Climate moderates release from nutrient limitation in natural annual plant communities
Abstract
Aim
To assess the combined influences of nutrient enrichment, invasive species and climate on assembly processes in natural annual plant communities.
Location
South-west Western Australia.
Methods
A comprehensive survey of winter annual plant communities (more than a thousand communities sampled in total) was undertaken across a natural moisture availability gradient in phosphorus (P)-limited York gum woodlands exposed to different levels of anthropogenic P enrichment. Three key functional traits (height, seed mass and specific leaf area) were measured incorporating intraspecific variation. Community richness, dominance of exotic species and trait distributions were investigated along local nutrient and regional climate gradients using hierarchical linear models. Results were assessed against expectations of moisture-regulated release from nutrient limitation based on trade-off theories and experimental findings of synergistic water and nutrient effects.
Results
Consistent with theoretical expectations, we identified significant interactions between local P levels and regional moisture availability for many of the response variables examined. Specifically, in communities exposed to both high P and high moisture we found: (1) reduced species richness, (2) high dominance of exotic species, (3) increases in community mean trait values and (4) strongly narrowing trait ranges. These results are consistent with competitive exclusion (via light competition). In naturally low-P situations we also identified shifting trait distributions and narrowing ranges as moisture stress increased, a finding consistent with environmental filtering.
Main conclusion
In this P- and water-limited system, plant community responses to P enrichment are contingent on regional moisture availability in a non-additive fashion. The most dramatic changes are seen under high-P and high-moisture conditions, i.e. where productivity is high and light has become a major limiting resource. By empirically validating theory, this study enhances our ability to predict ecological responses to multifaceted drivers of global change.
Introduction
Global environmental change is having an impact on biodiversity via multiple drivers including climate change, nutrient enrichment, habitat loss and biological invasions (Sala et al., 2000). Explicit investigation of the interactive effects of these drivers has revealed complex and often system-specific ecosystem responses (e.g. Reich et al., 2006). Here, we assess how regional climate interacts with eutrophication and species invasions to drive changes in plant community diversity and composition. We do this in natural (rather than experimental) annual plant communities whose productivity is limited to a large extent by water and phosphorus (P), two common limiting resources that have been, to date, understudied in this context.
Changes to abiotic conditions can directly affect the persistence of plant species in communities by acting on their physiological tolerances and indirectly by altering the way species interact (Tilman & Lehman, 2001). In relation to species interactions, ecological theory predicts that increased resource supply can reduce diversity by accelerating the rate of competitive displacement by superior competitors (Grime, 1979; Huston, 1979) and by reducing trade-off opportunities among competing species (Tilman, 1982; Chesson, 2000). Results from fertilization experiments provide support for these theories by showing that increased productivity can increase the relative importance of asymmetric light competition (e.g. Hautier et al., 2009). Because of trade-offs in the ability of species to compete for light and soil resources (Dybzinski & Tilman, 2007), intense light competition can lead to the exclusion of poor light competitors.
Community-level responses to anthropogenic nutrient enrichment will depend strongly on the composition of the regional species pool. Species pools can be augmented via biological invasions or reduced by regional (or complete) extinction of native species. Biological invasions are especially important in this context, as successful invaders are often pre-adapted to the ‘novel niches’ created by human activities (MacDougall & Turkington, 2005). In particular, nutrient enrichment is likely to favour non-native species with distinct fitness advantages under enriched conditions (MacDougall et al., 2009).
Moisture availability is also likely to influence the responses of herbaceous plant communities to nutrient enrichment, particularly in systems where productivity is strongly co-limited by water and soil nutrients. Experimental water addition has been shown to increase richness in low-rainfall (xeric) ecosystems (e.g. the initial response reported in Suttle et al., 2007). These experimental results are consistent with positive temporal associations between rainfall and richness in several xeric systems dominated by annual species (Cleland et al., 2013; Dwyer et al., 2014). Herbaceous species richness also increases along some spatial gradients of long-term average precipitation (e.g. Cleland et al., 2013; Zhang et al., 2014). Greater richness in less xeric environments may result, at least in part, from larger regional species pools (Zobel, 1997) and from greater numbers of species being able to maintain viable populations compared with drier regions (Wright, 1983). As conditions become drier, certain species may no longer maintain viable populations because they lack the required physiological tolerances (they are abiotically filtered; Chaves et al., 2002) or they experience strong competition from species with superior water use efficiency (Tilman, 1982).
