The potential role of species and functional composition in generating historical constraints on ecosystem processes
Abstract
Aim
Biogeographical processes and past environmental conditions are known to constrain current patterns of species, functional and phylogenetic diversity. An unanswered question is whether such constraints to biodiversity also affect ecosystem processes.
Location
Global.
Time period
Neogene and Quaternary.
Major taxa studied
Vascular plants.
Methods
We propose that evolution, migration-lag in response to environmental changes, and extinction may result in historical legacies in current ecosystem composition (species and functional) that can lead to historical legacies in ecosystem processes – that is, deviations from the potential rates or magnitudes of ecosystem processes, given current environmental conditions and the habitat-suitable native species and trait pools that exist under such circumstances.
Results
Historical legacies in ecosystem processes may begin with changes in species richness and composition in response to environmental shifts. These changes are mediated by response traits that determine how species react to environmental changes and track suitable environmental conditions. Response traits are often associated with effect traits, which determine the rates and magnitude of ecosystem processes. Via this association, environmentally driven response-trait determined changes in species diversity may cascade to ecosystem processes through effect trait compositional changes. These changes could lead to ecosystem processes deviating from a potential state defined by current environmental conditions and the overall species pool. We populate this conceptual framework with cases that show how historical legacies in biodiversity translate to historical legacies in ecosystem processes, and we discuss the implications of the concept for global change ecology.
Main conclusions
We need to rethink our expectations of future ecological dynamics as past, and current, environmental changes may push ecosystem processes away from their environmentally and compositionally defined potential state.
1 INTRODUCTION
A central pillar of ecological research is defining the factors that determine current biodiversity patterns. Many studies have shown a strong association between current biodiversity and water–energy dynamics (Hawkins et al., 2003; Kreft & Jetz, 2007). However, past climatic conditions have also been shown to shape modern biodiversity as a result of biogeographical processes, the cumulative effect of changing environmental conditions, and time-dependent ongoing processes (see Table 1 and references therein).
| Pattern | Effect of past events and dynamics | References |
|---|---|---|
| Mechanism | ||
| Species ranges | Contemporary species ranges fill a restricted portion of the potential ranges, due to the secondary effects (after limited migration ability) of the location of past ranges (areas or origin) and barriers (vicariance events) | Baselga, Lobo, Svenning, Aragon, and Araujo (2012); Bjorholm, Svenning, Baker, Skov, and Balslev (2006); Davies, Purvis, and Gittleman (2009); Normand et al. (2011); Saltré et al. (2013); Smith, Stephens, and Wiens (2005); J.-C. Svenning and F. Skov (2007); Svenning and Skov (2004) |
| Biogeographical processes | ||
| β-diversity (species turnover) | Dispersal limitation and demographic stochasticity (ecological drift) are key constraints for the exchange of individuals within a region. Ecological drift, the emergence of non-analogue communities, and connectivity history of local species assemblages would determine the nestedness of the assemblages, which increase via isolation and non-random extinctions or decrease via connectivity and secondary contact | Baselga (2008, 2010); Baselga, Gomez-Rodriguez, and Lobo (2012); Dobrovolski, Melo, Cassemiro, and Diniz (2012); Eiserhardt, Svenning, Baker, Couvreur, and Balslev (2013); Harrison, Ross, and Lawton (1992); Lenoir et al. (2010); Nekola and White (1999); Svenning, Flojgaard, and Baselga (2011); Williams, Shuman, Webb, Bartlein, and Leduc (2004) |
| Biogeographical processes | ||
| γ-diversity (total number of species occurring in a region) | Large-scale species richness patterns can be defined by the rate at which biogeographical processes act (i.e., immigration to a region from the centres of origin, and in situ speciation/extinction). Thus, differences in the total number of species occurring in a region can be the result of divergent rates of diversification and the influx of species, changing the number of species in a region | Bjorholm et al. (2006); Fritz et al. (2013); Lenoir et al. (2010); Smith et al. (2005); Svenning (2003); Svenning et al. (2014); Urban, Zarnetske, and Skelly (2013); Wiens (2011); Wiens and Donoghue (2004) |
| Biogeographical processes | ||
| Functional composition/ecosystem processes | Biogeographical determinants of the past distribution of functional groups (area of origin, geographical disjunction, and dispersal mechanisms) determine the functional traits at a location and thereby potentially also change the rates and magnitude of different ecosystem processes (e.g., biomass accumulation, nutrient sequestration, water balance, and fire regimes) | Davis, Webb, Wurdack, Jaramillo, and Donoghue (2005); Dutta et al. (2011); Liebergesell et al., (2016); Linder et al. (2013); Popp, Mirre, and Brochmann (2011); Richardson and Rejmanek (2004); Swenson et al., (2017, 2016) |
