further study and even better knowledge.
Main types of variables contained
Morphological, ecological, life history, geographical and conservation-related traits.
Spatial location
Global.
Time period
Late Holocene to recent.
Major taxa and level of measurement
Squamata, species.
Software format
xlsx.
1 INTRODUCTION
With over 11,700 recognised species (Uetz, 2023, and species described since) squamates (Reptilia: Squamata) are the largest clade of land vertebrates in terms of the number of species. It is an old clade, with the oldest known crown group members dating to the late Triassic (~206–202 million years ago; Simoes et al., 2018, Whiteside et al., 2022) but modern diversity mostly results from several major adaptive radiations occurring much later (e.g. Burress & Munoz, 2022; Grundler & Rabosky, 2021; Klein et al., 2021; Pincheira-Donoso et al., 2015). Squamates have a nearly global distribution on land (Roll et al., 2017), as well as in most warm oceans and seas (Lillywhite et al., 2017). This remarkably successful group shows immense variation in morphology, spanning forms with four, two or no legs (Camaiti et al., 2021)—and both digit loss and limbs losses are more common in squamates than in any vertebrate taxon (e.g. Sites et al., 2011; Skinner et al., 2008). Squamates also show great variation in limb, head, and trunk size (e.g. Camaiti et al., 2023; Pianka et al., 2017), as well as in body length, body mass, and length-mass relationships (Feldman et al., 2016; Feldman & Meiri, 2013). Some forms can lose—and regenerate, their tails. In others tails are prehensile, and many use their tails as a fat storage organ—and of course a locomotory organ (e.g. Arnold, 1984; Fleming et al., 2013; Price, 2017; Sheehy et al., 2016).
Squamates are likewise highly ecologically diverse—with different species and clades having vastly different diets, activity times, microhabitat preferences, population densities, and home range sizes (Rodda, 2020; Vidan et al., 2019; Vitt et al., 2003). While most squamates are solitary, and almost all are independent since birth, different levels of parental care and social groupings are well known (Whiting & While, 2017). Squamate life histories are very diverse. The sex of the developing embryo can be determined via diverse genetic mechanism, temperature dependent sex determination, or a combination of genetic and environmental conditions (Cornejo-Paramo et al., 2020; Mezzasalma et al., 2021; Sabath et al., 2016). While most species retain the ancestral egg laying mechanism, ovoviviparity, as well as true viviparity, with diverse degrees of placentation (Blackburn, 1995, 2006), evolved more often in squamates than in all other vertebrate clades put together (Griffith et al., 2015; Recknagel et al., 2021; Sites et al., 2011; Wright et al., 2015). Even a case of reversal from viviparity back to oviparity is known (Lynch & Wagner, 2009). Squamates are likewise diverse in their reproductive effort: many clades (most notably the Gekkota, but also anoles, gymnophthalmids, etc.) have fixed clutch sizes of one or two eggs, while in the others clutch (or litter) size usually increases with maternal body size to over 100 neonates: values unknown in endotherm amniotes (Meiri et al., 2021). The pace of life is highly varied in squamates, with reproduction taking place every few days (e.g., in many anoles) to once every 3–4 years (e.g. Andrews & Rand, 2022; Otsuka et al., 2020; Schwarzkopf, 1993), maturity reached in a few months to several years (e.g. Alcala, 1966; Andrews, 1976; Iverson et al., 2004), and longevities ranging from less than 1 year to well over 50 years (Stark et al., 2018, 2020). Lastly, squamate physiology shows much flexibility, with species active at body temperatures often showing variation of tens of degrees, and associated variation in metabolic rates, over the course of a single day of activity (I have recorded, for example, temperatures ranging from 16.0 to 37.0°C in active Phoenicolacerta laevis and from 16.6 to 37.9°C in Mesalina olivieri, in the field in Israel).
With such great variation in basic biological attributes, it is unsurprising that squamates are ubiquitous in all terrestrial biomes, except the coldest ones, across all continents and tiny, often very remote, islands. Indeed, squamates are relatively more successful than other vertebrates in colonising and persisting in warm deserts and islands (Raz et al., 2024; Roll et al., 2017).
Much of squamate diversity is threatened, often highly threatened, with extinction. Indeed, some species and populations are already extinct (Slavenko et al., 2016). A recent global evaluation conservatively estimated that 1829 species, some 20% of squamates, fall into a threatened category (Cox et al., 2022). A substantial body evidence suggest that the number of species actually threatened is much higher (because data deficient and non-assessed species are more likely to be threatened; e.g. Borgelt et al., 2022; Caetano et al., 2022; Gumbs et al., 2020). Future changes, especially climate change, likely threaten many species that are currently thought of as safe (Murali et al., 2023).