The above-mentioned studies indicate a positive association between species richness and water availability in low-rainfall herbaceous systems; however, the positive effects of moisture availability can be reversed when soil nutrients are artificially enriched. Experimental additions of water and soil nutrients to low-rainfall (380 mm year−1) California grassland revealed synergistic effects, such that the greatest species losses occurred where all resources were supplied (Harpole & Tilman, 2007). While such synergistic effects do not apply in all herbaceous systems (Goldberg & Miller, 1990), the results from California provide at least some basis for predicting community responses to resource addition in low-rainfall systems, particularly those with similar mediterranean climates. If synergistic effects apply, then nutrient enrichment in xeric systems is expected to boost productivity most where rainfall is higher, and for these higher-rainfall areas to suffer the greatest declines in richness due to intensifying competition for light (Fig. 1a). These productive situations are also where exploitative invasive species are expected to have the greatest fitness advantages and become dominant (Fig. 1b). Importantly, under natural (nutrient-limited) conditions, we expect local richness to be greatest in higher-rainfall areas, and to decline with increasing moisture stress (Fig. 1a).
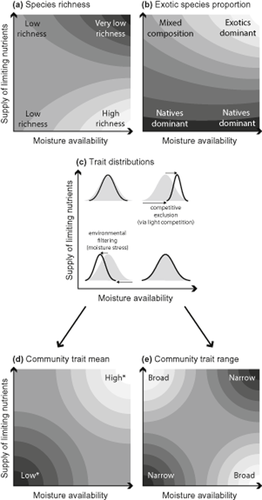
Expectations of climate-regulated release from nutrient limitation in low-rainfall herbaceous systems: (a) species richness, (b) exotic species proportions and (c) associated functional trait distributions. Axes are the same in each panel. Where nutrients and water are abundant (top right corner of each panel) we expect lowest richness, highest exotic species dominance and shifts in trait distributions due to the exclusion of weaker competitors for light. Under naturally low-nutrient conditions we expect native species to dominate regardless of moisture availability. However, we also expect moisture availability to act as an environmental filter, such that fewer species will be found in more xeric regions, and trait distributions will contract accordingly. In (a), (b), (d) and (e), shading indicates expected interaction surfaces from statistical models. Light colours indicate high values and dark colours indicate low values. In (c), bell curves represent community trait distributions; grey curves indicate expected distributions where richness is highest (lower right) and black curves indicate distributional shifts associated with either competitive exclusion (upper right) or environmental filtering (lower left). *Note that the direction of the distributional (mean) shifts associated with competitive exclusion and environmental filtering may vary by trait.
Few studies in diverse, natural systems have demonstrated the extent to which environmental filtering and competitive exclusion alter communities following environmental change. Functional trait-based approaches offer promise in this regard (Lavorel & Garnier, 2002), but require careful application and interpretation because the same trait patterns can result from different processes (Mayfield & Levine, 2010). Environmental filtering will be most evident under stressful abiotic conditions and in traits that reflect the physiological tolerances of species (Fig. 1c–e). Competitive exclusion will occur in situations where stabilizing niche differences have been reduced and fitness differences among species are substantial (Chesson, 2000; MacDougall et al., 2009). In the context of anthropogenic nutrient enrichment, competitive exclusion will be evident in resource-enriched situations (though not exclusively; Tilman, 1982) and in traits that reflect the ability to compete for light (Fig. 1c–e).
In this study we focus on the annual plant communities within York gum woodlands, a once common woodland type in the mediterranean climatic region of south-west Western Australia (Fig. 2). This region of Western Australia is now intensively and extensively farmed and York gum woodlands are restricted to fragments in a matrix of agricultural fields (primarily wheat and canola) and sheep pasture. This region spans considerable gradients of mean annual precipitation and temperature. Soils are deficient in P, and to a lesser extent nitrogen (N), and cropped areas are routinely fertilized to maintain agricultural production. Over the past 50 years, fertilizer run-off into the remaining woodland fragments has resulted in localized enrichment of P and N across the region (Prober & Wiehl, 2012). Many remnant York gum communities are now composed of native species and exotic agricultural commensals, but native-dominated ‘original’ communities also persist in some reserves providing reference communities with which to assess the drivers of community change. Importantly, there are no native annual grasses in this woodland ecosystem (only native perennial grasses), but a range of exotic annual grasses have invaded and persist in woodlands (Prober & Wiehl, 2011), including species that are known to capitalize on nutrient enrichment in other systems (e.g. Brooks, 2003). The considerable climatic gradients that the system spans, combined with local-scale abiotic gradients (both natural and anthropogenic), make this an ideal system for testing theoretical expectations about the processes driving community responses to environmental change.
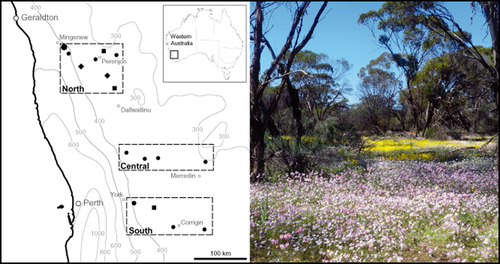
Location of the study region within the wheatbelt of Western Australia. Locations of sampled remnants within each of the latitudinal blocks are indicated with solid symbols. Symbol shapes indicate the sampling effort: round, three native–exotic mixture sites and three native-dominated sites; square, three native-dominated sites; diamond, three native–exotic mixture sites; hexagon, one native-dominated and one native–exotic mixture site. The large round symbol in the northern block shows the remnant that was grazed by sheep in the last decade. Also shown are mean annual rainfall isohyets (mm year−1). The photo depicts a typical woodland in bloom (photo J. Dwyer).
