Systemic review for the use of biochar to mitigate soil degradation
Abstract
Biochar, a black carbon material produced by high-temperature, low-oxygen pyrolysis of organic solids, can improve soil properties and realize carbon neutrality. However, how to effectively produce and apply biochar in the face of the complex soil environment and intractable widespread land degradation is still uncertain. This review is based on 1073 sets of data in 316 publications to address this issue. Firstly, the impact of different process parameters, namely feedstocks, pyrolysis temperature and activation on physicochemical properties of biochar are systematically summarized. Secondly, the effect of biochar on different soil degradation problems are reviewed from the perspective of the interaction between the physicochemical properties of biochar and soil characteristics. The “matching” of biochar properties, level of degradation and environmental factors can be used to design the desired biochar. Finally, future research should focus on biochar aging and costs and benefits of using biochar. The concept of “artificial intelligence designed biochar” is discussed to improve the degree of automation in biochar production and the predictability and suitability of its application for specific cases.
1 INTRODUCTION
Soil health plays a vital role in sustaining humans, plants, animals, and the environment (Doran, 2002). However, due to natural and anthropogenic factors, land degradation is currently spreading globally, with negative impacts on soil, including erosion, organic carbon depletion, nutrient imbalance, water deficiency, acidification, alkalinization, salinization, and pollution, which threaten ecosystem service functions. Recently, researchers in the very fertile Terra Perta of the Amazon Basin found it to contain human-burned wood carbon, ceramic carbon, crop residues, and bone residues from various animals (Marris, 2006; Sombroek et al., 2002). Evidently, black carbon was added to soil thousands of years ago, and, today, it is believed to be the main reason for the fertile soil (Chen et al., 2019).
With time, biochar was defined as a solid carbon material produced by pyrolysis of biomass under high temperature and limited oxygen (Lehmann, 2009). The International Biochar Initiative revised the definition of biochar, stating that it is a solid product obtained by thermochemical conversion of biomass under hypoxic conditions, which can be used alone or as an additive to improve soil fertility and resource utilization efficiency, mitigate environmental pollution, and reduce greenhouse gas emissions (Chen et al., 2019). This definition recognized the role of biochar in agriculture and environment improvement, differentiating its application from other carbon products. Biochar is being used widely as a multi-beneficial soil amendment to improve soil quality (Brtnicky et al., 2021; Cheng et al., 2020; He et al., 2019; Yuan & Xu, 2011) by reducing the hazards of heavy metals and organic pollutants (Peng et al., 2017 ), improving soil nutrients (Olmo et al., 2015; Prasad et al., 2018), increasing soil water content (Razzaghi et al., 2020), altering soil structure, stimulating microbial activity (Lehmann et al., 2011), and promoting crop growth. However, the use of biochar in commercial agriculture has been minimal, often focusing on quick energy gains, while often overlooking the long-term benefits of soil improvement (Chia et al., 2012; Maroušek et al., 2019).
The restoration of degraded soil by biochar depends on its specific properties, which are determined by the method in which biochar is produced. The effect of biochar was optimized by controlling pyrolysis temperature and feedstock type, and biochar with different characteristics was obtained (Das et al., 2021; Lataf et al., 2022; Tomczyk et al., 2020). Different thermochemical pathways can also be employed, such as slow, fast, vacuum, and microwave pyrolyses, and hydrothermal carbonization (Bruun et al., 2011; Carrier et al., 2011; Mašek et al., 2013). The pyrolysis processes, including temperature, heating rate, environment and residence time, influence the properties of biochar (Safarian, 2023). In addition, activation biochar can be prepared by introducing physical, chemical and/or biological treatments (Chu et al., 2018). Alternatively, a variety of functional materials such as metal oxide particles, organic matter, clay minerals, fertilizers, and microorganisms (Agegnehu et al., 2016; Awasthi et al., 2020; Chen et al., 2018; Yao et al., 2014) can be combined with biochar to produce new biochar-based materials with different properties. The production process of biochar has been developed innovatively, and the combination of different parameters, in addition to feedstock characteristics and pyrolysis temperature, has resulted in a great heterogeneity of biochar properties (Zhao et al., 2013). More comprehensive studies are warranted on the effects of various combinations of different parameters (pyrolysis methods, feedstocks, pyrolysis temperatures and activation methods) on the physicochemical properties of biochar (Aller, 2016; Tomczyk et al., 2020).
Biochar added to soil has the potential of providing a range of benefits, however, the benefits cannot always be optimized, and, inevitably, there are trade-offs (Joseph et al., 2010; Singh et al., 2023). For example, alkaline biochar can improve soil acidification (Fidel et al., 2017), but can also cause alkaline soil nutrient precipitation and reduce plant utilization of nutrients (Novak et al., 2014). To maximize the improvement effect of biochar, the appropriate biochar is critical, because biochar properties are highly heterogeneous, and different types of biochar have different effects on the soil. In addition, the spatial characteristics of the soil environment and the temporal changes of the physical and chemical properties of the biochar should be considered (Singh et al., 2022). Adding biochar to coarse grained soil improves soil hydrology (Razzaghi et al., 2020), while adding excessive biochar to clay results in very low soil water content (Castellini et al., 2015). Zhelezova et al. (2017) reported that aged soil-biochar mixtures reduced the adsorptive capacity of two herbicides (glyphosate and diquat) over time. In summary, the improvement effect of biochar on soil is dependent on the nature of biochar, the properties and the level of degradation of the soil, the local environmental conditions and the aging of the biochar (Li et al., 2023). Due to the wide range of combinations of biochar, soil, plants, degradation levels and regional environments, much research is still needed to understand the wide variety of interactions that are generated and their implications (Joseph et al., 2021).
At present, most studies on the improvement effect of biochar on soil only focus on the two factors of pyrolysis temperature and raw material type, which will limit the application conditions and reduce the effect of biochar (Lataf et al., 2022; Tomczyk et al., 2020). Some studies have verified that engineered biochar, using more treatment methods (change of pyrolysis mode, activation, combination of functional materials), has more prominent physical and chemical properties, less environmental risks and better remediation effects on soil than pristine biochar (Gusiatin & Rouhani, 2023; Mohit et al., 2024; Rostamian et al., 2015). However, engineered biochar is used mostly in environmental (water or soil) pollution remediation or energy storage research (Bao et al., 2022; Lee & Park, 2020; Liu, Lawluvy, et al., 2022; Mohit et al., 2024). There is a lack of applied research on improvement of degraded soil with multiple problems. In the face of more complex soil multiple degradation problems, it is necessary to designed biochar characteristics to mitigate the problems, control costs and improve efficiency. This paper summarizes the use of biochar to improve degraded soil from three aspects: production and properties of biochar, mechanism and effect of biochar improvement, and future research trend. This is the first review to employ a large dataset for examining the effects of feedstocks, pyrolysis temperature, and activation methods on the physicochemical properties of biochar. The characteristics of biochar prepared from different feedstocks and by different processes are listed and compared, while the effects of different biochars on soil degradation problems such as nutrient deficiency, organic carbon loss, water shortage, acidification, alkalization, salinization, heavy metal, and organic pollution, are compared and discussed. Material is presented on methods to customize “designed biochar” for the desired characteristics to achieve soil-biochar “matching”. The concept of “artificial intelligence (AI)-designed biochar” in combination with machine learning is examined.
2 EFFECTS OF DIFFERENT PROCESSES ON THE PROPERTIES OF BIOCHAR
2.1 Pyrolysis method
Pyrolysis is a common method for preparing biochar, and its products include biochar, bio-oil, and syngas. Slow pyrolysis is used commonly in the early stage, with the temperature ranging between 350 and 800°C. The heating rate is slow (3–10°C min−1), and the residence time is more than 1 h (Tomczyk et al., 2020). The advantage is the high yield of biochar, while the disadvantage is the high energy consumption. To reduce energy loss, a variety of pyrolysis techniques have been examined including fast pyrolysis, vacuum pyrolysis, microwave pyrolysis, and hydrolysis (Carrier et al., 2011; Gabhane et al., 2020; Gasim et al., 2022; Haeldermans et al., 2018; Mumme et al., 2011). These technologies reduce energy consumption cost of biochar preparation, while also producing biochar with different characteristics. Table 1 compares the physical and chemical properties of slow pyrolysis biochar and other pyrolysis types of biochar under the same conditions (same feedstock or pyrolysis temperature).
