The global abundance of tree palms
Robert Muscarella and Thaise Emilio are joint first author.
Abstract
Aim
Palms are an iconic, diverse and often abundant component of tropical ecosystems that provide many ecosystem services. Being monocots, tree palms are evolutionarily, morphologically and physiologically distinct from other trees, and these differences have important consequences for ecosystem services (e.g., carbon sequestration and storage) and in terms of responses to climate change. We quantified global patterns of tree palm relative abundance to help improve understanding of tropical forests and reduce uncertainty about these ecosystems under climate change.
Location
Tropical and subtropical moist forests.
Time period
Current.
Major taxa studied
Palms (Arecaceae).
Methods
We assembled a pantropical dataset of 2,548 forest plots (covering 1,191 ha) and quantified tree palm (i.e., ≥10 cm diameter at breast height) abundance relative to co-occurring non-palm trees. We compared the relative abundance of tree palms across biogeographical realms and tested for associations with palaeoclimate stability, current climate, edaphic conditions and metrics of forest structure.
Results
On average, the relative abundance of tree palms was more than five times larger between Neotropical locations and other biogeographical realms. Tree palms were absent in most locations outside the Neotropics but present in >80% of Neotropical locations. The relative abundance of tree palms was more strongly associated with local conditions (e.g., higher mean annual precipitation, lower soil fertility, shallower water table and lower plot mean wood density) than metrics of long-term climate stability. Life-form diversity also influenced the patterns; palm assemblages outside the Neotropics comprise many non-tree (e.g., climbing) palms. Finally, we show that tree palms can influence estimates of above-ground biomass, but the magnitude and direction of the effect require additional work.
Conclusions
Tree palms are not only quintessentially tropical, but they are also overwhelmingly Neotropical. Future work to understand the contributions of tree palms to biomass estimates and carbon cycling will be particularly crucial in Neotropical forests.
1 INTRODUCTION
Palms (Arecaceae/Palmae) are an iconic and diverse group (>2,500 recognized species worldwide) that have long delivered a wide range of provisioning services to humankind (Cámara-Leret et al., 2017; Eiserhardt, Svenning, Kissling, & Balslev, 2011; Levis et al., 2017; Tomlinson, 2006). Many palms are considered ecological keystone species because large numbers of animals depend on their fruit and flower resources (Onstein et al., 2017). In some areas, palms are also remarkably abundant. For instance, six of the 10 most common tree species in the Amazon rain forest are palms (ter Steege et al., 2013). Given the morphological and physiological distinctiveness of palms (which are monocots; Renninger & Phillips, 2016), palm abundance can have important consequences for tropical forest ecosystem function, including carbon sequestration. However, we currently lack a quantitative analysis of the biogeographical patterns and conditions associated with palm abundance.
As a family, palms exhibit a variety of growth forms, ranging from small shrubs to lianas and large trees. Based on available data (Kissling et al., 2019), c. 40% of palm species are capable of growing stems ≥10 cm in diameter at 1.3 m above the ground (here defined as “tree palms”). Tree palms have often been pooled with non-palm trees in forest inventory plots that are commonly used to measure terrestrial carbon stocks and parameterize vegetation models (Phillips et al., 2013). This raises several issues. First, biomass estimates are typically based on allometric equations developed for non-monocot trees (e.g., Chave et al., 2014; Feldpausch et al., 2012). These equations tend to perform poorly for palms because they lack secondary growth, which decouples diameter–height relationships (Goodman et al., 2013). Second, physiological and morphological differences between tree palms and other trees suggest the potential for large differences in terms of the responses of tree palms to drivers of environmental change compared with non-palm trees (Emilio et al., 2014; Renninger & Phillips, 2016). From an ecosystem functioning perspective, these issues are clearly most crucial in areas where palms account for a relatively high proportion of forest biomas, such as some swamp forests (Dargie et al., 2017). Here, we provide the first global analysis of tree palm abundance relative to other co-occurring trees to help reduce uncertainty about tropical ecosystem function.
Patterns of tree palm abundance may be associated with variation in contemporary ecological conditions that favour the establishment and persistence of palms over other types of trees (the contemporary conditions hypothesis). Most existing evidence for palm abundance distributions along contemporary environmental gradients comes from studies in Amazonian forests, where palms tend to be more abundant in areas with ample soil moisture and relatively high soil fertility (Castilho et al., 2006; Costa, Guillaumet, Lima, & Pereira, 2009; Emilio et al., 2014; Kahn & Mejia, 1990; Schietti et al., 2014; Svenning, 1999, 2001). Palm root architecture is the most likely explanation for the observed patterns in relationship to hydrological and soil properties for at least two reasons. First, palms tend to have dense, superficial root systems that may provide them with better anchorage compared with relatively deep-rooted trees in dynamic fluvial systems. In an Ecuadorian forest, for example, Gale and Barford (1999) found that uprooting was c. 50% higher for dicotyledonous trees than for the dominant palm, Iriartea deltoidea. Second, higher investment in root biomass towards the soil surface might be beneficial in terms of competition for nutrients but could represent a limitation for growth in regions where plants must rely on seasonal access to deeper water. In these conditions, high annual precipitation and low precipitation seasonality should maximize the advantages of shallow root systems, and therefore, promote relative abundance of palms (Eiserhardt et al., 2011).
However, local edaphic conditions in the Amazon region are also correlated with longer-term landscape evolution (Higgins et al., 2011; Hoorn et al., 2010) and forest stem turnover rates, which may also affect palm abundance indirectly (e.g., Emilio et al., 2014). For instance, several studies have shown that at least some palm species can be successful in relatively dynamic forests by capitalizing on high-resource conditions after disturbance (Eiserhardt et al., 2011; Emilio et al., 2014; Salm, 2005). Additionally, some palms are resilient to certain types of disturbance, including hurricanes and blowdowns (Lugo & Scatena, 1996) and, in some cases, fire. As a result, we might expect tree palm relative abundance to exhibit a positive association with contemporary rates of forest turnover. In general, the extent to which relatively local ecological factors can help to explain biogeographical scale variation in tree palm abundance remains unknown. In summary, under the contemporary conditions hypothesis, we expect significant associations between local ecological conditions and tree palm relative abundance. In particular, we expect higher tree palm relative abundance in areas with higher annual and dry season rainfall, more fertile soils with shallower depth to the water table, and faster stem turnover rates.
