A 30% reduction in switchgrass rhizome reserves did not decrease biomass yield
Abstract
A long-standing question in perennial grass breeding and physiology is whether yield improvement strategies could compromise winter survival. Since perennial grasses rely on stored carbohydrates for winter maintenance and spring regrowth, yield improvement strategies could reduce winter survival if they increase biomass and grain yields at the expense of carbon allocation to storage. Therefore, it is crucial to comprehend the dependence of regrowth on storage reserves. We experimentally depleted switchgrass (Panicum virgatum L.) rhizome reserves by storing rhizomes for 2 weeks at 5°C (control treatment) and 25°C (reserve-depleted treatment). During the storage period rhizome respiration was 5.3× higher at 25°C (0.010 μmol CO2 g−1 min−1 at 5°C vs. 0.054 μmol CO2 g−1 min−1 at 25°C; p < 0.0001) and the starch content was depleted by 30% by the end of storage. Surprisingly, reserve-depleted switchgrass had 60% larger leaf area (LA; LAcontrol = 149 cm2 pot−1 vs. LAdepleted = 239 cm2 pot−1; p = 0.013) and produced ~40% more aboveground biomass than control plants (9.46 g pot−1 vs. 6.63 g pot−1; p = 0.112). In addition, reserve-depleted switchgrass restored its rhizome starch reserves to pre-storage levels. Switchgrass showed a large plasticity among its source-sink components to buffer the imposed reserve depletion. It increased plant photosynthesis by increasing the photosynthetic leaf area while keeping photosynthesis constant on a leaf area basis and readjusted the timing and activity of sink organs. These results suggest that switchgrass, and potentially other perennial grasses, largely over-invest in storage reserves. Therefore, current breeding strategies in perennial grasses aimed to extend the aboveground growing season should not compromise crop persistence. Our study also has implications on long-term yield dynamics as it highlights sink limitations as potential driver of the yield decline commonly observed in perennial grasses 5+ years after cultivation.
1 INTRODUCTION
Perennial plants have the unique challenge of balancing growth and development during the growing season with off-season persistence (Lubbe et al., 2021; Preston & Sandve, 2013; Rohde & Bhalerao, 2007). To achieve persistence in temperate regions, deciduous perennials need to not only assimilate enough CO2 during the growing season to complete growth and development throughout challenging conditions (e.g., water deficit, grazing, fire), but also to meet the anticipated demand of winter maintenance and spring regrowth. This implies that during the growing season, perennial grasses allocate carbon to growth, development, and storage organs. The accumulation of storage reserves in storage organs (e.g., rhizomes, crowns, stolons, and ratoons) is crucial for winter maintenance and spring regrowth, (Sarath et al., 2014; Sheikh et al., 2022; Smith, 1975; Zhang et al., 2021) and it is also an important driver of carbon assimilation and growth during the same growing season (De Souza et al., 2018; Palmer et al., 2017; Palmer et al., 2014; Ruiz-Vera et al., 2021; Tejera-Nieves et al., 2023; Tejera et al., 2022; Van Heerden et al., 2010), however, it is less understood how changes in the storage reserve affect growth and development during the next season.
As perennial grasses are important forage and potential alternative crops for a more sustainable agriculture, it is crucial to better understand the dependence of regrowth on storage reserves to inform breeding strategies, and to better understand the crops' response to climate change. For example, if breeding efforts increase biomass and grain yields at the expense of carbon allocation to storage, it may increase the risk of carbon starvation, winter mortality, and reduced long-term persistence.
Herbaceous perennials and deciduous trees largely depend on the pool of carbohydrates accumulated in storage organs from the previous summers for spring regrowth (George et al., 1989; Kabeya & Sakai, 2005; Lu et al., 2018; Piper & Paula, 2020; Smith, 1975; Van der Heyden & Stock, 1996). Past work in shrubs and trees indicates that in these cases, there is little over-investment in storage reserves and shoot regrowth declines with depleted the storage reserves (Kabeya & Sakai, 2005; Lu et al., 2018; Mitchell et al., 2020; Piper & Paula, 2020; Van der Heyden & Stock, 1996). In perennial grasses the role of carbohydrates reserves on regrowth is less clear and regrowth tends to decline or show no response to depleted storage reserves (Anderson et al., 1989; Benot et al., 2019; Lee et al., 2008; Volaire, 1994; Volaire & Gandoin, 1996). In these experiments, reserves are often manipulated by defoliating plants and allowing them to regrow. While this method successfully depletes carbohydrate reserves, it is hard to control the degree of the depletion, and also removes nutrients and changes light conditions, ultimately confounding the results (Gastal & Lemaire, 2015). These results also indicate that the storage reserve may not be much larger than what is needed for regrowth and any decreases in carbon investment during the growing season may hinder persistence. This could present a challenge for perennial grass breeding programs as relocation of carbon could then reduce following-year performance; however, direct carbon storage manipulations are yet to be tested in perennial grasses.
