The regional species richness and genetic diversity of Arctic vegetation reflect both past glaciations and current climate
Abstract
Aim
The Arctic has experienced marked climatic differences between glacial and interglacial periods and is now subject to a rapidly warming climate. Knowledge of the effects of historical processes on current patterns of diversity may aid predictions of the responses of vegetation to future climate change. We aim to test whether plant species and genetic diversity patterns are correlated with time since deglaciation at regional and local scales. We also investigate whether species richness is correlated with genetic diversity in vascular plants.
Location
Circumarctic.
Methods
We investigated species richness of the vascular plant flora of 21 floristic provinces and examined local species richness in 6215 vegetation plots distributed across the Arctic. We assessed levels of genetic diversity inferred from amplified fragment length polymorphism variation across populations of 23 common Arctic species. Correlations between diversity measures and landscape age (time since deglaciation) as well as variables characterizing current climate were analysed using spatially explicit simultaneous autoregressive models.
Results
Regional species richness of vascular plants and genetic diversity were correlated with each other, and both showed a positive relationship with landscape age. Plot species richness showed differing responses for vascular plants, bryophytes and lichens. At this finer scale, the richness of vascular plants was not significantly related to landscape age, which had a small effect size compared to the models of bryophyte and lichen richness.
Main conclusion
Our study suggests that imprints of past glaciations in Arctic vegetation diversity patterns at the regional scale are still detectable today. Since Arctic vegetation is still limited by post-glacial migration lag, it will most probably also exhibit lags in response to current and future climate change. Our results also suggest that local species richness at the plot scale is more determined by local habitat factors.
Introduction
The Arctic is warming twice as fast as any other region on Earth, potentially triggering a range of ecological responses (Callaghan et al., 2005; CAFF, 2013). Shrubs are encroaching on sub-Arctic habitats and some cold-adapted plant species have shifted their distributional ranges towards higher latitudes and elevations (Lenoir & Svenning, 2013). Bounded to the north by the Arctic Ocean, the surface available to the terrestrial Arctic ecosystem is shrinking globally as sub-Arctic climates and habitats are moving north (Crawford et al., 2003; CAFF, 2013). This may ultimately cause the extinction of species for which suitable habitat disappears (Thuiller et al., 2005). Because parts of species ranges are lost, climate change is expected to lead to a loss of intraspecific genetic diversity (Alsos et al., 2012). Consequently this is expected to limit the potential of species for adaptive responses to climate change (Jump et al., 2009).
Studying ecosystem responses to past climate changes can help us to understand and forecast the response of current biodiversity to on-going climate change (Sandel et al., 2011). In this context, a key aspect to consider is disequilibrium dynamics, since equilibrium conditions are often assumed when the relationship between biodiversity and current climate is being investigated (Araújo & Pearson, 2005). This may lead to inaccurate forecasts of species distributional ranges if species show delayed responses to changing conditions, including, for example, migration lag (migration here referring to Quaternary range shifts as opposed to seasonal movements) (Bertrand et al., 2011; Sandel et al., 2011) or extinction debt (Tilman et al., 1994). In the Arctic, Quaternary climatic fluctuations and the associated displacement of ice sheets have been substantial and frequent (Svendsen et al., 2004). Arctic biodiversity, including species richness and intraspecific genetic diversity, may therefore still be in disequilibrium with climate (Brubaker et al., 1995; Normand et al., 2013) which may influence the rate of response of biodiversity to future climate change (Svenning & Sandel, 2013).
During the Quaternary climatic fluctuations, conditions affecting the distribution of species changed, causing the spatial displacement of plant assemblages (Brubaker et al., 1995). Pollen records suggest that there have been strong shifts in vegetation structure between glacial and interglacial periods (de Vernal & Hillaire-Marcel, 2008) with especially rapid advancement of shrub tundra during ice retreat as the climate became milder (Funder et al., 2001). Following the Last Glacial Maximum (LGM), plant species migrated from refugia to reach the distributional ranges that they currently occupy (Hultén, 1937; Abbott & Brochmann, 2003). This recolonization of the Arctic after the LGM occurred in several stages and its rapidity varied markedly among regions (Birks et al., 1994; Bennike et al., 1999; Kienast et al., 2011). Because of barriers to dispersal such as fjords, mountains and oceans, as well as the stochasticity of this recolonization process, species may not have reached all currently suitable habitats (Hoffmann et al., 2010). On the other hand, the recurrent glacial cycles may have selected for a highly mobile flora able to track its potential niche through frequent long-distance dispersal events (Alsos et al., 2007; Brochmann et al., 2013).