It is also possible that patterns of palm abundance are associated with historical distributions of conditions that allowed the persistence of palm species and populations over long time periods (the climate stability hypothesis). Previous work on macroecological patterns of palm diversity provides context for hypotheses about historical drivers of tree palm abundance at biogeographical scales (Eiserhardt et al., 2011; Kissling, Eiserhardt, et al., 2012; Svenning, Borchsenius, Bjorholm, & Balslev, 2008). For example, Kissling, Eiserhardt, et al. (2012) reported that the extent of tropical rain forest biome in different biogeographical realms during the Cenozoic period (based on palaeoclimate reconstructions) was positively associated with current palm diversity. Relatively low palm species richness in Africa has been attributed to extinctions during rain forest contraction (Faye et al., 2016; Kissling, Eiserhardt, et al., 2012). However, Baker and Couvreur (2013) argued that higher speciation rates outside Africa (as opposed to higher extinction rates in Africa) might better explain contemporary richness patterns. In Madagascar, Rakotoarinivo et al. (2013) reported higher palm diversity in areas that had higher precipitation during the Last Glacial Maximum (LGM; 21,000 years ago) compared with present-day precipitation. In general, palaeoclimatic variability could also be associated with spatial variation of palm abundance. For example, larger areas with more stable tropical climates could facilitate larger populations of palms, which could also be associated with higher diversification rates (Blach-Overgaard, Kissling, Dransfield, Balslev, & Svenning, 2013; Couvreur et al., 2015; Kisel, McInnes, Toomey, & Orme, 2011; Rakotoarinivo et al., 2013; Rosenzweig, 1995). Therefore, under the climate stability hypothesis, we expect a positive association between metrics of long-term climatic stability and tree palm relative abundance.
In this study, we use a large pantropical dataset of forest plots to quantify global-scale variation in tree palm abundance (quantified as the basal area and the number of stems) relative to co-occurring trees. We examine spatial patterns of tree palm relative abundance across major biogeographical realms, in addition to correlations with abiotic and biotic variables in light of the contemporary conditions and climate stability hypotheses outlined above. As a step towards assessing the potential ecosystem-level consequences of tree palm relative abundance patterns, we estimate the amount of error introduced to standard calculations of above-ground biomass (AGB) when tree palms are pooled with other trees versus treated separately. Finally, in light of the broad range of palm growth forms, we assess how the diameter size threshold commonly used in forest inventory plots (10 cm) affects inferences of tree palm relative abundance in different biogeographical realms. Our overarching aim is to develop a quantitative understanding of patterns and drivers of tree palm relative abundance across broad geographical and environmental scales that can help us to gain a better understanding of this important and unique group and to reduce uncertainty about tropical ecosystem functioning and dynamics.
2 METHODS
2.1 Forest inventory data
Our analysis is based primarily on data from ForestPlots.net (Lewis et al., 2009; Lewis et al., 2013; Lopez-Gonzalez et al., 2009; Lopez-Gonzalez, Lewis, Burkitt, & Phillips, 2011; Malhi et al., 2002), which integrates data from research plot networks active in the Neotropics (RAINFOR and PPBio), Africa (AfriTRON) and Southeast Asia (T-FORCES), in addition to other networks and researchers, and also uses the pan-tropical Gentry 0.1-ha transect dataset (Phillips & Miller, 2002) and the database compiled by Slik et al. (2018).
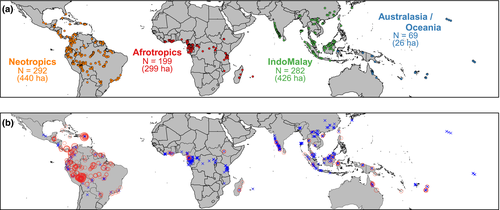
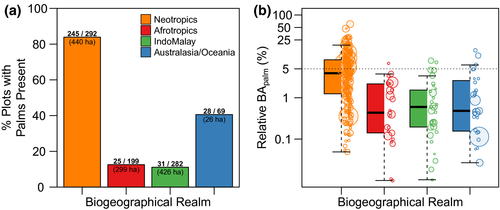
We assembled data for 2,624 individual forest plots located in the subtropical and tropical moist broadleaf forest biomes as defined by Olson et al. (2004). A list of publications associated with data used in this paper is found in the Supporting Information Appendix S1. In each plot, all individual stems with a diameter of ≥10 cm in diameter at breast height (d.b.h., 1.3 m above the ground) were identified and measured for d.b.h. Our analyses focus on arborescent palms that reach ≥10 cm d.b.h. (henceforth, “tree palms”), because smaller-diameter stems are excluded in the standard protocol of most forest inventories. Although palms occur in a wide range of tropical ecosystems (including rain forests, savannas and dry forests), we focused on moist forests because this biome houses the greatest palm diversity and it is where the majority of plots in our dataset are located. We restricted our analyses further to plots reported as “old-growth”, “primary” or “undisturbed” by the original data collectors and excluded 57 plots described as swamps or “monodominant” palm forests. We analysed a total of 2,548 plots (covering a total of 1,191 ha; Supporting Information Appendix S2). To reduce spatial autocorrelation, we pooled data from plots within the same 10 km × 10 km grid cell; hereafter, we refer to these as aggregated plots as “locations”. After aggregation, our dataset included 842 locations (Figure 1a). The area sampled per location ranged from 0.1 to 51.8 ha (median ± SD = 0.4 ± 3.5 ha), with 95% of the locations sampled were ≥0.1 ha. We assigned each location to one of the biogeographical realms defined by Olson et al. (2004), but we combined locations in Oceania with Australasia because of their strong historical connection (Muellner, Pannell, Coleman, & Chase, 2008) and their relatively low sample sizes (n = 32 and 37, respectively).
For each location, we calculated the relative basal area (BApalm) and the relative abundance (RApalm) of tree palms as the sum of the tree palm basal area or number of tree palm individuals divided by the total basal area or total number of stems. These relative metrics of tree palm abundance reduce variation caused by differences in sample area and stem density across locations. Results based on BApalm and RApalm were highly correlated, and we present and discuss BApalm results in the main text (results based on RApalm are provided in the Supporting Information Figure S4).