A dataset of ecological, morphological, physiological, life history and other traits, if accurate and up to date, can form a basis for the study of ecological, biogeographical and evolutionary questions, among others. It can thus serve to enhance basic and even applied science, conservation policy and action (Gallagher et al., 2021). Furthermore, in the current biodiversity crisis, such a dataset can help us identify the taxa, geographical areas, and areas of eco-space and morphospace, under the greatest current and future threat. Identifying these should be the first stage towards protecting them. Large squamate trait datasets have been assembled decades ago, for example, in the seminal works of Fitch (1970, 1981, 1985), Tinkle et al. (1970) and Dunham et al. (1988), among others. Such datasets were highly valuable and impressive for the age preceding the internet. They were, however, understandably limited in geographical and taxonomic scope, mainly to taxa inhabiting Western Europe (which has few squamates) and the USA. Often, they were also limited by design, to only encompass lizards. I have previously published global squamate trait datasets—especially of body size (Feldman et al., 2016; Meiri, 2008), and for life-history traits (e.g. Meiri, 2016, 2018; Meiri et al., 2012, 2013, 2020). These were, however, either restricted to lizards, or incorporated relatively few, mostly life-history traits. Furthermore, it is my belief that datasets claiming global coverage have sell-by-dates, after which advances in our understanding of both the traits themselves, as well as the geographical distribution of species, and especially, their taxonomic status, render them less useful, if not completely obsolete. This is especially true for taxa such as the Squamata, which experiences great taxonomic upheavals. Squamate species are being re-defined, elevated from, and less frequently, relegated to synonymy, elevated from subspecies to species status (but almost never back), and especially described, at very fast, and accelerating rates (Uetz, 2023). The present work stems from, and expands on, a dataset I published on lizard traits (Meiri, 2018). The database presented herein contains more than 1000 species of lizards and amphisbaenians that were not recognised in 6 years ago (Meiri, 2018). The 2018 dataset has data on 6657 species of lizards (including amphisbaenians) compared to 7654 here (a 997 species difference). Several species in Meiri (2018) are no longer considered valid today (Uetz, 2023), and thus the actual increase is of over 1000 species. Furthermore, my 2018 lizard dataset (Meiri, 2018; which) omits a major sub-order of squamates, the snakes (Serpentes), which, with ~4090 recognised species (as of January 2024), are one of the most remarkable of the vertebrate radiations.
Over the last 5 years I have been expanding the lizard dataset to include not only newly recognised lizard species, and more and better trait data and metadata for species already present, but also data on snake traits. My aim here is simply to present this database in a way that is both easy to use, and easy to verify (validate, refute or improve). I include data on multiple traits of all currently recognised species of squamates (including the few that are known to have gone extinct in recent centuries; Slavenko et al., 2016), as well as the associated metadata (sources and how I interpreted them—for each trait). I hope these data will enhance our basic understanding of these wonderful creatures, and lead to exciting new studies of them. I also hope that the shortcomings and biases of the dataset will enhance future efforts to rectify them by studying little known taxa and regions. Finally, I hope the dataset can serve conservation studies and efforts.
2 METHODS
I gathered data for several (often interrelated) traits—mainly from the literature (Supplementary Table S1). This consisted of books such as field guides and reptile biology texts, journal articles, theses, reports and other grey literature, and dedicated websites such as the reptile database (Uetz, 2023). I regularly (every week to every year, depending on the frequency of publications) scanned over 50 herpetological journals (e.g. Amphibia Reptilia, Journal of Herpetology, Sauria, Herpetology Notes, Revista Latinoamericana de Herpetologia), and journals publishing taxonomic or other data pertaining to reptiles (e.g. Zootaxa, Zookeys, Molecular Phylogenetics and Evolution). I checked two literature databases (http://vipersgarden.at/ and http://www.lacerta.de) once a year. I supplemented this by scanning general biological journals (e.g. Biological Journal of the Linnean Society, Evolution), and dedicated searches for species with little data. The literature database contains 10,285 sources (Supplementary Table S2). To these I added data from museum databases, my own observations in the field (mainly in Israel), and personal communication with reptile biologists (especially members of the GARD working group: http://www.gardinitiative.org/).
Snake data were initially gathered by Anat Feldman as part of her PhD project (Feldman, 2015) and supplemented by me since 2015. Some additional trait data for snakes were also collected lately by Anuj Shinde (activity times), Chen Donghe (substrate), and Anna Zimin (reproductive mode). I manually recorded trait data for each species, using actual data and ignoring imputed or guessed at values (I still list those in the metadata, but do not use them in the data columns). Data reported at higher levels (e.g. genus and family) were excluded, unless a focal species was discussed in the context of such a larger group, in which case I used the data for this species alone. Large datasets (e.g. Rodda, 2020 [from which only data having high confidence: 3 or 4 stars, were used]; Wang et al., 2023) were usually only used for species lacking data for the relevant traits. I noted values from Myhrvold et al. (2015) in the metadata only when no data for the focal trait were available in other sources. However, I did not use the data, as they are largely derived from imputations, and are often unreliable (Meiri et al., 2020). When sources were in conflict as to a categorical character state I either applied a more inclusive category (e.g. if one source gave microhabitat preferences as terrestrial, another as fossorial, I tallied the species as fossorial and terrestrial)—or decided based on my own assessment of the quality of the evidence (e.g. sample size, date, whether a datum was based on direct observation or a secondary compilation of data, etc.). For numerical traits I report the range and the range of means (i.e. the largest and smallest observation, and the largest and smallest mean).
