Cascading Effects Within Soil Food Web Amplify Fungal Biomass and Necromass Production
Funding: This study was funded by the National Natural Science Foundation of China (42077046), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA28020401 and XDA28080000), the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2021228), the Young Scientist Group Project of the Northeast Institute of Geography and Agroecology (2022QNXZ04), and the RUDN University Strategic Academic Leadership Program.
ABSTRACT
Soil food webs regulate microbial biomass and necromass production and are therefore critical for carbon sequestration. The mechanisms by which top predators regulate microbial necromass formation across multitrophic levels in the real-world soil food web remain nearly unknown. This study investigates how top-down forces—from omnivorous-predaceous nematodes to microbivorous nematodes and microbes—affect the formation of microbial necromass within tritrophic food webs under contrasting tillage regimes (tillage (till) vs. no-tillage (no-till)) on black soils (Mollisols), using a 1-year 13C-labeled straw in situ tracing experiment integrated with a long-term (> 5 years) tillage trial. The fungal-to-bacterial necromass ratio increased strongly in the no-till soil compared to the till soil, with omnivores-predators being the key factor for these changes. In the no-till soil, abundant and diverse omnivores-predators (46% and 67% higher in abundance and richness than in the till soil) created a typical predator–prey relationship with fungivores. This relationship was characterized by heavy predation on fungivores (51% of omnivore-predator diet) and opposite 1-year dynamics of 13C content between omnivores-predators and fungivores. Such a predator–prey relationship substantially reduced fungivore activity (73% and 90% decrease in 13C content and enrichment rate), while accompanied by increased fungal activity (64% and 50% increase in 13C content and enrichment rate) in the no-till soil compared to the till soil. This predator-driven cascade down the food chain amplified the fungal contribution to the fungal-to-bacterial necromass ratio. Conversely, these interactions, disrupted by continuous tillage, weakened fungal functions by interrupting the trophic cascade. In conclusion, these tiny yet ubiquitous omnivorous-predaceous nematodes exert a disproportionate impact on necromass formation by boosting fungal biomass and activity. Further manipulative experiments targeting multi-trophic interactions are essential to disentangle the mechanisms of microbial necromass formation, given the inherent complexity of soil food webs and the observational nature of this study.
1 Introduction
Soil carbon (C) storage plays a dual role in mitigating global warming and bridging the larger soil health gap (in line with the ethos of “4 per 1000” initiative) (Lal 2018; Minasny et al. 2017). The accumulation of microbial necromass has been conceptually and empirically recognized as a crucial mechanism for organic C storage in soils (Lehmann and Kleber 2015; Liang et al. 2017; Sokol et al. 2022). Fungal necromass, being more recalcitrant than bacterial necromass, is particularly effective in contributing to long-term soil C storage by associating within or on soil particles to form the stable soil C pool (Klink et al. 2022; Whalen et al. 2022; Yang et al. 2022). Necromass formation is closely linked to microbial growth-death cycles, which are driven by the bidirectional forces of resource-dependent bottom-up control and predator-induced top-down control (Buckeridge et al. 2020; Camenzind et al. 2023; Liang et al. 2017). The impact of basal resources (e.g., their bioavailability and input levels) on the microbial community composition, along with the quality and quantity of their necromass, has been extensively studied (Angst et al. 2024; Hu et al. 2022; Yang et al. 2022; Zhou et al. 2023). In contrast, the influence of soil fauna on the microbe-microbial necromass (i.e., both living microbes and microbial necromass) dynamics via top-down control remains underexplored.
Top-down control propagates through the soil food web in the opposite direction to bottom-up control, and these two opposing forces often interact synergistically (Dyer and Letourneau 2003; Riggi and Bommarco 2019). Once bottom-up processes increase predator abundance, diversity, or energetics, top-down processes triggered by predators could ensue. In this way, soil fauna can influence microbial growth-death turnover and the subsequent necromass formation through direct predation and indirect trophic cascades. The latter regulation is known as ‘cascading effects’, in which indirect effects of top predators on microbial activities are exerted via intermediate microbivores in the soil (Buchkowski 2016; Thakur and Geisen 2019). For example, microbivores such as nematodes and protozoa can reduce microbial biomass by up to 16% through direct feeding (Hou et al. 2023; Trap et al. 2016), lowering necromass accumulation. However, predators that feed on microbivores can alleviate this reduction by limiting microbivore activity, thereby reducing predation pressure on microbes and fostering microbial growth and necromass formation (Camenzind et al. 2023; Kou et al. 2023). Such consumer-induced trophic interactions are very common in soil ecosystems (Pausch et al. 2016) and, although not always directly observed, collectively shape the microbe-microbial necromass dynamics through top-down control.