2.2 Environmental variables
To address our hypothesis about the link between long-term climatic stability and tree palm abundance (the climate-stability hypothesis), we used several variables that are likely to have had major impacts on the distributions of palms and their habitats (e.g., Melo, Freitas, Bacon, & Collevatti, 2018). Specifically, we calculated the absolute difference (or “anomaly”) between the LGM (21,000 years ago) and modern climatological averages (1979–2013), for both the mean annual precipitation (in millimetres per year) and the precipitation in the driest quarter (in millimetres per quarter) using 30 arc s (c. 1 km2) resolution data from CHELSA (Karger et al., 2017). We used LGM variables based on data from the Palaeoclimate Modelling Intercomparison Project (PMIP3) and output from the Community Climate System Model (CCSM4) (Karger et al., 2017).
We used several datasets to address our hypothesis about contemporary ecological controls on tree palm relative abundance (the contemporary conditions hypothesis). For contemporary climate, we extracted the mean annual precipitation (in millimetres per year) and precipitation in the driest quarter (in millimetres per quarter) for each location from the 30 arc s (c. 1 km2) resolution CHELSA dataset (Karger et al., 2017). For edaphic conditions, we extracted cation exchange capacity (CEC; a general proxy for soil fertility; cmol+ kg−1) at 250 m resolution from the SoilGrids website (https://soilgrids.org/). We focused on CEC because prior work has shown associations between forest composition (including palm diversity) and soil fertility (Muscarella et al., 2019). We extracted the depth to the water table (in metres) from the 30 arc s (c. 1 km2) resolution database of Fan, Li, and Miguez-Macho (2013). We examined how conditions at the study locations reflect the range of conditions in their respective biogeographical realms by sampling each variable at 10,000 random points in each realm (Supporting Information Figure S1). We extracted values for the predictor variables listed above based on the mean latitude and longitude of plots in each location.
We calculated two proxies for turnover rates in each location. First, because forests with faster turnover rates tend to have shorter canopies (Feldpausch et al., 2011), we estimated the maximal canopy height for each location based on the tree inventory data. More specifically, given that most plots do not include measured data on tree height, we used the “BIOMASS” package (Réjou-Méchain, Tanguy, Piponiot, Chave, & Hérault, 2017) to estimate the height of each individual tree based on its diameter using the geographically based allometric equation of Chave et al. (2014). We used the 95th percentile of estimated tree height in each location as a metric of maximal canopy height (comparable results were obtained when using the 99th percentile). Second, we calculated the basal area-weighted mean (CWM) wood density for each location by matching wood density data from the global wood density database (Chave et al., 2009; Zanne et al., 2009) with the relative basal area of each species in each location. The CWM wood density reflects life-history strategies of trees, and stands with lower values of CWM wood density tend to have more rapid turnover rates (Chave et al., 2009; Phillips et al., 2004, 2019). Given that we were interested in the relationship between local environmental conditions and palm relative abundance patterns, we excluded palms when calculating CWM wood density. However, note that CWM wood density values per location were highly correlated whether or not palms were included (Pearson's r = .98). Species-level mean wood density values were available for 51% of the individual stems [representing 61% of the total basal area (BA)], genus-level mean values were used for 37% of the individuals (30% of the total BA), family-level mean values were used for 9% of the individuals (6% of the total BA), and we excluded the remaining 3% of individuals (representing 3% of the total BA) that were not identified to the family level. The computed CWM wood density values were highly correlated with CWM wood density values computed based only on species-level data (Pearson's r = .87), genus-level data (Pearson's r = .99) or family-level data (Pearson's r = .99).
2.3 Statistical analyses
As a first step to assess general patterns of tree palm occurrence, we used t tests to compare each environmental covariate described above between locations with and without tree palms, separately for each realm. We log10-transformed all covariatesbefore analysis, except for CWM wood density.
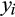 ~ BeZI (
~ BeZI (
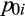 ,
,
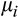 ,
,
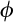 ), which represents a mixture of beta and Bernoulli distributions defined as:
), which represents a mixture of beta and Bernoulli distributions defined as:
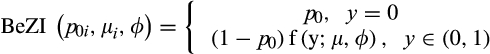
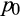 is the probability of tree palms being absent from a location, and
is the probability of tree palms being absent from a location, and
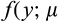 ,
,
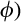 is the beta density function of BApalm at locations where tree palms are present:
is the beta density function of BApalm at locations where tree palms are present:
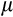 is the expected value and
is the expected value and
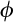 is a precision parameter (Ospina & Ferrari, 2010). For both components of the model (occurrence and relative basal area), we modelled the relationship between the response variable, y, at each location, i, including biogeographical realm as a random grouping variable,
is a precision parameter (Ospina & Ferrari, 2010). For both components of the model (occurrence and relative basal area), we modelled the relationship between the response variable, y, at each location, i, including biogeographical realm as a random grouping variable,
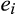 . We used the following model:
. We used the following model:
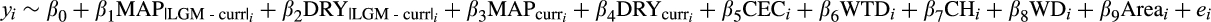
Where y is palm occurrence (for the Bernoulli part of the model) and BApalm (for the beta part of the model),
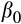 is the intercept, and the terms
is the intercept, and the terms
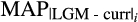 and
and
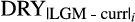 correspond to the anomalies between the LGM and contemporary mean annual and dry quarter precipitation, respectively;
correspond to the anomalies between the LGM and contemporary mean annual and dry quarter precipitation, respectively;
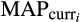 and
and
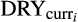 represent current climatological means for mean annual and dry quarter precipitation;
represent current climatological means for mean annual and dry quarter precipitation;
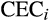 ,
,
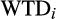 ,
,
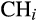 and
and
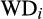 correspond to cation exchange capacity, water table depth, canopy height and CWM wood density, respectively; and
correspond to cation exchange capacity, water table depth, canopy height and CWM wood density, respectively; and