| Biogeographical processes | ||
| α-diversity (endemism) | Globally and regionally, areas that experienced little Quaternary climate change are areas with high endemic species richness. | Jansson (2003); Sandel et al. (2011) |
| Cumulative effect of changing environmental conditions | ||
| α-diversity (richness) | Geographical patterns of overall species richness reflect the range transformations and severe regional extinctions due to changes in environmental conditions. As a result, richness hotspots will be concentrated in areas where the glacial climate was least stressful or where the topographic relief permitted short-distance climate tracking | Araújo et al. (2008); Hortal et al. (2011); Jetz, Rahbek, and Colwell (2004); Kissling, Baker, et al. (2012); McGlone (1996); Svenning et al. (2010); J. C. Svenning and F. Skov (2007); Webb and Bartlein (1992) |
| Cumulative effect of changing environmental conditions | ||
| β-diversity (species turnover) | Compositional similarity patterns among local species assemblages within a region result from non-stationary environmental gradients (location of environmental refugia, and the spatio-temporal rate of change of climate change). In this way, the spatial turnover of species increases via their persistence in environmental refugia and long-term climatic stability | Baselga (2008); Baselga, Gomez-Rodriguez, et al. (2012); Dexter, Terborgh, and Cunningham (2012); Fitzpatrick et al. (2013); Leprieur et al. (2011); Nekola and White (1999); Svenning et al. (2011) |
| Cumulative effect of changing environmental conditions | ||
| γ-diversity (total number of species occurring in a region) | The total number of species occurring in a region is defined by the geological and environmental history of a region (e.g., tectonic movements, temperature, precipitation, energy, productivity, or long-term climatic stability), the available area, and rates of diversification. In this way, species-rich regions are located where conditions have promoted the generation and survival of a large number of species over a long period, and/or where the speciation rate is fast | Francis and Currie (2003); Hawkins et al. (2003); Hawkins and Porter (2003); Kreft and Jetz (2007); Zobel et al. (2011) |
| Cumulative effect of changing environmental conditions | ||
| Phylogenetic diversity | Phylogenetic richness and structure (clustering/dispersion) are related to changes in the availability of suitable environmental areas over long geological times (e.g., across the Cenozoic for palm species) and/or the cumulative or minimum area or productivity of particular bioregions over geological time | Eiserhardt et al. (2013); Forest et al. (2007); Kissling, Eiserhardt, et al. (2012) |
| Cumulative effect of changing environmental conditions | ||
| Functional composition/ecosystem processes | Past environmental conditions such as the palaeoclimate impose filtering effects on functional traits (e.g., freezing tolerance) in a species assemblage, beyond the filtering caused by current conditions. This filtering could potentially impose a lasting effect on ecosystem processes (e.g., biomass accumulation, nutrient sequestration, water balance and fire regimes) | Ackerly (2003); Ordonez and Svenning (2015, 2016, 2017); Reich et al. (2012); Svenning (2003); Zanne et al. (2013) |
| Cumulative effect of changing environmental conditions |
Assessing the importance of past conditions as a determinant of biodiversity dimensions other than richness, particularly functional composition (the variability in species traits cf. Díaz et al., 2007), has been the focus of multiple recent studies (Table 1 and references therein). Nonetheless, to the best of our knowledge, no explicit framework connects historical legacies in the species and functional composition, or how these legacies translate into ecosystem processes. We propose such a framework here, summarized under the term historical ecosystem legacies, defined as phenomena in which historical legacies in species and functional composition influence current ecosystem processes.
We start by describing the series of processes by which historical legacies in species and functional composition would percolate into ecosystem processes. Second, we present examples showing how historical legacies in species composition and functional composition have, or could, alter ecosystem processes, resulting in historical ecosystem legacies. Third, we discuss how the proposed framework provides testable hypotheses of when and where can we expect historical legacies in ecosystem processes. Fourth, we describe how the historical ecosystem legacies concept can be used to evaluate differences in trait–function relationships across regions and for assessing the risks and consequences of invasion by non-natives. Finally, we discuss why models predicting future ecosystem processes need to incorporate the impact of trait-mediated lags in species and functional composition responses to changing environments and the limitations to doing so.
