Biomass yield in a genetically diverse Miscanthus sinensis germplasm panel evaluated at five locations revealed individuals with exceptional potential
Abstract
To breed improved biomass cultivars of Miscanthus ×giganteus, it will be necessary to select the highest-yielding and best-adapted genotypes of its parental species, Miscanthus sinensis and Miscanthus sacchariflorus. We phenotyped a diverse clonally propagated panel of 569 M. sinensis and nine natural diploid M. ×giganteus at one subtropical (Zhuji, China) and five temperate locations (Sapporo, Japan; Leamington, Ontario, Canada; Fort Collins, CO; Urbana, IL; and Chuncheon, Korea) for dry biomass yield and 14 yield-component traits, in trials grown for 3 years. Notably, dry biomass yield of four Miscanthus accessions exceeded 80 Mg/ha in Zhuji, China, approaching the highest observed for any land plant. Additionally, six M. sinensis in Sapporo, Japan and one in Leamington, Canada also yielded more than the triploid M. ×giganteus ‘1993-1780’ control, with values exceeding 20 Mg/ha. Diploid M. ×giganteus was the best-yielding group at the northern sites. Genotype-by-environment interactions were modest among the five northern trial sites but large between Zhuji, and the northern sites. M. sinensis accessions typically yielded best at trial sites with latitudes similar to collection sites, although broad adaptation was observed for accessions from southern Japan. Genotypic heritabilities for third year yields ranged from 0.71 to 0.88 within locations. Compressed circumference was the best predictor of yield. These results establish a baseline of data for initiating selection to improve biomass yield of M. sinensis and M. ×giganteus in a diverse set of relevant geographies.
1 INTRODUCTION
Miscanthus is a C4 perennial grass native to East Asia, and a promising biomass crop for many applications, including lignocellulosic ethanol production, gasification or direct combustion to generate electricity or heat, producing paper, building materials, biodegradable plastic, animal bedding, mulch, and livestock feed (Acikel, 2011; Clifton-Brown & Lewandowski, 2002; Heaton, Clifton-Brown, Voigt, Jones, & Long, 2004; Johnson, Tucker, Barnes, & Kirwan, 2005; Sacks, Juvik, Lin, Stewart, & Yamada, 2013). All of these applications depend on high-yielding cultivars in order to be commercially viable. Nearly all Miscanthus biomass production currently uses a single high-yielding clone of M. ×giganteus (Głowacka et al., 2015), despite its insufficient winter hardiness in USDA zone 5b environments and colder (<−26.1°C average annual minimum air temperature; Dong, Green et al., 2018), as well as the risk of disease and pest susceptibility associated with monoculture (Ahonsi et al., 2010; Arnoult & Brancourt-Hulmel, 2015; Bradshaw, Prasifka, Steffey, & Gray, 2010; Clifton-Brown & Lewandowski, 2002; Prasifka et al., 2009). We refer to this clone as M. ×giganteus ‘1993-1780’ after the accession number of the type specimen at Kew Royal Botanic Gardens Herbarium (Hodkinson & Renvoize, 2001); it is also commonly referred to as M. ×giganteus ‘Illinois’ in North America (Głowacka et al., 2015). Although M. ×giganteus ‘1993-1780’ is a triploid, the species name can refer to hybrids of any ploidy between Miscanthus sinensis and Miscanthus sacchariflorus (McNeill et al., 2012; Sacks et al., 2013).
Of all temperate-adapted Miscanthus species, M. sinensis has the broadest native range (Clifton-Brown, Chiang, & Hodkinson, 2008; Dwiyanti, Stewart, & Yamada, 2013; Sacks et al., 2013), suggesting high genetic diversity for environmental adaptation. In previous population genetics studies, we identified six major genetic groups of M. sinensis, with three in China, one in China and Korea, and two in Japan (Clark et al., 2014), with the South Japan (S Japan) group being further subdivided into S Japan and Central Japan in a subsequent study (Clark et al., 2015). Ornamental M. sinensis cultivars represent a narrow portion of the genetic diversity of the species, originating almost exclusively from two small regions in southern Japan with subsequent introgression from diploid M. ×giganteus ‘Purpurascens’ in about half the cultivars currently marketed (Clark et al., 2014, 2015, 2018). US-naturalized M. sinensis were derived from non-admixed ornamentals (Clark et al., 2014, 2015). Despite this narrow genetic base, and artificial selection for short stature (Kaiser, Clark, Juvik, Voigt, & Sacks, 2015), ornamental M. sinensis have been used in the breeding of new biomass cultivars simply due to their availability in Europe and North America (Clifton-Brown et al., 2008). However, it is also widely recognized that better yields can be obtained with a broader germplasm base than that represented by the ornamental cultivars (Clark et al., 2014; Clifton-Brown et al., 2001, 2008; Jensen et al., 2011). The selection of high-yielding M. sinensis clones derived from crosses indicates the potential for yield improvement through breeding (Arnoult & Brancourt-Hulmel, 2015; Clifton-Brown et al., 2001).
To date, few studies have evaluated large, diverse germplasm panels of M. sinensis for yield, and these field trials were conducted only at single locations (Nie et al., 2016; Slavov et al., 2014; Zhao et al., 2013). In contrast to the single-location studies, previous multi-location trials of M. sinensis have included relatively few genotypes, yet consistently found significant genotype-by-environment (G × E) effects on yield (Arnoult & Brancourt-Hulmel, 2015; Clifton-Brown et al., 2001; Kaiser et al., 2015; Yan et al., 2012).
In the current study, we present field evaluations of the largest and most genetically diverse panel of M. sinensis evaluated to date, with phenotypic data from three field trial locations in East Asia and three in North America. In particular, we phenotyped 569 M. sinensis genotypes previously characterized for population structure (Clark et al., 2014), representing six genetic groups from nearly the entirety of the species’ natural geographic range. Biomass yield and 14 yield-component traits of M. sinensis were studied to (a) determine the range of genotypic diversity for yield in this species, and how performance varied with location of origin and genetic group, (b) quantify G × E effects and how well performance at one trial site predicted performance at other trial sites, and (c) identify yield-component traits that are strong predictors of yield.
2 MATERIALS AND METHODS
2.1 Plant materials and field trials
In total, 589 Miscanthus accessions were studied (Data S1 and S2). We previously assigned the 566 M. sinensis and three M. floridulus genotypes evaluated in the current study to one of the eight genetic groups (hereafter referred to as M. sinensis for a total of 569 genotypes) (Clark et al., 2014): 77 Ornamental, 38 US naturalized, 28 S Japan, 84 N Japan, 157 Korea/N China, 25 Sichuan, 75 Yangtze-Qinling, and 85 SE China/tropical. Due to the small number of individuals in the S Japan group, for the purpose of this study we did not divide it into the S Japan and Central Japan groups identified by Clark et al. (2015). Six of the genetic groups for M. sinensis were identified previously via discriminant analysis of principle components (Jombart, Devillard, & Balloux, 2010) and by the software Structure (Falush, Stephens, & Pritchard, 2003); the ornamental group and the US naturalized group were found to be subsets of the S Japan group but we label them independently to denote their unique provenances (Clark et al., 2014, 2015, 2018). Moreover, about half the accessions in the ornamental group had most of their ancestry from M. sinensis and ≤30% ancestry from diploid M. sacchariflorus, presumably the result of plant breeding efforts to introgress greater winter hardiness via crosses with diploid M. ×giganteus ‘Purpurascens’, while the other half were pure M. sinensis (Clark et al., 2014, 2015, 2018). Collection locations of all the natural and naturalized accessions are listed in Data S1 and those collected in Asia are shown in Figure 1. In addition to the M. sinensis accessions, we phenotyped seven diploid and three tetraploid M. sacchariflorus accessions from Korea and China, eight natural diploid M. ×giganteus from China, the ornamental diploid M. ×giganteus ‘Purpurascens’, and the biomass cultivar triploid M. ×giganteus ‘1993-1780’. All accessions were maintained as clonal stock plants in pots at a greenhouse in Ontario, Canada and vegetatively propagated. Ramets of each accession were distributed to each field trial location during January–March of 2012.