| Pyrolysis process | Feedstocks | BET (m2 g−1) | Ash content | Fixed carbon | C | Yield | pH | O/C | H/C | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | ||||||||||||
| Slow pyrolysis vs. fast pyrolysis | SP | 525°C/6°C min−1/120 min | Wheat straw | 0.6 | 19.8 | 69.6 | 34 | 10.1 | 0.08 | 0.36 | Bruun et al. (2012) | |
| FP | 525°C/250–1000°C/s | 1.6 | 21.6 | 49.3 | 36 | 6.8 | 0.36 | 0.90 | ||||
| SP | 300°C/10°C min−1/30 min | Municipal sewage sludge | 38.3 | 11.9 | 45.4 | 51.23 | 0.12 | 1.11 | Barry et al. (2019) | |||
| SP | 400°C/10°C min−1/30 min | 44.0 | 17.7 | 42.1 | 36.79 | 0.10 | 0.91 | |||||
| SP | 500°C/10°C min−1/30 min | 50.4 | 23.7 | 40.5 | 31.88 | 0.01 | 0.59 | |||||
| FP | 400°C | 53.7 | 10.7 | 29.9 | 29.60 | 0.21 | 0.96 | |||||
| FP | 500°C | 64.7 | 9.5 | 23.4 | 25.66 | 0.15 | 0.76 | |||||
| FP | 550 ± 25°C | Rice husk | 78.2 | 50.9 | 33.4 | 37.7 | 7.96 | 0.18 | 0.58 | Wang et al. (2014) | ||
| FP | 550 ± 25°C | 0.22 | 12.0 | 56.7 | 68.0 | 7.62 | 0.17 | 0.67 | ||||
| FP | 200°C | Elm sawdust | 2.2 | 46.0 | 95.1 | 0.77 | 1.56 | |||||
| FP | 300°C | 3.4 | 50.6 | 78.6 | 0.64 | 1.33 | ||||||
| FP | 400°C | 6.8 | 65.1 | 35.8 | 0.34 | 0.79 | ||||||
| FP | 500°C | 17.3 | 68.7 | 28.2 | 0.30 | 0.55 | ||||||
| FP | 600°C | 11.3 | 73.1 | 25.7 | 0.25 | 0.34 | ||||||
| Slow pyrolysis vs vacuum pyrolysis | SP | 420°C/21.3°C min−1 | Sugar cane bagasse | 29 | 13 | 32.6 | Carrier et al. (2011) | |||||
| VP | 500°C/12 kPa/12°C min−1 | 418 | 16.4 | 19.4 | ||||||||
| SP | 800°C/150 mL min−1/21.3°C min−1 | Cotton rose wood | 486 | 26.54 | Ma et al. (2021) | |||||||
| VP | 800°C/5–20 kPa/21.3°C min−1 | 355 | 28.13 | |||||||||
| SP | 350°C/20°C min−1 | Ashe juniper | 9.7 | 2.1 | 34.5 | 67.7 | 0.31 | 0.66 | Choi et al. (2017) | |||
| SP | 450°C/20°C min−1 | 10.8 | 2.3 | 44.3 | 75.9 | 0.19 | 0.55 | |||||
| SP | 520°C/20°C min−1 | 22.7 | 2.6 | 55.5 | 78.8 | 0.12 | 0.28 | |||||
| VP | 350°C/0.09 kPa/20°C min−1 | 144.7 | 5.2 | 55.7 | 73.5 | 0.24 | 0.47 | |||||
| VP | 450°C/0.09 kPa/20°C min−1 | 395.4 | 6.2 | 57.0 | 77.1 | 0.20 | 0.37 | |||||
| VP | 520°C/0.09 kPa/20°C min−1 | 516.2 | 3.7 | 62.7 | 83.9 | 0.10 | 0.27 | |||||
| VP | 520°C/0.7 kPa/20°C min−1 | 529.4 | 4.6 | 63.3 | 86.3 | 0.11 | 0.27 | |||||
| VP | 520°C/3 kPa/20°C min−1 | 560.2 | 6.1 | 71.4 | 85.8 | 0.17 | 0.46 | |||||
| Slow pyrolysis vs microwave pyrolysis | SP | 350°C/15°C min−1/30 min | Medium density fiberboard | 5.2 | 71.2 | 5.6 | 28.4 | Haeldermans et al. (2018) | ||||
| SP | 450°C/15°C min−1/30 min | 5.0 | 74.6 | 7.2 | 27.0 | |||||||
| MP | 750 W/40–80 mbar/30 min | 2.9 | 44.6 | 28.1 | 44.3 | |||||||
| MP | 1000 W/40–80 mbar/30 min | 3.0 | 47.8 | 28.1 | 44.5 | |||||||
| MP | 1250 W/40–80 mbar/30 min | 2.9 | 53.3 | 30.6 | 43.8 | |||||||
| SP | 300°C/10°C min−1/60 min | Biosolids | 4.7 | 46.16 | 58.15 | Brickler et al. (2021) | ||||||
| SP | 500°C/10°C min−1/60 min | 37.6 | 39.0 | 42.0 | ||||||||
| SP | 700°C/10°C min−1/60 min | 27.7 | 50.4 | 40.9 | ||||||||
| MP | 1320 W/60 min | 1.3 | 57.7 | 66.6 | ||||||||
| MP | 1650 W/30 min | 2.4 | 60.3 | 67.7 | ||||||||
| MP | 1320 W/60 min | 8.1 | 61.8 | 58.5 | ||||||||
| SP | 300°C/10°C min−1/60 min | Switchgrass | 2.1 | 37.5 | 37.3 | |||||||
| SP | 500°C/10°C min−1/60 min | 0.6 | 40.9 | 25.6 | ||||||||
| SP | 700°C/10°C min−1/60 min | 17.0 | 37.7 | 22.3 | ||||||||
| MP | 1320 W/60 min | 0.6 | 35.7 | 56.8 | ||||||||
| MP | 1650 W/30 min | 2.1 | 26.2 | 55.2 | ||||||||
| MP | 1320 W/60 min | 0.7 | 25.2 | 41.2 | ||||||||
| Slow pyrolysis vs. hydropyrolysis | SP | 600°C/30 min | Ensiled whole crop maize | 18.42 | 9.89 | Reibe et al. (2015) | ||||||
| SP | 850°C/30 min | Wood | 16.64 | 9.35 | ||||||||
| HP | 210°C/23 bar/640 min | Ensiled whole crop maize | 3.19 | 5.25 | ||||||||
| SP | 550°C/15 min | Corn stover | 10.8 | 74.3 | 28 | 9.89 | 0.12 | 0.44 | Fuertes et al. (2010) | |||
| HP | 250°C/4 MPa/240 min | 2.1 | 67.8 | 36 | 4.70 | 0.27 | 0.94 | |||||
| SP | 300°C/10°C min−1/60 min | Freshwater sludge | 104.67 | 54.88 | 6.08 | 15.78 | 60.26 | 1.39 | 0.11 | Zhang, Qin, et al. (2021) | ||
| SP | 500°C/10°C min−1/60 min | 109.9 | 67.2 | 5.21 | 10.9 | 47.8 | 1.67 | 0.02 | ||||
| SP | 700°C/10°C min−1/60 min | 108.9 | 81.3 | 4.57 | 1.47 | 43.0 | 0.45 | 0 | ||||
| HP | 140°C/240 min/300 mL water | 156.9 | 51.6 | 0.41 | 12.3 | 67.2 | 2.46 | 0.15 | ||||
| HP | 160°C/240 min/20 mL water | 230.7 | 56.6 | 0.56 | 10.1 | 52.3 | 2.81 | 0.17 | ||||
| HP | 180°C/240 min/20 mL water | 285.8 | 60.1 | 3.35 | 8.62 | 51.9 | 3.27 | 0.09 | ||||
| HP | 200°C/240 min/20 mL water | 197.6 | 62.8 | 1.75 | 8.63 | 51.2 | 2.97 | 0.10 | ||||
- Abbreviations: BET, Brunauer, Emmett and Teller Test Methods; FP, fast pyrolysis; HP, hydropyrolysis; MP, microwave pyrolysis; SP, slow pyrolysis; VP, vacuum pyrolysis.