Although soil food web energy modeling predicts that predators at higher trophic levels can disproportionately influence the C cycle with only small changes in the predator–prey relationships (De Ruiter et al. 2005; Holtkamp et al. 2011; Reyns et al. 2020), robust experimental evidence supporting this prediction remains scarce. Empirical studies of top-down regulation predominantly rely on a simplified bitrophic approach—manipulating the presence or absence of predators under controlled conditions—and focus on microbial community changes (e.g., abundance, biomass, and community composition) (Asiloglu et al. 2021; Erktan et al. 2020b; Lenoir et al. 2007; Lucas et al. 2020). Functional outcomes like necromass formation are neglected. Such reductionist approaches, though mechanistically informative, fail to capture the context-dependent complexity of real-world soil food webs, which typically comprise three or four trophic levels and are strongly shaped by agricultural management (van der Putten et al. 2004; Wyckhuys et al. 2021).
Tillage (till) and no-tillage (no-till; a classic form of conservation tillage) systems, two contrasting and widely used agricultural management practices, have long been known to profoundly affect soil biodiversity and food web structure (Betancur-Corredor et al. 2022; Hendrix et al. 1986). Compared to the till system, no-till not only increases the abundance of higher trophic groups, but also expands the length and complexity of the food chain (Henneron et al. 2015; Kladivko 2001; Zhang et al. 2021). A growing body of evidence has further demonstrated that the no-till system is an effective approach to stimulate fungal growth and their necromass accumulation, which is critical for long-term soil C stabilization (Sae-Tun et al. 2022; Yang et al. 2022; Zhou et al. 2023). These features in trophic interactions and predator–prey relationships resulting from contrasting tillage practices are expected to impose varying degrees and patterns of top-down control over microbe-microbial necromass dynamics.
- Omnivores-predators have a more pronounced effect on microbial necromass formation in no-till than in till soils. This is mainly due to a cascading effect of omnivores-predators on fungi, resulting in a greater fungal contribution to microbial necromass in no-till compared to till soils.
- This cascade effect in the no-till soil primarily involves the top-level omnivores-predators limiting the activity of intermediate-level fungivores, thereby indirectly stimulating fungal growth and necromass formation at lower trophic levels.
To test these hypotheses, long-term (> 5 years) field experiments including conventional till and no-till management were used to construct a natural and stable bottom-up, resource-driven network of functional groups to assess the role of omnivores-predators within the soil food webs. In parallel, a 1-year 13C in situ field tracing experiment using 13C-labeled straw was overlaid on the long-term field experiment to investigate the top-down control exerted by omnivores-predators. During the tracing period, the predation strategies of omnivores-predators were assessed, and their effects on microbial necromass formation and lower trophic groups (i.e., microbivores and microbes) were monitored. We developed a realistic framework to understand how top predators regulate fungi and fungal necromass by amplifying cascading effects. The results also highlight that active soil management can harness these tiny yet ubiquitous omnivorous-predaceous nematodes to support the 4 per 1000 goal (COP21 2015) by reinforcing fungal function in necromass accumulation for soil C sequestration.
2 Materials and Methods
2.1 Long-Term Field Experiment
2.1.1 Study Site, Experimental Design and Soil Sampling
The experimental site is located at the Changchun Agroecology Observation Station (44°00′ N, 125°24′ E) of the Northeast Institute of Geography and Agroecology. This region features a continental monsoon climate, with a mean annual temperature of 4°C and a mean annual precipitation of 550 mm. The soil is derived from loamy loess and is classified as a black soil (Hapudoll according to USDA Soil Taxonomy; Soil survey staff 1999). The long-term experiment began in autumn 2011, with initial soil pH at 7.3 and soil C and nitrogen (N) contents at 17.1 g C kg−1 and 1.5 g N kg−1, respectively. Prior to the trial, the field had been continuously cropped with maize (Zea mays L.) for 30 years.