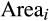 , corresponds to the sum of area sampled in component plots at location i. We included the area term to account for possible correlation between the area sampled and BApalm. Before fitting the model, we centred and scaled each predictor variable by subtracting its mean and dividing it by its standard deviation. This procedure facilitates model convergence and allows for the direct comparison of relative effect sizes across predictor variables (Gelman & Hill, 2006). The majority of model covariates were little correlated (Pearson's |r| < .3), but historical climate anomalies and current climate conditions were moderately correlated (Pearson's r = .47–.71; Supporting Information Table S1). Nonetheless, models fitted separately with either historical climate anomalies or current climate conditions gave similar estimates for all parameters, and we report results from the full model. We fitted models using STAN (Stan Development Team, 2016) via the “brms” R package (Bürkner, 2017) and with default (uninformative) priors (see model code in the Supporting Information Appendix S3). We used four chains with 1,000 burn-in samples and 1,000 sampling iterations per chain. All parameters had
, corresponds to the sum of area sampled in component plots at location i. We included the area term to account for possible correlation between the area sampled and BApalm. Before fitting the model, we centred and scaled each predictor variable by subtracting its mean and dividing it by its standard deviation. This procedure facilitates model convergence and allows for the direct comparison of relative effect sizes across predictor variables (Gelman & Hill, 2006). The majority of model covariates were little correlated (Pearson's |r| < .3), but historical climate anomalies and current climate conditions were moderately correlated (Pearson's r = .47–.71; Supporting Information Table S1). Nonetheless, models fitted separately with either historical climate anomalies or current climate conditions gave similar estimates for all parameters, and we report results from the full model. We fitted models using STAN (Stan Development Team, 2016) via the “brms” R package (Bürkner, 2017) and with default (uninformative) priors (see model code in the Supporting Information Appendix S3). We used four chains with 1,000 burn-in samples and 1,000 sampling iterations per chain. All parameters had
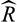 values < 1.1, indicating successful convergence. Plots showing posterior distributions, trace plots and a posterior predictive check are provided in the Supporting Information (Figures S5 and S6). We computed R2 of the model using the “add_ic” function of the “brms” R package (Bürkner, 2017). To evaluate spatial autocorrelation, we computed Moran's I for model residuals using geographical coordinates of locations. Observed and expected values did not differ significantly (p = .83), indicating a lack of spatial autocorrelation among residuals. All analyses were conducted in R v.3.5.1 (R Development Core Team 2019).
values < 1.1, indicating successful convergence. Plots showing posterior distributions, trace plots and a posterior predictive check are provided in the Supporting Information (Figures S5 and S6). We computed R2 of the model using the “add_ic” function of the “brms” R package (Bürkner, 2017). To evaluate spatial autocorrelation, we computed Moran's I for model residuals using geographical coordinates of locations. Observed and expected values did not differ significantly (p = .83), indicating a lack of spatial autocorrelation among residuals. All analyses were conducted in R v.3.5.1 (R Development Core Team 2019).
2.4 Implications for above-ground biomass estimates
To quantify the magnitude of error introduced to estimations of AGB if tree palms are pooled with other trees, we first used the geographically based allometric equation (i.e. using the environmental factor, E) of Chave et al. (2014), implemented in the BIOMASS R package (Réjou-Méchain et al., 2017) to estimate the total AGB for each location (including tree palms and non-palm trees): AGBChave-only. Ideally, we could compare these estimates with values where tree palm biomass was calculated using species-specific allometric equations developed specifically for palms that include information on plant height (Feldpausch et al., 2012; Marshall et al., 2012). Unfortunately, allometric equations have not yet been developed for most palm species, and most ground-based forest inventory datasets do not include height measurements. As an alternative, we calculated tree palm AGB using the family-level allometric equation (based on diameter only) from the study by Goodman et al. (2013). We then added this value of tree palm AGB to non-palm tree AGB calculated with the method of Chave et al. (2014) described above to arrive at a hybrid estimate of AGB for each location: AGBGoodman+Chave. We report the ratio of AGBGoodman+Chave to AGBChave-only as a step towards quantifying the error in AGB estimates introduced by palms.
2.5 Effect of size threshold on tree palm abundance
To examine how the 10 cm d.b.h. threshold (commonly used by forest inventories) could affect conclusions about palm abundance patterns, we analysed separately Alwyn Gentry's transect data (Phillips & Miller, 2002), which includes all woody stems ≥2.5 cm d.b.h. in 144 locations (0.1 ha each) distributed globally throughout the (sub)tropical moist broadleaf biome (Supporting Information Figure S2). For each transect, we compared BApalm (and RApalm) based on the full dataset (i.e., all stems ≥2.5 cm d.b.h., n = 80,712 individual stems) and a subset of the data using a ≥10 cm d.b.h. threshold (n = 16,665 individual stems).
3 RESULTS
Across all 842 locations, tree palms (≥10 cm d.b.h.) accounted for a total of 20,029 out of 661,194 individual stems (3%). The majority of tree palms (95%) belonged to 126 species and 71 genera (representing c. 5 and c. 39% of globally accepted palm species and genera, respectively). The remaining 5% of tree palm individuals in our dataset were identified to genus only. Across all locations, tree palms accounted for c. 1.4% of the total basal area sampled.
We found a striking pattern across biogeographical realms in terms of tree palm occurrence, relative basal area and relative abundance (Figures 1b and 2). The proportion of locations where tree palms were recorded was, by far, highest in the Neotropics (84%) compared with any other realm (41% in Australasia/Oceania, 13% in Afrotropics and 11% in IndoMalaya; Figure 2a). On average, locations in the Neotropics had higher BApalm (5.5%) and RApalm (8.4%) than locations in all other realms (mean of BApalm across other realms ranged from .15 to .84%; Figure 2b). In other words, palms represent <5% of the total basal area for trees ≥10 cm d.b.h. in 99% of the sampled locations outside of the Neotropics. Nonetheless, especially within the Neotropics, BApalm was highly variable, ranging globally from 0 to 60%.
3.1 Tree palm occurrence, abundance and environmental conditions
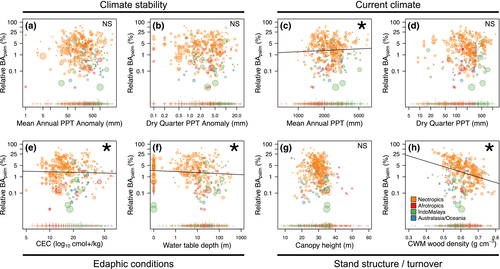
Based on t tests, there were some significant differences in environmental variables between locations where tree palms were present versus those where they were absent, but these were not always consistent or significant across realms (Supporting Information Figure S3). For example, soil fertility (CEC) and palm occurrence were negatively associated among Neotropical locations and positively associated among Afrotropical locations. Palms were more likely to occur in locations with shallower water tables in Neotropical locations and deeper water tables in IndoMalayan locations. Notably, tree palm occurrence was associated with higher dry season rainfall in all realms, although the relationship was not statistically significant in Australasia/Oceania. Results from the occurrence component of our zero-inflated model were consistent, in part, with results from the t tests (Figure 3a). Specifically, palms were more likely to occur in locations with higher precipitation in the driest quarter and in locations with smaller anomalies between historical and contemporary dry season precipitation. Palms also tended to be recorded in locations with larger total area sampled.