Fast pyrolysis increases the heating rate at 10–200°C/s, while flash pyrolysis reaches a heating rate of greater than 1000°C/s (Gabhane et al., 2020). Fast pyrolysis produces more bio-oil and has higher market value (Frank et al., 2020). However, a very fast heating rate and a short residence time do not allow sufficient condensation time for biochar production and leads to the incomplete conversion of biomass and high levels of available carbon (volatiles) (Bruun et al., 2011). In vacuum pyrolysis, the pressure ranges between 0.05 and 0.20 MPa and the temperature ranges between 450 and 600°C (Carrier et al., 2011). Vacuum/low pressure reduces the thermal degradation of organic matter, and, unlike the gas purging technique, it reduces the vapor residence time and decreases the secondary carbonation reaction time, thus shortening the residence time of volatiles that can block pores in the secondary carbonation reaction (Ma et al., 2021). Concomitantly, removal of steam in the vacuum/low-pressure prevents the desorption of inorganic materials from the feedstock, resulting in relatively high ash content in the biochar. Compared with slow pyrolysis biochar, vacuum pyrolysis biochar has greater porosity, specific surface area, stability, and carbon yield, and a more sensitive surface to oxidants (Bardestani & Kaliaguine, 2018). Compared with slow pyrolysis, microwave pyrolysis is faster, and selective volume heating from inside to outside can achieve better pyrolysis of biomass raw materials with less energy (Abas & Ani, 2014; Haeldermans et al., 2018; Wahi et al., 2017). Compared with slow pyrolysis, microwave pyrolysis can obtain more aromatic biochar at a lower pyrolysis temperature (Gronnow et al., 2013; Haeldermans et al., 2018; Mašek et al., 2013). The pyrolysis process retains more functional groups on its surface because of the inside-out heating mode. Brickler et al. (2021) reported that microwave pyrolysis biochar has better NO3− adsorption capacity than slow-pyrolysis biochar at the same pyrolysis temperature, most likely because the surface of microwave pyrolysis biochar contains more hydrophilic functional groups. Hydrolysis places the feedstocksin a subcritical liquid water environment of 160–350°C and 2–10 MPa (Fang et al., 2018; Mumme et al., 2011). The aqueous environment reduces the activation energy required for the pyrolysis of organic matter. The decomposition temperature of hemicellulose is 180–200°C, of lignin is 80–220°C, and of cellulose is above 220°C (Bobleter, 1994). Reaction temperature, residence time, solid-to-liquid ratio and liquid acidity/alkalinity are some of the key factors that have been proposed to determine the hydrothermal digestion of biomass (Ghanim et al., 2017; Liu, Fan, et al., 2022; Saha et al., 2019).
2.2 Feedstock and pyrolysis temperature
The type of feedstocks and pyrolysis temperature are one of the key factors that determine the physicochemical properties of biochar. The raw materials of biochar can be used locally. There are many possible feedstocks for biochar, such as agricultural straw, rice husk, wood chips, waste wood, coconut husk, animal manure, municipal waste, sewage sludge and animal residues (Bednik et al., 2022; Dai et al., 2018; Gholizadeh et al., 2019; Tsai et al., 2018). The characteristics of biochar are determined by the composition of organic macromolecules in the feedstocks and can be divided into lignocellulosic and non-lignocellulosic feedstocks. Lignocellulosic feedstocks are composed mainly of three organic macromolecules: hemicellulose, cellulose and lignin (Yaashikaa et al., 2019), and can be further divided into woody biomass and agricultural waste (Ji et al., 2022). Woody biomass has high lignin content, low ash content and low calorific value (Jafri et al., 2018), and it produces biochar of low ash content, high carbon content (Figure 1) and high stability (Bednik et al., 2022). Compared with woody biomass, agricultural waste contains relatively more ash (Figure 1), and agricultural stalks as raw materials have been used to prepare silicon-rich (Si) biochar (Li & Delvaux, 2019). Non-lignocellulosic biomass, including fecal matter, municipal refuse and sewage sludge, have complex organic matter, high ash content and low calorific value. Non-lignocellulosic biomass biochar has good fertilizer value (Hossain et al., 2020), but also has great potential environmental risks due to heavy metals, antibiotics, and pathogens (Ji et al., 2022), and high pyrolysis energy consumption (Jafri et al., 2018).
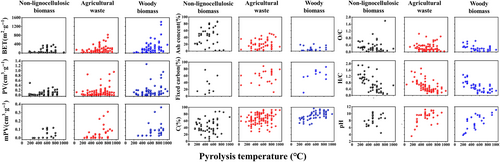
The highest pyrolysis temperature indicates the degree of pyrolysis of the organic matter in the feedstock. With the rise of pyrolysis temperature, organic macromolecules decompose gradually (Ding et al., 2014), and the specific surface area, pore volume, pH, ash content and stability of biochar increase, while the yield, H/C, O/C, cation exchange capacity (CEC) and surface oxygen-containing functional groups decrease. However, the thermal decomposition temperature of different organic macromolecules is different. For example, the pyrolysis temperature of hemicellulose ranges from 220 to 315°C, of cellulose ranges from 315 to 400°C, and of lignin ranges from 160 to 900°C (Yang et al., 2007). As a result, biochar properties of different feedstocks differ with pyrolysis temperature (Figure 1), with 400–500°C the dividing line. When the pyrolysis temperature reaches 400–500°C, cellulose and hemicellulose are completely pyrolyzed (Ding et al., 2014), and the physical structure of biochar begins to change substantially (Figure 1). Sizmur et al. (2017) used 450°C as a cut-off, with those above 450°C being high-temperature pyrolysis biochar, while those below 450°C being low-temperature pyrolysis biochar. Dai et al. (2014) concluded that the overall performance of manure biochar at 300, 400, and 500°C differed from that at 600 and 700°C. High-temperature pyrolysis biochar results in a larger specific surface area, better pore structure and carbon stability, easier carbon sequestration, and better physical adsorption of the biochar than low-temperature pyrolysis biochar. The incomplete pyrolysis of low temperature results in more functional groups and nutrient substances retained on the surface of biochar (Li et al., 2019, 2023).
2.3 Activation
Factors such as the composition of raw materials and production methods affect the properties of biochar. However, it is difficult to achieve the expected improvement effect by only adjusting these parameter (Abhishek et al., 2022; Lin et al., 2024). Therefore, different processes, called activation, are applied to modify the biochar, provide it with more functions or changes, and improve the efficacy and reduce the negative impact of the biochar. Different combinations of biochar feedstock, pyrolysis temperature and activation change biochar properties and enable more possibilities for its application (Gusiatin & Rouhani, 2023; Sajjadi et al., 2019).
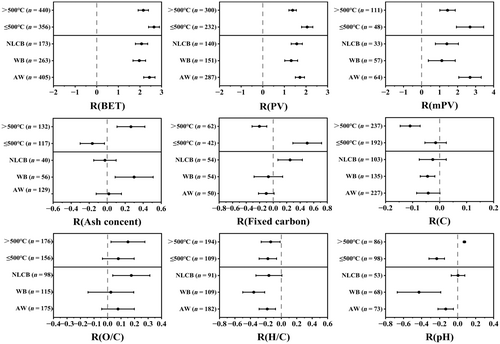
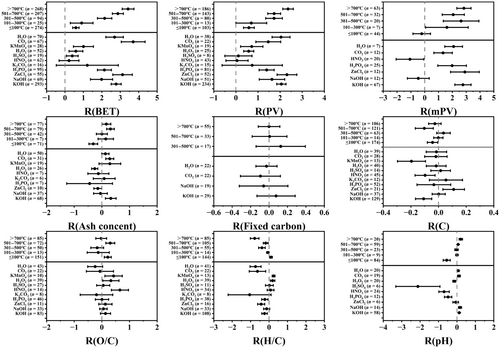
Many studies have examined the effects of activation parameters on biochar (Bao et al., 2022; Gusiatin & Rouhani, 2023; Mohit et al., 2024), but few have examined whether the parameters in the initial biochar (feedstock and pyrolysis temperature) affects the changes of physicochemical properties of biochar by activation. This paper analyzes the response of initial biochar to activation from three types of raw material (agricultural waste, woody biomass and non-lignocellulosic lignin biomass) and two pyrolysis temperatures (low temperature: ≤500°C and high temperature: >500°C). Activation alters the properties of pristine biochar, but the magnitude and direction of the changes differ with different feedstocks and pyrolysis temperatures (Figure 2). For example, activation increases the specific surface area, pore volume, and micropore volume of biochar more at low-temperature than at high-temperature pyrolysis. Activation increases the micropore volume of agricultural waste biochar more than in wood and non-lignocellulosic biomass biochars; increases the ash content of wood biochar, but not of agricultural waste and non-lignocellulosic biomass biochars; decreases the pH of lignocellulosic biomass biochar, but has no effect on non-lignocellulosic biomass biochar; and increases the fixed carbon content of non-lignocellulosic biomass biochar. Some physical and chemical properties of pristine biochar at different pyrolysis temperatures are altered in different directions due to activation (Figure 3). For example, activation reduces ash content and pH of low-temperature pyrolysis biochar and increases fixed carbon content; however, the effects are in the opposite directions with high-temperature pyrolysis biochar (Figure 2). These differences may be related to the initial properties of the pristine biochar, and the type and amount of organic macromolecules and minerals involved in the activation reaction. As illustrated in Figure 3, differences in the effects of activation parameters (activation temperature and activation reagents) on the physicochemical properties of biochar are usually divided