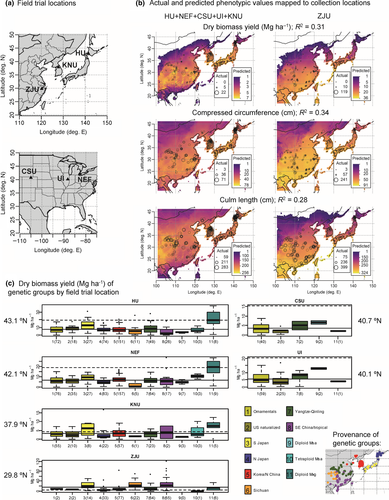
In the early summer of 2012, field trials were planted at five northern locations (Sapporo, Japan by Hokkaido University (HU); Leamington, Ontario, Canada by New Energy Farms (NEF); Fort Collins, Colorado, USA by Colorado State University (CSU); Urbana, Illinois, USA by the University of Illinois (UI); and Chuncheon, Korea by Kangwon National University (KNU)); plus one southern location (Zhuji, China by Zhejiang University (ZJU)) (Table 1). Except for CSU, which has an arid climate, supplemental irrigation was only provided in year 1 to facilitate establishment. Nitrogen fertilizer was applied to the field trials in the spring in the following amounts: 100 kg/ha at HU, NEF, and UI each year; none at CSU; 80 kg/ha at KNU each year; and 14 kg/ha at ZJU in the first year only. Not all accessions were successfully established at all six locations (Table 1 and Data S1). Further, at the two locations in the US we were limited largely to ornamental and US naturalized accessions due to quarantine limitations on importation of Miscanthus from Asia (Table 1 and Data S1). In spring of 2013, an additional planting was made at UI to include plant materials that had been newly released from USDA quarantine. Field trials at each site had from three to four replications in a randomized complete block design with single-plant plots equally spaced within and between rows on 1.5 m centers (Table 1). Harvesting was conducted in late autumn or early winter, after dormancy or the first killing freeze led to dry-down, with stems being cut 15–20 cm from the ground. To determine dry biomass yield, at some sites the entire plant was oven dried before weighing, while at other sites the fresh weight was measured, then a subsample was weighed before and after oven drying in order to estimate dry weight of the whole plant.
| Trial site code | Location | Latitude | Longitude | Elevation (m) | Hardiness zonea | Year planted | Total number of genotypes | Number of M. sinensis genotypes | Number of replicates | Number of yearsb |
|---|---|---|---|---|---|---|---|---|---|---|
| HU | Sapporo, Japan | 43.1 | 141.4 | 11 | 7b | 2012 | 529 | 509 | 4 | 4 |
| NEF | Leamington, Ontario, Canada | 42.1 | −82.6 | 196 | 6b | 2012 | 487 | 467 | 4 | 3 |
| CSU | Fort Collins, Colorado, USA | 40.7 | −105.0 | 1,556 | 5b | 2012 | 61 | 57 | 4 | 3 |
| UI | Urbana, Illinois, USA | 40.1 | −88.2 | 230 | 5b | 2012 | 112 | 108 | 4 | 3 |
| 2013 | 92 | 84 | 4 | 2 | ||||||
| KNU | Chuncheon, S Korea | 37.9 | 127.8 | 75 | 6b | 2012 | 206c | 194d | 3 | 4 |
| ZJU | Zhuji, China | 29.8 | 120.2 | 58 | 9a | 2013 | 290 | 282 | 3 | 3 |
| Growing season length (d)e | Growing degree days (d °C)f | Minimum air temperature preceding winter (°C) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year 1 | Year 2 | Year 3 | Year 4 | Year 1 | Year 2 | Year 3 | Year 4 | Year 2 | Year 3 | Year 4 | Soil type | |
| HU | 133 | 200 | 244 | 215 | 1,193 | 1,462 | 1,492 | 1,404 | −13.5 | −14.3 | −10.1 | Humic andosol |
| NEF | 124 | 223 | 190 | 888 | 1,556 | 1,251 | −18.0 | −27.0 | Brookston clay sand spot phaseg | |||
| CSU | 83 | 210 | 185 | 800 | 1,421 | 1,269 | −22.8 | −29.9 | Fort Collins loamh | |||
| UI 2012 | 126 | 210 | 192 | 1,055 | 1,789 | 1,651 | −15.9 | −25.2 | Drummer silty clay loami | |||
| UI 2013 | 185 | 192 | 1,718 | 1,651 | −25.2 | Drummer silty clay loam | ||||||
| KNU | 117 | 221 | 228 | 211 | 1,068 | 2,133 | 2,169 | 2,153 | −23.1 | −15.6 | −20.1 | 2:3 Alfisol:Inceptisol |
| ZJU | 262 | 267 | 281 | 3,209 | 3,173 | 3,159 | −5.0 | −4.0 | Silty loam | |||
Note
- HU = Hokkaido University; NEF = New Energy Farms; CSU = Colorado State University; UI = University of Illinois at Urbana-Champaign; KNU = Kangwon National University; ZJU = Zhejiang University.
- a USDA hardiness zone estimate based on plantmaps.com.
- b Phenotypic data were collected in years 2–4 only; year 1 was just an establishment year.
- c At KNU, a set of 86 genotypes was planted in all three replications, but in one replication an additional 120 genotypes were included for a total of 206.
- d At KNU, a set of 82 genotypes was planted in all three replications, but in one replication an additional 112 genotypes were included for a total of 192.
- e Measured from planting date (year 1) or first date of spring emergence (years 2–4) to last date of autumn dormancy.
- f For each day during the growing season, a number from 0 to 20 was calculated indicating the number of °C by which the average daily temperature exceeded 10°C. These were summed across the days of the growing season as estimated for growing season length.
- g Richards, Caldwell, and Morwick (1949).
- h US Dept. of Agriculture – Natural Resources Conservation Service (2005).
- i US Dept. of Agriculture – Natural Resources Conservation Service (2015).
2.2 Phenotypic data collection and analyses
Biomass yield (dry weight) and 14 yield-component traits were measured in the second and third year after planting at each trial location (Table 2). Additionally, phenotypic data were collected in the fourth year at KNU for all traits and at HU for biomass yield only. Only data on establishment were taken during the first year because year 1 yields of Miscanthus are not expected to be strongly associated with yield in years 2 and 3 but data from years 2 and 3 are typically strongly correlated (Clifton-Brown et al., 2001). At UI, year 2 was 2013 for the trial planted in 2012, and 2014 for the trial planted in 2013. To estimate dry biomass yield per area, yield per plant was divided by the plot area (2.25 m2) and expressed as Mg/ha. For plants on which basal circumference exceeded the square plot perimeter (mostly M. sacchariflorus accessions in years 3 and 4), we instead estimated plot area from basal circumference, assuming a circular footprint. Data on flowering time and winter hardiness were measured but are not presented here.
| Trait | Abbreviation | Descriptiona and notes |
|---|---|---|
| Dry biomass yield (g/plant or Mg/ha) | Yld | Single-plant plots on 1.5 m centers were harvested in late autumn by cutting the stems 15−20 cm above the soil surface. Samples were dried at 60°C until constant weight. Estimates are reported per area based on plot dimensions (2.25 m2) or per plant. |
| Compressed circumference (cm) | CC | Stems were compressed at the middle height of the plant such that all the culms were in close contact without air gaps; then the circumference of the compressed bundle was measured. |
| Basal circumference (cm) | BC | Circumference of the base of the plant, without compression. |
| Compressed circumference/basal circumference | CC/BC | Compressed circumference divided by basal circumference, to estimate the proportion of the plant's footprint filled by stems. |
| Culm length (cm) | CmL | Length of the tallest culm in late autumn, measured from the base of the stem to the tip of the panicle if present, otherwise to the highest part of the highest leaf. |
| Culm node number | CmNdN | Number of nodes on the tallest culm of each plant in late autumn. |
| Internode length (cm) | IntL | Culm length divided by the number of nodes for the tallest culm of each plant in late autumn. |
| Culm dry weight (g) | CmDW | Mass of the tallest culm of each plant in late autumn, after removal of leaves and drying at 60°C until constant weight achieved. (Not recorded at KNU in year 3 or 4 or at ZJU.) |
| Culm volume (cm3) | CmV | Estimated from culm length, culm diameter at first internode, and culm diameter at last internode, assuming the stem was shaped like the frustum of a cone:CmL * π * [(DBI/2)2 + (DBI/2)*(DTI/2) + (DTI/2)2]/3 |
| Culm density (g cm−3) | CmDW/V | Culm dry weight divided by culm volume. (Not estimated for KNU in year 3 or 4 or at ZJU.) |
| Diameter of basal internode (mm) | DBI | Measured on the tallest culm of each plant in late autumn. |
| Diameter of topmost internode (mm) | DTI | Measured on the tallest culm of each plant in late autumn. |
| Total number of culms | TCmN | Counted for each plant. |
| Proportion of reproductive culms | RCmN/TCmN | Number of reproductive culms divided by the total number of culms. (Not estimated at HU in year 2 or at KNU.) |
| Culms per footprint (# cm−2) | TCmN/A | The total number of culms divided by the area of the plant's footprint. The footprint area was estimated from the basal circumference, assuming a circular base. |
- a All traits were measured at the end of the growing season.