For each species I gathered data on the following traits, and their associated metadata (see Supplementary Table S2 for literature sources and Supplementary Table S3 for full field definitions):
Body size and morphology: 1. Total length; 2. Snout–vent length; 3. Body mass (observed); 4. Body mass (calculated; and the equations used to calculate it); 5. Limb development. B. Geography: 6. Insularity; 7. Country of description; 8. Elevation (minimum and maximum). C. Ecology: 9. Activity time; 10. Substrate/microhabitat use; 11. Diet; 12. Foraging mode; 13. Population density; 14. Home range size; 15. Yearly activity period; 16. Body temperature in the field; 17. Preferred body temperature in the lab. D. Life history: 18. Age at first breeding; 19. Maximum longevity; 20. Mode of reproduction; 21. Brood Size; 22. Brooding frequency; E. Range and extinction risk: 23. Major Biome; 24. Major biogeographical realm; 25. Geographical range size; 26. Extant or extinct; 27. IUCN threat status; 28. Population trend; 29. Threat criteria; 30. Year assessed by the IUCN.
2.1 Taxonomy and names
I provide the species name in the reptile database (Uetz, 2023), or the proposed name if the species was described after the publication of the latest version of the reptile database (in October 2023). I note whether the species appears in the latest version of the reptile database (October 2023; Uetz, 2023) or was described, revived from synonymy and elevated from subspecies status—or has been synonymised since (‘new’, ‘pending elevation’ and ‘pending synonymisation’ respectively). I tally for each species its sub-order (Amphisbaenia, Sauria or Serpentes), infraorder, family and year of description (according to the Reptile Database and the primary descriptions). I then provide a list of synonyms encountered while gathering trait data for the species. I consider the reptile database name (Uetz, 2023) as valid and also list for each species, the name used for it in the two largest available squamate phylogenies: of Tonini et al. (2016) and Zheng and Wiens (2016), to facilitate finding names that have changed while conducting phylogenetic analyses. I also provide the name used for the species in the IUCN redlist (IUCN, 2023), and its id numbers in the Reptile Database (Uetz, 2023) and in the GARD database. This is followed by a list of the sources I used for obtaining data for each species.
2.2 Body size and morphology
I provide for each snake species data on the maximum reported total length, mean (or midpoint) adult female total length, and mean or range midpoint hatchling or neonate length (all in mm). Here, and in other fields reporting single means, if more than one mean was reported I averaged the highest and lowest means. For all species I then provide the same types of data (maximum, mean female, mean hatchling/neonate) for snout–vent lengths (SVLs). I deleted data on total lengths of species that had higher SVLs for the same type (maximum, female and hatchling).
Squamates are rarely weighed—but mass data are probably much more comparable across taxa and body plans than the lengths commonly reported. Therefore, I provide data on observed (actually measured) mean adult non-gravid (preferably post-partum) female mass and mean, or midpoint, hatchling or neonate body mass. I then list for each species the sources used to derive length and mass data, and often the values reported in these sources. This is followed by data on body mass derived from SVLs. When SVL data were unavailable masses are calculated from total length (only for snakes). These calculations are based on allometric equations I developed for different families or higher-level taxa (if families do not have enough species, or enough species with mass available data to develop equations). Equations are from Feldman et al. (2016) (which, in turn, are based on Feldman & Meiri, 2013; Meiri, 2010 and subsequent publications). However, since there were several important taxonomic rearrangements since these works were published (e.g. Burbrink et al., 2020; Das et al., 2023; Georgalis & Smith, 2020), and more and better data became available for some clades, I also use equations from Chapple et al. (2023) (for skinks) and developed here new equations for other taxa (mostly of snakes) based on published literature, personal communications from GARD members, my own data from the field, and Data from the collections of the Steinhardt Museum of Natural history. Equations and underlying data are presented in Supplementary Table S4. New equations are only based on cases where mass and length were reported in the same source from the same specimens (although in some cases authors report slightly different sample sizes for mass and length, for example, when tails are damaged and total lengths cannot be measured). The equations are based on OLS regression of log10 transformed length (in mm) and mass (in g) data. I also define whether each species is fully limbed (have four well developed limbs—usually but not always with 5 digits in each), or has reduced limbs (at least one pair of limbs very short relative to SVL, often with fewer than 5 digits), has only front or hindlimbs, or lacks limbs completely (including species for which a tiny, non-functional vestige is present [e.g. Chalcides guentheri or Pseudopus apodus]).