The degree to which biodiversity is able to track the changing climate is expected to vary depending on the dispersal abilities of species (Lenoir et al., 2012). Because species generally show phylogenetic trait conservatism (Ackerly, 2009), taxonomic groups of closely related species may show comparable dispersal abilities. Similarly, functional traits may strongly vary across distantly related taxonomic groups with contrasting life histories. Arctic plant communities are largely dominated by three groups of primary producers: vascular plants, bryophytes and terricolous lichens. Compared with vascular plants, bryophytes and lichens have small spores or asexually produced diaspores which are light enough to be dispersed by wind, thus favouring long-distance dispersal (Munoz et al., 2004). Also, lichens and bryophytes can tolerate harsher climatic conditions (Kappen, 1993) and probably survived glacial periods closer to the ice margins. This means that they would have shorter distances to disperse into ice-free areas following deglaciation. As a consequence, we hypothesize a differential effect of the time since ice retreat on the survival, and thereby the distribution, of these three taxonomic groups.
The glacial–interglacial cycles have also left imprints on the current spatial structure of intraspecific genetic diversity in Arctic species (Brochmann et al., 2003; Eidesen et al., 2013). The most obvious signature of historic range expansions is reduced genetic diversity in the populations inhabiting more recently colonized areas, due to founder effects (Excoffier et al., 2009). In contrast, areas that have not recently been covered by ice and with less fluctuation in climate since the LGM have preserved a higher genetic diversity in populations (Eidesen et al., 2013; Yannic et al., 2014; Pellissier et al., 2015). As species richness and genetic diversity have been expected to be shaped by the same historical processes (Vellend & Geber, 2005), they have been hypothesized to covary (Vellend, 2005; Vellend & Geber, 2005). Several studies have investigated this expectation, and while a number of them have found a relationship (He et al., 2008; Adams & Vellend, 2011) others reported no correlations between these two measures of diversity (Puscas et al., 2008; Taberlet et al., 2012). It is generally supposed that species diversity is determined by different processes at different spatial scales (Crawley & Harral, 2001; Willis & Whittaker, 2002). In their review, Willis & Whittaker (2002) agued that at the broadest spatial scales the distribution of species is driven by historical processes acting over thousands to millions of years, while at local scales species richness is rather influenced by fine-scale biotic and abiotic interactions such as competition, habitat structure and availability. We therefore hypothesize a stronger effect of landscape age on regional than on local species richness.
- How does local (plot) and regional species richness relate to the age of arctic landscapes (number of years since ice retreat after the LGM)?
- How do vascular plants, bryophytes and lichens differ in their responses to time since deglaciation?
- Are patterns of species richness correlated with patterns of genetic diversity, indicating that they have been shaped by similar historical processes?
Materials and Methods
Species richness
As a measure of regional-scale species richness we used data on vascular plants from the Panarctic Flora (PAF; http://nhm2.uio.no/paf) (Elven et al., 2011). This gives a measure of diversity within 21 floristic provinces in the Arctic as defined by the Circumpolar Arctic Vegetation Map (CAVM; Appendix S1 in the Supporting Information) (CAVM Team, 2003; Walker et al., 2005). Studies of Arctic biodiversity at the local scale rarely span the entire Arctic region, mostly because the circumpolar region is composed of many different countries with different scientific methodologies and traditions. Data on plant relevés were compiled from existing sources and spanned all circumpolar countries including Canada, the USA (Alaska), Greenland, Iceland, Norway, Russia and Finland (references to data sets on species richness are given in Appendix 1). Most plots included were from the Arctic as defined by the CAVM (CAVM Team, 2003; Walker et al., 2005). Furthermore, plots from the northern part of Scandinavia (Norway and Finland) were included. We compiled georeferenced plots for which lists of vascular plants and terricolous bryophytes and lichens had been recorded. For each plot we computed the total number of species for each of the three taxonomic groups. In total, we compiled data from 6215 vegetation plots distributed around the Arctic (Fig. 1) and with a mean plot size of 4.22 m2 (SD = 18.00 m2).