The dependency of regrowth on storage reserves is commonly studied by measuring the initial regrowth during spring, however, the capacity of perennials to restore reserves to pre-reserve-depletion levels across the whole season is less understood. After reserve depletion, perennials would likely need to readjust their source-sink strategy to assimilate enough CO2 to complete growth, meet the anticipated winter demand, and compensate for the initial reserve depletion to avoid carbon starvation. Perennial grasses have a plastic source-sink system that responds to changes in sink demand and rapidly affects photosynthesis. For example, experimental shading of leaves (e.g., increase sink demand) increased leaf photosynthesis of unshaded leaves by 32% in sugar cane (McCormick et al., 2006) and cold-girdling leaves or placing leaves in sucrose solution (decreased sink demand) decreased leaf-level photosynthesis by 30%–45% (McCormick et al., 2008). These studies reveal a short-term coordination between sink demand and source activity but only investigated the immediate response after a rapid source-sink perturbation. Alternatively, our understanding of the longer, seasonal response of perennials' source-sink strategy is limited. Field observations show that the seasonal build-up of starch reserves in rhizomes (decreased sink demand) limited photosynthesis by up to 30% (De Souza et al., 2018; Tejera-Nieves et al., 2023), however, no experimental manipulation has confirmed this response.
The aim of this study was to evaluate the role of the storage reserve in spring regrowth and to quantify the capacity of perennial grasses to readjust source and sink activities when reserves are artificially depleted. To this end, we experimentally decreased rhizome reserves of the perennial grass, switchgrass (Panicum virgatum L.), and quantified the changes in switchgrass source strength (i.e., source activity [leaf photosynthesis] × source capacity [leaf area]) and the sink demand of carbohydrates in source (leaves) and sink (rhizome) organs across an entire growing cycle. Interestingly, reserve-depleted switchgrass achieved a similar final biomass despite an initial ~30% reduction in initial rhizome starch pools. To compensate for the initial reserve depletion without reduced biomass, switchgrass increased its leaf photosynthetic area, and shifted main sink activities earlier in development while keeping carbon allocation to the storage reserve constant.
2 MATERIALS AND METHODS
Rhizomes used in this experiment were harvested from a 12-year-old switchgrass stand in the Michigan State University Agronomy Farm (42.713 N, −84.467 W) in East Lansing, Michigan, USA. Rhizomes were harvested during winter, in early January, and stored at 5°C for 2 weeks before the start of the reserve-depletion treatment. Reserve depletion was imposed by storing switchgrass rhizomes at 25°C for 14 days, control treatment rhizomes remained at 5°C for the same period. Rhizomes smaller than 1 g or bigger than 7 g were not included in the experiment. A total of 80 rhizomes were randomly assigned to each treatment. Rhizomes were thoroughly washed with water, and all lateral roots were removed before the start of the treatments.
During storage, each rhizome was assigned a rhizome ID and weighed, placed on the individual cells of planting trays, and covered with paper towels. Water was sprayed on paper towels weekly to keep rhizomes moist. No signs of fungi infection or disease were found at the end of storage. Rhizomes weighing less than 1 g were discarded from the experiment.
Immediately after the storage period, rhizomes were planted individually in 2.54 L pots filled with a peat and perlite mix (SureMix™; Michigan Grower Products) and kept in a greenhouse at 27°C and 16 h photoperiod supplemented with artificial lights for the length of the growth cycle (~180 days). Rhizomes were randomly assigned to a position in the greenhouse bench. During the first 2 weeks after planting, pots were irrigated every 2 days to ensure emergence. Irrigation drip lines kept water at soil capacity for the rest of the experiment. No extra fertilizer was applied throughout the rest of experiment.