For each trait × year combination, phenotypic values were transformed using the Box–Cox method (Box & Cox, 1964) as implemented in the R package MASS (Venables & Ripley, 2002) in order to make the data approximate a normal distribution. Linear models for determining optimum lambda values for Box–Cox (where the transformation to be performed is log(x) if λ = 0, and (xλ – 1)/λ otherwise) were fit in R with field trial location, genotype, and their interaction as fixed effects, using the 569 M. sinensis individuals from the field trial. Lambda values ranging from −2 to 2, at intervals of 0.1, were tested. Least square means (LS means) were estimated for all entries using the R package lsmeans (Lenth, 2016) with linear models fit in R using genotype and replication as fixed effects for within-location estimates, and using genotype and replication within location as fixed effects for multi-location estimates. Back-transformed LS means were then calculated and presented in Table 2, Table S2 and Data S1.
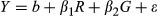 (1)
(1)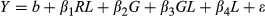 (2)
(2)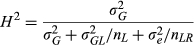 (3)
(3)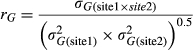 (4)
(4)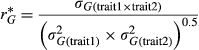 (5)
(5)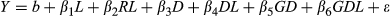 (6)
(6)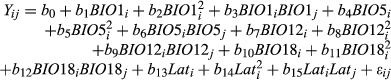 (7)
(7)All data and code are available in the Illinois Data Bank, https://doi.org/10.13012/B2IDB-0790815_V2.
3 RESULTS
3.1 Yield performance
The commercially important biomass cultivar control, triploid M. ×giganteus ‘1993-1780’, typically yielded more at the northern trial sites (LS means for year 3 dry biomass yields were 19.7, 18.7, 15.2, and 20.4 Mg/ha at HU, NEF, CSU and UI, respectively; Figure 1 and Table 3) than at the southern site (3.8 Mg/ha at ZJU), though performance at KNU was also low (4.2 Mg/ha) although compressed circumference and culm length at KNU were similar to those at other northern sites (Data S2). In comparison to the northern locations in the current study, prior studies reported similar third year yields for triploid M. ×giganteus of 13.7–37.3 Mg/ha in IL, USA (Arundale et al., 2014; Heaton, Dohleman, & Long, 2008; Kaiser et al., 2015) and 13.8–37.8 Mg/ha in England, Germany and Portugal (Clifton-Brown et al., 2001), though the prior trials were planted at higher density (1–2 plants m−2) than the current study (1 plant 2.25 m−2). Thus, data from this and prior studies indicate that small and large plots provide similar estimates of Miscanthus biomass yield when expressed in Mg/ha (Dong, Liu et al., 2018; Kaiser et al., 2015; Zhao et al., 2013). Consistent with the poor performance of M. ×giganteus ‘1993-1780’ at ZJU, we have previously observed this genotype to have low yields at similar latitudes in the southern coastal plain of the US due primarily to early flowering (e.g., in August, unpublished).
| Species | Genotype | Genetic group | Latitude | Longitude | HU | NEF | CSU | UI | KNU | ZJU | Northern trial locations | All trial locations |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M. sinensis | JM0119.001 | S Japan | 33.56 | 133.38 | 12.3 | 0.2 | 50.9 | 3.9 | 9.3 | |||
| JM0125.001 | S Japan | 31.53 | 131.24 | 30.9 | 0.4 | 7.5 | 15.9 | 11.1 | ||||
| JM0232.001 | S Japan | 35.05 | 133.72 | 20.7 | 10.6 | 34.2 | 11.7 | 15.1 | ||||
| JM0232.002 | S Japan | 35.05 | 133.72 | 23.0 | 12.6 | 11.9 | 12.7 | |||||
| JM0361.002 | S Japan | 33.02 | 131.06 | 21.0 | 7.8 | 9.1 | 9.7 | |||||
| PMS−014 | Sichuan | 29.66 | 109.12 | 25.4 | 40.2 | 20.6 | 26.3 | |||||
| PMS−053 | Sichuan | 30.22 | 103.48 | 69.6 | 57.4 | |||||||
| PMS−054 | Sichuan | 30.08 | 103.08 | 51.7 | 41.1 | |||||||
| PMS−257 | SE China/tropical | 23.26 | 104.42 | 52.8 | 42.5 | |||||||
| PMS−314 | Yangtze-Qinling | 26.53 | 119.63 | 8.5 | 4.0 | 66.1 | 7.6 | 19.9 | ||||
| PMS−397 | SE China/tropical | 24.40 | 109.98 | 63.7 | 51.6 | |||||||
| PMS−399 | SE China/tropical | 24.39 | 110.13 | 0.3 | 81.1 | 0.1 | 35.1 | |||||
| PMS−404 | SE China/tropical | 24.69 | 110.47 | 54.1 | 43.2 | |||||||
| PMS−427 | Yangtze-Qinling | 29.77 | 114.04 | 27.6 | 18.2 | 22.3 | 17.9 | |||||
| PMS−496 | Korea/N China | 41.91 | 124.67 | 2.2 | 3.8 | 81.9 | 2.3 | 13.0 | ||||
| PMS−558 | SE China/tropical | 28.35 | 109.55 | 53.8 | 43.5 | |||||||
| PMS−576 | SE China/tropical | 25.77 | 109.59 | 88.8 | 74.7 | |||||||
| PI417947 | SE China/tropical | −6.00 | 144.00 | 119.1 | 101.2 | |||||||
| PI423566 | SE China/tropical | 24.00 | 121.00 | 1.1 | 28.2 | 7.0 | 7.6 | |||||
| M. ×giganteus triploid | ‘1993−1780’ | M. ×giganteus | 35.44a | 139.64 | 19.7 | 18.7 | 15.2 | 20.4 | 4.2 | 3.8 | 14.9 | 14.5 |
| M. ×giganteus diploids | PMS−111 | M. ×giganteus | 32.96 | 108.32 | 15.8 | 28.1 | 7.6 | 17.8 | 18.7 | |||
| PMS−162 | M. ×giganteus | 35.71 | 112.61 | 21.3 | 28.3 | 20.7 | 21.8 | |||||
| PMS−279 | M. ×giganteus | 29.10 | 117.08 | 36.0 | 14.0 | 9.5 | 12.7 | 19.1 | 16.6 | |||
| PMS−300 | M. ×giganteus | 30.87 | 120.14 | 23.8 | 19.1 | 14.9 | 17.6 | 15.7 | ||||
| PMS−430 | M. ×giganteus | 29.90 | 114.29 | 38.3 | 23.1 | 12.5 | 33.4 | 24.8 | 25.9 | |||
| PMS−459 | M. ×giganteus | 36.22 | 120.54 | 17.4 | 24.6 | 4.9 | 16.7 | 11.5 | ||||
| PMS−467−8 | M. ×giganteus | 37.17 | 121.74 | 14.5 | 17.7 | 6.5 | 4.3 | 13.0 | 10.5 | |||
| PMS−533 | M. ×giganteus | 31.02 | 108.87 | 1.2 | 16.7 | 0.7 | 5.5 | |||||
| ‘Purpurascens’ | M. ×giganteus | 0.8 | 1.9 | 2.3 | 3.9 | 0.0 | 2.0 | 2.2 | ||||
| M. sacchariflorus | KMS053 | M. sacchariflorus | 35.42 | 129.06 | 5.5 | 10.7 | 1.0 | 0.3 | 5.5 | 4.7 | ||
| KMS062 | M. sacchariflorus | 36.34 | 129.06 | 3.3 | 7.9 | 3.0 | 4.0 | 4.4 | ||||
| PMS−071 | M. sacchariflorus | 40.39 | 117.01 | 4.7 | 7.2 | 5.2 | 5.6 | |||||
| PMS−074 | M. sacchariflorus | 40.13 | 116.17 | 4.0 | 3.4 | 2.5 | 2.8 | |||||
| PMS−075 | M. sacchariflorus | 40.13 | 116.18 | 4.4 | 6.0 | 7.6 | 13.5 | 1.5 | 6.5 | 7.1 | ||
| PMS−076 | M. sacchariflorus | 40.16 | 116.05 | 4.3 | 4.9 | 3.4 | 3.7 | |||||
| PMS−077 | M. sacchariflorus | 40.15 | 115.90 | 2.1 | 5.2 | 3.3 | 3.6 | |||||
| PMS−458 | M. sacchariflorus | 36.17 | 120.50 | 12.4 | 12.5 | 10.0 | 10.4 | 11.1 | ||||
| PMS−512 | M. sacchariflorus | 41.33 | 123.69 | 4.3 | 3.0 | 0.8 | 2.4 | 2.7 | ||||
| ‘Hortico’ | M. sacchariflorus | 2.4 | 4.7 | 5.6 | 12.4 | 1.2 | 4.5 | 5.0 |
Note
- Original data were Box–Cox transformed before estimating least-squared means; back-transformed values are shown here. Genotypes outperforming the triploid M. ×giganteus ‘1993-1780’ control at a given site are highlighted in bold. Collection locations are indicated for wild accessions.