2 WHAT ARE HISTORICAL LEGACIES IN SPECIES COMPOSITION, FUNCTIONAL COMPOSITION AND ECOSYSTEM PROCESSES?
Historical legacies in biodiversity is a phenomenon in which current patterns are influenced by past conditions (Ricklefs, Latham, & Qian, 1999). Two kinds of processes could introduce historical legacies in biodiversity. The first is time-dependent ongoing processes (migration-lag in response to environmental changes, or lasting imprints of past environmental filtering). The second is a lasting effect of biogeographical processes (extinctions due to glaciations or other climatic excursions, or lags in the spread from areas of origin). Invasions by non-natives (removal of biogeographical barriers) can also introduce historical legacies in biodiversity via the introduction of new environmentally suitable species. These introductions would supersaturate local diversity, resulting in the deviation from what is expected based on only those species that are ecologically suitable and biogeographically related to a particular habitat.
A key point when defining the existence of a historical legacy is how to assess the cumulative effects of biogeographical processes and/or time-dependent ongoing processes on current diversity. One way to do this is defining a theoretical potential state where these processes have not occurred. However, providing an unequivocal definition of such a theoretical potential state is difficult because no uniformly accepted characterization exists of the expected diversity and composition of an assemblage. Discussing how a potential state could be generated from first principles or particular theory has been the focus of many studies (see Enquist & Niklas, 2001; Hubbell, 2001; Loreau, 2000; Storch & Okie, 2019) and is beyond the scope of this work.
For simplicity, here we take a phenomenological approach to describing a theoretical potential state. The goal here is describing an expectation of the species pool that could potentially be represented in a focal local assemblage if no migration-lag in response to environmental changes, or past environmental filtering had occurred (Figure 1). For this, we equate the theoretical potential state to the habitat-specific species pool (list of species that could hypothetically be part of a particular local assemblage given the specific habitat conditions; cf. Partel, Szava-Kovats, & Zobel, 2011). This approach allows the characterization of a species and trait composition pattern in the absence of historical legacies similarly as classic null-model approaches used to test hypotheses of community dynamics.
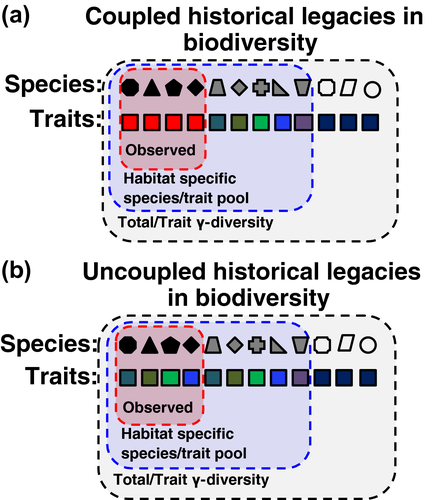
Describing a theoretical potential state where the effects of regional diversification or extinctions had not taken place is more complex, as these are the diversity generating mechanism at regional scales. Defining the habitat-specific species pool based on species occurring in similar habitats across different biogeographical regions could potentially generate a theoretical potential state without the effects of biogeographical processes on the region of interest; but these processes will still shape the species pool within each region.
An important point of clarification is that the habitat-specific species pool is not the regional species list (hereafter, total γ-diversity). Specifically, the habitat-specific species pool corresponds to those species affiliated with specific habitat conditions (e.g., a grassland, wetland, woodland, forest) regardless of the region. In contrast, the total γ-diversity is the list of native species within a region, encompassing species in all existing habitats. Furthermore, given our phenomenological approach, environmental filtering (pool of abiotic factors that prevent the establishment or persistence of species in a particular location cf. Chesson, 2000), and migration-lags in response to environmental changes on a site could potentially remove species from the habitat-specific species pool defined potential sate.
In the case of species composition, our definition of the theoretical potential state means that all sites with a similar habitat would have the same subset of species from the total γ-diversity – that is, the habitat-specific species pool (blue oval, Figure 1). However, species composition of any place is, almost by definition, different from the habitat-specific species pool expectation (red oval, Figure 1). As a result, the observed species poll will show biodiversity deficits (fewer species than the theoretical potential state) due, for example, to lagging responses of ecological factors (e.g., species interactions). It can also show surpluses (more species than the theoretic potential) due, for instance, to invasions by non-natives. However, species–area relation and competitive interactions can also result in local pools being a subset of the habitat-specific pool.