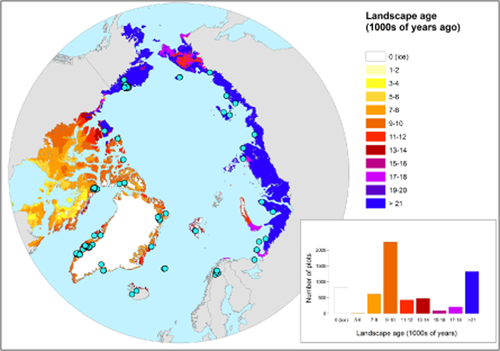
Map of time since ice retreat since the Last Glacial Maximum (LGM) across the Arctic. Modified from Raynolds & Walker (2009) where landscape age was constructed based on information on the most recent deglaciation, emergence from sea, or drainage of proglacial lakes. The 1000-year time steps used in the simultaneous autoregressive (SAR) models have here been merged to make a clearer presentation. Landscape ages older than 21,000 years were compiled in the category > 21. Dots show the distribution of vegetation plots collected. One dot may indicate several vegetation plots at this location. The projection used is North Pole Lambert Azimuthal Equal Area 180. The insert shows the number of plots sampled in each age category. The 828 plots sampled in ice were not covered in ice but sampled so close to permanent ice cover that the resolution of the map is not fine enough to display their true category. When analysed in the SAR models these plots were given the age 0 since it is assumed that these areas emerged from ice more recently than all other areas.
Genetic diversity
We compiled data on vascular plant species from published work (Alsos et al., 2012; references to data sets on genetic diversity are given in Appendix 2), choosing species occurring in the Arctic bioclimatic zone at the tree line and beyond and leaving out species with very restricted ranges. This left 23 plant species (including 16 of the 17 analysed by Eidesen et al., 2013) for which we analysed the intraspecific genetic datasets. These datasets were constructed by genotyping 9416 individual plants from 1362 local populations using amplified fragment length polymorphisms (AFLPs) (78–334 markers per species). Within a local population, leaves from 11 individuals were collected 25 m apart along a line transect as in Eidesen et al. (2013). To create maps of genetic diversity we transformed the estimates of diversity (D, estimated as the average proportion of pairwise differences between individual AFLP profiles) in order to enable the comparison between species following the formula: transformed value = (value)/(max. value). Full details on data collection and genetic structuring have been published elsewhere (Alsos et al., 2012). For each species the transformed genetic diversity was assigned to the grid cells where samples were available. A grid cell size of 50 km was chosen because this scale is small enough to separate the genetic groups into different areas but still large enough to obtain coarse-scale diversity patterns (Eidesen et al., 2013). The genetic diversity was then spatially interpolated to fill the unsampled grid cells within the outlined distribution range of each species using the rgdal package (Keitt et al., 2013) in R (Team, 2015) and an average of all species was computed to produce a metamap for genetic diversity (Fig. 2).
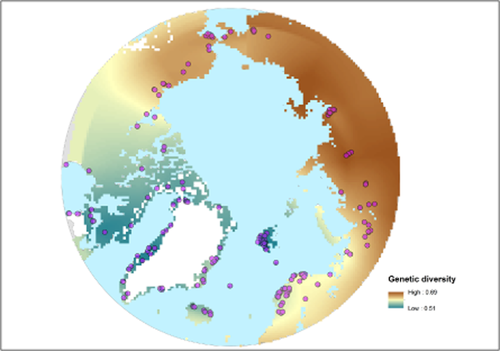
Map of interpolated genetic diversity across the Arctic. We assessed levels of genetic diversity inferred from amplified fragment length polymorphism (AFLP) variation in 23 relatively common Arctic species from already published work (Appendix 2). Estimates of diversity (D) were transformed in order to enable the comparison between species following the formula: transformed value = (value)/(max. value). For each species the standardised genetic diversity was assigned to the grid cells where samples were available. The genetic diversity was then spatially interpolated to fill the unsampled grid cells within the outlined distribution range of each species, and an average of all species was computed to produce the metamap for genetic diversity. Dots show where genetic data have been sampled. The projection used is North Pole Lambert Azimuthal Equal Area 180.