- HU = Hokkaido University; NEF = New Energy Farms; CSU = Colorado State University; UI = University of Illinois at Urbana-Champaign; KNU = Kangwon National University; ZJU = Zhejiang University.
- a Assuming origination from Yokohama, Japan.
Among the M. sinensis genotypes, large and highly significant differences in yield and yield components were observed at each location (Tables 4 and 5). For example, over all trial sites, LS means for M. sinensis genotypes in year 3 ranged from 0.004–119 Mg/ha for dry biomass yield, 4–708 culms per plant, 2–241 cm for compressed circumference, and 28–399 cm for culm length (Data S1). Nineteen M. sinensis genotypes were observed to have year 3 yields exceeding 20 Mg/ha at HU or NEF, more than the highest-producing planting of the M. ×giganteus ‘1993-1780’ control in this study, and/or 50 Mg/ha at ZJU (Table 3). Three M. sinensis genotypes at HU and one at NEF exceeded 25 Mg/ha, and four exceeded 80 Mg/ha at ZJU (Table 3). Average yield of M. sinensis in year 3 at the southern trial location, ZJU, was 15.4 Mg/ha, which was 2–5-fold greater than at the five northern locations (Figure 1, Table 4). Location main effects for year 3 yield of M. sinensis accounted for 23% of the total variation if all trial locations were included in the ANOVA and 20% for just the northern trial locations (Table 5, Equation 6). However, genotype × location interactions were modest among the five northern trial sites (37.9–43.1° N) for year 3 yield but large between ZJU (29.8°N) and the northern sites (Table 5). The combined genetic group × location and genotype within genetic group × location effects were only 10% of the total variation for yield in the five northern locations but were 32% of the total variation when ZJU was included in the analysis (Table 5). Similarly, in years 2 and 3, genetic correlations for yield between pairs of locations were always negligible between ZJU and northern locations (except for a moderately negative correlation between UI and ZJU in year 2) but generally much higher among northern locations (Table 6, Equation 4). Lower genetic correlations for yield among the northern trial locations in year 3 relative to year 2 may have been due to drought stress at HU in year 3 and an unusually cold winter prior to year 3 at UI that caused winter damage in some genotypes (Table 1 and Figure S1). Genetic correlations among sites tended to be high (>0.7) for culm node number, culm dry weight, and diameter of basal internode (Data S3).
| Trait | HU | NEF | CSU | UI | KNU | ZJU | Northern locations | All locations |
|---|---|---|---|---|---|---|---|---|
| Dry biomass yield (Mg/ha) | 7.8 ± 0.2 | 6.5 ± 0.2 | 3.5 ± 0.4 | 5.7 ± 0.4 | 3.2 ± 0.2 | 15.4 ± 1.0 | 5.1 ± 0.1 | 7.6 ± 0.4 |
| Dry biomass yield (g/plant) | 1744 ± 50 | 1,477 ± 35 | 793 ± 80 | 1,289 ± 89 | 723 ± 40 | 3,494 ± 223 | 1,157 ± 29 | 1728 ± 80 |
| Compressed circumference (cm) | 26.1 ± 0.5 | 37.3 ± 0.5 | 31.1 ± 1.7 | 37.5 ± 1.6 | 56.7 ± 2.6 | 60.0 ± 1.7 | 35.0 ± 0.5 | 40.2 ± 0.7 |
| Basal circumference (cm) | 97.2 ± 1.4 | 139.6 ± 4.4 | 98.3 ± 4.7 | 145.3 ± 3.8 | 130.3 ± 3.6 | 159.8 ± 2.7 | 123.9 ± 1.9 | 131.1 ± 1.7 |
| Compressed circumference/basal circumference | 0.27 ± 0.00 | 0.28 ± 0.00 | 0.32 ± 0.01 | 0.26 ± 0.01 | 0.44 ± 0.02 | 0.36 ± 0.01 | 0.29 ± 0.00 | 0.31 ± 0.00 |
| Culm length (cm) | 218.2 ± 1.8 | 210.6 ± 1.7 | 151.3 ± 6.8 | 198.5 ± 4.2 | 212.9 ± 3.0 | 241.1 ± 3.4 | 202.9 ± 1.6 | 217.7 ± 1.7 |
| Culm node number | 11.9 ± 0.1 | 11.3 ± 0.1 | 10.0 ± 0.4 | 10.7 ± 0.3 | 10.5 ± 0.2 | 13.8 ± 0.2 | 10.9 ± 0.1 | 11.7 ± 0.1 |
| Internode length (cm) | 18.8 ± 0.2 | 19.6 ± 0.2 | 15.6 ± 0.7 | 19.3 ± 0.5 | 21.0 ± 0.4 | 18.3 ± 0.3 | 19.0 ± 0.2 | 19.0 ± 0.2 |
| Culm dry weight (g) | 10.5 ± 0.2 | 13.9 ± 0.3 | 8.6 ± 0.4 | 11.2 ± 0.6 | 12.2 ± 0.3 | 12.2 ± 0.3 | ||
| Culm volume (cm3) | 45.6 ± 1.0 | 47.8 ± 1.0 | 24.1 ± 2.2 | 42.4 ± 2.2 | 44.1 ± 2.3 | 54.7 ± 2.0 | 44.0 ± 0.9 | 49.4 ± 1.1 |
| Culm density (g cm−3) | 0.23 ± 0.00 | 0.30 ± 0.00 | 0.35 ± 0.02 | 0.27 ± 0.01 | 0.27 ± 0.00 | 0.27 ± 0.00 | ||
| Diameter of basal internode (mm) | 6.4 ± 0.1 | 5.7 ± 0.0 | 5.4 ± 0.1 | 5.6 ± 0.1 | 6.7 ± 0.2 | 5.4 ± 0.1 | 6.2 ± 0.0 | 6.2 ± 0.1 |
| Diameter of topmost internode (mm) | 3.4 ± 0.0 | 4.7 ± 0.0 | 3.2 ± 0.1 | 4.5 ± 0.1 | 2.6 ± 0.1 | 4.7 ± 0.1 | 3.9 ± 0.0 | 4.2 ± 0.0 |
| Total number of culms | 123.0 ± 3.7 | 112.5 ± 2.8 | 130.7 ± 8.7 | 170.5 ± 10.0 | 118.3 ± 5.2 | 213.9 ± 6.4 | 112.9 ± 2.3 | 123.5 ± 2.2 |
| Proportion of reproductive culms | 0.58 ± 0.01 | 0.69 ± 0.01 | 0.45 ± 0.02 | 0.55 ± 0.02 | 0.53 ± 0.01 | 0.50 ± 0.01 | 0.53 ± 0.01 | |
| Culms per footprint (# cm−2) | 0.18 ± 0.01 | 0.08 ± 0.00 | 0.20 ± 0.02 | 0.11 ± 0.01 | 0.11 ± 0.01 | 0.11 ± 0.00 | 0.10 ± 0.00 | 0.10 ± 0.00 |
Note
- Data from individual plots were Box–Cox transformed, then used to estimate least-squared means for each genotype; back-transformed values shown.
- HU = Hokkaido University; NEF = New Energy Farms; CSU = Colorado State University; UI = University of Illinois at Urbana-Champaign; KNU = Kangwon National University; ZJU = Zhejiang University.
| Source | All locations | ZJU excluded | ||||||
|---|---|---|---|---|---|---|---|---|
| df | Variance | Prop. total variance | p | df | Variance | Prop. total variance | p | |
| Location | 5 | 1.01 | 0.23 | 0.0905 | 4 | 0.64 | 0.20 | 0.0915 |
| Rep within location | 15 | 0.58 | 0.01 | 0.0108 | 13 | 0.06 | 0.02 | 0.0157 |
| Genetic group | 7 | 0.05 | 0.01 | 0.3901 | 7 | 0.42 | 0.13 | 0.0549 |
| Genetic group × location | 25 | 0.93 | 0.20 | 0.0008 | 18 | 0.05 | 0.01 | 0.0636 |
| Genotype within genetic group | 548 | 0.70 | 0.15 | <0.0001 | 489 | 0.57 | 0.18 | <0.0001 |
| Genotype within genetic group × location | 902 | 0.56 | 0.12 | <0.0001 | 690 | 0.29 | 0.09 | <0.0001 |
| Residual | 1.33 | 0.28 | <0.0001 | 1.21 | 0.37 | <0.0001 | ||
Note
- All effects were treated as random. Data were transformed by the Box–Cox method before fitting the model.