into low temperature activation (≤100°C) and high temperature activation (>300°C) (Park et al., 2015; Wang & Kaskel, 2012). Low temperature activation is the impregnation of biochar in some activating reagent solution, and its physicochemical properties depends mainly on the chemical characteristics of the activation reagent. The activation reagents are classified into alkalization (NaOH, KOH, NH4OH), acidification (HCl, HNO3, H2SO4, H3PO4 and organic acidifiers) and oxidation (H2O2 and KMnO4) (Sajjadi et al., 2019). As a relatively mild modification method, low-temperature activation can effectively change the specific surface area, ash content, surface functional groups and pH (Figure 3). High-temperature activation provides sufficient activation energy to induce a thermochemical reaction between the activating reagent and the substrate (Table S1). The temperature ranges between 400 and 1200°C (Wang & Kaskel, 2012), and, depending on the type of activation reagent, it can be divided into physical high temperature activation and chemical high temperature activation. Physical activation commonly uses reactive gases, including CO2, H2O, oxygen, air, ammonia and their mixtures, while the reagents for chemical activation include KOH, K2CO3, NaOH, Na2CO3, AlCl3, ZnCl2, MgCl2, and H3PO4 (Feng et al., 2018; Sajjadi et al., 2019). The temperature of chemical high-temperature activation ranges between 400 and 900°C and of physical high-temperature activation ranges between 600 and 1200°C (Wang & Kaskel, 2012). High temperature activation occurs mainly through the reaction of chemical agents with the carbon structure in biochar, corrosion of biochar matrix and production of volatile gases, resulting in increased pore volume and specific surface area of the biochar (Wang et al., 2021). The activation reagent can react with the carbon structure in the biochar only at a sufficiently high temperature (Table S1). Anthonysamy et al. (2021) concluded that the decomposition reaction of KOH and K2CO3 occurred above the activation temperature of 600°C. When the temperature exceeded 650°C, the reduction reaction between K2CO3 and K2O occurred and mainly carbon was produced (Chen et al., 2020). The Boudouard reaction temperature needs to exceed 710°C. The specific surface area of the pristine biochar increased when CO2 activated at 750°C, but decreased when CO2 activated at 650°C (He et al., 2021). Activation at low temperatures can open the pores and increase the specific surface area of biochar by cleaning ash impurities and corrosive materials in the pores (Zhang, Zhu, et al., 2021), but the increase of oxygen-containing functional groups on the surface can decrease the specific surface area by blocking the pores due to oxidation (Saini et al., 2022; Shahib et al., 2022). When the pristine biochar was modified with 30% H2O2 by Pradhan and Sandle (1999), the specific surface area decreased by 9.2%, but by Yakout et al. (2015), the specific surface area increased by 55%. Therefore, the change in physical parameters such as specific surface area, pore volume and micropore volume of biochar depends not only on the activation reagent, but also on other factors, such as temperature activation and feedstock. Because KOH, NaOH, ZnCl2, CO2 and H2O are used mainly for high temperature activation, their effect is generally greater than other activation reagents (Figure 3).
Activation also alters the composition of biochar (Figure 3). Most of the inorganic salts in biochar, such as oxides and salts of Ca, Mg, Mn, Fe, Pb, Cd, Ni and Cr, can be removed by concentrated acids, while Si-O and Al can be converted to soluble SiO32− and AlO2−, respectively, with strong alkaline solutions (Tang et al., 2018; Ibrahim et al., 2022). Low temperature activation reduces ash content; whereas, high temperature activation increases ash content of biochar. Jiang et al. (2019) concluded that during the preparation of straw biochar activated by alkaline reagents at high temperature, NaOH and KOH reacted with mineral elements in straw to form metallic alumino-silicate compounds, which increased the ash content of biochar. The effect of activation on the fixed carbon content in biochar is uncertain (Figure 3); however, high temperature activation can increase the stability of biochar. Co-synthesis of K2CO3 with sewage sludge and corn stover produced CaO, CaCO3 and aluminosilicates during activation, which increased the resistance of biochar to degradation and altered the heavy metals into a more stable form, thus reducing its leachability (Wang et al., 2021). H3PO4 reacts with biomass carbon at high temperatures to form stable substances, such as C-O-PO3 and (CO)2PO2 as a protective layer, thus retaining more carbon and limiting its oxidation (Rosas et al., 2012). O/C in biochar represents the oxygen-containing functional groups on the surface of biochar. Most reagents in low temperature activation oxidize. HNO3 low temperature activation resulted in an increase of carboxyl and carbonyl groups, and with H2O2 resulted in an increase of hydroxyl groups (Guo et al., 2013), while with H3PO4 resulted in the esterification of -OH and -COOH groups on the biochar (Sahin et al., 2017). Coconut fiber biochar modified with HNO3 led to an increase in the number of weakly acidic lactone groups and carboxylic acids (Wu et al., 2017). Oxalic acid is used to esterify hydroxyl functional groups to enhance carboxylic acid groups. In addition, surface modification of biochar with HNO3, H2SO4, and H3PO4 promotes nitro, sulfate, and phosphate groups into biochar, respectively (Sahin et al., 2017; Vaughn et al., 2017). When the activation temperature is ≤100°C or when KMnO4, H2O2 and HNO3 are used as activation agents, there is an increase in the O/C of biochar (Figure 3). High temperature leads to the reduction of oxygen-containing functional groups, while different activation reagents introduce different types of functional groups to the biochar with high temperature activation. Reactions between KOH, oxygenates and carbon fragments create a large number of vacancies in the biochar. The OH- from KOH occupies these vacancies rapidly and forms new oxygen-containing groups such as C=O, -OH, C-O, O-C=O, and -COOH (Chen et al., 2020). The H/C ratio represents the aromatics of biochar, and the smaller the H/C ratio, the stronger the aromatic structure. High temperature activation increases the aromaticity of biochar, as with an increase in temperature, H/C and O/C decrease due to the thermal conversion of organic matter into carbonated organic matter and the formation of aromatic rings containing condensed carbon atoms (Magid et al., 2021). Low temperature activation reduces the pH of biochar under the dual action of increasing surface acidic oxygen-containing functional groups and removing ash (Figure 3). The acidic reagents HNO3, H2SO4, and H3PO4 with dual action have a greater reduction effect than H2O2 with single action, which increases the surface acidic oxygen-containing functional groups. High temperature activation leads to a decrease in surface acid functional groups and an increase in ash content, which increases the pH of biochar (Dou et al., 2022). Biochar activated by KOH at 500°C contained the least number of basic groups and the greatest concentration of acidic groups and, at 800°C, the number of basic groups increased by 2 to 3 times (Lü et al., 2022).
2.4 Functional material binding
Biochar composites are combined with other functional materials by extrusion, agitation, co-pyrolysis, electrolysis, ball milling, inoculation and other means (Shan et al., 2016; Tang et al., 2018; Tran et al., 2022; Zhang et al., 2019; Wang, et al., 2019). Functional materials can compensate for some shortcomings in the application of biochar in soil improvement. Biochar can play a role in soil as a platform supporting functional material (Figure 4), with metal/metal oxide biochar-based materials as the most common composite materials. The main reason for preparing metal oxide biochar-based composites is because of the poor anion adsorption capacity of pristine biochar. The biochar surface contains a large number of phenol and carboxylic acid groups, while fulvic acid and humic acid cause a negatively charged surface, which is not conducive to the adsorption of anions (Agegnehu et al., 2016). Metal/metal oxide biochar-based composites rely mainly on the precipitation, complexation and electrostatics of metal oxides to trap anions, while porous biochar acts as an intercalation platform to increase the contact area between metal oxides and anions (Jung & Ahn, 2016; Long et al., 2019). The developed MgO biochar can absorb a large amount of phosphate ions that can act as a phosphate slow-release fertilizer (Chen et al., 2018). Zhang et al. (2020) reported that the soil CEC increased by 9.4%–164.1% after applying nano-Fe3O4-modified biochar, which promoted the formation of iron spots and enhanced root retention of Cd, while soil Cd availability decreased by 6.8%–25.0%. The attachment of zero-valent iron (nZVI) enhanced the reducibility of biochar, which reduced the more toxic As (V) to the less toxic As (III) (Zhou et al., 2014).