Extending the habitat-specific species pool to the functional composition realm requires some minor modifications compared to the species composition case. As a start, climatic (Ordonez & Olff, 2013; Wright et al., 2005) and edaphic (Ordonez & Olff, 2013; Ordonez et al., 2009) factors are known to modulate trait variation across sites. This relationship indicates the local filtering of the functional variability observed in a region (i.e., trait γ-diversity). Based on this idea, the potential functional composition of a site can be defined based on trait conditions that can exist under specific habitat conditions (i.e., habitat-specific trait pool; Figure 1). As for the species composition case, biogeographical processes, time-dependent ongoing processes, or invasions/colonizations will induce historical legacies in the functional composition of a site by removing traits from the expected habitat-specific trait pool.
Historical legacies in ecosystem processes are a new idea because, traditionally, contemporary environmental factors are considered the primary determinants of ecosystem processes (biological, physical and geochemical processes that take place within an ecosystem, cf. Hooper et al., 2005). Nonetheless, various experimental studies (Huang et al., 2018; Isbell et al., 2011; Reich et al., 2012) and field studies (De Deyn, Cornelissen, & Bardgett, 2008; Pakeman, 2011) show the existence of a relationship between biodiversity and ecosystem processes such as productivity, water balance, fire regulation, or carbon/nutrient cycling and accumulation. The link between biodiversity and ecosystem processes underscores how historical legacies in biodiversity could also affect the rates at which ecosystems accumulate and cycle biomass, elements and energy.
Historical legacies in ecosystem processes would then not be the result of the direct effects of contemporary environmental conditions on the physiological performance of species in a location. These legacies would be the indirect result of changes in the species and functional composition (Morin et al., 2018). For example, the productivity of a particular ecosystem may be typical for some combination of environmental variables (the theoretical potential state), but deviations from this expectation can be explained based on the effects of historical legacies upon the species and functional composition of the site. Therefore, a deviation from environmentally defined rates and magnitudes of ecosystem processes could be used to indicate the role of historical legacies in biodiversity as a determinant of observed ecosystem processes. The question remaining is how historical legacies in species and functional composition translate into historical legacies in ecosystem processes.
3 NOT ALL TRAITS ARE CREATED EQUAL, BUT SOME ARE MORE EQUAL THAN OTHERS
Not all traits describing the functional composition of an assemblage are operationally equivalent. Traits can be grouped into two categories: response traits and effect traits (Lavorel & Garnier, 2002). Response traits mediate how species occurrences, abundances and ranges respond to changes in biophysical conditions. By comparison, effect traits determine ecosystem processes by controlling how species modulate the movement and accumulation of biomass and nutrients in an ecosystem. Therefore, not all changes in the functional composition of an assemblage would result in ecosystem-level responses.
Although conceptually independent, response and effect traits can be associated (Lavorel & Garnier, 2002; Suding et al., 2008). The strength of the association between response and effect traits depends on whether a trait determines both the response to environmental and biotic filters and the rates and magnitudes of ecosystem processes. Terrestrial plants present a clear example of the synchronization that exists between trait classes. Specifically, response traits that determine species performance and persistence to changes in environmental conditions and resource (e.g., the specific leaf area and leaf chemical composition) show a strong association with the effect traits that determine primary productivity (e.g., rates of carbon and nutrient uptake). Root growth is another plant attribute that determines both the susceptibility of aquatic macrophytes to flooding and determines the sediment nutrient dynamics (Engelhardt, 2006), hence acting as a response and effect trait.
Linkages and trade-offs among traits can also introduce an association between response and effect traits. Such is the case for plant size, a trait linking different ecological strategies, like the wood economics spectrum (Chave et al., 2009) and the leaf economics spectrum (Wright et al., 2004). In this case, the response to an environmental change such as a reduction in water availability is modulated by traits in the wood economics spectrum (e.g., wood density) that vary based on plant size. Meanwhile, traits in the leaf economics spectrum that also change based on plant size (e.g., photosynthetic rate and leaf size), determine how ecosystem processes (productivity) respond to changing environmental conditions. Therefore, plant size can be used as a measure of water-stress response and of changes in productivity in a site.