2.3 Geography and elevation
A full polygonal range for each species will be made available elsewhere. For most species, however, ranges are already available from an updated version of Roll et al. (2017) GARD database, that is, Caetano et al. (2022; GARD version 1.7). Here I list only whether species are insular endemic (have their ranges only on landmasses smaller than mainland Australia) or not (present in mainland Australia, South or North America, Africa or Eurasia). I further note which country (according to 2023 country borders) the species was described in. For each species I provide data on the minimum and maximum recorded elevation as recorded from observations (above or, in a few cases such as Ptyodactylus guttatus I observed near Ein Gedi, Israel, below sea level). I note that elevation data are often very partial (e.g. based on just one observation or just part of the range on a wide-ranging species). I provide minimum (rounded down at 10 m intervals) and maximum (rounded up at 10-m intervals) elevation data and the sources used to derive them. Elevation data are not derived from polygonal range maps or even from the elevation of a point locality—unless the values are provided as elevational data in the sources of these maps.
2.4 Ecology
For each species, I provide data on the following traits: activity times, substrate use, diet, population density, home range size, activity period and body temperature. Activity times are categorised into Diurnal, Nocturnal or Cathemeral (active both day and night). Species are categorised as diurnal or nocturnal according to their predominant activity time (see Slavenko et al., 2022). Species that spend considerable time active in both day and night are classified as cathemeral. This includes species that shift their activity time over the course of the year (i.e. nocturnal in summer and diurnal in colder seasons), or have different activity times in different parts of their geographical range (usually more diurnal in colder regions). Few species (33) are defined as Crepuscular—active only around dawn and dusk. Otherwise, crepuscular and diurnal species are categorised as diurnal while crepuscular and nocturnal species are categorised as nocturnal. Substrate or microhabitat uses I classify into Arboreal (above the ground in vegetation), Saxicolous (in and on rocks), Terrestrial (on the ground), Semi-Aquatic (spend part of the time in fresh water), Aquatic or Marine (both categories have only snake species), Fossorial (digging underground) or Cryptic (almost always found under objects, almost never in the open; for example, Chalcides ocellatus, pers. obs.). Many species are classified as having a combination of these categories (e.g. Arboreal and Saxicolous or Fossorial and Terrestrial).
Diet is categorised as a herbivore/omnivore/carnivore trichotomy following Meiri (2018): herbivorous species are those that mainly consume (>50% in volume, if quantitative data are available), carnivorous species are those feeding predominantly on animal matter (including eggs; >90% by volume if quantitative data are available), and omnivorous species are those eating mostly animal matter but with considerable percentage of plants (10%–50% plant matter, if quantitative data exist). I note that, while data are not available for many snakes, all snakes can be safely categorised as carnivorous. Therefore, I have spent much less effort in obtaining dietary data for snakes than I did for other taxa and other traits, despite an abundance of easily available data. I provide more detailed data on diet (e.g. types of prey species taken, quantitative data when available) in the metadata field. Foraging mode is defined as either Active foraging or Sit and wait (=ambush predators). Species that can display either tactic are classified as Mixed. Strict herbivores are not assigned a foraging mode (though a case could be made for them to be active foragers).
Population density is given for adults (males and females together) in individuals per hectare, and reported as minimum, maximum and mean. When more than one mean was calculated I report the smallest and largest means. I note that such ‘naked’ values of population density are extremely sensitive to their context. Factors such as detectability, weather, method and especially, the area over which density is measured, have profound influence on density estimates. Thus, for example, in a large dataset of tetrapod densities (Santini et al., 2018), 68% of the variation in density can be explained by the area over which it was sampled alone (Meiri, 2022). Density estimates should therefore best be associated with such data (not provided here) or compared to those calculated over similar areas. Home range size is given for adults, in square meters. Home ranges are reported as minimum, maximum and mean. When more than one mean was calculated I report the smallest and largest means. Yearly activity period is the minimum and maximum number of months a species is recorded active in the field. For species that are mainly active in warmer months and are only occasionally seen (usually warm days) during colder seasons I report the main activity in the minimum column, and all activity in the maximum column. Thus, a species (obviously in the Northern Hemisphere) that is most active from March to November, but can also occasionally be seen active between December and February, will have an activity season of 9–12 months.
Body temperature (in °C, cloacal) is given as means (highest and lowest reported) for animals measured while active in the field. I also report the mean preferred body temperatures recorded in a thermal gradient in the lab.