The long-term parent field experiment followed a randomized complete block design, with three tillage treatments, including no-till, reduced till, and conventional till, and two cropping systems such as maize-soybean rotation and continuous maize cropping. Each treatment had four replicates with each plot area of 7.8 × 25 m. In this study, only the plots with maize monoculture under conventional till and no-till, combined with straw return to the field, were considered (Figure 1A).
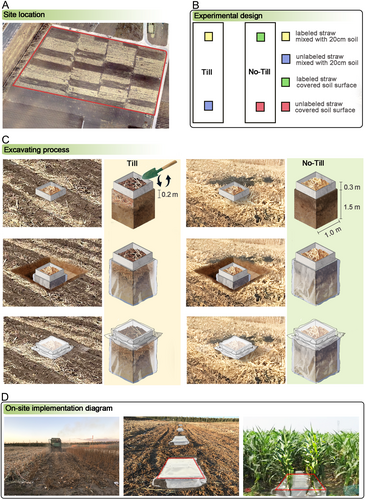
For the no-till treatment, there was no soil disturbance except for planting, which was performed using a no-till planter (KINZE-3000NT, Williamsburg, IA, USA). For the till treatment, the soil was annually fall moldboard ploughed to a depth of 20 cm after maize harvest, followed by discing (7.5–10 cm in depth), harrowing, and ridge-building during the seedbed preparation process in the following spring. After harvest, maize straw in all treatments was returned to the soil to prevent water and wind erosion during the winter and early spring. In the no-till plots, maize straw was cut into about 30 cm pieces and manually placed among 30–35 cm standing stubble. The straw in the till plots was removed before and manually replaced on the soil surface after fall moldboard ploughing, and then mixed with the plow layer by discing and cultivation in the following spring. The treatments were applied each year since the initiation of the parent experiment in 2011. Based on the maize yield and harvest index, the annual straw return amount to the field was approximately 11 Mg ha−1 in both no-till and till treatments. Consequently, this straw amount was used for the follow-up in situ 13C straw tracing experiment (Section 2.2).
Soil samples were collected in late April of 2017 and 2018, as this timing best reflects the impact of tillage practices without being influenced by the growing season of maize. Five soil cores were randomly taken from the center of each plot using a hand auger (4.8 cm inner diameter) to a depth of 20 cm. These cores were then combined to form one representative soil sample for each plot.
2.1.2 Soil Nematode and Microbial Community Analysis
Nematodes were extracted from 50 g of fresh soil using the cotton wool filtration method (Liang et al. 2009). In each soil sample, the total number of nematodes was counted, and at least 100 nematodes were identified to the genus level (see Table S1 for taxa list). Nematodes were classified into four trophic groups: bacterivores (BV), fungivores (FV), plant-parasites (PP), and omnivores-predators (OP) (Bongers 1994).
The soil microbial community was assessed using phospholipid fatty acid (PLFA) analysis. PLFAs were extracted, fractionated, and purified according to Bossio et al. (1998), and their content was quantified using an Agilent 6850 series Gas Chromatograph with MIDI peak identification software (Version 4.5; MIDI Inc., Newark, DE, USA). PLFA biomarkers were categorized into three groups: actinomycetes, general bacteria (including gram-negative, gram-positive, and non-specific bacteria), and fungi. Detailed classification of biomarkers is provided in Table S2.
2.1.3 Soil Microbial Necromass Determination
Soil microbial necromass was assessed based on amino sugars, following the extraction and quantification methods of Zhang and Amelung (1996). The content of amino sugars was determined by gas chromatography-flame ionization detection (GC-FID 6890A; Agilent Technologies). Muramic acid served as a biomarker for bacterial necromass, while fungal glucosamine was calculated by subtracting bacterial glucosamine from the total glucosamine to estimate fungal necromass. The C content of bacterial and fungal necromass was calculated from their respective glucosamine masses and the C portion of the glucosamine molecular structure (Luo et al. 2021; see Data S1 for detailed formulae).