We can sometimes also expect little association between response and effect traits. For example, traits related to ecosystem disturbances show how changes in response traits can be uncorrelated to shifts in effect traits modulating the intensity of said disturbance (Lavorel & Garnier, 2002). Such is the case for traits determining a species’ response to fire (e.g., fast growth rate, seed banks, thick bark, and resprouting ability), which are decoupled from traits controlling ecosystem flammability (e.g., water content, drought resistance, chemical composition, and decomposition rates). A decoupling between response and effect traits highlights how species responses to environmental forcing are not always related to the way species transform the ecosystem and/or regulate the intensity of said environmental forcing (Suding et al., 2008). One possible reason for the decoupling between trait classes is that the effect trait distribution is random compared to the response trait distribution. As a result of this random distribution, multiple species represent each of the effect trait states in the region, so that selective species extinctions (Eiserhardt, Borchsenius, Plum, Ordonez, & Svenning, 2015; Svenning, 2003) or colonization lags (Normand et al., 2011; Svenning & Skov, 2004) would not impose a historical ecosystem legacy.
4 HOW DO HISTORICAL LEGACIES IN BIODIVERSITY LEAD TO HISTORICAL ECOSYSTEM LEGACIES?
Four ideas ground the historical ecosystem legacies concept. First, biogeographical processes introduce historical legacies in the total γ-diversity and subsequently the habitat specific species pool. Second, time-dependent ongoing processes introduce historical legacies upon the species and functional composition at a site, reshaping the observed species and attributes within an assemblage. Third, response traits determine the rates and magnitude of the processes shaping regional and local-scale species richness and composition. However, this is a bidirectional relationship as changes in species richness and composition also affect the response trait space composition and topological attributes. Fourth, local shifts in species richness and composition may restructure the effect trait composition, and subsequently, alter the rates and magnitudes of ecosystem processes.
We now present a framework (Figure 2) describing how response-trait modulated historical legacies in species composition may determine the effect trait composition of an assemblage and introduce historical legacies into one or more ecosystem processes. The processes leading towards historical ecosystem legacies proceed in two steps. The first step is the filtering of the habitat-specific species pool resulting in a lower proportion of the potential species inhabiting the evaluated assemblage. Such filtering can occur via response-trait mediated regional extinctions, local filtering by transient climatic changes or changes in the resource availability (Figure 2a, removal of elements in the centre and right panels), and migration-lag in response to environmental changes (Figure 2a, empty elements in the central panel). Invasions by non-natives could also set the stage for historical ecosystem legacies, as these allow the introduction of species and traits that otherwise would not have been present at a location, resulting in an observed composition that differs from the habitat-specific species pool defined by native species.

The second step is the removal or introduction of effect traits (change in shape types of the elements shown in each panel of Figure 2a) as a result of response-trait mediated changes in species composition. Therefore, if the species composition of an assemblage shows prevalent historical legacies from past events, the effect trait composition of such an assemblage will deviate from a habitat determined theoretical expectation (Figure 2a). Legacies in the effect trait composition may consequently introduce a historical legacy in ecosystem processes according to our definition of historical ecosystem legacies.
The strength of the association between the response and effect traits will determine how likely historical ecosystem legacies are to emerge. When response and effect traits are uncorrelated, historical legacies in species composition due to the limited spatial tracking of suitable environments (Figure 2b central panel), biogeographical processes, or trait-mediated species filtering by past environments (Figure 2b right and left panel) will not alter the effect trait composition (all the shapes are observed in all three time-periods, Figure 2b). As a result, we should not expect ecosystem processes to deviate from their theoretical potential states in this situation, even though the species in the system are a subset of the habitat-specific species pool.
The prevalence (how many species that belong to a particular habitat-specific species pool are missing) and the pervasiveness (how long the disparity between observed and potential states lasts) of biodiversity deviations from a theoretically predicted state will determine the strength of historical ecosystem legacies. Prevalent and/or pervasive historical legacies in biodiversity imply a substantial and enduring deviation from the potential species and functional composition, which can translate into a long-lasting historical ecosystem legacies process.
5 EMPIRICAL EVIDENCE FOR HISTORICAL ECOSYSTEM LEGACIES
Current species and functional composition can differ from a theoretical potential state due to (a) cumulative biogeographical processes, such as extinction-speciation (Ricklefs, 2007), or colonization from centres of origin (Wiens & Donoghue, 2004), (b) range shift lags (Normand et al., 2011; Svenning & Skov, 2004), (c) local filtering of environmentally unsuitable species (Blonder et al., 2015; Jackson & Weng, 1999; Svenning, 2003), and (d) current human movement of species across biogeographical barriers (Hobbs, Higgs, & Harris, 2009). These processes have been shown to introduce lasting historical legacies in species ranges (e.g., the limited filling of the potential range; Table 1) that translate to historical legacies in species and functional α-diversity (e.g., low potential-to-realised local species richness; Table 1), β-diversity (e.g., increased spatial turnover in species and functional traits; Table 1), and γ-diversity (e.g., slow or accelerated rates of diversification and extinction; Table 1). We now present a series of examples showing how do historical legacies in the species and functional composition associated with the processes described above translate into historical ecosystem legacies.