Predictor variables
We compiled a set of macroclimatic predictor variables from WorldClim (Hijmans et al., 2005) (http://www.worldclim.org/) that are expected to contribute to explaining the variation in species richness across the Arctic. We chose mean temperature of the warmest quarter (hereafter temperature, Appendix S2), since it has previously been shown to be a strong predictor for plant diversity in the Arctic (Nilsen et al., 2013). Further we selected annual precipitation (hereafter precipitation, Appendix S2) as a measure of precipitation, since some areas in the Arctic are strongly influenced by summer rain (e.g. west Greenland; Jensen, 2012), while others that experience low levels of summer precipitation are more affected by the amount of snow during winter (e.g. Zackenberg; Hansen et al., 2008). As a metric of landscape age since the LGM we used the map already published by Raynolds & Walker (2009) (Fig. 1). Landscape age was constructed on the basis of information on the most recent deglaciation, emergence from sea or drainage of proglacial lakes. The information was obtained from a compilation of Quaternary glaciations, available in digital format (Ehlers & Gibbard, 2004). Areas of age 21,000 years and older were regrouped into one age class. To test the importance of fine-scale habitat we analysed a homogeneous subset of the data covering Russia and Svalbard for which local-scale habitat information was recorded in the field (Appendix S3).
Statistical analyses
Because residuals from models of plot species richness are assumed to be spatially autocorrelated, given the aggregated structure of vegetation plots along smooth climatic gradients (Fig. 1), we used a simultaneous autoregressive (SAR) error model (SARerr) as suggested by Kissling & Carl (2008). We used a spatial weight matrix with neighbourhoods defined as all cells within 2000 km of the focal cell, and applied a Moran's I global test to determine whether residual autocorrelation persisted in the SAR models. To account for potential nonlinear associations between richness and climatic variables, we tested the quadratic term of temperature and precipitation in the SAR models. The plot area variable was log-transformed prior to analyses, because this variable showed a power relationship with species richness that was better fitted with a log transformation. Based on the full SAR models, information theoretic-based model selection was undertaken by comparing all competing models, including all possible combinations of variables. To asses the fit of the model, we used the Akaike information criterion (AIC) (Burnham & Anderson, 1998) as well as a metric of R2 as suggested by Kissling & Carl (2008) (hereafter called R2). We computed the R2 as the squared Pearson correlation between predicted and observed values to quantify the support for each model. To quantify the relative importance of variables in the model, we computed the mean squared error (sum of the squared differences between predicted and observed values divided by the number of observations) of the full model and the model with only a given environmental variable. Before running the SARerr models, all variables were scaled to zero mean and unit variance to be able to compare the effect size of the predictors. The SARerr model was used both to relate regional richness of vascular plants within the CAVM subregions of the Arctic (PAF data) as well as to plot species richness of the three taxonomic groups and total species richness to all predictor variables. It was also used to relate regional species richness to the genetic diversity of vascular plants and finally to relate genetic diversity to landscape age as well as current temperature. Before running the models we tested for collinearity between all predictors (for models at the plot level variance inflation factors were never greater than 1.42 and for the regional model 2.60). We further quantified the predictive power of all models beyond the calibration region, using a geographically independent validation approach. For this, the study region was divided into a training and a testing region (Appendix S4). A SAR model fitted on the training half of the data was then projected in the testing half using the estimated parameters. Pearson correlations between predicted and observed values were used as a measure of predictive ability. Because the genetic diversity map was created using spatial interpolation, the model aiming to explain patterns in genetic diversity is expected to contain a particularly large amount of spatial autocorrelation. To test the importance of environmental variables relative to a pure spatial component we calibrated the full SAR model, an intercept-only SAR model as well as an ordinary linear model (Appendix S5). Analyses were performed using R and the package ‘spdep’ (Bivand, 2014).