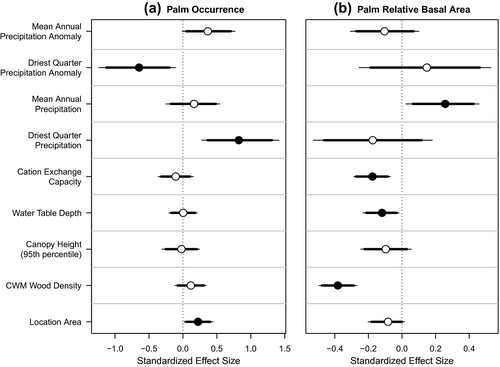
In contrast, palm relative basal area (BApalm) was not significantly associated with either palaeoclimate stability or current precipitation in the driest quarter (Figures 3b and 4). However, BApalm was positively associated with current mean annual precipitation (Figures 3b and 4c). There were also significant negative associations between BApalm and CEC (soil fertility), depth to water table and CWM wood density (Figures 3b and 4e–h). The relationships between BApalm and canopy height and area sampled were not statistically significant. Together, these results indicated that tree palms accounted for a greater proportion of total basal area in locations with lower soil fertility, closer access to groundwater and lower CWM wood density. The R2 of the full model was .26 [95% credible intervals (CIs) = .21–.32; Supporting Information Tables S2-3].
3.2 Implications for above-ground biomass estimates
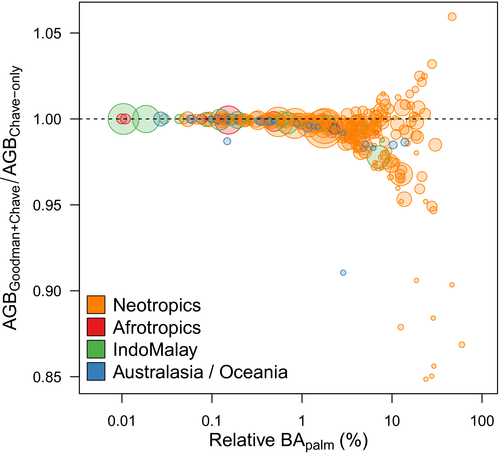
As expected, the difference in AGB estimated by the two methods (AGBGoodman+Chave versus AGBChave-only) increased with BApalm (Figure 5). However, the direction and magnitude of the change in AGB predictions were not consistent. For example, where palms accounted for >10% of total basal area, the ratio of AGB calculated using the two methods ranged from 0.84 to 1.05. Notably, for non-Neotropical locations with BApalm > 1%, values of AGBChave-only were always higher than those of AGBGoodman+Chave (Figure 5).
3.3 Effect of size threshold
In total, 116 of the 144 Gentry transects considered included at least one palm. Across all transects and using a 2.5 cm d.b.h. threshold, 4,502 palm individuals (from 177 species) accounted for 5.5% of the total individuals (80,712) and 2.8% of the total basal area. When using a 10 cm d.b.h. threshold, there were 880 palm individuals (from 76 species) that accounted for 5.3% of the total individuals (16,665) and 2.5% of the total basal area. Among Neotropical transects, changing the size threshold from 2.5 to 10 cm d.b.h. caused palm relative basal area (BApalm) to increase or decrease in 60 and 40% of the transects, respectively (Figure 6). In the other realms, however, BApalm increased in 16 out of 17 transects (94%) when stems down to 2.5 cm d.b.h. were included. In fact, outside of the Neotropics, 53% of the transects with at least one palm present at the 2.5 cm d.b.h. threshold had no palms recorded above the 10 cm d.b.h. threshold. These results indicate that the majority of palm abundance in locations outside the Neotropics occurs in the 2.5–10 cm d.b.h. size class. The different d.b.h. thresholds changed the value of BApalm in each transect by ≤ 14% (Figure 6).
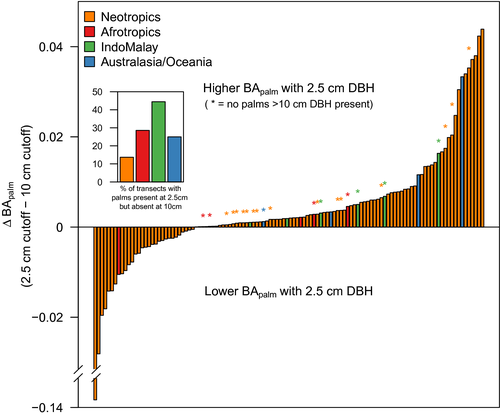
4 DISCUSSION
4.1 Patterns and drivers of tree palm abundance and diversity
Tree palms were clearly most abundant in Neotropical forests, where they composed ≤ 60% of the total forest basal area and stem abundance. We consider these figures to be conservative estimates because we did not consider areas of tree palm monodominance, which will require a separate and focused treatment. Reinforcing prior work (e.g., Dransfield et al., 2008; Moore, 1973; ter Steege et al., 2013, 2019), tree palms are particularly abundant components of forests in western Amazonia, which is also a hotspot of palm diversity (Svenning et al., 2008). In contrast, tree palms play a relatively minor role in terms of abundance in other biogeographical realms, especially the Afrotropics and IndoMalaya [with notable exceptions of tree palm monodominance in some swamps of these regions (Dargie et al., 2017)]. Tree palms do, however, reach relatively high levels of relative abundance in some plots in Madagascar and on the northeast coast of Australia (≤ 14% of the total basal area), but these locations were exceptional in comparison to most locations in those realms.