5.1 Extinction-speciation
Climatically driven extinctions can introduce historical legacies into ecosystem processes via the selective removal of certain species groups. The documented regional extinctions of species as a result of the Neogene cooling exemplify how the selective and permanent removal of cold-sensitive genera from temperate floras can result in historical ecosystem legacies (Eiserhardt et al., 2015). The selective removal of cold-sensitive species reshaped the richness and composition of the temperate forest. As condition returned to a warmer state, the taxonomic and trait composition of the local assemblages differed from the habitat-specific species pool expected before the Neogene cooling. This reduced species pool could affect stand productivity (Morin, Fahse, Scherer-Lorenzen, & Bugmann, 2011) as the effect trait composition of current assemblages will lack those attributes of species belonging to genera lost during the Neogene but that are climatically viable in the region today.
5.2 Colonization from centres of origin
Differences in the movement of species across biogeographical barriers can have a lasting impact on ecosystems’ structure and function, as shown by the comparison of similar habitats in disjunct biogeographical regions. The lack of dipterocarpoids in the Neotropics (Dutta et al., 2011) is a classic example of this. This group of highly productive species has an eastern Gondwana origin, expanding into the Asian tropical forests in the Eocene after India’s collision with Asia (Dutta et al., 2011). After their arrival in the Asian tropical forest, dipterocarpoids became a crucial determinant of the tropical forest vertical structure (Banin et al., 2012) and therefore the biomass in the region (Banin et al., 2014; Slik et al., 2013). The lack of dipterocarpoids in the Neotropics offers a putative explanation for the much higher average height (Banin et al., 2012) and productivity differences (Banin et al., 2014; Slik et al., 2013) of Southeast Asian forests when compared to forests in South America in similar habitats.
5.3 Range shift lags
Range dynamics lags can be the result of direct (physiological constraints and low dispersal ability) and/or indirect (changes in species interactions) mechanisms regulated by response traits. Migration-lags would make local assemblies a subset of the habitat-specific species and functional pool. Studies of the forest composition of Arctic regions in response to future climates (Normand et al., 2013) predict the prevalence of short-term lag responses (102–104 years) in the range expansion of herbaceous species in this region. These lag responses would result in a shift from a herbaceous to a woody plant-dominated ecosystem (shrubification, cf. Myers-Smith et al., 2011), as non-woody species of the habitat-specific pool are lost. This structural change is expected to increase productivity (Fridley, Lynn, Grime, & Askew, 2016), affect the atmospheric and soil CO2 content (Zhang et al., 2013), and alter the surface albedo of the region (Betts, 2000). Moreover, the shift in the vertical structure of Arctic ecosystems (herbaceous-to-woody change) would change ecosystem processes (i.e., productivity), and shift the primary food sources for herbivores (Wheeler, Hoye, & Svenning, 2018).
The palaeoecological record also provides clear examples of lags in range tracking introducing historical legacies into ecosystem processes. For example, the dispersal-limited immigration of lodgepole pine (Pinus contorta) from glacial refugia into north-western American boreal spruce-dominated forests has introduced a historical ecosystem legacy for the fire regime, forest productivity and carbon storage in those climatically suitable areas where lodgepole pine is missing (Johnstone & Chapin, 2003). Likewise, interspecific differences in range-edge shifts after the Younger Dryas onset (14,000 to 12,0000 years BP) for North American woody species (Ordonez, 2013) has resulted in current communities deviating from their environmentally expected species (Blonder et al., 2015) and functional composition (Ordonez & Svenning, 2016), and possibly a lower productivity than expected based on the habitat-specific species pool.
Examples of indirect mechanisms are rare, but as shown by Pither, Pickles, Simard, Ordonez, and Williams (2018), the speed at which range edges for North American plants responded to changes in climatic conditions over the last 16,000 years was modulated by the type of mycorrhizal association; that is, ectomycorrhizal fungal symbionts move faster than species with arbuscular mycorrhizal associations. Contemporary climate change examples show how the degree of functional similarity between current and novel competitors has an asymmetric effect on range changes, with functionally distinct species moving at faster rates (Alexander, Diez, & Levine, 2015). This differential effect of biological interactions across species would make the realised diversity a subset of the habitat-specific species pool and also would introduce lasting historical legacies in ecosystem processes via changes in the effect trait composition.