2.1 Data collection
Rhizome respiration, development, and photosynthesis were measured repeatedly over the course of the experiment. These non-destructive measurements were taken on randomly assigned rhizomes weekly or biweekly. Rhizome respiration was measured during the storage period every 3–4 days after the 5th day of storage. We used an open gas exchange system (LI-6800; LI-COR Biosciences) attached to a 49.9 cm3 insect respiration chamber (LI-6800-89; LI-COR Biosciences) equipped with a thermocouple. Air temperature and relative humidity (RH) inside the respiration chamber were set to mimic ambient conditions at each treatment. Rhizome respiration was measured in a cold room and a laboratory for the 5°C and 25°C storage treatments, respectively. The CO2 concentration was maintained at 400 μmol mol−1 in the reference channel. Respiration was computed on a mass basis.
After planting, switchgrass development was assessed weekly during the experiment. At each sampling date, the number of tillers, number of fully expanded leaves per tiller, and senescence was recorded for each individual plant. Plants were considered dead and removed from the experiment when plants were 90% senescent.
Leaf net CO2 assimilation rate () and stomatal conductance to water () were measured biweekly in the middle portion of the youngest fully expanded leaf with a LI-6800. For the instrument was equipped with an integrated modulated chlorophyll fluorometer and a light source. Air temperature, photosynthetic photon flux density (PPDF), and relative humidity (RH) inside the leaf chamber were set to mimic ambient conditions at each sampling time. PSII quantum efficiency (PSII) was calculated as: , where is the steady-state fluorescence, and is the maximum fluorescence after a saturating light flash.
Aboveground biomass, rhizome and root biomass, and total leaf area were sampled 5 times during the experiment. Rhizome biomass was also sampled three times during the storage period on the first, 8th, and 14th day of storage. These destructive measurements were taken on 10 randomly preassigned rhizomes per treatment every 30–40 days. At each sampling date, stems were cut at soil surface, leaves were removed from stems and scanned with a leaf area meter (LI-3100C; LI-COR Biosciences). Leaves and stems were then oven-dried at 60°C for 72 h or until constant weight. Aboveground biomass was recorded after weighing the dried samples. Senesced leaves were not scanned but weighed for aboveground biomass. The upper-most fully expanded leaf of each plant was not included in the aboveground biomass, and instead placed in an aluminum pouch and flash frozen in liquid nitrogen for glucose, sucrose, and starch content analyses.
To harvest belowground tissues, soil was rinsed using tap water and roots were removed from rhizomes. Roots were dried and weighted separately as with aboveground biomass. All rhizome samples were weighed fresh, placed in an aluminum pouch and flash frozen in liquid nitrogen for glucose, sucrose, and starch content analyses. Total belowground biomass was estimated as the sum of root and rhizome biomass. We are aware that by not drying them first, rhizome biomass was overestimated, but drying would have increased starch degradation.
2.2 Sample processing
Leaf and rhizome samples were stored at −80°C until further processing. Leaf samples were ground to a fine powder with a mortar and pestle. Rhizome samples were ground with a spice mixer (Cuisinart; SG-10). All samples stayed in contact with liquid nitrogen during grinding and were then freeze-dried for at least 48 h in a lyophilizer. Leaf and rhizome starch, sucrose and free glucose were measured at the Biomass Analytics Facility at Michigan State University according to Santoro et al. (2010) and Sekhon et al. (2016). In brief, glucose content was assayed using the glucose oxidase/peroxidase (GOPOD) method (K-GLUC, Megazyme). For sucrose and starch, samples were first treated with a combination of alkaline buffer and high heat to degrade all pre-existing free glucose. Then samples were treated with invertase (Sigma-Aldrich), or amyloglucosidase (K-TSTA, Megazyme) for sucrose extraction and 5 μL α-amylase (K-TSTA, Megazyme) for starch extraction. The resulting glucose was measured as in the free glucose assay.