In our study, local conditions, including current climate, edaphic properties and proxies for turnover rates, were more strongly related to tree palm abundance within biogeographical realms than our metrics of palaeoclimate stability. In particular, tree palms had greater relative abundance in locations with higher mean annual rainfall, lower soil fertility, shallower water tables and lighter CWM wood density. These results are broadly consistent with a number of previous studies at smaller spatial scales that have linked spatial distributions of palms to hydrology, edaphic conditions and stand turnover rates (Costa et al., 2009; Eiserhardt et al., 2011; Emilio et al., 2014; Svenning, 2001).
Our finding that palms were relatively more abundant in locations with low soil fertility, shallow water tables and lighter CWM wood density might reflect related underlying processes. For instance, Schietti et al. (2014) reported a strong relationship between water table depth and palm community composition in forests of central Brazilian Amazonia. Likewise, Castilho et al. (2006) found greater palm biomass in sites with poorly drained, sand-rich soils. Quesada et al. (2012) reported that forests with lighter CWM wood density are associated with shorter stem residency times, and palms may be more likely to be abundant at sites that face more severe or frequent disturbances. In fact, some areas regularly exposed to wind disturbance, for example, show high levels of tree palm abundance (e.g., Caribbean islands, east coast of Australia). Emilio et al. (2014) linked palm abundance in Amazonian forests with aspects of soil structure and suggested that edaphic properties, such as soil depth and texture, might affect plant composition and abundance indirectly by influencing stem turnover rates. Additionally, CWM wood density is geographically structured in our dataset; locations in western Amazonia tend to have lighter CWM wood density than locations in eastern Amazonia, potentially reflecting gradients in drought and nutrient stress (ter Steege et al., 2006).
We hypothesized that long-term climatic conditions could also influence current patterns of tree palm abundance based on the climatic sensitivity of palms (Reichgelt, West, & Greenwood, 2018; Tomlinson, 2006). In some respects, our results are broadly similar to global patterns of palm diversity (i.e., high diversity and relative abundance of tree palms in the Neotropics but not the Afrotropics). However, variation of tree palm relative abundance within realms was not significantly related to the anomaly of either mean annual precipitation or dry season precipitation between the LGM (21,000 years ago) and the current period. It appears that local environmental heterogeneity mediates palm relative abundance more strongly within biogeographical realms, whereas legacy effects of palaeoclimate might be more apparent at larger scales. Interestingly, palaeoclimate stability (anomaly of dry quarter precipitation) was (negatively) associated with palm occurrence. We might generally expect abundance to exhibit less coupling to historical legacies than diversity, given that species present in a locality have the potential to respond in a relatively rapid manner to current environmental conditions through population growth. In other words, processes governing abundance are likely to occur along shorter time-scales than processes affecting species richness (i.e., speciation, extinction, immigration).
In this study, we were unable to assess the role of several potentially important drivers of palm abundance directly. First, humans have affected tropical landscapes for millennia (Roberts, Hunt, Arroyo-Kalin, Evans, & Boivin, 2017) and, especially given the many uses of palms (Cámara-Leret et al., 2017), past and recent human activity could influence observed contemporary patterns of palm abundance. Levis et al. (2017) showed that domesticated tree species, including several tree palms, were more abundant in forests near archaeological sites in Amazon forests, suggesting long-term human impacts on the composition and structure of tropical forests. In contrast, Piperno, McMichael, and Bush (2019) reported a lack of evidence for ancient human impacts on palm abundance in Amazonian terra firme forests. Humans have clearly impacted forests in other biogeographical realms also (Hunt & Rabett, 2014; Malhi, Adu-Bredu, Asare, Lewis, & Mayaux, 2013; Roberts et al., 2017), but we do not expect human activity to have altered the broad biogeographical patterns we report. The degree to which human activities have impacted populations of palms and other trees requires more study.
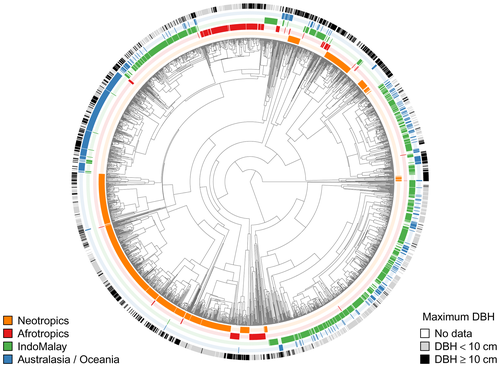
Second, we cannot rule out the possibility that the historical presence or absence of certain palm lineages influences patterns of palm abundance. Palms display strong spatial phylogenetic structure (Kissling, Baker, et al., 2012; Figure 7), showing that different regions are characterized by different lineages. If certain lineages have a particular tendency to evolve highly abundant tree palms, this could drive patterns like the ones observed here. For example, the palm tribes Euterpeae, Iriarteae and Cocoseae, which include the most abundant palms in Amazonia (ter Steege et al., 2013), are absent outside the Neotropics (except for a few Attaleinae species in South Africa and Madagascar, which, interestingly, are not particularly abundant there). Also, non-human animal seed dispersers influence palm diversification and diversity via eco-evolutionary interactions (Onstein et al., 2017), and these mutualisms could also potentially influence contemporary palm abundance patterns. For instance, Onstein et al. (2018) argued that palms with megafaunal fruits (some of which could also be large tree palms) may have reduced abundance and increased extinctions owing to the extinction of their megafaunal dispersers (also see Doughty et al., 2016). Quantifying these effects will require additional phylogenetic analyses, which will be attempted soon, once a comprehensive species-level phylogeny of the palm family is available.
Finally, our dataset does not include swamp habitats, and some tree palms are known for forming monodominant stands, especially in swampy conditions associated with large carbon pools. For example, palm swamps in the Congo basin, Amazonia and southeast Asia cover millions of hectares and store huge amounts of biomass both above and below ground (Dargie et al., 2017; Kahn & Mejia, 1990). Better understanding of these unique habitats requires additional work; recent progress has been made using various remote sensing techniques (e.g., Dargie et al., 2017).