Materials and Methods
Study region
This study was conducted in south-west Western Australia, between the eastern edge of the Yilgarn block plateau east of Perth and the western edge of the great western woodlands (Fig. 2). This mainly agricultural region referred to as the ‘wheat belt’ occupies a transition zone between mesic mediterranean in the west and semi-arid climates in the east. Rainfall is winter dominant and generally declines with distance from the coast, though this gradient is steeper in the north than the south (Fig. 2). Average daily temperatures during the winter–spring growing season (June–October) vary from 16 °C in the south to 19 °C in the north. Soils of the region are ancient, highly weathered and characterized by low plant-available P (McArthur, 1991). This study focuses on the herbaceous understorey of York gum woodland fragments dominated by Eucalyptus loxophleba (York gum) and the N2-fixing Acacia acuminata (jam). Herbaceous vegetation consists of sparse perennial tussock grasses interspersed with diverse annual and perennial forbs that emerge during the growing season.
Phosphorus enrichment has been widely reported in woodlands surrounded by fertilized crop fields or pastures and the residual effects of the addition of P have been shown to last decades under some circumstances (Standish et al., 2006). Livestock grazing can also alter and homogenize woodland soils, so we adopted the approach of Prober & Wiehl (2011) and targeted long-ungrazed or ungrazed remnants. Most of the surveyed remnants are nature reserves that have been protected since before 1963 or remnants on private property that are known to have been free of livestock for at least 30 years. One property in the north of the study region was grazed by sheep until 2005 (Fig. 2), though the intensity of grazing in the woodland portion of the property was very low.
Data collection
Within the natural distribution of York gum woodlands, we selected three blocks (referred to as northern, central and southern) spanning more than 3° latitude. Across the three blocks, we selected 16 remnants from this region's natural mean annual precipitation gradient (Fig. 2). In each remnant, multiple 15 m × 15 m (225 m2) sites were established in forb-dominated patches no less than 80 m apart. In most remnants we established six sites composed of three native–exotic mixture sites and three native-dominated sites. In some remnants it was only possible to capture one of these site ‘types’, but we ensured that each site type was represented along the latitudinal and precipitation gradients (Fig. 2). The native–exotic mixture sites tended to be closer to reserve edges but exhibited no obvious signs of anthropogenic disturbance beyond variable levels of anthropogenic nutrient enrichment, which we explore in this study.
Within each site, communities were sampled in 15 randomly located quadrats (each 0.3 m × 0.3 m). We applied a minimum herbaceous plant cover threshold of 30% and where necessary moved quadrats to the nearest suitable patch to avoid large trees, shrubs or bare patches. The identity and abundance (counts of individuals) of all species was recorded and the tallest individual of each species was collected and pressed while fresh in the field. Following the species inventory a small soil sample (0–70 mm depth, litter excluded) was collected from the centre of each quadrat. Woody (tree and shrub) canopy cover was recorded using a spherical crown densiometer (Forestry Supplies Inc., Jackson, MS, USA). Grass litter (from exotic annual grass species) and sclerophyllous leaf litter were recorded as binary variables (present or absent). Soil samples were air dried and later analysed for ammonium, nitrate and extractable P by CSBP Limited (Bibra Lake, WA, Australia). Ammonium and nitrate were combined into one variable as an approximation of plant-available N.
Above-ground biomass was harvested from five additional quadrats within each site. These quadrats were systematically placed to capture compositional variation within sites, but were limited to areas of > 90% herbaceous plant cover to permit comparison of biomass values among sites and remnants. The survey was undertaken between 25 August and 10 October 2011, starting in the warmer northern block where peak biomass occurs first. At the end of the growing season we revisited a number of remnants to collect bulk seed from as many species as possible. A total of 1155 communities were sampled and 248 useable biomass samples were harvested.
Interpolated climate data (SILO; Jeffrey et al. 2001, available online) were used to calculate an index of moisture availability for each remnant. This index was calculated as the ratio of mean growing season precipitation to mean growing season potential evapotranspiration.