Results
Species richness
The regional diversity of vascular plants increased with increasing area of the floristic province as well as with increasing precipitation and landscape age (Table 1). Landscape age was the variable with the largest slope coefficient and the lowest partial mean squared error (MSE; Table 1), indicating that it had the strongest contribution in terms of explanatory power (Fig. 3). Temperature was not significantly correlated with regional vascular plant species richness. Nevertheless, the standardized coefficients for temperature and precipitation were very similar, which suggests a similar correlation between these two predictors and regional diversity. The explanatory power of the model for regional species richness was high compared with those for plot species richness. This is supported by our analysis of a subset of our dataset showing that fine-scale habitat heterogeneity is an important driver of species richness at the plot scale (Appendix S3). The predictive ability in the geographically independent validation was high for species richness at the regional scale. The model calibrated in eastern North America–western Eurasia explained 75% of the variation in richness in the other half of the Arctic (Appendix S4).
| Response | Predictor | Slope | P-value | SE | R2 for the full model | MSE full model | Partial MSE | Sample size (regions) |
|---|---|---|---|---|---|---|---|---|
| Vascular richness | Landscape age | 0.61 | << 0.001 | 0.16 | 0.76 | 0.23 | 0.69 | 23 |
| Precipitation | 0.47 | << 0.001 | 0.14 | 0.83 | ||||
| Temperature | 0.39 | 0.87 | 0.17 | 0.64 | ||||
| Area | 0.60 | << 0.001 | 0.12 | 0.85 | ||||
| Vascular richness | Genetic diversity | 0.46 | 0.02 | 0.19 | 0.30 | 0.84 | ||
| Area | 0.45 | 0.02 | 0.19 | 0.85 |
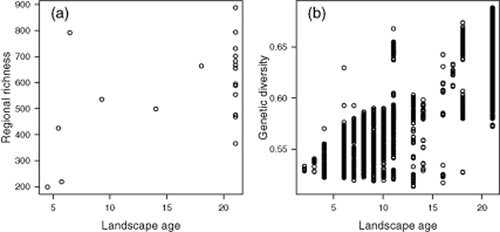
Relationship between landscape age and (a) regional species richness and (b) genetic diversity. Both regional species richness (R2 = 0.27) and genetic diversity (R2 = 0.78) increases with age of the landscape.
At the plot scale the number of bryophytes and lichens increased with the number of years since the ice retreated, while vascular plants showed no significant relationship and a low standardized coefficient (Table 2). All three groups of species showed significant correlations with both precipitation and temperature. The standardized coefficients were larger for precipitation than for temperature, but the partial MSEs were lower for temperature, suggesting that both variables contribute to explaining plot richness. All groups except bryophytes (i.e. vascular plants, lichens, total species richness) were positively correlated with the size of the plots (Area, Table 2). At the plot scale, models trained in eastern North America–western Eurasia explained 44%, 64%, −2% and 25% of richness in the testing region for total, vascular, bryophyte and lichen richness respectively (Appendix S4). Species richness at the plot level was not found to covary with regional species richness (Appendix S8), as has previously been found by others (Loreau, 2000; Lenoir et al., 2010).