5.4 Local filtering of environmentally unsuitable species
The removal of unfit phenotypes from local assemblages due to transient climatic events (perturbations in the system that push it towards a new state cf. Hastings, 2004) can result in local diversity patterns deviating from the habitat-specific species and functional pool. For example, species composition across 471 New World forest plots has been shown to differ from a habitat-specific species pool defined by theoretical expectations (Blonder et al., 2015); the magnitude of climate change over the last 21,000 years partially predicts this difference. Likewise, Ordonez and Svenning (2017) showed that long-term palaeoclimate variability imposes consistent supplementary constraints on the functional composition of six widely distributed orders of European angiosperms via local filtering during the last glaciation and colonization lags from climatic refugia.
The balance between filtering and colonization dynamics is a central aspect when determining the prevalence of historical ecosystem legacies. For example, when trait-mediated colonization dynamics are slow and coupled with fast filtering of ‘unfit’ species, we expect to observe biodiversity levels that broadly differ from the habitat-specific species pool (Ordonez & Svenning, 2015; Svenning et al., 2010). Under this set of conditions, a lasting historical ecosystem legacy would be introduced via the non-random removal of effect traits. However, if the colonization occurs at a faster rate than ‘unfit’ species are filtered out, historical legacies in biodiversity and ecosystems processes would not be as prominent, given that species and functional composition could fulfil their full theoretical potential after a filtering event.
5.5 Human intervention effects on historical legacies
Human movement of species across regions can reduce the prevalence of historical legacies in biodiversity by compensating for local extinctions and colonization lags and returning the system to an expected state. For example, the introductions of Quercus cerris, Prunus laurocerasus and Rhododendron ponticum into Britain (Prance, 1985; Svenning & Skov, 2004) have compensated for dispersal constraints on the postglacial re-colonization of these species into north-western Europe from southern European refuge regions. These successful introductions into areas that were part of these species’ pre-glacial ranges provide indirect evidence of past biogeographical legacies (extinctions) in the habitat-specific species pool. Furthermore, the successful colonization by non-natives in some (but not all) instances could be considered a ‘replacement’ of a species lost from the habitat-specific species pool. Such replacement potentially removes a biogeographical legacy and (re)introduces a lost set of effect traits to the evaluated assemblage.
The introduction of fit phenotypes from new species that do not have a biogeographical or historical linkage to a region (cf. non-natives) could lead to historical ecosystem legacies, as the observed diversity at a site would not represent the expected habitat-specific species pool based only on the native species pool. The introduction of Morella faya from Macaronesia to Hawaii (Creighton, 1975) and Australian Acacia species to South Africa (Richardson et al., 2011) are classic examples of how the removal of biogeographical barriers introduce new effect traits not observed in the habitat-specific species pool defined by native species. The introduction of new effect traits by non-native invasives has resulted in changes in water availability and hydrology to a new non-native state. Furthermore, these introductions have also led to changes in soil nutrient and biogeochemical cycling rates via nitrogen fixation in nutrient-poor soils (Creighton, 1975; Richardson et al., 2011).
6 IMPLICATIONS OF HISTORICAL ECOSYSTEM LEGACIES FOR ECOLOGICAL THEORY AND GLOBAL CHANGE ECOLOGY
The historical ecosystem legacy idea provides testable hypotheses for ecological theory. One such hypothesis is that the differential effects upon local biodiversity of biogeographical and time-dependent ongoing processes between climatically similar regions would result in regional differences in ecosystem processes. The null expectation would be that similar current environmental conditions would result in a convergent potential state (i.e., similar species richness and functional composition). However, differences between regions in the type and magnitude of processes introducing historical legacies in the richness, species composition, and functional composition in each region, would translate into regional differences in the magnitudes and rates of ecosystem processes. To test this hypothesis, one could contrast the composition (species and functional) and/or ecosystem processes across a diversity anomaly (Ricklefs, 2004). Such a contrast would establish how differences in the magnitude of past climatic changes and biodiversity disparities explain ecosystem process differences between regions (for examples of this approach see Liebergesell et al., 2016; Ordonez & Svenning, 2018; Swenson et al., 2016).