2.5 Life history
Age at first breeding (in months) is reported as a range. If data are reported separately for females and males, I listed only the data for females; Maximum longevity is the longest an individual of a species is recorded as having lived (in years). I also report whether data originate from nature or from captive animals, as this was found to greatly influence on maximum longevity estimates (Stark et al., 2018, 2020). Maximum reported longevities are often associated with small samples and are thus unreliable. Not infrequently published longevity estimates are lower than reported age at maturity for the same species. I did not record such entries (but sometimes report them in the metadata, leaving the data column with ‘NA’) and generally disregarded longevity data of less than 2 years unless the authors of the relevant study specifically mentioned they are reliable (e.g. Furcifer labordi, Karsten et al., 2008). I report mode of reproduction as either Oviparous or Viviparous. Viviparity here includes ovoviviparity (Feldman et al., 2015; Zimin et al., 2022) because they are difficult to distinguish for poorly studied species, and because I am interested more in mother ecology than in embryo physiology. The few species showing a bimodal reproduction—either with different populations showing different modes, or where mode differs within populations and even for an individual (e.g. Saiphos equalis; Laird et al., 2019), are reported as having a ‘Mixed’ mode. Brood size is the size of a clutch or a litter. It is reported as minimum, maximum and mean. When more than one mean was calculated I report the smallest and largest means. Brooding frequency is the number of clutches or litters a female produces in a year. It is reported as minimum, maximum and mean. When more than one mean was calculated I report the smallest and largest means.
2.6 Range and extinction risk
I record the Major Biome inhabited by each species as the largest one of the biomes in Olson et al. (2001) that intersect a species GARD 1.7 (Caetano et al., 2022) range. Similarly, I report the major biogeographical realm inhabited by each species as the largest one of the realms in Falaschi et al. (2023) that intersect a species range (from GARD 1.7, Caetano et al., 2022, and later sources, mostly the species descriptions). For this I consider the Antillean, Neotropical, Central American and Chilian realms of Falaschi et al. (2023) together a single ‘Neotropic’ Realm, their Tibetan and Palearctic realms as a single ‘Palearctic’ realm, and the New Guinean, Australian and Oceanian realms as a single ‘Australo-Pacific’ realm. I further designate all marine snakes as inhabiting a single ‘Marine’ realm (resulting in a total of nine realms). I report Geographical range size (in km2) from both GARD 1.7 (Caetano et al., 2022) and the IUCN (2023). Extinction status (Extant or Extinct) are based on data and methods in Slavenko et al. (2016), with necessary updates, rather than on the, more restrictive, EX category in the IUCN dataset. I nevertheless consulted the Red List status when revising these data. Finally, I report the Threat status, Population trends, and Threat criteria (for threatened species only), as assessed by the IUCN (2023), and also report the year each species was assessed by the IUCN.
3 RESULTS AND DISCUSSION
Data are presented for 11,744 squamate species (7453 lizard, 202 amphisbaenian and 4089 snake species) (Supplementary Table S2). Data availability varies greatly across the studied traits, with completion rates varying from less than 1% (for home range size) to 99.5% and more (lizard and amphisbaenian SVL, calculated mass, biogeographical realm, limb status; Table 1)—with a median of 29% and a mean of 41%.
| Maximum total length (snakes only) | 3735 | 91 |
| Mean female total length (snakes only) | 341 | 8.3 |
| Hatchling/neonate total length (snakes only) | 398 | 9.7 |
| Maximum SVL (squamata) | 9229 | 79 |
| Mean female SVL (squamata) | 5803 | 49 |
| Hatchling/neonate SVL (squamata) | 2483 | 21 |
| Maximum SVL (lizards and amphisbaenians) | 7623 | 99.6 |
| Mean female SVL (lizards and amphisbaenians) | 5413 | 71 |
| Hatchling/neonate SVL (lizards and amphisbaenians) | 2240 | 29 |
| Maximum SVL (snakes) | 1606 | 39 |
| Mean female SVL (snakes) | 389 | 9.5 |
| Hatchling/neonate SVL (snakes) | 242 | 5.9 |
| Mean female mass | 1164 | 9.9 |
| Hatchling/neonate mass | 1244 | 11 |
| Maximum mass (from allometric equations) | 11,690 | 99.5 |
| Mean female mass (from allometric equations) | 6252 | 53 |
| Mean hatchling/neonate mass (from allometric equations) | 2811 | 24 |
| Limbs | 11,744 | 100.0 |
| Elevation (both min and max) | 8218 | 70 |
| Activity time | 9185 | 78 |
| Substrate | 10,155 | 86 |
| Diet | 5925 | 50 |
| Foraging mode | 2824 | 24 |
| Minimum population density | 563 | 4.8 |
| Mean population density | 320 | 2.7 |
| Maximum population density | 607 | 5.2 |
| Minimum home range size | 203 | 1.7 |
| Average home range size | 89 | 0.8 |
| Maximum home range size | 211 | 1.8 |
| Length of activity season | 886 | 7.5 |