| Response | Predictor | Slope | P-value | SE | R2 for the full model | MSE full model | Partial MSE | Sample size (number of plots) |
|---|---|---|---|---|---|---|---|---|
| Total richness | Landscape age | 0.27 | << 0.001 | 0.07 | 0.46 | 3.44 | 1.12 | 4414 |
| Precipitation | 4.06 | << 0.001 | 0.32 | |||||
| Precipitation2 | −3.55 | << 0.001 | 0.25 | 1.63 | ||||
| Temperature | 1.54 | << 0.001 | 0.18 | |||||
| Temperature2 | −1.08 | << 0.001 | 0.14 | 1.00 | ||||
| Area | 0.16 | << 0.001 | 0.02 | 0.91 | ||||
| Vascular richness | Landscape age | 0.08 | 0.17 | 0.06 | 0.46 | 1.54 | 1.02 | 6192 |
| Precipitation | 1.39 | << 0.001 | 0.25 | |||||
| Precipitation2 | −1.43 | << 0.001 | 0.21 | 1.07 | ||||
| Temperature | 0.33 | << 0.001 | 0.06 | 1.18 | ||||
| Area | 0.13 | << 0.001 | 0.02 | 0.94 | ||||
| Bryophyte richness | Landscape age | 0.19 | 0.006 | 0.07 | 0.28 | 1.05 | 1.01 | 4427 |
| Precipitation | 2.17 | << 0.001 | 0.25 | |||||
| Precipitation2 | −2.23 | << 0.001 | 0.23 | 1.49 | ||||
| Temperature | 0.72 | << 0.001 | 0.18 | |||||
| Temperature2 | −0.83 | << 0.001 | 0.15 | 0.98 | ||||
| Area | 0.01 | 0.68 | 0.03 | 0.99 | ||||
| Lichen richness | Landscape age | 0.23 | 0.001 | 0.07 | 0.41 | 1.18 | 1.17 | 4431 |
| Precipitation | 2.39 | << 0.001 | 0.26 | |||||
| Precipitation2 | −2.09 | << 0.001 | 0.22 | 1.57 | ||||
| Temperature | 0.69 | << 0.001 | 0.17 | |||||
| Temperature2 | −0.68 | << 0.001 | 0.14 | 0.93 | ||||
| Area | 0.16 | << 0.001 | 0.02 | 0.94 | ||||
| Genetic diversity | Landscape age | 0.02 | << 0.001 | 0.003 | 0.99 | 0.52 | 0.57 | 7509 |
| Temperature | 0.06 | << 0.001 | 0.007 | |||||
| Temperature2 | 0.09 | << 0.001 | 0.005 | 1.05 |
Genetic diversity
The interpolated genetic diversity map (Fig. 2) indicated that genetic diversity in Eurasia increased from west to east, with lower levels in northern Fennoscandia and western Russia and higher levels in central and eastern Siberia (Beringia). In North America, genetic diversity decreased from west to east. Genetic diversity was relatively high in Alaska (Beringia), while north-eastern Canada and Greenland displayed the lowest values of genetic diversity. Interpolated genetic diversity generally corresponds to individual genetic diversity for the 23 species (Appendix S6). The SAR models showed a positive significant relationship between genetic diversity and landscape age (Fig. 3) as well as temperature (Table 2). The MSE was lower for landscape age than temperature, suggesting a stronger explanatory power of landscape age. The SAR model showed a high R2 (Table 2), which is probably a consequence of the spatial interpolation of the genetic diversity allowing the spatial component of the SAR to account for most of the variation. Nevertheless, a non-spatial model only based on environmental variables also showed a high R2 (R2 = 0.80, Appendix S5). In addition, the MSE of the full model SARerr including all variables was almost half (MSE = 0.52) that of the intercept-only model (MSE = 1.22) accounting only for spatial covariation, suggesting a clear model improvement when environmental variables are included. The model for genetic diversity calibrated in eastern North America–western Eurasia explained 83% of the variation in richness in the testing region (Appendix S4). The genetic diversity of vascular plants was positively correlated with regional species richness of vascular plants (Table 1).
Discussion
Here we evaluated whether landscape history in the Arctic is correlated with current Arctic vegetation diversity at two spatial scales, the plot and the regional species pool. Our results indicate that regional species richness and coarse-scale patterns of genetic diversity are associated with landscape age. This macroecological pattern is supported by high model transferability across two main regions of the Arctic. In contrast, relationships at the plot scale are weaker, with lower model transferability. Plot richness of bryophytes and lichens was correlated with landscape age, while richness of vascular plants was not. Our results therefore suggest a link between landscape age and vegetation diversity in the Arctic that depends on the spatial scale investigated.