Functional trait measurements
Given the large number of communities sampled, and our interest in capturing interspecific trait variation, we focused on three continuous functional traits that represent important axes of functional variation (Westoby, 1998), namely specific leaf area (SLA), maximum plant height and seed mass. SLA is an important determinant of relative growth rate and net photosynthetic rate (Shipley et al., 2005). Plant height, in the context of annual herbaceous species, captures the ability to intercept light, and seed mass captures the trade-off between stress tolerance and colonization ability (Muller-Landau, 2010). Details of measurements of SLA are described in Dwyer et al. (2014). Height was measured on the tallest specimen of each species in each quadrat as the distance between the base of the plant and the tallest leaf. Seeds were collected opportunistically from seeding populations, and for most species this resulted in only one population being sampled. Seed mass was measured for most species by randomly selecting 100 seeds from bulk collections excluding seeds with obvious damage or deformities. Dispersules were removed where required and the subset of seeds was oven dried at 60 °C for 72 h. Seeds were then weighed in ten batches of ten seeds each using a microbalance (Sartorius AG, Goettingen, Germany), providing a mean and variance for each species. For some species no seeds were available at the time of collection so we used published mean estimates (Moles et al., 2004 and the studies cited therein); in the case of orchids we applied a realistic low value (0.0046 mg) to all species based on estimates of Arditti & Ghani (2000).
Generating community trait distributions that incorporate intraspecific variation
We generated continuous trait distributions for each quadrat and calculated the mean and range from each distribution. For SLA, we modelled each species' SLA as a function of environmental variables. These models were used to predict SLA values for each species' occurrence and to estimate a variance term for each prediction. We then used these species-specific predictions and variances to simulate community SLA distributions, with the number of draws from a species' distribution equal to its abundance in each quadrat. For each of the 999 simulated distributions we calculated the mean and range and then used the medians of these metrics as the community mean and community range of SLA values (see Dwyer et al., 2014, for details). We used a similar approach for seed mass, except that each species' mean seed mass was constant (it did not vary by quadrat). For height we measured the tallest individual of each species in each quadrat (c. 13,000 separate height measurements) and then took the maximum measured height for each species in each remnant to generate community height distributions. This approach captures the height potential of each species in a given locality as well as regional-scale intraspecific variation in height potential. For clarity, we refer to community trait means as community-weighted means (CWMs).
Data analysis
Biomass–resource relationships
Biomass data were analysed first to identify relationships between productivity and measured resource levels. The biomass response was ln-transformed and modelled as a function of sqrt(P), ln(plant available N), growing season moisture availability (calculated for the 2011 growing season) and their interactions using linear mixed effects models, with sites nested in remnants as random effects. All explanatory variables were standardized to a mean of zero and SD of 0.5 (Gelman, 2008) to permit comparison of coefficient estimates. Model averaging was used, and all combinations of explanatory variables were considered as candidate models, respecting marginality constraints associated with interaction terms. Models were ranked according to the corrected Akaike information criterion (AICc) and those within 7 AICc units of the best model were selected for model averaging (Grueber et al., 2011).
Models of richness, exotic species proportions, trait CWMs and trait ranges
Richness was numerically constrained in low-density quadrats, and the trait range responses were similarly influenced by low plant densities (Fig. S1), so we chose to adjust these responses using a null modelling approach to account for density. However, because plant density can strongly influence diversity along productivity gradients (densities can decrease as average plant size increases; Oksanen, 1996), we first assessed relationships between plant density and environmental variables (Methods S1). This preliminary analysis revealed that 66% of the variation in plant density occurred among quadrats (within sites), and almost none of this local density variation was explained by soil and moisture availability variables, though 10% was explained by the other environmental covariates (Appendix S1). We therefore concluded that demographic processes contributed substantially to the observed local-scale density variation, and that it was appropriate to account for this variation when assessing support for our various hypotheses. Details of null model adjustments are provided in Methods S2 and Fig. S2. In brief, we used the abundance-based ‘quasiswapcount’ algorithm (Oksanen et al., 2014) to generate 499 randomized community data matrices. For each randomized community matrix, trait values were assigned to each species by drawing from a pool of possible trait values for each species (incorporating intraspecific trait variation where available). Community richness, trait mean and trait range values were expressed as deviations from the randomized community expectations using standardized effect sizes, thus creating ‘abundance-adjusted’ response variables.
The exotic species proportion (proportion of exotic individuals in each quadrat) was not abundance-adjusted. This response was logit transformed after replacing all zeros with the lowest non-zero values and replacing all ones with the highest value less than one (Warton & Hui, 2011). This involved modifying around 17% of the observations (mainly zeros), so we also tried converting zeros to half of the lowest non-zero values, but this had a very minor influence on the results (not shown). Refer to Methods S3, Appendix S2 and Fig. S3 for details about modelling this response variable.
Linear mixed effects models were used to model the adjusted responses. Explanatory variables corresponded to the quadrat scale, namely sqrt(P), ln(available N), woody canopy cover, grass litter (binary) and sclerophyllous leaf litter (binary), or the remnant scale, namely moisture availability. In all models remnant and site were included as nested random effects (varying intercepts only) to represent the spatially nested sampling design. For all responses we also detected significant spatial dependence in the within-site residuals. Spherical correlation structures proved effective at modelling this spatial dependence, as indicated by likelihood ratio tests on nested model fits using restricted maximum likelihood (Pinheiro & Bates, 2004). Thus, all candidate models for a given response variable had the same random effects and spatial correlation structures. Given our interest in fixed effect parameter estimates, all candidate models were fitted using maximum likelihood estimation (Pinheiro & Bates, 2004).