| Average body temperature | 1273 | 11 |
| Average preferred temperature | 665 | 5.7 |
| Age at first breeding | 923 | 7.9 |
| Maximum longevity | 12,008 | 10 |
| Mode of reproduction | 7947 | 68 |
| Brood size range | 5590 | 48 |
| Mean brood size | 3410 | 29 |
| Yearly broods | 1537 | 13 |
| Major biome | 10,370 | 88 |
| Major realm | 11,740 | 99 |
| Geographical range size (GARD 1.7) | 10,266 | 87 |
| Geographical range size range size (IUCN) | 9551 | 81 |
| Threat status (IUCN) | 9772 | 83 |
| Population trend (IUCN; excluding unknown) | 4915 | 42 |
Squamates SVLs range from 17 mm (in the shield-snouted geckolet, Sphaerodactylus elasmorhynchus) to 1570 mm in the longest lizard, Komodo dragon, Varanus komodoensis and to over 6000 mm (the reticulated python; Malayopython reticulatus) with modes at ~80 mm for lizards and at ~650 mm for snakes (Figure 1). Snake total lengths range from 91 mm (in the scolecophidians Indotyphlops veddae and Myriopholis yemenica) with a wide mode between 500 and 1000 mm (Figure 1). The literature on maximum length of the largest snakes is plagued with often dubious claims (Barker et al., 2012; Murphy, 2020; Penning et al., 2013; Platt & Rainwater, 2015), and I therefore took a very conservative approach here. I list the length of the longest snake, M. reticulatus, as 7900 mm. The green anaconda is second largest (6700 mm). Python bivittatus, P. sebae and P. bivittatus all have reliable recorded maxima of 5700–5740 mm that, given the difficulty of measuring the lengths of snakes in general and large snakes in particular (Barker et al., 2012; Cundall et al., 2016), are virtually identical. The median ratio between mean female total length and maximum total length is 80.7% (n = 292 species with both types of data), whereas the median total length of a hatchling snake is 29.2% of that of an adult female (but n is only 47 species).

For SVL the corresponding values are 85.6% and 42.0%, respectively (n = 5578 and 1976 respectively). The median ratio of neonate/hatchling to female weight in squamates is 6.2% (based on actual, non-imputed mass data, n = 267 species). All snakes, and 199 of 202 amphisbaenians, are limbless, as are 233 lizard species. Three species of amphisbaenians (Bipes biporus, B. canaliculatus and B. tridactylus) and five lizard species have forelimbs but no hindlimbs, and 62 lizard species have hindlimbs but no forelimbs. I classify the other 6397 lizard species as fully limbed. Other classification schemes (e.g. Camaiti et al., 2022) may arrive at slightly different numbers of limb-reduced and fully limbed lizards.
A large proportion of squamates are insular endemic (27%, 3170 species), perhaps unsurprisingly given their impermeable skin and low metabolic demands that could facilitate trans-oceanic dispersal. For some taxa (e.g. geckos, Novosolov & Meiri, 2013) laying highly calcified eggs could have made island colonisation easier (because dispersal could happen in the egg stage). This could explain the high frequency of reptiles on islands relative to other tetrapods (Roll et al., 2017).
Squamate descriptions have clearly been accelerating, with 28% of species described this century alone, and fully 23.5% in the 17 years since 2007 (see also Uetz & Stylianou, 2018). Interestingly, while in the first 16 years of the century Australia and Argentina have seen the most new species descriptions (at least for lizards; Meiri, 2016), in each year since 2018 India had more new species described than any other country (185 species in total).
Squamates range from near the shores of the Dead Sea (in Israel, the Palestinian Authority and Jordan, the lowest land on Earth, see above) to 5490 m a.s.l. (Ablepharus ladacensis; Kastle et al., 2013; Nanhoe & Ouboter, 1987). Few species, however inhabit very high altitudes: 129 species (1.1%) reach 4000 m or above, and only 10 (all of them lizards) reach or surpass 5000 m, while most species have their lower altitudinal limit at or near sea level (Supplementary Figure S1).
Most squamates (58.6%) with known activity times are diurnal, and less than a third (32.6%) are nocturnal. Interestingly, most lizards (68%) are diurnal and only 25.5% are nocturnal—while most snakes (51.1%) are nocturnal and just over a third (34.2%) are diurnal. Only 34 amphisbaenian species (17%) have known activity times, with a relatively similar spread across all categories (15 nocturnal, 11 diurnal and 7 cathemeral species). That said, except skinks (8.3% nocturnal, 398 species with unknown activity time, see Slavenko et al., 2022), the taxa with the largest numbers of species with unknown activity times, Alethinophidian and Scolecophidian snakes (1244 and 338 species with unknown activity times respectively), amphisbaenians (168 species) and geckos (159 species), have high proportions of nocturnal species (50%, 75%, 44% and 72% of species with known activity times, respectively). Furthermore, these four taxa comprise 70.4% of the squamate species described in 2020–2023 (59 of 838 species), thus the true proportion of nocturnality in squamates is likely to be higher than is known today.