Regional species richness of vascular plants was associated with landscape age (Fig. 3), and both effect size and R2 for this model were relatively high, suggesting that regional richness of vascular plants may still be limited by post-glacial migration lag. Our results provide evidence that in the Arctic flora, as in other ecosystems (Ricklefs, 2004; Qian et al., 2007), glacial histories leave strong legacies in current species richness at coarse spatial scales. In contrast, landscape age explained little of the variation in species richness patterns at the plot scale (Appendix S3). Coarse-scale climatic and historical variables used in the models are not sufficient to explain richness patterns at the plot scale because they are influenced more by local-scale environment such as microtopography (Crawley & Harral, 2001; Willis & Whittaker, 2002; Vellend et al., 2014). This conclusion is supported by the analysis of local-scale microtopography, which had a larger effect size and smaller confidence intervals than landscape age in models from the subset of data covering Russia and Svalbard (Appendix S3). Note, however, that the remoteness and the large spatial extent of the study area constrained the use of existing plot data which are not equally distributed in the different landscape age categories (Fig. 1). A stratified vegetation sampling based on glaciation history might have provided stronger relationships to landscape age.
As expected, vascular plants, bryophytes and lichens showed differential responses to time since deglaciation that may be linked to different dispersal abilities and proximity of glacial refugia. A growing number of studies suggest that the spatial diversity of various groups of species within different ecosystems may be structured by dispersal-based processes (Qian, 2009; Beaudrot & Marshall, 2011). Lenoir et al. (2012) found a high degree of similarity between lichen communities across the Eurasian Arctic tundra, a result they attribute to the combination of effective wind dispersal in northern circumpolar areas as well as the higher dispersal capacities of lichens compared with vascular plants or even bryophytes. Hence, lichens are more likely to be at equilibrium with their current climatic conditions than vascular plants or bryophytes (Araújo & Pearson, 2005). Lenoir et al. (2012) investigated macroscopically identifiable lichens whereas we only looked at terricolous species. Species with different life-forms may have very different dispersal strategies or experience various degrees of competition from vascular plants. Bültmann (2010) found a high degree of similarity for terricolous lichens along a south–north gradient from Italy to Greenland, a fact that she attributes to lichens being a common element of the periglacial tundra vegetation. We found landscape age to be positively correlated with lichen and bryophyte richness but not with richness of vascular plants within plots. The higher species richness of lichens in older landscapes may result from an indirect edaphic effect. During succession over thousands of years, a maximal biomass stage is reached after which there is significant reduction in soil nutrients, reducing the productivity of vascular plants (Wardle et al., 2004). To what extent this is plausible for the Arctic is unknown, but reduction in vascular plants has been found to favour the occurrence of cryptogams (Pajunen et al., 2011). The fact that vascular plants, bryophytes and lichens have had different survival rates in climatically extreme microrefugia at higher latitudes and areas close to the ice sheets during the LGM (Rull, 2009) could also add to the taxon-specific patterns.
As expected based on the findings by Eidesen et al. (2013), Abbott et al. (2000) and Tollefsrud et al. (1998) the pattern of genetic diversity corresponds closely to the pattern of glaciation during the LGM. Ice-covered areas host significantly lower diversity than areas that were ice free during glacial periods. The strong correlation of genetic diversity with time since ice retreat fits the model of loss of genetic diversity during successive bottlenecks during leading-edge expansion (Hewitt, 1996). As early as 1937, Hultén (1937) hypothesized the existence of a major refugium, Beringia, around the Bering Strait, and that the majority of Arctic species spread from there following deglaciation. This idea has been supported by several later studies using phytogeographical, fossil (Abbott & Brochmann, 2003) and molecular evidence (Abbott & Brochmann, 2003; Eidesen et al., 2013). Our data corroborate Hultén's hypothesis of initial radiation from Beringia, since this region displays a high degree of genetic diversity, which gradually declines in North America when moving east into the more recently glaciated areas. Moving west from Beringia into Eurasia, the genetic diversity remains high throughout Asia and then gradually declines westwards from the Urals, where glaciations were also more severe (Astakhov, 2008). Moreover, the pattern we see of larger areas of high genetic diversity in Eurasia than in North America (Fig. 2) is consistent with the overall pattern of ice retreat after the LGM (Ehlers & Gibbard, 2004).