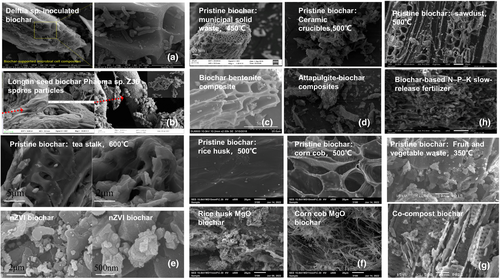
Biochar can combine with fertilizer to form biochar-based slow-release fertilizer, which can increase the supply of nutrients and compensate for the shortcomings of rapid release in common fertilizers (Table S2). Common methods to combine biochar with chemical or organic fertilizers include blending (Zheng et al., 2017), adsorption (Khan et al., 2007), reaction (El Sharkawi et al., 2018) and coating (Chen et al., 2018). The organic mineral biochar mimics the highly fertile Amazonian black soil (Cheng et al., 2020). A new slow-release fertilizer, known as enriched biochar, is prepared by mixing biochar, acid, clay, minerals, and organic nutrients and heated at low temperature (Chia et al., 2014; Farrar et al., 2018; Joseph et al., 2015). The addition of biochar during composting can improve the compost by promoting compost maturation, increasing microbial diversity and activity (Wei et al., 2014), and reducing nutrient losses (Agyarko-Mintah et al., 2017), greenhouse gas emissions, pathogens (Czekala et al., 2016) and pollutants (Oleszczuk et al., 2014). Concomitantly, the physicochemical properties of biochar are subject to change; due to the blockage of composting, the surface area of biochar decreases, while, due to oxidation that occurs during composting, the surface oxygen-containing functional groups increase and form an organic mineral coating on the surface (Hagemann et al., 2017). Various clay biochar materials, such as chitosan/clay/biochar, bamboo-derived montmorillonite biochar composites, bentonite, and potato stem-derived intaglio biochar, have been developed (Ahmad et al., 2017; Chen et al., 2017; Ismadji et al., 2016; Lu et al., 2020) (Figure 4). The clay-biochar not only increases the adsorption of biochar and possesses certain nutrient properties, but the adhesion of clay also enhances the stability of biochar (Ahmad et al., 2017). Li et al. (2012) reported that the combined modification of clay minerals can improve the redox activity of biochar, increase CEC, enhance the biological and abiotic reactions of biochar and soil, and promote soil nutrient cycling and plant nutrient absorption. The clay biochar is used as a mineral fertilizer or combined with common fertilizer to prepare enriched biochar (Chia et al., 2014). The temperature of biochar and clay co-pyrolysis should not be too high as too high a temperature can break the spatial structure of some clays and reduce the CEC of clay (Rawal et al., 2016). In addition, it should be noted that the high ash content of clay biochar can have adverse effects when applied in excessive amounts (Xiang et al., 2021). In general, clay biochar composites are efficient, low-cost, versatile and resource-stable soil ameliorants.
Microbial inoculated biochar is an emerging biochar composite, as it can alleviate the low survival and growth of exogenous functional microorganisms inoculated into soil (Azeem et al., 2021; Zheng et al., 2019). Inoculating microorganisms into biochar improves the environmental tolerance and storage stability of microorganisms (Liu, Tie, et al., 2020). As a carrier, biochar possesses good water holding capacity and permeability, while the complex pore structure provides a suitable habitat for microorganisms and promotes their activities (Joseph et al., 2018). The small amount of available carbon sources and mineral elements provide energy for microorganisms and sustain their survival and growth (Awasthi et al., 2020). The chemical properties of biochar, particularly nitrogen and pH, are important for the survival of the initial microorganisms. However, once incorporated into the soil, physical characteristics including surface area, pore diameter and water-filled pores are more important to their survival (Hale et al., 2015). Lehmann et al. (2011) determined that the most suitable biochar pore size for microbial inoculation is 2–4 mm, while adhesion may be reduced in larger and smaller pores. Research on soil remediation with biochar inoculated with microorganisms is still in its infancy; however, the immobilization of heavy metals (Liu, Feng, et al., 2020), degradation of organic pollution and promotion of soil nutrient supply (Zheng et al., 2019) have reported positive results. Biochar can further enhance the ameliorative efficacy of functional microorganisms. The interaction between thermophilic microorganisms and biochar acting together on compost accelerated soil organic carbon (SOC) degradation resulting in greater bacterial abundance and diversity than single microbial inoculation (Sun et al., 2021). Furthermore, combining rhizobia with appropriate levels of biochar could improve the nitrogen fixing effect of rhizobia (Ahmad et al., 2015).
3 REMEDIATION OF DEGRADED SOIL BY BIOCHAR
In the face of different soil degradation problems, the improvement effects of biochars with different characteristics are different (Table 2). In the following sections, we describe the reported effects of biochar on soil organic carbon deficiency, lack of nutrients, water shortage, acidification, alkalization, salinization, heavy metal pollution and organic pollutants. The proven or potential remediation mechanisms of biochar for different degradation problems are also discussed.
| Feedstock | Pyrolysis temperature (°C) | Modified method bonding material | Effects on soil | References |
|---|---|---|---|---|
| Wood chips | 750 | Compost | Improves plant water status and plant ion (K+, Mg2+, and Ca2+) contents | Abideen et al. (2020) |
| Poultry manure | 300 | HNO3 HTA | Increases water-soluble P, K, Ca, Mg, Fe, Zn, Cu and Mn concentrations and plant-available nutrients | Sahin et al. (2017) |
| H3PO4 HTA | ||||
| HNO3 + H3PO4 HTA | ||||
| Reed straw | 900 | CaO/MgO/shell powder | Three mineral loaded biochars decrease the leaching amount of phosphate-P | Cui et al. (2021) |
| Peanut | 600 | MgO | Increases soil available P content and results in higher rice yields in field experiments in saline soil | Wu et al. (2019) |
| Soybean straw | Inorganic phosphate solubilizing bacterial community | Improves rape growth and phosphate uptake | Zheng et al. (2019) | |
|
Bamboo Pig manure Cow manure |
500 | De-ashing | Enhances soil N retention, higher bacterial colonization | Ibrahim et al. (2020) |
| Rice straw | 200 | Fe-montmorillonite combination | Improves stability of biochar | Lu et al. (2020) |
| 300 | ||||
| 400 | ||||
| 500 | ||||
| Pig manure/peach shell | 700 | H3PO4 HTA | H3PO4-modified biochar has been found to have a higher impact on water retention as compared to KOH-modified biochar | An et al. (2020) |
| KOH HTA | ||||
| Sunflower husk | 650 | Causes a 30% increase in the available water content | Gluba et al. (2021) | |
| Maize straw | 700 | Ball-milling Phosphorus LTA | Decreases soil pH and alkalinity, enhances organic carbon content, cation exchange capacity, soil nutrients (e.g., N, P, K), and soil enzyme activities | Zhang et al. (2022) |
| Rice straw | 300 | H2O2 LTA | Increases soil pH and soil pH buffering capacity | He et al. (2022) |
| HNO3/H2SO4 LTA | ||||
| Compost combination | Available nutrients and sequestered C | Oldfield et al. (2018) | ||
| Chicken-litter, barley-straw and iron oxide. Mist with water, homogeneity and sundry | High concentrations of exchangeable cations, available phosphorus and high acid neutralizing capacity | Chia et al. (2014) | ||
| Rice husk | 700 | Si particles combination | Decreases As(III) concentration in the rice rhizosphere by 76% and 73% | Herath et al. (2020) |
| Montmorillonite combination | ||||
| Rice straw | 300 | Fe-oxyhydroxy sulfate combination | Immobilizes As | Wu et al. (2018) |
| Corn residue | 550 | Fe2O3 combination | Immobilizes Cd, increases soil pH, the cation exchange capacity of soil and soil organic matter content | Moradi and Karimi (2021) |
| Corn stalks | 500 | Gram-negative bacterium Delftia sp. B9 combination | Higher remediation Cd efficiency | Liu, Tie, et al. (2020) |
| Tea waste | 500 | HNO3/H2SO4 LTA | The dry weight of onion is increased, and the absorption of Pb by the root is reduced by 8.3 times | Peiris et al. (2022) |
| Apple tree | 500 | Glucose combination | Enhances reduction of Fe (III) in soil | Jia et al. (2018) |
| Bacillus Zhanjiangensis strain TJTB48, Bacillus Pseudofirmus TJTB58 and Oceanobacillus kimchii TJTB66 | Promotes degradation of cypermethrin in soil | Liu, Ding, et al. (2017) | ||
| Corn stalks | 600 | MnO/Fe2O3 combination | Better bioavailability of phthalate esters | Liu, Feng, et al., 20202020 |
- Abbreviations: HTA, high temperature activation; LTA, low temperature activation.