4.2 Implications for above-ground biomass estimates
One motivation for our study was to gain a better understanding of the potential error introduced to estimates of AGB when tree palms are pooled with other trees. Specifically, standard allometric equations generated for trees that estimate AGB from stem diameter perform in a relative poor manner when estimating biomass for palms because, unlike other trees, growth in diameter and growth in height of palms are largely decoupled. The magnitude of error this introduces clearly depends, in part, on tree palm relative abundance. In our dataset, pooling tree palms with other trees added error to AGB estimates, but the direction of the error was not consistent. Pooling palms with dicots most often led to an overestimation of AGB [compared with estimates based on using the family-level model from the study by Goodman et al. (2013) for palms and the dicot models of Chave et al. (2014) for non-palm trees]. Variation in the magnitude and direction of these estimates emerged, in part, from the geographical variation in the equation of Chave et al. (2014). The differences in AGB estimates between the two methods used here (AGBGoodman+Chave and AGBChave-only) were <1% in >90% of locations where BApalm was <5%, indicating that error from palms is probably negligible when palms are minor components of the total forest basal area. However, the difference between the two methods increases with BApalm, and the difference ranged from −15 to 6% in our dataset. Future analyses using data on stem height are needed to refine these estimates, and additional species- and region-specific palm allometric models will be important to improve AGB estimates in many Neotropical forests where tree palms are abundant. Fortunately, increasingly available remote sensing data on canopy height (e.g., NASA GEDI) will improve this situation when paired with appropriate ground-based data. Additionally, recent advances in remote sensing to identify palms (Tagle Casapia et al., 2020) will also help to identify areas where attention to palm abundance is important to reduce the error in biomass estimates.
4.3 Palm life-forms and the effect of size threshold
To some extent, the patterns we report reflect these differences in palm life diversity across biogeographical realms. For example, clades that assume a climbing form (e.g., rattans in the subfamily Calamoideae) constitute a major component of palm diversity in the IndoMalayan and Australasian biogeographical realms (Couvreur et al., 2015). In the Neotropics, in contrast, many abundant palm species grow as large canopy trees (e.g., Astrocaryum chambira, Euterpe precatoria, Iriartea deltoidea, Oenocarpus bataua), which are among the hyperdominant species of Amazonia (ter Steege et al., 2013). Meanwhile, understorey species also make up a large portion of palm diversity in the Neotropical, IndoMalayan and Australasian biogeographical realms (e.g., Bactris and Geonoma species in Amazonia; Dypsis in Madagascar; Pinanga and Licuala in IndoMalaya; Pritchardia in parts of Oceania).
Contrasting patterns of life-form diversity influence the patterns of abundance we report, because the forest inventory datasets we used only include trees with a large diameter (e.g., 10 cm). On the one hand, plots censused with this size threshold are adequate for quantifying important forest properties (Bastin et al., 2018; Lutz et al., 2018; Slik et al., 2013; Stephenson et al., 2014). For example, Lutz et al. (2018) showed that half the above-ground living biomass in a global dataset of forest plots was stored in the largest trees, which made up 1% of the total stems. On the other hand, major components of biodiversity are neglected by using a size threshold of 10 cm d.b.h., because a substantial amount of tropical rain forest diversity exists as understorey and slender climbing plants (Cicuzza et al., 2013; Hubau et al., 2019). For palms, we estimate that c. 40% of all species reach a diameter of 10 cm (Kissling et al., 2019; Figure 7). The plot dataset we analysed included a total of 126 palm species, which represent c. 5% of the total known palm species and nearly 20% of the species known to reach 10 cm d.b.h. Meanwhile, lowering the diameter cut-off for the Gentry transect dataset from 10 to 2.5 cm resulted in the inclusion of 51 additional palm species despite containing < 25% the number of palm individuals.
Our analysis of the Gentry transect data provides additional confirmation that biogeographical differences in palm life-form diversity can affect the perception of palm abundance. For example, small-diameter climbing palms are diverse and more common in IndoMalaya compared with arborescent palms (Couvreur et al., 2015). In fact, many IndoMalayan palm species (e.g., rattans) are unlikely to reach even the 2.5 cm d.b.h. used by Gentry; a major part of palm diversity in the IndoMalayan realm is contributed by the subfamily Calamoideae, which comprises mostly climbers. Notably, acaulescent (or stemless) palms can also be common in Neotropical forests. Overall, a comprehensive understanding of the abundance of palms across the entire phylogenetic tree will require additional data that include small size classes and different life-forms.
We propose several additional potential reasons for generally low tree palm abundance in the Afrotropics and IndoMalaya in addition to the differences in life-forms described above. The first reason may be related to the generally tall forest canopies in these realms, especially IndoMalaya, compared with the Neotropics (Banin et al., 2014). These conditions may favour the evolution of alternative life-forms, including climbers (Couvreur et al., 2015). In fact, Couvreur et al. (2015) reported a link between diversification of the climbing life-form in palms and forest canopy height. Additional synthetic work integrating abundance patterns with evolutionary drivers of palm life-form variation could be a fruitful research avenue. Second, for IndoMalaya, we note that dry quarter precipitation is greater in locations with palms than in locations where palms were absent (Supporting Information Figure S2d). On average, locations in this realm without palms have only c. 50 mm rain during the driest quarter, whereas sites with palms have >100 mm. It is possible that the minimum monthly rainfall generally limits palm occurrence in many areas that have months of rainfall <100 mm, which includes much of mainland Southeast Asia.
4.4 Conclusions and future directions
The results of our analysis of tree palm abundance show that tree palms are not only quintessentially tropical, but also overwhelmingly Neotropical. Although palaeoclimatic conditions appear to have a strong influence on global palm diversity, tree palm relative abundance was more strongly related to current ecological conditions, and tree palm abundance patterns might be particularly sensitive to future climate change. Future research should focus on specific drivers (and interactions among drivers) linked to tree palm abundance within biogeographical realms. We suggest that stronger consideration of the influence of palms can reduce uncertainty in biomass estimates. Especially in the Neotropics, improvement of our understanding of carbon cycling will require additional fieldwork to measure palm height and develop new allometric equations for palms. High tree palm relative abundance in locations with low average wood density (presumably, high-turnover forests) might also dramatically impact field estimates of forest productivity, because measurements of palm height growth are typically neglected. We also show that understanding the general patterns of abundance across the entire palm family will require additional work to understand macroevolutionary drivers of palm life-form diversity. Finally, our study illustrates the synergistic research potential of large data-sharing networks such as RAINFOR in South America and AfriTRON in Africa (Hubau et al., 2013; Lewis et al., 2013, Lopez-Gonzalez et al., 2009; Lopez-Gonzalez et al., 2011). Besides storage and organization of data to facilitate research, these networks strengthen the entire research chain (seeking field funding, fieldwork, developing protocols, allometries, collaborations, training, database design and quality control). Networks such as these are essential for conducting broad-scale and data-rich analyses. As development of these networks continues, it will be especially useful if they are even denser than today and even more balanced in terms of biomes, biogeographical realms and natural and disturbed sites.