2.3 Data analysis
The datasets generated and/or analyzed during the current study are available at Dryad (https://doi.org/10.5061/dryad.4b8gthtj1). We used R (R Core Team, 2017) for all analyses and plots. We used lm() to fit linear models, gnls() in the nlme package to fir non-linear models (Pinherio & Bates, 2000), and emmeans() in the emmeans package (Lenth et al., 2018) for mean and parameter comparisons. Different linear and non-linear models were used to fit the different variables. For rhizome respiration and rhizome carbohydrates during the storage period (i.e., glucose, sucrose, and starch), sampling dates and treatments were considered fixed effects. The number of tillers per pot and total number of leaves (tillers/pot × leaves/tiller) time series were analyzed using non-linear models fitted to an asymptotic exponential and logistic response functions, respectively. Then treatment effects were studied based on their effects on function parameters. Aboveground and belowground biomass, and total leaf area per plot were analyzed as a two-way ANOVA with sampling dates and treatments as fixed effects. Because each sampling was destructive, no correlation was added to the models. Treatment effects were tested based on treatment differences at each sampling date. To estimate change rates, as proxy for sink activity, of the main sink activities (i.e., aboveground, rhizome and root biomass, total number of leaves, number of tillers per pot, and starch content) we fitted treatment means at each sampling date to a logistic response curve (or an asymptotic exponential for number of tillers per pot), predicted values for Days 1–180 of the experiment, and estimated the change rates as the difference between consecutive observations.
3 RESULTS
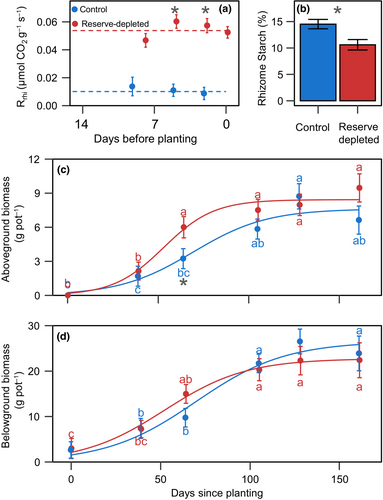
3.1 A 14-day storage period at 25°C reduced rhizome reserves by 30% due to 5.3× higher rhizome respiration
Rhizome respiration remained constant during the 14-day storage period. On average, rhizome respiration was 0.010 μmol CO2 g−1 min−1 at 5°C and 0.054 μmol CO2 g−1 min−1 at 25°C (p < 0.0001; Figure 1a). This 5.3×-fold change in rhizome respiration over a 20°C temperature difference indicates a Q10 of 2.31. Starch in rhizomes at 5°C remained constant during the storage phase (p = 0.742), while rhizomes at 25°C lost ~3 mg Starch g Rhi−1 day−1 (p = 0.002; Figure S1a). Sucrose content showed a similar dynamic to starch content, while the glucose content remained unchanged during the storage period for both treatments (p > 0.190; Figure S1). At planting, rhizomes at 25°C had lost 57% and 32% of their initial sucrose and starch content, respectively, (psucrose = 0.0074, pstarch = 0.002) and had 32%, 53%, and 27% less glucose, sucrose, and starch than rhizomes stored at 5°C (pglucose = 0.0019, psucrose = 0.0057, pstarch = 0.005; Figure 1b; Figure S1). Plants grown from rhizomes at 25°C and 5°C are referred to as reserve-depleted and control plants, respectively, for the rest of the manuscript.
The amount carbon lost due to starch content depletion (i.e., starch difference between consecutive samples) was proportional to the total amount of CO2 that was respired up to the sampling points (R2 = 0.99; p = 0.0023; Figure S2). In reserve-depleted plants, rhizome respiration accounted for almost 90% of the starch depletion.
3.2 Depletion of rhizome reserves did not affect above- and belowground biomass at the end of the growth cycle
Average switchgrass final aboveground biomass was 9.46 g pot−1 and 6.63 g pot−1 for reserve-depleted and control plants, respectively (p = 0.112; Figure 1c). Both treatments showed a logistic growth pattern. Reserve-depleted plants peak aboveground biomass around 60 days after planting, ~40 days sooner than control plants.
Belowground biomass was similar between treatments. Reserve-depleted plants reached 22.4 g pot−1 and control plants 23.9 g pot−1 at the end of the experiment (p = 0.783; Figure 1c). Both treatments had similar rhizome biomass at the end of the experiment (p = 0.4152; Figure 2a). Reserve-depleted plants reached peak rhizome biomass around the same time they peaked aboveground; however, control plants continued to increase rhizome biomass almost linearly until the end of the experiment (Figure S3a). Treatment differences in root biomass were more dynamic. Both treatments had similar root biomass at the end of the experiment (p = 0.609), but reserve-depleted plants had 82% more roots in 3rd sampling (p = 0.0293) and control plants had 80% more roots on the fifth sampling (0.0004; Figure S3b).