3.1 Organic carbon deficiency
Biochar with high carbon content can increase soil organic carbon content, which is especially important in poor soil. The organic carbon pool of biochar is divided into labile and stable organic carbon. The labile organic carbon is mineralized rapidly in the short term, while the remaining stable organic carbon is mineralized at an extremely slow rate. The direct input of biochar to soil organic carbon depends on the stable organic carbon content. The raw materials selected for the preparation of highly stable biochar should be of the lignocellulosic type. To maintain carbon stability in biochar, the preparation process should avoid procedures that result in the incomplete pyrolysis of the feedstock, such as low temperature pyrolysis, fast pyrolysis or hydrolysis (Bruun et al., 2012; Li et al., 2019; Malghani et al., 2015; Song et al., 2020). Ash content also contributes to improving the stability of biochar. In 36 samples, a negative correlation emerged between the ash content of biochar and the carbon content of biochar oxidized by hydrogen oxide (H2O2). The ash content explained 60% of the variation of biochar resistance to H2O2 oxidation (Brtnicky et al., 2021). In addition, modifications such as high-temperature activation of KOH (Xiao et al., 2018), high-temperature activation of phosphoric acid (Zhao et al., 2014), synergistic pyrolysis of metal compounds (Fu et al., 2019) and clay mineral binding can further improve the stability of biochar. Biochar stability is also affected by some characteristics of the soil, such as soil active mineral content, pH, temperature, and moisture (Fang et al., 2015; Yang et al., 2018).
3.2 Lack of soil nutrients
Biochar can improve soil fertility by providing nutrients such as organic carbon, nitrogen, phosphorus, and potassium (Gul & Whalen, 2016; Kim et al., 2014). The direct supply of mineral elements from biochar are usually in the form of phosphate, carbonate, silicate, or alkaline earth metal oxides (Kim et al., 2018; Xu et al., 2017), and, during pyrolysis, most are retained. During application, the soluble mineral element fraction of biochar is released rapidly and absorbed by the plant, while the other fraction is stored in the biochar. Limwikran et al. (2018) determined the dissolved mineral content of biochar after 8 weeks in tropical soils; 0%–75% of K and P were dissolved and released into the soil, while most of the Ca was insoluble. Compared to conventional fertilizers, biochar has a greater soil nutrient retention capacity. The element content of biochar can be increased by preparing raw materials with different elemental characteristics such as feces, algae, and animal residues (Bird et al., 2011; Rathnayake et al., 2023), or biochar-based fertilizers (Table S3), and the increase in available elements promotes the absorption and utilization by plants. The content of mineral elements in biochar increases with an increase in pyrolysis temperature, but the minerals crystallize and their solubility decreases, especially above 500°C. Some studies reported that high-temperature activation modification improves the utilization of mineral elements. KOH high-temperature activation promoted the decomposition of phytoliths in plant feedstock and increased the content of available silica (Wang, Wang, et al., 2018), and after pyrolysis modification of corn stover with nitric acid, phosphoric acid, or a combination of nitric acid and phosphoric acid, water-soluble P, K, Ca, Mg, Fe, Zn, Cu, and Mn increased (Sahin et al., 2017).
Biochar, through its unique properties, alters the geochemical cycle of soil elements, thereby allowing more soil nutrients to be retained in the soil or to be used for plant growth (Olmo et al., 2015; Prasad et al., 2018). Biochar increases the interaction between soil organic matter and minerals, which protects soil organic carbon and increases soil nutrient content (Weng et al., 2018). Biochar reduces leaching of NH4+, NO3− and PO3− from soils (Park et al., 2015; Yin et al., 2018). For example, biochar adsorbs phosphate by surface precipitation, ligand exchange and electrostatic adsorption to reduce leaching. Shepherd et al. (2017) suggested that phosphorus adsorption by biochar depends mainly on surface mineral phases rather than surface functional groups. The modification treatment of metal oxides can improve the adsorption of phosphate ions on biochar, especially the attachment of MgO (Zhang et al., 2012). Table S2 presents the maximum adsorption capacity of biochar with different characteristics for the three nutrients. The “electron shuttle” reduction of biochar inhibits denitrification in soil and reduces nitrous oxide (N2O) emission to the atmosphere (Yuan et al., 2019). Biochar promotes solid phase phosphorus dissolution in soil (He et al., 2014) and improves the ability of roots to absorb nutrients (Xiang et al., 2017), while Fe attachment (Zhou et al., 2014) and KOH high-temperature (Lü et al., 2022) improves the reducibility of biochar. In addition, biochar alters the physical and chemical properties of the soil (soil texture, CEC, pH and water content) and improves soil nutrients (Fang et al., 2015; Yang et al., 2018).
3.3 Water shortage
Biochar has positive effects on some hydraulic properties of the soil such as water availability, saturated water content, water holding capacity, soil moisture absorption coefficient and transpiration (Omondi et al., 2016; Qian et al., 2020); however, in some cases, it has little or even a negative effect (Hardie et al., 2014). The effect of biochar on soil moisture depends mainly on the physicochemical properties of the biochar, application rate and soil texture (Table S4). Biochar exerts its unique properties, namely, specific surface area, pore volume, particle size, surface oxygen-containing functional groups, hydrophobicity, and organic carbon content to improve the water retention capacity of soil. There are three main mechanisms by which biochar affects soil water content: (1) alters the physical structure of soil. The internal porosity of biochar particles and the formation of new pore structures between biochar and soil can alter the soil structure and, thus, increase the water content capacity of soil. The greater the porosity of biochar, the greater the effect (Liu, Dugan, et al., 2017). Alghamdi et al. (2020) concluded that biochar with particle size less than 1 mm could enhance the physical and hydrological properties of light soils and improve the water retention capacity of soils. (2) hydrophilicity/hydrophobicity. The hydrophobicity of biochar is related to the content of alkyl and residual aliphatic compounds in the pores of biochar, specific surface area and ash content (Gray et al., 2014; Kinney et al., 2012; Mao et al., 2019), and it can change the water repellency of the soil (Mao et al., 2019). It can be improved by various methods, such as low temperature pyrolysis, low temperature oxidation and formation of organic mineral coatings (Duan et al., 2021; Suliman et al., 2017). Oxygenated functional groups on the biochar surface can act as water absorption nuclei and promote the formation of water clusters on the biochar surface (Cybulak et al., 2016; Wang, Gao, et al., 2018). A correlation between total oxygenated functional groups and soil water content emerged at different basal potentials (Suliman et al., 2017); and (3) promotes better soil structure between biochar and soil particles and changes soil water retention capacity. Biochar particles with fine size, large specific surface area and high organic carbon content can be the main binder of soil aggregates (Li et al., 2015), which wraps the surface of soil particles in the form of a gel film and stabilizes water-stable aggregates. Acidified biochar increases O/C and (O+N)/C values and hydrophilicity, and promotes soil water-stable macroaggregates to a greater extent than pristine biochar (Duan et al., 2021). The amount of biochar applied is an important factor affecting the effectiveness of biochar in improving soil water holding capacity. Application of 2%, 5% and 10% (w/w) biochar to the soil increased saturated water content and field capacity and increased plant available water content by 33%, 42% and 50%, respectively (Qian et al., 2020). An application rate of 2%–5% is recommended, as a high dose increases soil porosity greatly, resulting in a decrease of soil water potential, and the availability of water (Qian et al., 2020). Soil characteristics, such as particle size, can affect the effectiveness of biochar on soil hydropower remediation. Biochar increased relative plant water availability by 45%, 21%, and 14% in coarse-, medium-, and fine-textured soils, respectively (Razzaghi et al., 2020). Coarse-textured soils (especially sandy soils) benefit from the addition of highly porous biochar (Mohamed et al., 2016). Mao et al. (2019) reported that strongly hydrophobic biochar affected the water repellency of hydrophilic soils with low organic carbon content, but not with high organic carbon content (Mao et al., 2019), while Gao et al. (2020) reported that soil pH affected remediation of biochar, and emphasized the need to match biochar pH and soil pH.