The most common substrate categories for squamates are terrestrial (3177 species), arboreal (1682), saxicolous (1047), and fossorial (1008). Interestingly, while the terrestrial category is the most common in both lizards and snakes, arboreal lizards and saxicolous lizards are common, but fully fossorial ones less so, while in snakes fossorial is the second commonest category, arboreal third and only 11 species are categorised as fully saxicolous. All snakes, all amphisbaenians, and 81.7% of lizards with known diets are carnivores, while only 14.2% of lizards are omnivores and just 4.0% feed mostly on plants. Active foraging is more common (58% of species with known foraging mode) than sit and wait foraging (36%), and just 6% of species are classified as having a mixed mode. This is mainly driven by snakes, where 73.7% of the species are active foragers (20.9% sit and wait) while in lizards the proportions are roughly equal (45.4% active foragers 47.5% sit and wait).
Population densities range from 0.001 (Cyclodomorphus gerrardii, Williams et al., 2009) to 100,000 (Alsophylax pipiens) animals per hectare. However, these data are not calculated over a hectare: the datum for A. pipiens, for example, is derived from the finding of ‘up to l0 specimens in 1 m2’ (Szczerbak, 2003). Furthermore, several species have over 3 orders of magnitude range of reported densities (this can also characterise other taxa; see e.g., Meiri, 2022). Thus, while I report these data, I urge readers not to use them without additional qualifiers. Known adult squamate home range sizes range from just 0.4 m2 (Anolis aeneus, Henderson & Powell, 2018) to 18.3 km2 (Varanus albigularis; Bennett, 1995), but data are too scarce to provide reliable description of moments of central tendency.
The squamate reaching the highest latitudes worldwide, Zootoca vivipara, may only be active from the first half of June to the end of October (Sindaco et al., 2009), and some other squamates (e.g. in the Caucasus and southern USA; Arakelyan et al., 2011; Degenhardt et al., 1996) are active for as few as 3–4 months of each year. That said, the mode is year-round activity (Supplementary Figure S2). In fact, available data likely underestimate the proportion of squamates active year-round, as yearly activity data are often reported (e.g. in field guides) for the reptiles of cold regions, but seldom for tropical areas, where most squamates reside. I suspect this is because most tropical reptiles are active year-round and thus this information is considered uninteresting, resulting in under-reporting.
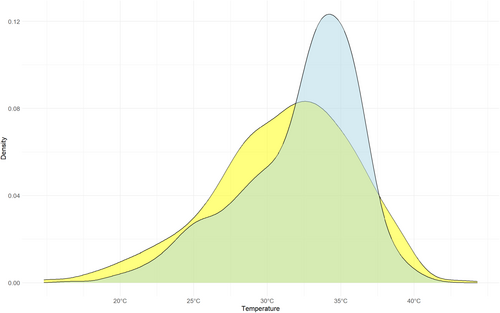
Squamates can be active over a remarkable range of mean body temperatures: from 11.5°C (in the New Zealand and Woodworthia maculate/brunnea; Cree, 1994, Hare & Cree, 2016) to 44.3°C (the Australian Diporiphora bilineata, Bradshaw & Main, 1968), with a mode of ~33°C. Preferred body temperatures can be as low as 16.6°C (in Goniurosaurus kuroiwae (Werner et al., 2005), and as high as 40.7°C (in Pholidoscelis polops; Angeli, 2017), but their distribution is more peaked, with a distinct mode at ~34°C (Figure 2). Of 533 species with both types of data, preferred temperatures are higher in 327 (61.3%). Only 53 snake species (and 10 amphisbaenians) have field body temperature data, and only 21 (and six amphisbaenians) have preferred temperature data (most of those were recently reported by Pettersen et al., 2023).
3.1 Life history
Squamates reach sexual maturity as fast as 30–40 days (in several East Asian gecko species, Alcala, 1966) or as slow as 15 years (in Galapagos land iguanas of the genus Conolophus), but most species for which data exist (543 of 923) reach maturity at minimum ages of 9–24 months. Squamate maximum natural longevities can be as short as ~1 year (e.g. in some Anolis species) and can exceed 60 years (e.g. in some iguanids) with a mode of about 5 years. About four in five squamate species (80.5%) are oviparous and a fifth (19.3%) are viviparous. Eighteen species are thought to have a mixed mode of reproduction. Numbers are similar for lizards (81.9% oviparous, 17.8% viviparous) and snakes (78.1% oviparous, 21.8% viviparous). The taxon with the greatest number of species with no data, however, the Gekkota (980 species with no data), is predominantly oviparous (98.2% of species with known mode are oviparous and all viviparous species are members of the New Zealand and New Caledonian Diplodactylidae radiation). Thus, true proportions of viviparity may be somewhat lower.