As for the biomass analysis, all explanatory variables were standardized and a model averaging approach was used. Statistical analyses were performed in R (R Development Core Team, 2014). Null modelling was executed using the Vegan package (Oksanen et al., 2014), mixed-effects models were fitted using the nlme package (Pinheiro & Bates, 2004) and model averaging was undertaken using the MuMIn package (Bartoń, 2012).
Results
Biomass–resource relationships
Only the main terms for P and moisture availability were significant, both being positively associated with biomass (Table 1). The biomass model explained considerable variance at the remnant and site levels, but only 6% among quadrats (Table 1).
| Model term | Estimate (95% CI) |
|---|---|
| Intercept | 2.86 (2.80, 2.93) |
| sqrt(P) | 0.31 (0.21, 0.42) |
| ln(available N) | −0.05 (−0.16, 0.06) |
| Moisture availability. | 0.17 (0.05, 0.30) |
| sqrt(P) × ln(available N) | −0.04 (−0.21, 0.13) |
| sqrt(P) × moisture availability | −0.10 (−0.25, 0.08) |
| ln(available N) × moisture availability | −0.04 (−0.28, 0.08) |
| sqrt(P) × ln(available N) × moisture availability | 0.12 (−0.15, 0.39) |
| Variance components | |
| Among remnants* | 0.01 |
| Among sites | 0.04 |
| Within sites | 0.12 |
| Marginal R2 | 0.21 |
| Conditional R2 | 0.35 |
- *‘Remnants’ are discrete patches of native vegetation within which multiple sites were surveyed.
Species richness
Only the main terms for P, grass litter and sclerophyllous litter were significant in the averaged model for richness (all negative, Table 2). The moisture availability × P interaction was close to significant and indicated that richness was lowest where water and P were in abundant supply (Fig. 3a). The model for abundance-adjusted richness yielded similar results, except that moisture availability was also strongly significant and sclerophyllous litter was not significant (Table 2). Therefore, accounting for local and regional density variation strengthened the positive relationship between richness and moisture availability.
| Explanatory variables | Richness | Adj. richness | Exotic species prop. | Adj. SLA CWM | Adj. SLA range | Adj. height CWM | Adj. height range | Adj. seed mass CWM | Adj. seed mass range |
|---|---|---|---|---|---|---|---|---|---|
| Intercept | 11.38 | 0.07 | −2.03 | 0.09 | −0.20 | 0.08 | −0.85 | 0.03 | 0.29 |
| sqrt(P) | −0.83 | −0.34 | 0.66 | 0.28 | −0.19 | 0.27 | −0.23 | 0.25 | −0.10 |
| ln(available N) | 0.09 | 0.02 | 0.11 | 0.22 | −0.01 | 0.05 | −0.03 | −0.09 | 0.14 |
| sqrt(P) × ln(available N) | −0.33 | −0.13 | 0.02 | 0.00 | −0.06 | −0.04 | −0.00 | 0.03 | 0.09 |
| Moisture availability | 1.10 | 0.85 | 1.11 | −0.1 | 0.42 | 0.57 | 0.33 | −0.38 | 0.13 |
| Moisture availability × sqrt(P) | −0.48 (Fig. 3a) | −0.32 | 0.54 (Fig. 3b) | 0.25 | −0.42 (Fig. 3c) | 0.17 | −0.06 | 0.22 | −0.47 (Fig. 3d) |
| Moisture availability × ln(available N) | −0.21 | −0.22 | 0.22 | 0.37 (Fig. 4a) | 0.41 (Fig. 3e) | −0.12 | 0.08 | −0.04 | 0.01 |
| Moisture availability × sqrt(P) x ln(available N) | −0.98 | −0.37 | 0.12 | −0.13 | −0.19 | 0.09 | NA | 0.18 | −0.48 |
| Canopy cover | −0.01 | 0.20 | −0.15 | 0.72 | 0.06 | 0.27 | −0.20 | 0.08 | −0.03 |
| Canopy cover × sqrt(P) | −0.18 | −0.29 | −0.05 | 0.35 (Fig. 4b) | −0.15 | 0.09 | −0.01 | 0.22 | −0.09 |
| Canopy cover × ln(available N) | −0.81 | −0.28 | −0.13 | 0.06 | −0.09 | 0.03 | 0.04 | −0.18 | −0.24 |
| Canopy cover × moisture availability | −0.28 | −0.04 | 0.48 | 0.15 | −0.05 | −0.27 (Fig. 4c) | 0.14 | 0.24 | −0.35 (Fig. 3f) |
| Grass litter | −1.57 | −0.44 | 1.14 | 0.18 | −0.24 | 0.44 | −0.22 | 0.69 | −0.28 |
| Sclerophyllous litter | −0.85 | −0.15 | 0.09 | 0.03 | 0.09 | 0.27 | −0.05 | 0.01 | −0.11 |
| Variance components | |||||||||
| Among remnants | 1.90 (4%) | 0.40 (46%) | 1.77 (34%) | 0.00 | 0.07 (71%) | 0.53 (26%) | 0.10 (20%) | 0.04 (61%) | 0.05 (0%) |
| Among site | 0.00 | 0.00 | 1.95 (23%) | 0.24 (42%) | 0.06 (0%) | 0.28 (29%) | 0.17 (3%) | 0.22 (31%) | 0.07 (0%) |
| Within sites | 14.73 (16%) | 2.09 (9%) | 4.06 (18%) | 1.33 (14%) | 0.57 (9%) | 0.84 (13%) | 0.87 (5%) | 1.30 (7%) | 1.07 (7%) |
- SLA, specific leaf area.