3.4 Acidification
Biochar can alleviate soil acidification (Table S5), as the impact of biochar improves with an increase in soil acidity (Tusar et al., 2023). For example, biochar: (1) raises soil pH; (2) improves soil pH buffering capacity to prevent re-acidification; and (3) reduces the toxicity of aluminum in acidic soils. However, not all biochars can raise soil pH. Yuan and Xu (2011) reported that the alkalinity of biochar is more relevant to the remediation of acidic soil than the pH of biochar. The alkalinity of biochar is derived from the four basic molecules of biochar, namely, surface organic functional groups, soluble organic compounds, carbonates (bicarbonates and carbonates), and other inorganic bases (oxides, hydroxides, sulfates, sulfides, and orthophosphates) (Dai et al., 2017). In biochar prepared by pyrolysis at 300–500°C, the surface COO- and O- contribute greatly to the alkalinity of the biochar (Yuan et al., 2011). In addition, oxygenated functional groups and phosphate ions in biochar undergo ligand exchange reactions with Al-OH and Fe-OH in soil, releasing OH- and raising the soil pH (Eduah et al., 2020). There is a strong correlation between total base cations and alkalinity, especially in lignocellulosic biochar, where inorganic bases account for the majority of total alkalinity (Fidel et al., 2017). In terms of feedstock, biochar prepared from beans had a higher alkalinity than from grain and was more effective in reducing soil acidity (Yuan & Xu, 2011).
Compared to treatments such as lime and Ca(OH)2 to increase soil pH, biochar also increases the pH buffering capacity of soil and retards soil re-acidification (Shi et al., 2020). CEC is one of the key physicochemical traits that determines the ability of biochar to alter soil pH buffering capacity, while the protonation-antiprotonation process of oxygen-containing functional groups on the surface of biochar is the main mechanism in altering the soil pH buffering capacity (Xu et al., 2012). Carboxyl groups are the primary exchange sites for cation adsorption or desorption and play an important role in altering soil pH buffering capacity (Chen et al., 2015). Organic mineral biochar has a better capacity to neutralize acid than pristine biochar when applied to soil (Chia et al., 2014). Kopittke and Blamey (2016) reported a 1000-fold increase in the activity of the toxic aluminum species Al3+ for every unit decrease in soil pH. During soil re-acidification, application of biochar reduced the concentration of active Al and altered the distribution of Al speciation in the soil solution (Shi et al., 2020). Biochar mitigated the phytotoxicity of aluminum through adsorption, precipitation, complexation, and cation exchange (Qian et al., 2013).
3.5 Alkalinization
Although most biochars are alkaline, some lower soil pH (Table S5). Liu, Feng, et al. (2020) concluded that one of the reasons for the decrease in soil pH was that biochar promoted the conversion of Na2CO3 and NaHCO3 to the neutral calcium salts CaCO3 and Ca(HCO3)2. The reduction of soda salts and the replacement of exchangeable calcium lowered the pH of alkaline soils. Another reason was due to the acidity produced by oxidative decomposition of soil organic matter. Zhang, Riaz, et al. (2019) reported that biochar with a pH lower than soil pH did not affect or reduce the pH. Typically, low temperature pyrolysis, hydrolysis, acidification, oxidation, aging and acid binding are used to produce acidic biochar for alkaline soils (Ippolito et al., 2016; Sahin et al., 2017). Acidic biochar with a pH of 5.8 was prepared by high temperature steam activation and when applied to an eroded calcareous soil, reduced the soil pH by 0.2–0.4 units (Ippolito et al., 2016). Although biochar is relatively ineffective in reducing pH in alkaline soils, there are controversies on the feasibility of applying biochar to alkaline soils and whether it can have positive effects (Yang et al., 2019).
3.6 Salinization
Most salts occurring in saline soils consist of four cations, Ca2+, Mg2+, K+ and Na+, and four anions, CO3−, HCO3−, Cl−, and SO42−. High Na content is one of the main characteristics of soil salinization (sodium adsorption >13%, exchangeable sodium percentage (ESP) >15%, and electric conductivity (EC) >1.5 dS/m) (Yu et al., 2019). Biochar can adsorb soil Na+ and reduce salinity by two mechanisms: (1) changing the exchangeable calcium content of the soil. Exchangeable calcium replaces sodium in the soil colloid and reduces ESP (Saifullah et al., 2018), and it was reported that high calcium content biochar reduced salinity (Lashari et al., 2013). To reduce soil salinization, Saifullah et al. (2018) prepared a designed high calcium biochar by controlling pyrolysis temperature, selecting feedstocks with more Ca and less Na, and modifying it with salt and acid; and (2) increasing the adsorbability of the sodium ion by increasing the specific surface area and surface functional groups of biochar. KOH high-temperature activated biochar with a large specific surface area has a very high Na+ adsorption capacity and can be used as an agent to reduce soil salinization (Rostamian et al., 2015). Mehmood et al. (2020) improved biochar with chitosan, introduced non-aromatic and nitrogen-containing functional group chitosan on the surface of biochar, and improved the polarity of biochar, which enhanced the adsorption capacity for sodium salt. H2SO4 acidification treatment enhanced the sodium adsorption capacity of biochar and decreased the salinity and the water-soluble Na+ content of soil (Duan et al., 2021). Lashari et al. (2015) also reported that acidified modified biochar reduced pH and ESP in saline soils, while increasing the CEC and organic carbon content of the biochar. Luo et al. (2016) verified that changing the CEC and organic matter content of biochar to improve the exchangeability for Na+ can also reduce the ESP of saline soil. Some initial biochars have a high ash content, and their applications could have negative effects on saline soil. Studies have reported that high application of biochar can lead to an increase in soil salinity (Mumme et al., 2018; Rajkovich et al., 2011), and, therefore, it is recommended to apply designed biochar with specific characteristics to optimize the effects.
3.7 Contaminants
3.7.1 Organic contaminants
Biochar reduces some organic pollutants such as polycyclic aromatic hydrocarbons (PAHs), antibiotics/drugs, agrochemicals, such as herbicides and pesticides, and aromatic cationic dyes, in soil mainly through adsorption and accelerated degradation (Mandal et al., 2021; Xiong & Bi, 2023). Faced with organic pollutants differing in relative molecular weight, molecular size, surface charge, functional groups, polarity/nonpolarity, hydrophobicity/hydrophilicity, and mobility, biochar relies on different physicochemical properties for adsorption mechanisms (Peiris et al., 2017; Taheran et al., 2016). The bioavailability of organic pollutants in soil can be reduced by improving the adsorption and immobilization capacity of biochar for organic pollutants (see Table S3). However, organic pollutants remaining in the soil persist for a long time, as the adsorption by biochar of some organic pollutants weakens with time (Ren et al., 2018). Biochar promotes the degradation of organic pollutants in soil indirectly by altering soil pH, albedo and aeration (Qiu et al., 2009; Zhu et al., 2017). Zhang et al. (2013) reported that biochar accelerated the HP of organophosphorus and carbamate pesticides in soil through an alkali-catalyzed mechanism. Biochar can also be considered as a “co-metabolite” (Dalton & Stirling, 1982), as application of biochar together with some nutrients can stimulate soil microbial activity and promote microbial utilization of organic pollutants (Lehmann et al., 2011; Qiu et al., 2009). The adsorption by biochar reduces the utilization of organic pollutants by soil microorganisms and inhibits the degradation of organic matter (Beesley et al., 2011). Modification can avoid this negative effect, as a biofilm-biochar can promote the biodegradation of organic pollutants (Frankel et al., 2016). Cypermethrin-degrading bacteria were inoculated onto biochar and applied to cypermethrin-contaminated soil. The inoculated biochar had the fastest degradation rate, the fastest removal rate, and the shortest degradation half-life of cypermethrin among all treatments (Liu, Ding, et al., 2017). In addition, solid materials that promote degradation such as compost, clay, and metal oxide were also effective.
3.7.2 Heavy metals
Biochar immobilizes some heavy metals in soil mainly by adsorption, redox and the ability to change the physicochemical properties of soil (Beesley et al., 2011; Cheng et al., 2020; He et al., 2019). Biochar can adsorb heavy metals through specific and non-specific adsorption mechanisms such as complexation, electrostatic adsorption, ion exchange and precipitation, and physical adsorption (Gholizadeh & Hu, 2021; Herath et al., 2020). Biochar can also affect heavy metal mobility by altering the soil properties such as dissolved organic carbon (DOC), pH, and redox. The application of composted biochar resulted in a decrease in Zn and Cd in soil pore water, a more than 30-fold increase in Cu and As concentrations, and an increase in soil DOC and pH (Beesley et al., 2011). An increase in soil pH can increase the availability of pH-dependent cation exchange sites (Gomez-Eyles et al., 2011), can lead to the precipitation of low-soluble minerals (Liang et al., 2006), and can enhance the movement of heavy metals in oxygen ions, such as As (Zhang et al., 2013). Heavy metals such as Cr and As are present in soils in multivalent forms and the toxicity and mobility of heavy metals with different valence levels differ (Choppala et al., 2016; Wu et al., 2018). For example, the toxicity and mobility of Cr(VI) at higher valence in soil are greater than those of Cr(III) at lower valence (Ding et al., 2021), but the toxicity and mobility of As are opposite to those of Cr (Xu et al., 2019; Yin et al., 2017). Biochar affects the fate of polyvalent heavy metal ions in soil by altering the redox conditions through its acidic functional groups, pH, DOC, iron, manganese, and sulfur (Beiyuan et al., 2017; Choppala et al., 2012). In acidic soils, chitosan/zero-valent iron modified biochar reduced hexavalent chromium in soil by up to 55%, compared to the 29% of manure biochar, and reduced the toxicity and mobility of Cr in soil (Mandal et al., 2017). However, biochar can increase the mobility and toxicity of arsenic in soil (Choppala et al., 2016). Depending on the mechanism action on different heavy metals, suitable modification methods can be employed on biochar to maximize the fixation of heavy metals in soils (Table S3).