We find a positive correlation between genetic diversity and regional species richness, thereby supporting the findings by Vellend & Geber (2005) who found that correlations between species diversity and genetic diversity were generally positive, but the strength seems more pronounced at the coarser spatial scale within carefully selected regional vegetation units. The effect of landscape age seen in regional species richness recurs in genetic diversity, which mainly reflects coarse-scale immigration patterns (Alsos et al., 2007). The bottleneck effect of post-glacial recolonization might be very similar at interspecific and intraspecific levels.
Finally, the variables from WorldClim (Hijmans et al., 2005) describing current climate conditions were significant for all species groups. Temperature (Odland & Birks, 1999; Nilsen et al., 2013) and precipitation (Odland & Birks, 1999) have previously been found to drive diversity patterns at various spatial scales. However, at all scales considered we found that temperature was not the main correlate of vegetation diversity, which is in contrast to other studies (Currie, 1991; Currie et al., 2004), and precipitation showed a stronger effect in several models. Both temperature and precipitation are expected to control species richness but possibly with different intensity in different areas (Grytnes, 2003). In addition, since we focus on an ecosystem beyond the tree line, the temperature will globally show lower variation and have a lower importance in models.
Our study shows that we can still detect imprints of past glaciations on the diversity patterns of Arctic vegetation, at least for regional diversity. Current patterns of regional species richness of vascular plants as well as genetic diversity are still strongly affected by historical processes associated with the time and habitat available for dispersal and colonization. This offers important implications for the response of Arctic vegetation to ongoing climate change as well as for models trying to forecast future distributions. If we mistakenly assume that species are at present in equilibrium, predictions of species responses to future changes will most certainly be inaccurate. Vascular plants have not been able to track climatic changes during the Quaternary, at least not at the regional scale, and they are therefore expected to have even more difficulty tracking ongoing climate change. The positive relationship seen for regional vascular plant species richness does not recur in plot species richness, indicating that richness at this scale is more determined by local and landscape-scale parameters (Lenoir et al., 2010). The impact of ongoing climate change on species diversity may thus vary largely across scales and monitoring of change at both regional and plot scales is essential to understand ongoing processes and future responses. Our study illustrates the strength of investigating vegetation diversity across different scales to understand the consequences of climate change on vegetation patterns.
Acknowledgements
Compilation of the species richness data was made possible through the TFI Networks grant to C.D., ‘Effect studies and adaptation to climate change’, under the Norforsk initiative (2011–14) which supported two CBIONET-AVA workshops held in Denmark during 2013. The genetic studies were funded by the Research Council of Norway (grant nos. 150322/720 and 170952/V40 to C.B.). We extend our sincere thanks to Mads C. Forchhammer and three anonymous reviewers for highly valuable comments on earlier versions of the manuscript
Appendix 1 Data sets used in analyses of species richness
References
Biosketch
Lærke Stewart is a PhD student at the Department of Bioscience, Århus University. Her research focuses on distribution of Arctic vegetation and how these patterns change with a changing climate. She works at fine as well as coarse spatial extents, using empirical data and spatial modelling to answer questions about species, community and diversity distributions.
The current project was a collaborative research effort to put together fine-scale data for an entire biome to analyse patterns of species diversity. Author contributions: the study was designed by L.S., J.C.S., M.S.W., N.M.S. and L.P; data were collected by L.S., I.G.A., C.B., A.L.B., C.B., N.B.L., H.B., F.J.A.D., D.E., P.B.E., I.S.J., E.L., M.L., J.N.N., P.S., A.T., L.U.T., R.V., D.A.W. and K.B.W. L.S. and L.P. conducted the analyses with the help of N.M.S., J.C.S., M.S.W., P.K.B. and C.D. L.S. led the writing with contribution from all co-authors.