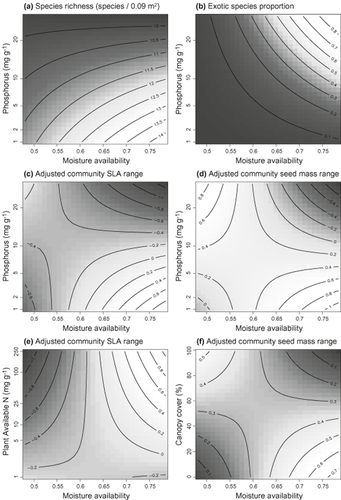
Plots of (a)–(d) moisture availability × soil P interactions from averaged models of (a) richness, (b) exotic species proportion, (c) adjusted community specific leaf area (SLA) range and (d) adjusted community seed mass range. Panels (e) and (f) show additional significant interactions from models of (e) adjusted community SLA range and (f) adjusted community seed mass range. In all plots shading and contours indicate levels of the response variables indicated above (dark colours for low values and light colours for high values). Note the sqrt scale on the y-axis for panels (a)–(d) and the ln scale for panel (e). All plotted interactions were significant (Table 2) except for (a) which was included to assess alignment with our expectations (Fig. 1). Units of response variables in (a) and (b) are as indicated. In (c–e) the shape and direction of the interaction surfaces are more important than the units of the response variables. These abundance-adjusted response variables are actually effect sizes (refer to methods) where positive values indicate broader community trait ranges than expected under the null model, and negative values indicate narrower than expected ranges.
Exotic species proportion
The moisture availability × P interaction was significant in the model of exotic species proportions (Table 2), and the fitted interaction surface matched our expectation of exotic species dominance in high-P, high-moisture situations (Fig. 3b). These conditions correspond to those where richness was lowest (Fig. 3a; refer to Fig. S4 for the relationship between adjusted richness and the proportion of exotic species). Exotics were also more dominant where grass litter was present (Table 2), as was expected given that exotic annual grasses produce almost all the grass litter in this system.
Models of trait CWMs and trait ranges
SLA
Moisture availability and P had significant associations with SLA CWMs (Table 2), and surprisingly the coefficient for moisture availability was negative. The interaction between available N and moisture availability was significant and indicated strong increases in SLA with increasing N in mesic regions (Fig. 4a). The interaction between woody cover and P was also significant, indicating stronger responses of SLA to P in shaded situations (Fig. 4b). SLA CWMs were higher in the presence of grass litter (Table 2). In the model of SLA ranges the moisture availability × P interaction was significant and the fitted surface was generally in agreement with our expectations for community trait ranges (Fig. 3c). The moisture availability × available N interaction was also significant (Table 2), but differed from the former interaction in which SLA ranges were narrowest in high-N situations in drier regions (Fig. 3e). Community SLA ranges were consistently narrower in the presence of grass litter (Table 2).
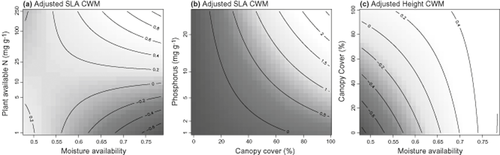
Significant interactions from averaged models of community weighted means (CWMs); (a) the moisture availability × available N interaction from the specific leaf area (SLA) CWM model, (b) the canopy cover × P interaction from the SLA CWM model and (c) the canopy cover × moisture availability interaction from the height CWM model. Shading and contours as per Fig. 3. In all panels, the shape and direction of the interaction surfaces are more important than the units of the response variables. These abundance-adjusted response variables are actually effect sizes (refer to methods) where positive values indicate higher community trait values (CWMs or ranges) than expected under the null model, and negative values indicate lower than expected values. Note the sqrt scale on the Y-axis in (a).
Height
Community height increased with P (irrespective of moisture availability), and was also greater in the presence of litter, particularly grass litter (Table 2). The moisture availability × woody cover interaction was also significant; cover had no discernible relationship with height in mesic regions and a positive relationship in dry regions (Fig. 4c). No interactions were significant in the averaged model for community height ranges, but some main terms were significant. Height ranges became narrower along local gradients of P and shade, and were also significantly narrower in the presence of grass litter (Table 2).