- The field trial locations were HU = Hokkaido University; NEF = New Energy Farms; CSU = Colorado State University; UI = University of Illinois at Urbana-Champaign; KNU = Kangwon National University; ZJU = Zhejiang University. df = degrees of freedom, estimated using the Satterthwaite method for fixed effects model; all other estimates based on random effects model. P = significance.
| HU | NEF | CSU | UI | KNU | ZJU | |
|---|---|---|---|---|---|---|
| Year 2 | ||||||
| HU | 0.68 | 1.03 | 0.53 | 0.89 | 0.01 | |
| NEF | 426 | 0.64 | 0.45 | 0.48 | 0.15 | |
| CSU | 56 | 57 | 1.00 | 1.62 | ||
| UI | 105 | 113 | 55 | 0.90 | −0.47 | |
| KNU | 187 | 183 | 46 | 74 | −0.03 | |
| ZJU | 242 | 200 | 4 | 18 | 67 | |
| Year 3 | ||||||
| HU | 0.61 | 0.77 | 0.22 | 0.48 | 0.11 | |
| NEF | 400 | 0.40 | 0.66 | 0.58 | 0.08 | |
| CSU | 44 | 47 | 0.47 | 0.94 | ||
| UI | 77 | 87 | 43 | 0.51 | ||
| KNU | 166 | 170 | 39 | 62 | 0.14 | |
| ZJU | 192 | 197 | 4 | 10 | 62 | |
Note
- Genetic correlations are shown in the top halves of the matrices; lower halves of the matrices indicate the number of individuals with yield data in common between each pair of sites. Correlation values are omitted for pairs of sites with fewer than 15 individuals in common. Genetic correlation was estimated as the genotypic covariance between two sites divided by the square root of the product of the genetic variance at each site (Equation 5).
- HU = Hokkaido University; NEF = New Energy Farms; CSU = Colorado State University; UI = University of Illinois at Urbana-Champaign; KNU = Kangwon National University; ZJU = Zhejiang University.
The M. sinensis genotypes that yielded best at a given trial location typically originated from a latitude that was similar to or more southern by ~5° than the trial location, though there were notable exceptions (Figure 1). Additionally, the M. sinensis genotypes that had the longest culms at a given trial location were typically from within ~10° south of the testing site (especially the Yangtze-Qinling group for the northern trial locations, and the Sichuan and SE China/tropical groups for ZJU; Figure 1). One of the largest exceptions to this geographic pattern was PMS-496 from 41.9° N in northeast China (Korean/N China genetic group), which yielded 86.4 Mg/ha in year 3 at ZJU, yet most accessions from this northern group yielded poorly at ZJU (Figure 1, Table 3). Linear modeling using bioclimatic variables (Equation 7) predicted that the highest yielding M. sinensis for temperate regions could be collected at high elevations from ~24–32° N in China (e.g., PMS-014; Table 3) followed by S Japan, whereas the highest yielding M. sinensis for subtropical regions could be collected from the Ryukyu Islands, Taiwan and the Philippines (primarily due to large predicted culm length), and to a lesser extent coastal southeast China (Figure 1b and Figure S2). Notably, our model predicted the presence of M. sinensis (yields greater than zero) along coastal southeast Russia, where it has been collected recently (Hodkinson et al., 2016), despite our study lacking genotypes of that provenance. Model terms and coefficients are provided in Table S1. Among the M. sinensis genetic groups, the S Japan group was especially notable for having produced relatively high and stable yields over all trial locations (Figure 1). For example, year 3 yields of JM0232.001 from the Hiruzen highlands in southwestern Honshu were 21.8, 11.4, and 35.6 Mg/ha at HU, KNU, and ZJU, respectively (Table 3). However, a comparison of all genetic groups over all species (not just within M. sinensis) revealed that the diploid M. ×giganteus genotypes collected in China from 29° to 37° N were the best-yielding entries at the northern trial sites HU, NEF and KNU, and some of these genotypes out-yielded the triploid M. ×giganteus ‘1993-1780’ control where it performed best, at NEF and HU (Figure 1 and Figure S3, Table 3, Data S2). The diploid M. ×giganteus genotypes that outperformed triploid M. ×giganteus ‘1993-1780’ also had compressed circumferences exceeding that of M. sinensis and M. ×giganteus ‘1993-1780’ across all sites where they were planted (Figure S3). Culm lengths of the diploid M. ×giganteus genotypes, however, were similar to those of M. sinensis (Figure S3).
Though some diploid M. ×giganteus hybrids had high yields at ZJU, the best performing groups at this southern trial location were the M. sinensis SE China/tropical, Sichuan basin, and S Japan (Figure 1, Table 3). Genotypes in the SE China/tropical and Sichuan basin groups were also especially tall at ZJU, with culm lengths of eight genotypes between 3.5 and 4.0 m (Figure 1, Figures S3 and S4; Data S1 and S2). In contrast to the ZJU results, relatively few genotypes of the M. sinensis SE China/tropical group and Sichuan basin group survived in the northern trial locations and most that did survive in the north performed poorly there. However at HU (43.1° N), PMS-014 was the second highest yielding M. sinensis genotype in year 3 (25.4 Mg/ha) and it was present in three replicates, which was exceptional given that it originated from the Sichuan group (29.7° N); PMS-014 also yielded well at ZJU, with 40.2 Mg/ha but it did not survive at NEF (Table 3). Similarly, PI423566 from the SE China/tropical group was the highest yielding M. sinensis entry at NEF with 28.2 Mg/ha in year 3, essentially in a tie for top yield with two diploid M. ×giganteus hybrids (Table 3).
Among the ornamental cultivars, there was a significant negative association between year 3 yield and the proportion of ancestry from M. sacchariflorus (via diploid M. ×giganteus ‘Purpurascens’) at HU but not at the other trial locations (Figure S5a); however, a similar negative association was observed at CSU, but too few non-hybrid individuals survived for the association to be significant (Figure S5a). Similarly, there was a negative association among the ornamental cultivars between the proportion of ancestry from M. sacchariflorus and culm length at HU, NEF, CSU, UI, and KNU (Figure S5b). In contrast, the total number of culms had a significant positive association with proportion of M. sacchariflorus ancestry at NEF, KNU, and UI for the ornamental cultivars (Figure S5c). Therefore, M. sacchariflorus ancestry did not affect yield at NEF, UI, or KNU because although hybrid ornamentals had shorter culms than the non-hybrids, they had more of them. At HU and CSU, hybrid and non-hybrid ornamental genotypes had similar numbers of culms, but because culms of hybrid ornamentals were shorter, they had lower yields than the non-hybrids. Less water availability at HU in year 3 and CSU in general (Figure S1) may have preferentially reduced the number of culms in the hybrids with greatest M. sacchariflorus ancestry, as in nature this species is typically found in riparian environments. A similar genotype-by-environment effect observed by Kaiser et al. (2015) was also associated with drought at a previous trial in southern Illinois, USA where hybrid ornamentals performed poorly.