ACKNOWLEDGMENTS
This study would not have been possible without the ambitious and dedicated work of many colleagues, including Emmanuel Akampulira, Miguel N. Alexiades, William Balée, Olaf Banki, Serge K. Begne, Desmo Betian, Wemo Betian, Michael I. Bird, Neil M. Bird, George A. Blackburn, Rene Boot, Roel J. W. Brienen, Foster Brown, Ezequiel Chavez, Eric Chezeaux, Manoela F. F. Da Silva, Douglas C. Daly, Kyle G. Dexter, Luisa Fernanda Duque, Jose Farreras, Nina Farwig, Toby Gardner, Alwyn Gentry, Francisco Gómez, Rachel Graham, René Guillén Villaroel, Olivier J. Hardy, Terese Hart, Miriam van Heist, Mireille Breuer Ndoundou Hockemba, Kathryn Brun-Jeffery, Valerie Kapos, Jeanette Kemp, Miguel Leal, Eddie Lenza, Antonio S. Lima, Maurício Lima Dan, Pedro Lisboa, Jon Lloyd, Jhon Mario Lopez, Ubirajara N. Maciel, Jean-Remy Makana, Antti Marjokorpi, Toby Marthews, Emanual H. Martin, James Franklin Maxwell, Irina Mendoza Polo, Edi Mirmanto, Kazuki Miyamoto, Franklin Molina, Sam Moore, Pantaleo K. T. Munishi, Helen Murphy, David M. Newbery, Vojtech Novotny, Navendu Page, Karla Pedra de Abreu, Maria C. Peñuela-Mora, Ghillean T. Prance, John Proctor, Wilfredo Ramirez Salas, Adela Reatigui Ismodes, Eliana Riascos, Terhi Riutta, Nelson A. Rosa, Philippe Saner, Lars Schmidt, Marcela Serna, Michael Swaine, James Taplin, Peguy Tchouto, Johan van Valkenburg, Peter van de Meer, Cesar Velasquez, Jason Vleminckx, George Weiblen and Roderick Zagt. We also depend on the centuries of work completed by palm taxonomists. W.L.E.'s contribution was supported by a research grant (00025354) from VILLUM FONDEN. J.C.S. considers this work a contribution to his VILLUM Investigator project “Biodiversity Dynamics in a Changing World” funded by VILLUM FONDEN (grant 16549). R.M. was supported by Vetenskapsrådet (2019-03758). This work was supported by the Danish Council for Independent Research Natural Sciences (grant 4181-00158) to H..B, the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No. 706011, and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. This paper is a product of the RAINFOR, AfriTRON and T-FORCES networks and other partner networks in ForestPlots.net, which together support long-term forest science and monitoring across tropical countries. These initiatives have been supported by numerous people and grants since their inception. We are particularly indebted to >1,400 field assistants for their help in establishing and maintaining the plots, in addition to hundreds of rural communities and institutions. Collection and management of data analysed here from the RAINFOR, AfriTRON and T-FORCES networks have been supported by multiple grants, most notably the European Research Council (ERC Advanced Grant 291585, “T-FORCES”), the Gordon and Betty Moore Foundation (#1656, “RAINFOR”), the David and Lucile Packard Foundation, the European Union's Fifth, Sixth, and Seventh Framework Programme (EVK2-CT-1999-00023, “CARBONSINK-LBA”; 283080, “GEOCARBON”; and 282664, “AMAZALERT”), the Natural Environment Research Council (NERC grants: NE/D005590/1, “TROBIT”; NE/F005806/1, “AMAZONICA”; E/M0022021/1, “PPFOR”; NERC Urgency Grants and NERC New Investigators Grants), the NERC/State of São Paulo Research Foundation (FAPESP) consortium grants “BIO-RED” (NE/N012542/1, 2012/51872-5) and “ECOFOR” (NE/K016431/1, 2012/51509-8), the Royal Society (University Research Fellowships and Global Challenges Awards “FORAMA”, ICA/R1/180100), the National Geographic Society, the Centre for International Forestry (CIFOR), Gabon's National Parks Agency (ANPN) and Colombia’s Colciencias. We thank the National Council for Science and Technology Development of Brazil (CNPq) for support to the Cerrado/Amazonia Transition Long-Term Ecology Project (PELD/403725/2012-7), the PPBio Phytogeography of Amazonia/Cerrado Transition project (CNPq/PPBio/457602/2012-0), PVE grants, and Productivity Grants to several colleagues. Atlantic Forest plots in Brazil were supported by the State of São Paulo Research Foundation (FAPESP 2003/12595-7 and 2012/51509-8, BIOTA/FAPESP Program) and by the Brazilian National Research Council (CNPq/PELD 403710/2012-0; Universal 459941/2014-3) under COTEC/IF 41.065/2005 and IBAMA/CGEN 093/2005 permits. Some of the data were provided by the Tropical Ecology Assessment and Monitoring (TEAM) Network, a collaboration between Conservation International, the Smithsonian Institution and the Wildlife Conservation Society, and partially funded by these institutions, the Gordon and Betty Moore Foundation and other donors. RAPELD plots in Brazil were supported by the Program for Biodiversity Research (PPBio), the National Institute for Amazonian Biodiversity (INCT-CENBAM) and BDFFP (INPA-STRI). Grant USM-RUI-1001/PBIOLOGI/8011031 also supported fieldwork. This is publication 788 of the BDFFP Technical Series and is an outcome of the ForestPlots.net approved research project #2, “Global Patterns of Palm Abundance”. We acknowledge the support of the European Space Agency. We thank several anonymous reviewers and the editor for help improving our manuscript.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
Raw data are available from ForestPlots.net and through contact with the authors. Restrictions apply to the availability of these data, which were used under licence for this study. Summarized data to replicate the main analyses presented in the paper are provided in the Supporting Information.
REFERENCES
BIOSKETCH
Our group represents a broad range of researchers and land managers interested in the composition and dynamics of tropical forests worldwide. All authors are responsible for long-term field data-collection efforts, which are crucial for global syntheses.