Seed mass
Only the main terms for P, moisture availability and grass litter were significant in the model of seed mass CWMs (Table 2). Community seed masses increased with P and grass litter, but decreased with moisture availability. The model for seed mass ranges included two significant interactions. The moisture availability × P interaction was similar to that for community SLA ranges (compare Fig. 3c and 3d) and the fitted surface matched our expectation for trait ranges. The moisture availability × canopy cover interaction was also significant, and showed that seed mass ranges in mesic regions were broadest in open situations, whereas in drier regions they were broadest in shaded situations (Fig. 3f). As in all other models of trait ranges, grass litter had a significant negative association.
Most variation in all of the response variables occurred at the local scale (within sites, Table 2). However, the amount of variance explained at this level was generally small (from 0.3−22% explained), suggesting that the explanatory power of all of the models was quite modest. Variance among remnants was much smaller, but this component was generally well explained (Table 2), suggesting that moisture availability captured regional differences for most responses.
Discussion
Using data from more than a thousand naturally assembled annual plant communities, we provide evidence that the negative effects of nutrient enrichment on community diversity and composition are amplified in higher-rainfall regions, consistent with some experimental findings from other systems with similar climates (Harpole & Tilman, 2007). Our hypotheses (Fig. 1) were supported by significant (or close to significant) interactions between moisture availability and P in models of species richness and exotic species proportions. Analyses of trait distributions provided additional support for all hypotheses, and provided evidence for the shifting relative importance of environmental filtering and competitive exclusion in different situations.
Climate-regulated release from nutrient limitation (H1a and H1b)
Community biomass was greatest in moist, P-enriched situations. These same conditions were associated with low richness and the highest exotic species proportions, consistent with hypothesis H1a (Fig. 1a). These trends in richness and exotic species were mirrored in functional trait distributions along the same environmental gradients, providing support for H1b (Fig. 1b). Specifically, SLA, height and seed mass distributions narrowed and means shifted toward much higher values in high-resource situations. Given that SLA is positively related to relative growth rate (Shipley et al., 2005) and is indicative of the abilities of species to exploit limiting resources (Angert et al., 2007), the observed shifts in SLA distributions highlight the importance of rapid growth under high-resource conditions where light has become a major limiting resource (Hautier et al., 2009; Laliberté et al., 2012). The shifts in community height distributions also reflect intensifying competition for light, and the narrowing height ranges indicate the exclusion of shorter species under P-enriched conditions. Increases in seed mass CWMs reflect the larger seed sizes of exploitative exotic species (Fig. S5). It is likely that larger seed size is important for the persistence of exotics, particularly grasses that produce persistent litter as discussed below.
Many of the exotic species that became dominant in high-resource situations were of Mediterranean or South African origin, including the annual grasses Avena barbata, Ehrharta longiflora and Bromus rubens, and the forbs Erodium botrys and Arctotheca calendula. These species have successfully invaded other mediterranean regions (e.g. California; Hickman, 1993), and are pre-adapted to exploit microsites with elevated nutrient levels (Brooks, 2003). While these species tended to be taller, have large seeds and average to high SLA, their trait space overlapped with that of many native species (Fig. S5). In addition, a considerable number of ‘less dominating’ exotic species had an almost complete overlap of trait space with the natives in our study system. Further investigation of trait differences among exotic and native species at more local scales, and the inclusion of traits not measured in this study, may yield much needed insights into the processes driving different invasion outcomes, from complete dominance to apparent coexistence.
In our study system, grass litter is produced almost entirely by exotic annual grasses. The presence of grass litter was consistently associated with reduced species richness, increasing trait CWMs and narrowing trait ranges. Of course litter-producing grass species were always recorded where grass litter was present, and this contributed substantially to the litter-associated trait shifts that we observed. However, the narrowing trait ranges also reflect that grass litter excludes short species with low SLA and small seeds, though there were some exceptions (e.g. the diminutive herbs Crassula colorata and Calandrinia eremaea were recorded in many littered quadrats). In a range of other systems, grass litter has been found to reduce germination and establishment of co-occurring species through a combination of direct suppressive effects and increased pathogen attack (Facelli & Pickett, 1991). As discussed above, most of the litter-producing exotic grasses in our system have large seeds (Fig. S5) that permit their germination and establishment under dense litter. Many of these species also germinate high proportions of their seed each season (Stevens et al., 2007), producing swards that are capable of persisting even during very dry growing seasons (temporal data not shown).
Available N was not associated with above-ground biomass in our observational dataset, nor was available N significant in models of richness, exotic species proportion and most trait CWMs and ranges. However, available N was probably underestimated because we collected soil samples at peak biomass when ammonium and nitrate had already been depleted to some extent, and in the case of nitrate leached by rainfall in the months leading up to soil sampling. Therefore N is likely to be more important than our results suggest, and this is supported by experimental data from this ecosystem showing that productivity is co-limited by P and N (S. Prober, unpublished data).