3.2 Heritabilities, and genetic correlations between years and traits
Genotypic heritabilities for most traits and locations were high, suggesting strong potential for improvement via clonal selection within M. sinensis (Table 7, Equation 3). For example, genotypic heritabilities for yield among the trial locations in year 2 ranged from 0.49 at KNU to 0.85 at ZJU, and in year 3 from 0.71 at HU to 0.88 at ZJU. Over all northern trial sites, heritability for yield was 0.78 in year 2 and 0.84 in year 3 (Table 7). Genetic correlations between year 2 and year 3 for each of the traits were moderate to strong (Table 8, Equation 5). For yield, genetic correlations between year 2 and year 3 ranged from 0.58 at UI to 0.95 at ZJU, and were 0.76 across all five northern sites. Traits with consistently strong genetic correlations between years 2 and 3 at each trial site were: culm length, culm node number, internode length, culm dry weight, culm volume, diameter of basal internode, and diameter of topmost internode.
| Trait | HU | NEF | CSU | UI | KNU | ZJU | Northern trial locations | All trial locations |
|---|---|---|---|---|---|---|---|---|
| Year 2 | ||||||||
| Dry biomass yield (g/plant) | 0.60 | 0.81 | 0.62 | 0.92 | 0.49 | 0.85 | 0.78 | 0.72 |
| Compressed circumference (cm) | 0.65 | 0.78 | 0.71 | 0.91 | 0.61 | 0.87 | 0.70 | 0.65 |
| Basal circumference (cm) | 0.72 | 0.81 | 0.80 | 0.89 | 0.68 | 0.83 | 0.80 | 0.79 |
| Compressed circumference/basal circumference | 0.71 | 0.78 | 0.72 | 0.80 | 0.65 | 0.83 | 0.73 | 0.72 |
| Culm length (cm) | 0.81 | 0.89 | 0.92 | 0.95 | 0.75 | 0.89 | 0.89 | 0.71 |
| Culm node number | 0.80 | 0.86 | 0.77 | 0.95 | 0.68 | 0.89 | 0.92 | 0.80 |
| Internode length (cm) | 0.82 | 0.86 | 0.87 | 0.96 | 0.59 | 0.83 | 0.87 | 0.81 |
| Culm dry weight (g) | 0.77 | 0.88 | 0.84 | 0.96 | 0.33 | 0.92 | ||
| Culm volume (cm3) | 0.83 | 0.88 | 0.87 | 0.95 | 0.53 | 0.92 | 0.92 | 0.82 |
| Culm density (g cm−3) | 0.81 | 0.83 | 0.74 | 0.89 | 0.84 | 0.91 | ||
| Diameter of basal internode (mm) | 0.80 | 0.87 | 0.84 | 0.95 | 0.27 | 0.83 | 0.93 | 0.90 |
| Diameter of topmost internode (mm) | 0.83 | 0.84 | 0.69 | 0.94 | 0.17 | 0.89 | 0.88 | 0.80 |
| Total number of culms | 0.67 | 0.82 | 0.63 | 0.90 | 0.53 | 0.77 | 0.77 | 0.77 |
| Proportion of reproductive culms | 0.84 | 0.64 | 0.92 | 0.72 | 0.85 | 0.45 | ||
| Culms per footprint (# cm−2) | 0.74 | 0.81 | 0.84 | 0.88 | 0.65 | 0.77 | 0.89 | 0.88 |
| Year 3 | ||||||||
| Dry biomass yield (g/plant) | 0.71 | 0.83 | 0.74 | 0.74 | 0.76 | 0.88 | 0.84 | 0.77 |
| Compressed circumference (cm) | 0.68 | 0.80 | 0.72 | 0.69 | 0.61 | 0.81 | 0.71 | 0.70 |
| Basal circumference (cm) | 0.66 | 0.91 | 0.70 | 0.79 | 0.44 | 0.81 | 0.73 | 0.71 |
| Compressed circumference/basal circumference | 0.63 | 0.79 | 0.73 | 0.60 | 0.59 | 0.79 | 0.66 | 0.69 |
| Culm length (cm) | 0.84 | 0.92 | 0.96 | 0.94 | 0.90 | 0.90 | 0.92 | 0.81 |
| Culm node number | 0.87 | 0.90 | 0.85 | 0.91 | 0.32 | 0.82 | 0.93 | 0.90 |
| Internode length (cm) | 0.79 | 0.90 | 0.83 | 0.93 | 0.30 | 0.68 | 0.89 | 0.83 |
| Culm dry weight (g) | 0.88 | 0.93 | 0.39 | 0.93 | 0.95 | |||
| Culm volume (cm3) | 0.86 | 0.93 | 0.94 | 0.93 | 0.83 | 0.92 | 0.93 | 0.89 |
| Culm density (g cm−3) | 0.74 | 0.92 | 0.56 | 0.74 | 0.86 | |||
| Diameter of basal internode (mm) | 0.85 | 0.90 | 0.94 | 0.90 | 0.65 | 0.87 | 0.92 | 0.92 |
| Diameter of topmost internode (mm) | 0.77 | 0.91 | 0.84 | 0.91 | 0.78 | 0.87 | 0.92 | 0.83 |
| Total number of culms | 0.82 | 0.78 | 0.64 | 0.75 | 0.59 | 0.70 | 0.80 | 0.78 |
| Proportion of reproductive culms | 0.74 | 0.78 | 0.74 | 0.79 | 0.83 | 0.66 | 0.00 | |
| Culms per footprint (# cm−2) | 0.79 | 0.78 | 0.91 | 0.87 | 0.50 | 0.76 | 0.83 | 0.83 |
Note
- HU = Hokkaido University; NEF = New Energy Farms; CSU = Colorado State University; UI = University of Illinois at Urbana-Champaign; KNU = Kangwon National University; ZJU = Zhejiang University.
| Trait | HU | NEF | CSU | UI | KNU | ZJU | Northern trial locations | All trial locations |
|---|---|---|---|---|---|---|---|---|
| Dry biomass yield (g/plant) | 0.85 | 0.69 | 0.77 | 0.58 | 0.68 | 0.95 | 0.76 | 0.99 |
| Compressed circumference (cm) | 0.69 | 0.53 | 0.65 | 0.66 | 0.94 | 0.74 | 0.92 | 1.23 |
| Basal circumference (cm) | 0.67 | 0.67 | 0.89 | 0.83 | 0.91 | 0.87 | 0.90 | 1.00 |
| Compressed circumference/basal circumference | 0.56 | 0.61 | 0.58 | 0.67 | 0.78 | 0.68 | 0.93 | 1.14 |
| Culm length (cm) | 0.90 | 0.85 | 0.91 | 0.94 | 0.76 | 0.89 | 0.94 | 1.02 |
| Culm node number | 0.84 | 0.97 | 1.02 | 0.89 | 1.20 | 0.90 | 0.94 | 0.98 |
| Internode length (cm) | 0.76 | 0.88 | 0.98 | 0.89 | 0.76 | 0.68 | 0.92 | 0.91 |
| Culm dry weight (g) | 0.91 | 0.90 | 1.19 | 0.90 | 0.95 | 0.95 | ||
| Culm volume (cm3) | 0.92 | 0.92 | 0.90 | 0.92 | 0.92 | 0.95 | 0.98 | 1.01 |
| Culm density (g cm−3) | 0.66 | 0.78 | 0.45 | 0.66 | 0.88 | 0.88 | ||
| Diameter of basal internode (mm) | 0.91 | 0.90 | 0.84 | 0.87 | 0.99 | 0.99 | 1.01 | 0.99 |
| Diameter of topmost internode (mm) | 0.94 | 0.82 | 1.05 | 0.88 | 0.98 | 0.96 | 0.90 | 0.95 |
| Total number of culms | 0.85 | 0.83 | 0.95 | 0.72 | 0.59 | 0.50 | 0.85 | 0.67 |
| Proportion of reproductive culms | 0.93 | 0.55 | 1.15 | 0.38 | 0.93 | 0.86 | ||
| Culms per footprint (# cm−2) | 0.56 | 0.85 | 0.94 | 0.86 | 0.96 | 0.70 | 0.82 | 0.78 |
Note
- All traits were Box–Cox transformed. Genetic correlation was estimated as the genetic covariance of two traits divided by the square root of the product of the genetic variance of each trait (Equation 5).
- HU = Hokkaido University; NEF = New Energy Farms; CSU = Colorado State University; UI = University of Illinois at Urbana-Champaign; KNU = Kangwon National University; ZJU = Zhejiang University.