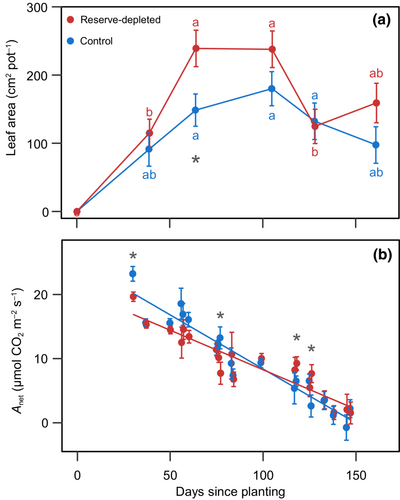
3.3 Plants grown from rhizomes with depleted reserves had 60% larger leaf area with similar leaf-level CO2 assimilation
Reserve-depleted switchgrass reached peak leaf area 40 days sooner than control plants and reached 60% larger leaf area than in control plants (p = 0.013; Figure 2a). The higher leaf area in reserve-depleted plants was driven by a faster emergence that led to 47% more tillers than control plants (p = 0.0092; Figure S4a).
Reserve-depleted plants had 51% more leaves than the control at the end of the experiment (p = 0.0119; Figure S4b). Reserve-depleted plants were able to continue producing leaves for an extra 12 days. On average, control plants produced more leaves per tiller early on development, but plateaued for the second half of the experiment, while reserve-depleted plants continue to increase the number of leaves per tiller until the end of the growth cycle.
Leaf-level CO2 assimilation declined during the growth cycle, from 25 to 2 μmol CO2 m−2 day−1 in both treatments (Figure 2b). During the growth cycle, the decline in and in the control plants was steeper than in reserve-depleted plants ( = 0.0419, = 0.0025; Figure 2b; Figure S5a). also showed a declining trend over the course of development, with no differences between treatments ( = 0.6617; Figure S5b).
3.4 Depleted rhizome reserves made switchgrass grow faster and sooner but not bigger or larger
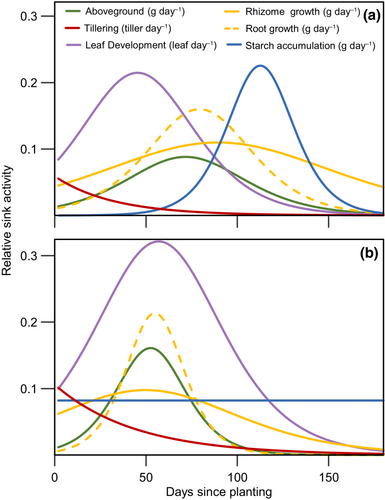
The daily slope of the predicted value of main switchgrass sinks (i.e., aboveground, rhizome and root, total number of leaves, number of tillers per pot, and starch content) was used to compare activities across treatments during the growth cycle. Overall, reserve-depleted switchgrass shifted growth and development towards earlier in the cycle and maintained starch synthesis constant (Figure 3). Reserve-depleted plants peaked aboveground, rhizome and root growth 20–40 days sooner than control plants. Leaf development had a similar span between treatments, but leaf development rates was 50% higher in reserve-depleted plants (Figure 3). Reserve-depleted plants first allocated resources to rhizome growth and tillering, followed by leaf development. Control plants replenished their reserves last, after below and aboveground growth had ceased (Figure 3). Rhizome starch content increased linearly during growth in reserve-depleted plants, while it lagged in control plants. Control plants reached initial rhizome starch content 32 days sooner than reserve-depleted but, by the end of the experiment, reserve-depleted plants reached similar rhizome starch content to the control plants (Figure S6a; p = 0.523). No differences were found in rhizome sucrose and glucose between treatments, and both remained relatively constant during the growth period (Figure S5b,c).