2.2 13C In Situ Tracing Experiment
2.2.1 Experiment Design and Soil Sampling
The 13C in situ tracing experiment was conducted in early spring 2017. Two 1.0 m × 1.0 m subplots were established in each plot of the no-till and till practices in the long-term parent experiment, with a minimum separation of 5 m to avoid interference between subplots. Each subplot was enclosed with PVC boards (3 mm thickness, 33 cm height), which were installed 25 cm depth into the soil. A 1.5 m deep trench was carefully excavated around each subplot, and the central undisturbed soil column was wrapped with polyethylene plastic film to prevent cross-contamination between labeled and unlabeled areas caused by lateral water flow or soil animal movement. Afterward, the excavated soil was back-filled into the trench in the reverse order of excavation to minimize disruption and maintain the original soil layering as much as possible. Photographs of the sequence of steps for the procedure are shown in Figure 1B–D.
In each plot, two subplots were randomly assigned to receive either 13C-labeled maize straw (mean δ13C = 272‰) or unlabeled maize straw (mean δ13C = −11.67‰). Based on the average annual field straw return (described in Section 2.1.1), 1.1 kg maize straw (cut into approximately 2 cm pieces) was added to each subplot. In the no-till subplots, the maize straw was evenly spread on the soil surface, whereas in the till subplots, it was thoroughly mixed with the top 20 cm of soil to simulate mixing by tillage. The labeled straw was produced using the 13CO2 pulse-labeling method in a sealed chamber, with 18 labeling events occurring from the maize jointing to maturing stages (see Zhang et al. 2022 for details). To prevent straw displacement and debris contamination, a nylon mesh (178 μm opening) was placed over the PVC frame during the 13C tracing period. Four seeding holes (5 cm deep) were punched in each subplot, and two maize seeds were manually sown in each hole. Once the seedlings reached the 2-leaf stage, one weak seedling was removed, leaving four seedlings with similar growth stages in each subplot.
Soil samples were collected at 1, 3, 6, and 12 months post-labeling. Samples were obtained from each subplot using the method described in Section 2.1.1. Each composite sample consisted of three soil cores taken to a depth of 20 cm.
2.2.2 Analysis of δ13C Signature in Nematode and Microbial Groups and Microbial Necromass
The δ13C signature of soil nematode trophic groups was determined using a modified combustion method (Crotty et al. 2013) with a Thermo Fisher MAT253 isotope ratio mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). Nematodes from each trophic group in each subplot were hand-picked using an eyelash under a dissecting microscope (Motic SMZ-168, Xiamen, China). They were then transferred to a tin capsule (8 × 5 mm, Elemental Microanalysis, Okehampton, UK) containing a drop of deionized water and dried at 60°C for 48 h. For each analytical replication, a minimum of 60 individuals was required for omnivores-predators and 100 individuals for other trophic groups.
The δ13C signatures of individual PLFAs were determined using gas chromatography-combustion-isotope ratio mass spectrometry (GC-c-IRMS) (Trace GC Ultra, GC Isolink and Delta V IRMS, Thermo Scientific, Bremen, Germany). The δ13C signatures were corrected for the additional C atoms introduced during the transesterification reaction (Sanaullah et al. 2016; see Data S1 for detailed formulae).
The δ13C signature of individual amino sugars was analysed using GC-c-IRMS (Delta plus, MAT 253; Thermo Finnigan). Similar to PLFAs, the δ13C signatures of amino sugars were corrected for additional C atoms introduced during the derivatization reaction (Glaser and Gross 2005; see Data S1 for detailed formulae).
2.2.3 Calculating 13C Pool and Its Enrichment Rate
The 13C isotope signature, initially expressed in δ notation (‰ relative to Pee Dee Belemnite), was converted to atomic fraction (%, AT) for subsequent calculations. The 13C content (ng 13C g−1 dry soil) enriched in nematode and microbial groups, as well as microbial necromass, was determined based on the C content (ng C g−1 dry soil) in each pool and its corresponding 13C-enrichment (%, AT). Detailed formulae are provided in the Data S1.
2.3 Data Analysis
All statistical analyses were performed in R 3.6.2, except for the structural equation model (SEM) analysis, which was performed using IBM SPSS Amos 22.0.