Genetic correlations between yield-component traits and yield in year 3 were mostly moderate, with some exceptions (Table 9, Equation 5). Among all yield component traits, compressed circumference had the highest correlation with yield, ranging, in year 3, from 0.91 at UI to >0.99 at KNU. In comparison, year 3 genetic correlations between culm length and yield ranged from 0.46 at NEF to 0.88 at ZJU, with 0.62 over all five northern trial sites. Mostly low or negligible year 3 genetic correlations with yield were observed for internode length, total culm number, and the proportion of reproductive culms (RCmN/TCmN). Negligible to moderate negative year 3 genetic correlations were observed between culms per footprint (TCmN/A) and yield at each trial location.
| HU | NEF | CSU | UI | KNU | ZJU | Northern trial locations | All trial locations | |
|---|---|---|---|---|---|---|---|---|
| Compressed circumference (cm) | 0.96 | 0.93 | 0.95 | 0.91 | 1.00 | 0.96 | 1.04 | 1.06 |
| Basal circumference (cm) | 0.95 | 0.24 | 0.79 | 0.71 | 0.61 | 0.76 | 0.64 | 0.50 |
| Compressed circumference/basal circumference | 0.43 | 0.64 | 0.57 | 0.59 | 0.93 | 0.84 | 0.71 | 0.96 |
| Culm length (cm) | 0.65 | 0.46 | 0.72 | 0.49 | 0.57 | 0.88 | 0.63 | 0.52 |
| Culm node number | 0.55 | 0.50 | 0.39 | 0.16 | 0.64 | 0.48 | 0.57 | 0.64 |
| Internode length (cm) | 0.01 | −0.16 | 0.29 | 0.16 | 0.43 | 0.38 | −0.11 | −0.41 |
| Culm dry weight (g) | 0.71 | 0.66 | 0.85 | 0.38 | 0.72 | |||
| Culm volume (cm3) | 0.61 | 0.48 | 0.68 | 0.33 | 0.51 | 0.85 | 0.62 | 0.58 |
| Culm density (g cm−3) | 0.36 | 0.38 | −0.87 | 0.32 | 0.51 | |||
| Diameter of basal internode (mm) | 0.54 | 0.47 | 0.36 | 0.07 | 0.52 | 0.83 | 0.54 | 0.67 |
| Diameter of topmost internode (mm) | 0.44 | 0.37 | 0.59 | 0.39 | 0.17 | 0.66 | 0.48 | 0.29 |
| Total number of culms | 0.39 | 0.42 | 0.39 | 0.55 | 0.29 | 0.46 | 0.23 | 0.08 |
| Proportion of reproductive culms | 0.02 | 0.12 | 0.23 | 0.56 | 0.52 | −0.19 | ZH | |
| Culms per footprint (# cm−2) | −0.64 | −0.04 | −0.53 | −0.08 | −0.13 | −0.18 | −0.50 | −0.53 |
Note
- All traits were Box–Cox transformed. Genetic correlation was estimated as the genetic covariance of two traits divided by the square root of the product of the genetic variance of each trait (Equation 5).
- HU = Hokkaido University; NEF = New Energy Farms; CSU = Colorado State University; UI = University of Illinois at Urbana-Champaign; KNU = Kangwon National University; ZJU = Zhejiang University. ZH=not calculated due to zero heritability.
Genotypes with relatively many culms typically compensated to varying degrees with thinner, less voluminous, and lighter culms than genotypes with fewer culms (Data S4, Equation 5). At the northern trial locations, culm basal and topmost diameters, culm volume, and culm dry weights were negatively correlated with the total number of culms in year 3 (Data S4). Genetic correlations in year 3 between total number of culms and diameter of topmost internode were negative but weak at all locations (−0.02 at ZJU and from −0.27 at HU to −0.40 at CSU for the northern trial locations). However, for diameter of basal internode, year 3 genetic correlations with total number of culms were strongly negative at CSU, UI, and KNU (−0.69, −0.72, and −0.80, respectively) and weakly negative at HU and NEF (−0.38 and −0.44, respectively). Year 3 genetic correlations between culm volume and total number of culms were negative and weak at HU, NEF, CSU, and UI (−0.28 to −0.49) and strongly negative at KNU (−0.70). Similarly, year 3 genetic correlations between culm dry weight and total number of culms were negative and weak at HU, NEF, CSU, and UI (−0.28 to −0.47). However, genetic correlations between total number of culms and culm length were negligible. Moreover, negative year 3 genetic correlations between basal circumference and number of culms per footprint were strong at NEF (−0.73) and CSU (−0.80), moderate at HU (−0.51) and UI (−0.42), but negligible at KNU (−0.16) and ZJU (−0.33), indicating that genotypes with larger basal circumferences typically had fewer stems per area (more space between stems and/or thicker stems) than those with smaller footprints (Data S4).
4 DISCUSSION
4.1 Yield potential, adaptation and implications for breeding
A key finding of this study has been the identification of which M. sinensis genetic groups yield the most, and in which production environments. Given that the M. sinensis genetic groups originate from known geographies (Figure 1, Data S1), the results of the current study are expected to provide useful guidance to researchers regardless of whether their M. sinensis genotypes of interest were included in the present study. Moreover, our estimates of genotype-by-location interactions and genetic correlations among trial locations for M. sinensis yield indicated that information from one northern trial location may be expected to be moderately informative about performance at other northern trial locations (e.g., HU and NEF), whereas performance of genotypes at southern trial locations may be substantially different from and not well predicted by performance at northern trial locations and vice versa. Notably, we found that the S Japan M. sinensis group had high and stable yields over the northern and southern trial locations tested, and was the overall best M. sinensis group at the northern trial sites, which will be useful for breeding biomass cultivars that have broad adaptation. Among all the non-ornamental M. sinensis groups, the S Japan group had on average the greatest total number of culms in year 3 at all locations tested (Data S2), although year 3 genetic correlations between total culm number and yield at each trial location were mostly low (Table 9). In contrast to the wild accessions from S Japan, the S Japan-derived ornamental group, which mostly included individuals selected for short stature, was with few exceptions not advantageous for yield (Figure 1), yet most prior breeding of Miscanthus in the US and Europe has been based on the ornamental germplasm. For subtropical environments (hardiness zone 8 or warmer) such as ZJU, we expect that the M. sinensis SE China/tropical, Sichuan basin, and S Japan groups will be the most promising source of M. sinensis parents to breed improved biomass cultivars. To the best of our knowledge, this is the first study to identify differences in yield potential among M. sinensis genetic groups for different production environments.
In addition to quantifying differences among M. sinensis genetic groups for yield, we also identified specific genotypes with outstanding yield potential for different production environments. At HU and NEF, two of the northern trial locations, where the biomass cultivar control triploid M. ×giganteus ‘1993-1780’ yielded well (~20 Mg/ha), we observed six M. sinensis genotypes at HU and one at NEF with greater yield than the control in year 3 (20.7–30.9 Mg/ha; Table 3); such high yields have commercial potential. Similarly, high yields (15.0–22.4 Mg/ha) have been previously reported for M. sinensis genotypes that were collected from Honshu, Japan and evaluated in Sweden, Denmark and Portugal (Clifton-Brown et al., 2001), and from a Chinese germplasm panel evaluated at a trial in Wuhan, China in which rare individuals had high year 2 yields (~20–38 Mg/ha) (Zhao et al., 2013). However, at our southern trial location, ZJU, dry biomass yields of the seven most productive M. sinensis genotypes (63.7–119.1 Mg/ha; Table 3) were similar to maximum experimental dry matter yields obtained for sugarcane in Australia, Hawaii, and Louisiana (Bischoff et al., 2008; Tew & Cobill, 2008) and for the C4 grass Echinochloa polystachya growing in the Amazon floodplain with no water or nutrient limitation, which has been suggested to represent the maximum productivity of a C4 crop (Piedade, Junk, & Long, 1991). Such high yields in Miscanthus have not been reported previously. The exceptionally high yields estimated for some M. sinensis genotypes at ZJU were likely due to a combination of highly conducive growing conditions and small-plot bias. In small plots and especially single-plant plots, a tall individual can lean out and take canopy space from neighboring plots containing shorter plants, which would otherwise be unavailable if the tall genotype had been grown in a large monoculture. Additionally, the growing conditions that favored high biomass production at ZJU included high soil fertility and a high water table associated with the land having previously been in rice production, a growing season of ~10 month duration with a large number of growing degree days (USDA hardiness zone 9; Table 1), and a carefully managed trial. Thus, exceptionally high yields were achieved for some entries at ZJU; however, determining exactly how exceptional the yield potential is for each of these entries will require further testing in large-plot trials. Overall, the results of this study suggest that some Miscanthus genotypes, when grown in a humid subtropical climate such as in Zhejiang province China or the southern coastal plain of the US, may be able to achieve dry matter yields similar to that of sugarcane grown in the tropics.
For subtropical production environments where light to moderate freezes are common, select Miscanthus genotypes or intergeneric hybrids between Miscanthus and Saccharum (i.e., miscanes), may have an adaptive advantage over sugarcane. In Tifton, GA (31.5° N), Knoll et al. (2013) observed that two new energycane selections (Ho 06-9001 and Ho 06-9002) had maximum dry matter yields greater than 34 Mg/ha, whereas maximum yields of the sugarcane cultivar controls were less than 15 Mg/ha, highlighting the importance of selecting biomass grass crops for adaptation to their potential production environments. Moreover, top yields of the energycane and sugarcane entries at Tifton were in year 2, followed by a substantial decline in year 3 (Knoll et al., 2013), which is typical for these short-lived (usually 3–5 years) crops. In contrast, Miscanthus yields in our study increased each year from years 2–4 (Data S2), which was consistent with previous studies documenting that Miscanthus typically reaches a yield plateau during years 2–5 and can remain highly productive for more than 10 years (Heaton et al., 2004; Jones & Walsh, 2001; Lewandowski, Clifton-Brown, Scurlock, & Huisman, 2000). Thus, for subtropical environments, some Miscanthus germplasm, such as the highest-yielding genotypes we observed at ZJU, may have both short-term and long-term biomass yield advantages over sugarcane. Moreover, the energycanes Ho 06-9001 and Ho 06-9002 had a high proportion of ancestry from Saccharum spontaneum, a wild species with thin stems (Tew & Cobill, 2008), which suggests that a similar breeding strategy of combining commercial sugarcane genotypes with M. sinensis that are highly productive in and adapted to subtropical environments may result greater gains still.