4 CONCLUSIONS AND FUTURE RESEARCH
As a soil amendment with multiple benefits, biochar can change soil properties through its unique physical and chemical properties, thus alleviating a variety of soil degradation problems. The properties of biochar depends not only on raw materials and pyrolysis conditions, but is also affected by the combination of activation and functional materials. The effectiveness of biochar to mitigate degraded soil depends mainly on the characteristics of the biochar, amount applied, soil characteristics and environmental factors. The application of biochar should be considered comprehensively, and a customized preparation scheme should be formulated to produce design biochar according to the degradation. Although biochar research has made great progress, there are still many knowledge gaps. For example, there is no standard system to characterize the physical and chemical properties of biochar, which are important factors in predicting the restoration effects of biochar. Long-term in-situ field studies of biochar remediation of soil degradation are lacking. Most current conclusions on biochar have been reached through pot experiments and mathematical simulations. Due to the complexity of the factors, there can be large differences between laboratory and field results. There is a difference between short-term agronomic benefits and the long-term soil amendments. Most studies have focused only on determining the effects of feedstocks and carbonization processes on the properties of biochar. Major breakthroughs are needed in the field of biochar activation and biochar-based materials, the development of biomass carbonization technology and modern equipment, and the improvement of carbonization efficiency and product quality. Further research is warranted in the following three areas.
4.1 Artificial intelligence designed biochar
At present, in the face of a wide variety of preparation processes, different climatic environments and soil characteristics, as well as complex soil degradation problems, many studies are needed to verify the characteristics of biochar and their effect in soil improvement. However, the large-scale or in-situ experiments needed to obtain these characteristics are very expensive and time-consuming (Yi et al., 2020). The question that arises is whether it is possible to accurately predict biochar and soil characteristics with a minimal of data collection. Intelligent modeling can learn and mime system features and effectively predict system output from limited data. Designed biochar needs upgrading; in combination with artificial intelligence (AI), more accurate, more effective and less expensive “AI-designed biochar” could be produced. Artificial intelligence has the ability to analyze complex relationships among variables and to optimize interactions. This can be done by modifying existing knowledge to improve biochar performance or by simulating and then generating new methods or processes. Machine learning is an important branch of artificial intelligence. Collected data are used with modeling algorithms, including artificial neural networks, decision trees, support vector machines, regression analyses, genetic algorithms, convolutional neural networks, and recurrent neural networks to optimize operations or make predictions (Lakshmi et al., 2021; Ukoba & Jen, 2022).
Currently, a little information is available on the application of artificial intelligence and machine learning in the fields of biochar preparation (Cao et al., 2016) and heavy metal remediation (Palansooriya et al., 2022). Various factors, such as biochar characteristics, levels of biochar application, complex soil degradation problems, soil properties, environmental climate and temporal conditions can affect the effectiveness of biochar in remediating degraded soils (Ma et al., 2023; Wang et al., 2024; Wei et al., 2024). Such complex conditions can be predicted and optimized by models generated by machine learning. Machine learning allows the construction of models to predict the effectiveness of biochar restoration, changes in biochar characteristics, and the timing of biochar restoration. Important characteristics of the desired biochar are presented and multi-task prediction models could be generated to predict biochar properties based on feedstock, pyrolysis, and modifications. A solid foundation could be established for the next step of building and optimizing the desired characteristics. The use of machine learning and artificial intelligence principles could lead to a better understanding of the effectiveness of biochar remediation of degraded soils as well as short- and long-term effects (Cao et al., 2016; Palansooriya et al., 2022). While biochar research has employed machine learning techniques, current work has focused on model training using laboratory-scale data (Wang et al., 2024).
4.2 Long-term effects of biochar in soil-aging
The properties of biochar determine its effectiveness in improving degraded soils. But, the properties of biochar exposed to soil tend to change with time. Biochar interacts with soil organisms, soil constituents, and environmental factors, including abiotic oxidation (light, rainfall, wind-drying, freeze–thaw), microbial degradation, macrofaunal activity, dissolution of unstable materials, chemisorption, and physical decomposition (Wang et al., 2016), over time in a process known as aging. Biochar tends to homogenize its physical and chemical properties due to aging when it enhances some restorative effects (Rathnayake et al., 2021), but weakens others (Zhelezova et al., 2017). Further systematic research on the aging of biochar is needed to determine how a certain type of biochar can be used in a particular environment in terms of remediation and duration. Most current conclusions on biochar have been drawn from basin studies and mathematical simulations, and there are discrepancies between ideal experimental conditions and in situ experiments with complex factors. Differences exist between the observed short-term agronomic benefits and the expectations of biochar as a lasting soil amendment, and these differences can explain, at least in part, the different results in studies. Most studies to date have examined the short-term remediation effects of biochar (Tsolis & Barouchas, 2023; Wang et al., 2020). however, the aging effect determines its timeliness to different levels of soil degradation. The strong stability of biochar ensures its long-term retention in soil, so it is recommended to carry out long-term in-situ studies on soil degradation remediation of biochar (Joseph et al., 2021).
4.3 The economics of biochar
The economics of biochar is the core issue that is hindering the promotion of biochar in its use for soil remediation. Most cost–benefit analyses have concluded that the use of biochar is not economically sound (Bach et al., 2016). In fact, the direct agricultural benefits of biochar are calculated to be low. Shackley et al. (2011) argued that the price of biochar would have to be reduced by approximately 90% of its current market price to make biochar profitable. Zilberman et al. (2023) suggested that biochar should be applied to marginal land that is producing high-value crops, where the availability of low-cost feedstock is nearby, and that carbon sequestration subsidies should be provided. The cost of biochar is mainly the source of raw material acquisition and labor. Shackley et al. (2011) estimated that the cost of producing, transporting, and disseminating biochar in the UK reaches £389 t−1. When there are abundant organic waste resources nearby, the costs of raw materials and transportation are negligible (Dickinson et al., 2015). The controllability of the cost means that it is not the key issue in the economics of biochar. Biochar has substantial carbon neutrality; however, an incomplete carbon trading market cannot directly realize the profit of biochar. The direct benefits of biochar are the greatest concerns to farmers and are also the core issue of its application at the farmer scale (Dickinson et al., 2015). To improve the economic attractiveness of biochar, more attention should be paid to the quality of biochar, especially the restoration of degraded soil, and the conversion of “unusable” degraded soil into “usable” fertile soil (Dickinson et al., 2015). Biochar should not be valued by conventional fertilizers or biofuels, but rather, it should be valued according to its ability to improve soil characteristics (Maroušek et al., 2019).
AUTHOR CONTRIBUTIONS
Shuai Qi: Conceptualization; data curation; formal analysis; supervision; writing – original draft. Allan Degen: Conceptualization; writing – review and editing. Wenyin Wang: Data curation; writing – review and editing. Mei Huang: Data curation; writing – review and editing. Dongmei Li: Data curation; writing – review and editing. Binyu Luo: Writing – review and editing. Jianhui Xu: Data curation. Zhiqiang Dang: Writing – review and editing. Ruiying Guo: Formal analysis; writing – review and editing. Zhanhuan Shang: Conceptualization; funding acquisition; project administration; resources; writing – review and editing.
ACKNOWLEDGEMENTS
This study was supported by the Natural Science Foundation of China (U21A20183, 31961143012), Chief Scientist Program of Qinghai Province (2024-SF-101), the Science-based Advisory Program of The Alliance of National and International Science Organizations for the Belt and Road Regions (ANSO-SBA-2023-02), the Second Tibetan Plateau Expedition (2019QZKK0302), and he ‘111’ Programme 2.0 (BP0719040).
CONFLICT OF INTEREST STATEMENT
We declare we have no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article [and/or its supplementary materials].