To examine the relationships between omnivores-predators and other nematode and microbial groups from a bottom-up resource-driven perspective, a functional group-based interaction network was built using data from the field experiment (Potapov et al. 2023). Seven functional groups were classified based on traits, resource use, and predation: primary consumers (fungi, actinomycetes, and general bacteria), secondary consumers (fungivores, bacterivores, and plant-parasites), and tertiary consumers (omnivores-predators). Plant-parasites were classified as secondary consumers because some genera (e.g., Boleodorus, Neothada, and Tylenchus; refer to Table S1) feed on both plant roots and fungal hyphae (Yeates et al. 1993). For consistency, all taxa were converted to their respective C content (ng C g−1 dry soil) prior to network construction (conversion details are provided in the Data S1).
Each functional group was represented as a matrix, and Bray–Curtis dissimilarity was calculated across all plots within the same treatment (Rzanny and Voigt 2012). Mantel tests were performed for each pair of functional groups across trophic levels, and significant correlations (p < 0.1) were used to create directional links from lower to higher trophic groups. Interaction complexity and strength were quantified using three metrics: connectivity, interaction diversity, and interaction strength, as described by Rzanny and Voigt (2012). Detailed formulae are provided in the Data S1.
To evaluate the feeding strategy of omnivores-predators under different tillage systems, two approaches were employed. First, δ13C signatures in organisms (refer to Table S3) were used to assess predator–prey relationships. A significant linear correlation between the δ13C signature of omnivores-predators and a particular group (e.g., microbivores, plant-parasites and microbial groups) indicates a strong predator–prey relationship, as the δ13C signature in an organism's body is responsive to the δ13C signature of its dietary resources (Potapov et al. 2018; Ruess and Chamberlain 2010).
These feeding proportions were subsequently subjected to a repeated measures analysis of variance (RMANOVA) to test for differences in feeding behavior of omnivores-predators between tillage practices (no-till vs. till).
To explore the differences in microbial necromass under contrasting tillage practices, Student's t-test and RMANOVA were used to analyze data from the 2017 to 2018 integrated dataset and the 1-year 13C in situ tracing experiment. To explore the role of omnivores-predators in explaining the variation in microbial necromass, Pearson correlation and random forest model analyses were conducted. Pearson correlation was first performed to assess the correlation between the fungal-to-bacterial necromass ratio and different soil nematode and microbial groups in both the field and 13C in situ tracing experiments. To further identify the relative importance of all soil nematode and microbial groups to microbial necromass within different tillage systems, a random forest analysis was performed based on data from the 13C in situ tracing experiment. Group importance was determined based on the percentage increase in mean square error in the random forest model.
To understand how omnivores-predators affect microbial necromass via top-down control within the food chain, general linear models (GLMs) and structural equation modeling (SEM) were applied based on the 13C enrichment rates of organisms. GLMs were used to examine the relationships between omnivores-predators, microbivores, and microbes. The analysis was initially performed on the full dataset and then on subsets grouped according to different tillage practices, with only p-values < 0.05 reported. Positive linear regression suggests that the rate at which higher trophic groups acquire 13C by consuming lower trophic groups is closely related to the 13C enrichment rate of the lower trophic groups. Negative linear regression indicates that the increased predation pressure from higher trophic groups decreased the 13C enrichment rate in the lower trophic groups. To verify the extent of top-down control exerted by omnivores-predators on adjacent trophic groups (microbivores), best-fit models were used to assess how the predation influences the temporal trajectories of 13C content in microbivores during the tracing period. Additionally, the relationships between various microbial groups (i.e., fungi, bacteria, and actinomycetes) and the fungal-to-bacterial necromass ratio were examined using the same GLMs approach. To improve model linearity, the ratio was ln-transformed. The negative linear correlation between the 13C enrichment rates of microbial groups and the 13C-fungal to 13C-bacterial necromass ratio was expected, as active microbes are the precursors of necromass formation (Liang et al. 2017).
SEM was conducted to partition the top-down control mechanisms of omnivores-predators on microbial necromass across distinct tillage systems. To increase the model fit, paths from plant-parasites to general bacteria or actinomycetes were included, as suggested by modification indices. These paths are justified because plant-parasitic nematodes can subsidize bacteria by releasing C and other nutrients through penetrating root cells (Yeates et al. 1993). Such modifications do not detract from the overarching regulatory role of omnivores-predators in exerting top-down control on other nematode and microbial groups. The final model was selected based on the p-value, comparative fit index (CFI), goodness-of-fit (GFI) and chi-square fit statistics/° of freedom (CMIN/DF).