Atypical M. sinensis genotypes that performed well at trial sites that were far north or far south of their collection sites were also observed, and these likely represent a valuable breeding opportunity. Though the M. sinensis SE China/tropical and Sichuan basin were the highest-yielding groups at ZJU, few genotypes of these southern groups performed well at the northern trial sites. However, rare individuals from the SE China/tropical and Sichuan basin groups were among the highest-yielding entries at some of the northern trial sites, pointing the way toward a useful breeding strategy. If high yield potential from the SE China/tropical and Sichuan basin groups can be combined with greater winter hardiness from more northerly adapted Miscanthus, it may be possible to make large gains in yield potential for northern production environments. Similarly, rare genotypes from northern M. sinensis groups that performed well at ZJU may have advantageous alleles that are rare or absent from southern groups. Thus, we might expect to obtain useful transgressive segregants by crossing individuals of differing provenance but each with complementary genes for high yield potential in a particular environment (Rieseberg, Archer, & Wayne, 1999).
The results of this study and previous studies indicate that interspecific progeny of M. sinensis and M. sacchariflorus were frequently, but not always, high yielding and vigorous, likely due to heterosis and/or transgressive segregation; this advantage has been conferred regardless of whether the progeny were diploid, triploid or tetraploid. In the current study, diploid M. ×giganteus F1 genotypes from 29° to 37° N in China were the highest-yielding entries at the northern trial locations, with yields at NEF and HU that were ~1.5- and ~2-fold greater than the high-yielding control, M. ×giganteus ‘1993-1780’ (Table 3, Figure 1). The best performing diploid M. ×giganteus, PMS-430 and PMS-279, were collected from even lower latitudes (29–30° N) than the putative origin of M. ×giganteus ‘1993-1780’ (~35.4° N; Table S2), with the M. sinensis portion of their genome originating from the SE China/tropical group (Clark et al., 2014); we hypothesize that sufficient winter hardiness for the northern trial locations was inherited primarily from the M. sacchariflorus parents. Yan et al. (2012) compared 31 M. sinensis and 48 M. sacchariflorus Chinese populations at Xilinhot, a cold-winter site in north China (43.9° N), and observed that for a given latitude of origin, overwintering survival of M. sacchariflorus accessions was substantially greater than for M. sinensis accessions. In contrast to the high yields of PMS-430 and PMS-279, the diploid M. ×giganteus ‘Purpurascens’ and the ornamental M. sinensis descended from it (Clark et al., 2015) were low-yielding compared to most M. sinensis (Table 3, Figure 1). Clifton-Brown et al. (2001) also found that diploid M. ×giganteus selected from a cross and grown at five locations in Europe typically, though not in all cases, had a yield advantage over selected M. sinensis genotypes collected in Japan. Uwatoko, Tamura, Yamashita, and Gau (2016) observed that seven new triploid M. ×giganteus genotypes collected from the wild in Japan had yields that were similar to the high-yielding control ‘1993-1780’, when grown in a field trial in Koshi, Japan (32.9° N). Matumura, Hasegawa, and Saijoh (1987), studying a cross between diploid M. sinensis and tetraploid M. sacchariflorus, observed that one triploid progeny had biomass yields intermediate to its parents but its tetraploid sibling yielded about twice as much as its highest-yielding parent. Thus, the development and testing of new M. ×giganteus genotypes are expected to be an important breeding strategy for obtaining new higher-yielding cultivars of Miscanthus.
4.2 Yield-component traits for predicting M. sinensis yields
Compressed circumference, which serves as an easily measured proxy for culm diameter multiplied by the square root of the number of culms (genetic correlation of 0.85 across all sites in year 3), was a strong predictor of yield in the current study (genetic correlations ≥0.91 for each trial location; Table 9) and the best predictor of yield among all the yield-component traits we evaluated. Both yield and compressed circumference had a larger environmental component than many other traits (Table 7), but the high genetic correlation between them indicated that the phenotypic variation attributable to genotype followed a very similar pattern between yield and compressed circumference. Culm length, which had a moderate to high genetic correlation with yield (0.46–0.88; Table 9), is also easily measured, and together with compressed circumference provides a three-dimensional model of overall plant size. Gifford, Chae, Swaminathan, Moose, and Juvik (2015) also observed a strong genetic correlation between compressed circumference and yield (0.88) and a moderate genetic correlation between plant height and yield (0.54) in an F1 population of M. sinensis ‘Grosse Fountaine’ × M. sinensis ‘Undine’ evaluated at Urbana, Illinois. Similarly, Slavov et al. (2014) observed a moderate genetic correlation between plant height and yield (0.65) in a panel of 138 M. sinensis genotypes phenotyped near Aberystwyth, UK. Previously published phenotypic correlations between height and yield for Miscanthus have also been mostly moderate, though less frequently high (Anzoua, Suzuki, Fujita, Toma, & Yamada, 2015; Clifton-Brown et al., 2001; Jezowski, 2008; Nie et al., 2016; Yan et al., 2012; Zhao et al., 2013) or low (Nie et al., 2016). Thus, compressed circumference and culm length (or height) are expected to be consistently good predictors of yield in M. sinensis populations, while being considerably less expensive to measure.
We also found that culm dry weight was a potentially useful predictor of yield, with genetic correlations ranging from 0.38 to 0.85 (Table 9). Similarly, Lim et al. (2014) observed a high phenotypic correlation between culm dry weight and yield (0.84) for 42 M. sinensis genotypes collected from South Korea, Kyushu, Japan, and southeastern Russia when evaluated in a field trial at Suwon, South Korea.
Genetic tradeoffs between total number of culms per plant, plant footprint, and culm diameter, volume and dry weight, were observed among the M. sinensis genotypes in this study. Genotypes with larger footprints tended to have fewer and sometimes thicker culms (Data S4). Also, genotypes with many culms typically had thinner and lighter culms. However, these tradeoffs were partial and varied in magnitude, sometimes substantially, by trial location. Such variability in partitioning tradeoffs is consistent with previous studies, which have reported highly diverse estimates for correlations between yield and number of shoots, and between yield and stem diameter for M. sinensis (Clifton-Brown et al., 2001; Gifford et al., 2015; Jezowski, 2008; Nie et al., 2016; Slavov et al., 2014; Yan et al., 2012). Moreover, exceptions to these tradeoffs may be important, as the broadly adapted and high-yielding S Japan group had the greatest average number of culms among the M. sinensis groups at all trial locations, but also the largest or nearly largest basal circumference at all locations tested (Data S2); diameter of basal internode was variable over locations but relatively high at HU, NEF, and ZJU. Thus a desirable ideotype for a biomass cultivar of M. sinensis may be a plant that has a large compressed circumference obtained via many thick and heavy culms, a large footprint, and long (>3 m) culms.
5 CONCLUSIONS
Large genotype-by-environment interactions between the southern trial site, ZJU, and the northern trial sites indicates the need to breed M. sinensis separately for southern and northern production zones. However, good concordance of M. sinensis genotypic performance among northern trial locations, even those on different continents, will facilitate breeding for northern production zones. To establish a new M. sinensis breeding program for high biomass yield, we recommend conducting field trials that are focused on genotypes belonging to the genetic groups that are expected to perform best in the target environment; these genetic groups are: S Japan for all environments, Sichuan and SE China/tropical for hardiness zones 8 or warmer, and Korea/N China and Yangtze-Qinling for hardiness zones 7 or colder. Our climate models suggest that additional germplasm collections should be performed in mountainous regions of China south of 32° N for hardiness zones 7 or colder, and in the Ryukyu Islands, Taiwan, and the Philippines for hardiness zones 8 or warmer.
ACKNOWLEDGEMENTS
This research was supported by the DOE Office of Science, Office of Biological and Environmental Research (BER), grant no. DE-SC0006634. New Energy Farms provided in-kind support. We especially thank our field crews at all institutions for many hours planting and maintaining field trials and taking measurements.