The proposed framework can also be used to compare the sensitivity to, and impact of, biological invasions for regions with similar environments. The working hypothesis is that locations with a smaller representation of the habitat-specific species pool would be more prone to invasions by species that can occupy the empty ecological space; an idea associated with the biotic resistance hypothesis in invasion biology (Elton, 1958; Levine, Adler, & Yelenik, 2004). The invasion by non-natives could also be used to determine historical legacies in the effect trait composition of an assemblage. One way to assess this is by establishing the missing traits re-introduced by the non-native species, and if these reintroductions reduce the deviance between observed and expected ecosystem processes. However, the removal of such a historical legacy would depend on the link between traits, as introductions in areas where response and effect traits are decoupled will affect ecosystem processes only if the non-native is an ecological novelty in a historical context.
The development of readily applicable tools that quantify and model historical ecosystem legacies is necessary to establish the magnitude and prevalence of biodiversity-driven legacies in ecosystem processes. One possible way to do this is by incorporating historical legacies in biodiversity into dynamic global vegetation models (DGVMs). One of the goals of DGVMs is to predict the distribution of terrestrial vegetation-defined ecosystems, and large-scale ecosystem attributes under different environmental conditions (Van Bodegom et al., 2012). The historical ecosystem legacies idea provides a new perspective about how DGVMs should consider the link between biodiversity and ecosystem processes, that is, via response-trait mediated legacies in species composition at regional and local scales that alter the effect trait composition. As currently implemented in DGVMs, historical legacies in biodiversity are only a result of population dynamics (Woodward & Lomas, 2004) and limited dispersal between patches (Higgins & Harte, 2006). Developing DGVMs that account for historical legacies in the species and functional composition due to biogeographical processes and time-dependent ongoing processes will provide a more realistic assessment of the spatio-temporal dynamics of vegetation-related ecosystem processes.
Incorporating the constraints imposed by long-term biodiversity dynamics into DGVMs will help the prediction of spatio-temporal dynamics of ecosystem processes in multiple ways. First, it would make it possible to incorporate a link between historical legacies in biodiversity and ecosystem processes at large scales through species and functional composition shifts. Second, it would allow for the development of a novel macroecological approach, focused on the links between multiple biodiversity dimensions, that would be useful for predictive ecosystem ecology. Last, when combined with the evaluation of functional variability as a continuous variable (i.e., variation along an ecological strategy axis, cf. Westoby, Falster, Moles, Vesk, & Wright, 2002), it would improve the mechanistic basis for predicting future climate change and its feedback with ecosystems.
We acknowledge that multiple difficulties exist in implementing the idiosyncrasy of historical legacies in earth system models. However, we consider that such integration can be possible, as some historical legacies can be highly predictable (e.g., if linked to past climate changes). Others, such as those related to lineage biogeographical histories have a level of idiosyncrasy, but they can still be incorporated into a model and predicted using detailed knowledge coming from phylogeographical studies describing the biogeographical history of a lineage.
7 CONCLUSIONS
Here, we have presented the idea of historical ecosystem legacies as a way to link past historical legacies in species and functional composition, and how these changes could affect the rates and magnitude of ecosystem processes. Historical ecosystem legacies are the result of response-trait mediated shifts in species composition that have the potential to introduce legacies in the effect trait composition of an assemblage. These changes in effect trait composition at a location would shift ecosystem processes away from a potential defined solely on current environmental conditions. The magnitude and prevalence of historical ecosystem legacies depend on the strength of the legacies of past events on current species and functional composition patterns and how close the link is between response and effect traits.
Studies focused on determining the effects of the legacies of past and contemporary environmental changes on ecosystem processes should pay particular attention to the relationships between response and effect traits. Evaluating how long it takes for differences between observed and expected effect trait composition and ecosystem processes to be minimized should be prioritized, notably to provide a better basis for predicting compositional and functional changes under future climate changes. Including the historical ecosystem legacies idea into quantitative frameworks such as DGVMs is paramount to provide a more realistic description of possible future ecosystem dynamics.
ACKNOWLEDGMENTS
We thank the European Research Council for their economic support (grant ERC-2012-StG-310886-HISTFUNC to JCS). AO was partly supported by a seed funds grant from Queen's University and an Aarhus University Research Foundation-starting grant during the preparation of this manuscript. JCS considers this work a contribution to his VILLUM Investigator project (VILLUM FONDEN grant 16549).
REFERENCES
BIOSKETCHES
Alejandro Ordonez and Jens-Christian Svenning are interested in understanding past and present changes in climatic conditions, long-term lags in range-shifts, and how lineage biogeography imposes lasting legacies in contemporary biodiversity and ecosystem patterns.