4 DISCUSSION
4.1 A 30% decrease on rhizome starch did not affect switchgrass final biomass or final rhizome starch content
These results show that up to 30% of the starch content in rhizomes at the end of the growing season was not needed to complete development and refill reserves in switchgrass for the following season. Despite this decreased initial starch, the reserve-depleted switchgrass showed no differences in above and belowground biomass compared with control plants or in rhizome starch content at the end of the experiment (Figure 1). In contrast to other perennials in the literature, such semiarid shrubs, trees and forage species (Kabeya & Sakai, 2005; Lu et al., 2018; Piper & Paula, 2020; Van der Heyden & Stock, 1996), these findings suggest that switchgrass and other perennial grasses may allocate more carbon to reserve pools than what is needed to cover the demand for winter respiration and spring regrowth. This surplus of storage may have provided an evolutionary advantage on its natural niche in case of fires or herbivory to overcome competition. A more fully domesticated species might have had these “insurance” storage reserves removed during selection by early farmers.
In practical terms, this carbon surplus in the reserve pool presents an opportunity for yield improvement, as it could be reallocated to aboveground growth and development without compromising long-term persistence of the crop. This finding confirms that current breeding strategies in perennial grasses aimed to extend the growing season (Casler, 2020; Schwartz & Amasino, 2013; Yang & Udvardi, 2018) should not compromise crop persistence, as long as the carbon relocation to growth does not deplete starch reserves below the 10% of rhizome biomass (last starch measurement of the reserve-depleted treatment before planting) by the end of the growing season. Similarly, perennial grain breeding programs facing a tradeoff between perenniality and grain yield (Cox et al., 2002; DeHaan et al., 2018) may be able to reallocate a similar portion of the reserve pool to grain yield without compromising biomass yield, or long-term persistence of the perennial.
So, how much could this carbon reallocated to aboveground tissues increase yield? To make a rough estimate, assuming a switchgrass field of 8 Mg ha−1 end-of-season yield, with ~6 Mg ha−1 of rhizomes and an end-of-season starch concentration of ~15% (first measurement of the storage period), then the 30% surplus of the starch would represent a carbon surplus of 270 kg ha−1. If all this carbon were allocated to aboveground tissue (assuming 40% carbon content), it could imply an extra 300 kg ha−1 of aboveground biomass. We understand these calculations oversimply carbon dynamics in perennial grasses, but they highlight the yield potential of this surplus of carbon. While this carbon relocation only represents a 5% increment in yield, a larger portion of the storage pool could be removed, and if combined with other strategies, such as extending the growing season, it could help achieve larger increments.
4.2 Switchgrass increased plant photosynthesis, produced more tillers, and extended their growth cycle under reserve depletion
After storage reserve depletion, switchgrass accelerated leaf development and reached peak leaf area 40 days sooner than control plants, had 60% larger leaf area and produced ~40% more aboveground biomass. This is a novel finding among perennials, as herbaceous perennials and deciduous trees commonly reduced regrowth after a depletion of storage reserves (Kabeya & Sakai, 2005; Lu et al., 2018; Piper & Paula, 2020; Van der Heyden & Stock, 1996). How does reserve-depleted Switchgrass make up the difference? Switchgrass relied on the large plasticity among its source-sink components to buffer the imposed reserve depletion (Figure 3). First, switchgrass increased carbon source strength (i.e., photosynthesis) by increasing the photosynthetic leaf area while keeping photosynthesis constant on a leaf area basis (Figure 2). Second, switchgrass readjusted the timing and activity of sink organs to maintain a constant allocation of carbon to storage that was greater than the control treatment (Figure S5). Reserve-depleted above and belowground growth peaked 20–40 days sooner than control plants. It also produced more tillers, faster, and with more leaves than control plants which led to up to a 60% larger leaf area (Figure 2; Figure S4). As a result, reserve-depleted plants successfully compensated for the initial starch depletion within one growing cycle (Figure S6).
Resolving this dual response to reserve depletion in switchgrass was possible due to our novel experimental approach that only manipulated storage reserves prior to the growth period. In contrast to cut-and-regrowth studies in forage studies our approach does not add the confounding effects of nutrient removal or changes in the light environment (Gastal & Lemaire, 2015; Lemus et al., 2008; Volaire, 1994; Volaire & Gandoin, 1996). A potential pitfall of our method is that part of the starch loss during the storage period could be mobilized to growth, and not directly consumed and respired. Then, the physiological response observed could be caused by plants being developmentally ahead, and not driven by a larger sink demand. We tested this hypothesis by comparing the amount of carbon that was mobilized from starch (as starch loss between consecutive samplings) and the total amount of carbon respired at each sampling (Figure S2). Rhizome respiration accounted for almost 90% of the carbon mobilized in the reserve-depleted treatment, confirming switchgrass physiological response was mainly driven by sink depletion.