3 Results
3.1 Role of Omnivores-Predators in Different Tillage Practices
The nematode community had clear changes at the community level in the no-till soil compared to the till soil, characterized by a 46% increase in abundance and a 67% increase in richness of omnivores-predators (Figure S1 and Table S4). Functional group networks displayed a distinct role of omnivores-predators in maintaining the structure of the soil food web between till and no-till treatments (Figure 2A). In the no-till soil, omnivores-predators, acting as tertiary consumers, interacted more diversely and strongly with secondary consumers of fungivores, bacterivores, and plant-parasites, as well as primary consumers of fungi, general bacteria, and actinomycetes (as indicated by the interaction diversity, strength, and connectivity; Figure S2). These changes led to a notable increase in the structural complexity of the no-till soil food web compared to the till soil food web. Specifically, interaction diversity, interaction strength, and connectivity in the no-till soil food web increased by 105%, 28%, and 200%, respectively (Figure 2B).
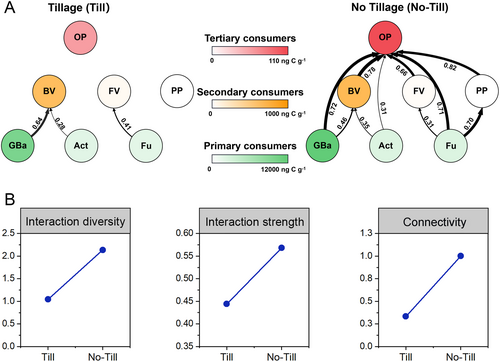
The structural differences in the soil food web between till and no-till treatments were reflected in corresponding changes in the 13C content and enrichment rate across food web components (Figure S3). Specifically, during the tracing period, the 13C content and enrichment rate increased by 310% and 402% for omnivores-predators and by 64% and 50% for fungi under the no-till treatment compared to the till treatment. However, the 13C content and enrichment rate of fungivores decreased by 73% and 90%, respectively, under the no-till soil (Figure S3).
3.2 Importance of Omnivores-Predators in Explaining Microbial Necromass
Tillage practices did not affect fungal and bacterial necromass as indicated by statistical analyses in both the long-term field and 13C in situ tracing experiments (Figure 3A,B). However, the fungal-to-bacterial necromass ratio was notably affected by tillage, with no-till treatment increasing this ratio by 20% in the long-term field experiment and by an average of 29% in the 13C in situ tracing experiment (Figure 3A,B).

Correlation analysis demonstrated a consistent positive correlation between omnivores-predators and the fungal-to-bacterial necromass ratio in both experimental settings (Figure 3C). Further random forest analysis identified the key components of the soil food web (Figure 3D) that influenced microbial necromass in the 13C in situ tracing experiment. Fungi and general bacteria were the two most important predictors of variation in fungal and bacterial necromass under till treatment (Figure 3D). In contrast, omnivores-predators emerged as the principal predictor of variation in both fungal and bacterial necromass under no-till treatment.
3.3 Feeding Strategy of Omnivores-Predators in Different Tillage Practices
In the till soil, the δ13C of omnivores-predators was closely associated with the δ13C of fungi (Figure 4A and Figure S4). Conversely, the δ13C of omnivores-predators in the no-till soil was primarily linked to the δ13C of fungivores (Figure 4B and Figure S4). The feeding proportions of omnivores-predators displayed distinct differences between till and no-till treatments (Figure 4C). In the till soil, omnivores-predators fed mainly on plant-parasites (46%) and fungi (14%), whereas in the no-till soil, fungivores and plant-parasites accounted for 51% and 29% of their diet, respectively. Among various food sources in the omnivores-predators' diet, fungivores and fungi were strongly affected by tillage practice (Figure c and Table S5). Compared to the till treatment, the proportion of fungivores in the diet increased by 292% and the proportion of fungi decreased by 64% under the no-till treatment (Figure 4C). This intense feeding by omnivores-predators on fungivores in the no-till soil reduced the feeding intensity of fungivores on fungi, with a 99% reduction compared to their feeding intensity in the till soil (Figure S5).