Climatic filtering of communities under low-nutrient conditions (H2a and H2b)
In unenriched locations, native species were dominant and richness increased along the regional moisture gradient, consistent with H2a and highly indicative of environmental filtering associated with moisture stress in drier locations. Trait distributions provided further evidence of filtering in accordance with H2b. Specifically, ranges of all traits expanded along the regional moisture availability gradient (but this was only mildly significant for height ranges). CWMs of all traits also shifted along this gradient, with height CWMs increasing (especially in sun-exposed situations; Fig. 4c) and SLA and seed mass CWMs decreasing (Table 2, Fig. 4). In relation to height, a number of low-statured native species were more frequently recorded in drier regions, including Velleia rosea, Bellida graminea, Pogonolepis muelleriana and Chthonocephalus pseudevax, though intraspecific height variation also contributed to the observed regional trend (not shown). Shorter plants tend to have lower energy requirements for water lift (Givnish, 1995), and even in herbaceous plant communities plant heights are known to decline with increasing moisture stress (Gross et al., 2008). The significant interaction between woody cover and moisture availability suggests that shading by woody perennial species can ameliorate moisture stress and permit taller herbaceous communities to develop in drier regions.
Moderate decreases in SLA along the moisture availability gradient are counter to observed trends in perennial species (e.g. Cornwell & Ackerly, 2009), but are not surprising for annual species that must complete their life cycles within discrete growing seasons. Higher SLA, and hence higher relative growth rates, permit annual species to reproduce sooner in dry regions where precipitation is also more variable. The finding that communities in drier regions had higher seed mass CWMs is consistent with findings from other mediterranean and semi-arid climates (Baker, 1972) and suggests greater importance of stored maternal resources for population persistence in drier, more variable regions.
It should be noted that other processes such as niche partitioning are almost certainly operating in this system. These additional processes might be detected with different analyses, or with additional traits. For example, Maire et al. (2012) used 28 traits to represent multiple axes of functional specialization in experimental grasslands and found that environmental filtering and niche partitioning act simultaneously on different axes. Even without such wide-ranging trait data, the results reported here provide indirect evidence that trade-offs in resource requirements are important for the maintenance of diversity under natural (low-nutrient) situations, because when the number of limiting resources is reduced, species are lost (Tilman, 1982; Harpole & Tilman, 2007).
Conclusions
The assembly of plant communities in this era of global change undoubtedly results from a complex interplay of processes, including environmental filtering and competitive dynamics mediated by resource limitation. In Australia's York gum woodlands, anthropogenic nutrient enrichment acts at the local scale to release communities from P limitation, and due to changes in the regional species pool, exploitative exotic species appear to be thriving at the expense of native species. However, the magnitude of these effects depends on regional moisture availability, implying that the negative impacts of eutrophication and invasion may be moderated by climate change. That said, our findings also indicate that under natural soil conditions, continued drying and warming in Australia's south-west is likely to exert a strong moisture stress filter. Given that York gum woodlands persist in only small isolated remnants, it remains unclear if species will be able to track their preferred climates. This study illustrates how the relative importance of abiotic filters and competitive interactions can shift along environmental gradients and supports theoretical expectations of community assembly in response to the addition of multiple limiting nutrients. Such empirical tests of theory are critical for enhancing our ability to predict the responses of natural communities to ongoing global change.
Acknowledgements
Thanks to Jenny Borger, Mike Griffiths and Chris Curnow at WWF for organizing access to Woodland Watch properties, the landholders for their cooperation and the local councils and the Government of Western Australia for permitting access to public reserves. We thank Bob Holt, Eric Seabloom, Elizabeth Borer, Joe Bennett and Scott Burgess for helpful discussions, and Suzanne Prober, Karel Mokany, Janneke Hille Ris Lambers, Benjamin Blonder, Deborah Goldberg and Brian Enquist for constructive comments on earlier versions of the manuscript. We also thank Delphia Manietta, Justine Gay-des-Combes, Hao Ran Lai, Caroline Oldstone-Moore, Emily Searle and Monica Radovski for field and lab assistance, Jenny Borger, Suzanne Prober and Michael Hislop for assistance with plant identification and Heather Gordon, Bec Parsons and Tim Morald for logistical support. This research was funded by an Australian Research Council grant (DP1094413) awarded to M.M.M. and R.J.H.
References
Additional references can be found at the end of Fig. S5 in the Supporting Information.
John Dwyer undertook this research as a post-doc in the labs of Professor Richard Hobbs at the University of Western Australia and Dr Margaret Mayfield at the University of Queensland, Australia. John is currently a lecturer at the University of Queensland and research scientist at CSIRO. He has strong interests in plant ecology, especially the assembly of plant communities experiencing global environmental change.