Conventional source-sink manipulations are applied during the growth cycle, limiting the response to changes in the rate of the activity of an organ. For example, shading of leaves (increased sink activity), leaf removal (decreased source capacity), tuber excisions and fruit removal (decreased sink strength), all successfully perturb the source-sink balance, show changes in leaf-level photosynthesis, but limit the plant response to structures that are already built (e.g., Basu et al., 1999; Hastilestari et al., 2018; McCormick et al., 2006, 2008). This may explain why changes in leaf photosynthesis were not shown in our reserve-depleted plants. The reserve-depleted switchgrass was able to respond at the whole-plant level, increasing the photosynthetic leaf area instead of the individual rates of the leaves. Because the larger sink demand was distributed between more leaves, each individual leaf would not perceive as large of a change in the sink demand and would not need to readjust its individual photosynthetic rates. Similarly, our results cannot support or deny that sink limitations drive the late season decline in leaf-level photosynthesis (De Souza et al., 2018; McCormick et al., 2006; Tejera et al., 2022; Tejera-Nieves et al., 2023) but confirm that whole-plant photosynthesis is affected by changes in sink strength.
4.3 Perennial grasses showed large adaptability to winter warming
To put our storage treatments into perspective, the two-week period at 5°C or 25°C increased average winter temperature from 0.95°C to 1.6°C and 4.7°C, respectively. As winter temperature are projected to increase (Chin et al., 2018; Seneviratne et al., 2021), our results also apply to climate change scenarios, where the 5°C treatment would represent mild warming and the 25°C storage an extreme winter warming event. In either case, switchgrass completed growth and development without biomass or storage reserve penalties. These findings highlight the potential adaptability of perennial grasses to climate change and winter warming.
Across species, perennial grasses show a clear yield decline after 5+ years of cultivation which limits profitability and farmers' adoption (e.g., Alexopoulou et al., 2015; Larsen et al., 2014; Lesur et al., 2013; Roozeboom et al., 2019). In this research we showed that 12-year-old switchgrass rhizomes have the potential for higher tillering and biomass production when storage reserves are depleted. In fact, the faster and sooner development seen in reserve-depleted switchgrass, is like the phenological response M. × giganteus during establishment (1–3 years of cultivation) (Tejera et al., 2021). While reserve depleting using higher temperatures may not be a feasible management practice to “rejuvenate” mature stands in commercial settings, it highlights sink limitations as a potential driver of the yield decline. We understand that there are trade-offs between the tractability of a greenhouse study and the relevance of a field-based approach. To bridge these trade-offs as much as possible, we used field-harvested rhizomes. Further research is needed to expand reduced sink limitations studies to field settings, and to confirm this mechanism in commercial settings. Ultimately, higher storage temperature could be used to prime miscanthus rhizomes prior to planting, or moderate vertical tillage, could be applied to increase sink strength in mature stands and potentially increase yield.
5 CONCLUSIONS
In switchgrass up to 30% of reserves stored in rhizomes was not needed to complete growth and development. The increased sink strength in rhizomes led to important changes in the switchgrass source-sink strategy that allowed switchgrass to restore reserves and complete growth without final biomass compensation. The main changes of reserve-depleted plants were increased carbon source by increasing source capacity (i.e., larger leaf area) and maintaining source activity (i.e., leaf photosynthesis), and shifting sink activities and timing to earlier in the growth cycle. Our results suggest that the yearly C balance of a perennial grasses may not be as constrained as previously thought and there may be a larger opportunity for yield and grain improvements. Our study also has implications on the long-term yield dynamics and persistence of perennial grasses and supports sink-limitations as potential driver of the yield decline.
ACKNOWLEDGMENTS
We thank Heather Roney for her help in pot maintenance, data collection, and sample processing. This work was supported in part by the Great Lakes Bioenergy Research Center, US Department of Energy, Office of Science, Office of Biological and Environmental Research under Award Number DE-SC0018409, and Basic Energy Sciences under Award DE-FG02-91ER20021.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
All primary data to support the findings of this study are openly available in Dryad at https://doi.org/10.5061/dryad.4b8gthtj1.