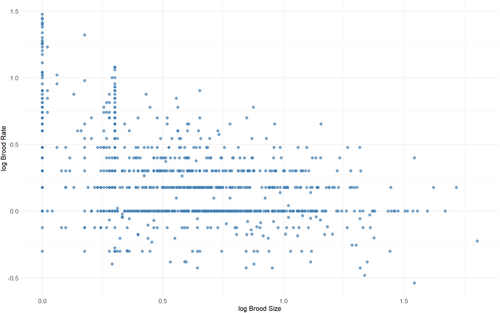
Brood size ranges from one egg (rarely, one neonate) in small geckos, anoles, and other taxa (this is also commonly the mean and maximum brood size) to 156 in the puff adder Bitis arietans. The largest mean is 63.7 neonates in the green anaconda, Eunectes murinus (Pizzatto & Marques, 2007), and the largest mean for a lizard (and second largest overall) is 52.2 eggs for the Senegal chameleon, Chamaeleo senegalensis (Cisse & Karns, 1978). A few squamate species (both snakes and lizards, oviparous and viviparous) may only reproduce every 4 years (though all these species may reproduce biennially or annually in good years). On the other side of the spectrum, several anole species can produce their single-egg clutch once a week in the reproductive season, and up to 30 times a year (see Andrews & Rand, 2022). Several geckos can produce one or two egg clutches (depending on the species) more than 10 times a year, and Rosler et al. (2017) recorded 78 one-egg clutches in 43 months in the Middle Eastern semaphore gecko, Pristurus flavipunctatus, in captivity. There is a clear trade-off between clutch size and number (Figure 3), with a (non-phylogenetic) log–log slope of −0.34 ± 0.02 (p < 0.0001, R2 = 0.155, n = 1516).
3.2 Range size and extinction risk
The two estimates of geographical range size (Gard 1.7: Caetano et al., 2022; Roll et al., 2017; and those of the IUCN) are tightly correlated: across the 9266 species with ranges in both datasets the log–log correlation has an intercept of 0.59 ± 0.02 and a slope of 0.857 ± 0.005 (R2 = 0.78, p < 0.0001). However, while GARD rages are larger than IUCN ranges in 59.6% of the species, the positive intercept and shallow (shallower than 1) slope mean that GARD ranges are actually smaller for small-ranging species. The IUCN classified the vast majority of threatened squamates based on its B criterion for extent of occurrence ranges (such as those used here; Meiri et al., 2023). If we count the number of species that fall under the thresholds for IUCN risk categories, we see that GARD ranges allow 4020 species with ranges under 20,000 km2 that can potentially fall under the VU category versus 3919 species in the IUCN dataset. The numbers are also similar for species with ranges <5000 km2 (fitting the EN category; 3118 species in GARD, vs. 3062 species in the IUCN) but GARD identifies 1887 species with ranges smaller than 100 km2, that can potentially fall under the threshold for the CR category, some 60% more than the IUCN (1175).
As of October 2023, the IUCN lists 1630 squamate species as threatened with extinction. A further 1428 are listed as data deficient, and species have not yet been assessed. Their population trends are worrying. First, they are only listed for 4912 of the 11,744 species (4076 species have ‘unknown’ population trends and the rest have not been assessed). Only 50 species have increasing populations while those of 1304 species (26% of those with known population trends) are decreasing—including those of 450 species classified as non-threatened. I list 56 squamate species as extinct, two as extinct in the wild, and 32 as possibly extinct.
4 CONCLUSIONS
I present data I have been collecting for 18 years that are highly relevant for squamate morphology, ecology, physiology, life history, geography and conservation biology. These data reflect, to the best of my knowledge, much of the publicly available data for this species rich, widespread and fascinating group. The database contains wide gaps (Table 1) that hopefully will get herpetologists motivated to fill. In general, I hope this dataset could facilitate further research and better understanding and enable better, more informed conservation of this wonderful clade of animals.
ACKNOWLEDGEMENTS
I want to thank Anat Feldman, who initiated the collection of snake trait data, for her meticulous and voluminous work. Anat refused an invitation to co-author the paper but, in my view, deserves much of the credit. I thank GARD members, my students present (especially Anna Zimin, Chen Donghe, and Anuj Shinde) and past, and colleagues for sharing hard-earned data and correcting errors. I thank two anonymous referees for important comments. A dataset of this scope, especially when collated by someone like me, obviously contains errors in recording (of both data and metadata) and interpretation, as well as erroneous data from the sources used to create it. I will be grateful for anyone pointing such errors to me so I could correct them. This project was supported by an ISF grant #611/23 and a BSF grant #2021030 to SM.
CONFLICT OF INTEREST STATEMENT
The author declares no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The entire dataset, and associated metadata, is fully available in Dryad doi:10.5061/dryad.76hdr7t3b.
REFERENCES
BIOSKETCH
Shai Meiri is a vertebrate zoologist mainly interested in evolution, and obviously in lizards, snakes, and data.