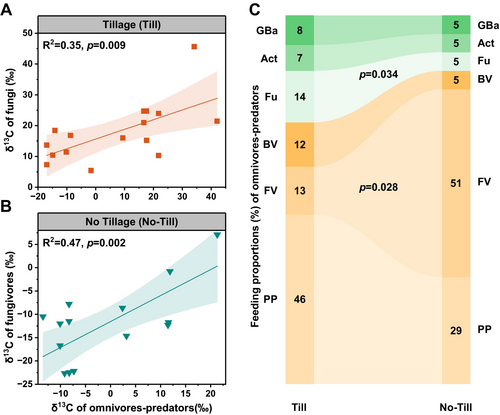
3.4 Top-Down Control of Omnivores-Predators on Microbial Necromass
In a top-down direction in the food chain, a relationship between omnivores-predators and fungivores was only found in the no-till soil, where the 13C enrichment rate of fungivores decreased as the 13C enrichment rate of omnivores-predators increased (Figure 5A). This inverse relationship was further highlighted by the opposite temporal changes in the 13C content of omnivores-predators and fungivores under the no-till soil, a pattern not present under the till soil (Figure 6A). For the relationship between fungivores and fungi, a positive correlation was found only in the till soil. For the relationship between omnivores-predators and fungi, a positive correlation was revealed in both the till and no-till soils (Figure 5A). For the relationships between microbial groups and fungal-to-bacterial necromass ratio, the strongest correlation was found between fungi and the fungal-to-bacterial necromass ratio under the no-till treatment (R2 = 0.44; Figure 5B).
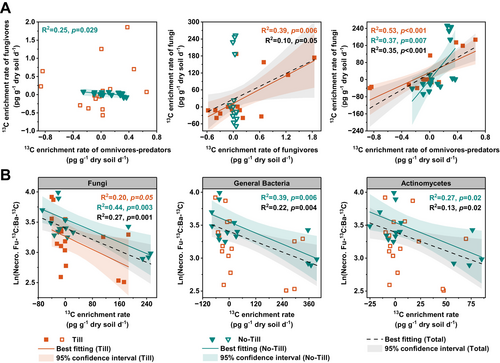
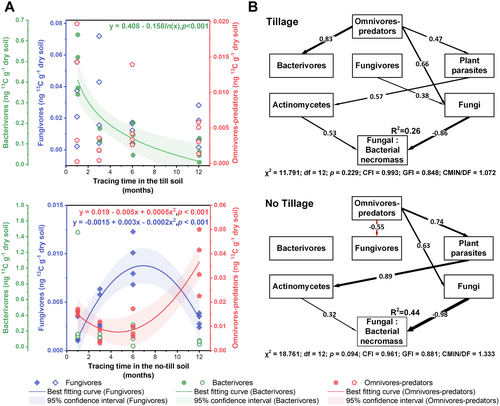
SEMs revealed that the regulatory pathways underlying the top-down effects of omnivores-predators on the fungal-to-bacterial necromass ratio differed between the two tillage systems (Figure 6B). In the no-till soil, an inverse relationship (path coefficient = −0.55) was revealed between omnivores-predators and fungivores, disrupting the direct pathway from fungivores to fungi. Instead, a strong pathway from omnivores-predators to fungi was established (path coefficient = 0.63). In the till soil, no such inverse relationship was found and the pathway from fungivores to fungi remained significant (path coefficient = 0.38). Moreover, a pathway from omnivores-predators to fungi was also present in the till soil (path coefficient = 0.66). As a result, the path coefficient from fungi to the fungal-to-bacterial necromass ratio was 14% higher under the no-till treatment compared to the till treatment.
4 Discussion
4.1 Omnivores-Predators Serve as Key Regulators of Microbial Necromass in No-Till System
Microbial necromass formation is typically influenced by microbial activity (Buckeridge et al. 2020; Wang et al. 2023; Yang et al. 2022), as shown in the till soil, where general bacteria and fungi emerged as the primary predictors of necromass (Figure 3D). In contrast, omnivores-predators were identified as the most important predictors of both fungal and bacterial necromass in the no-till soil (Figure 3D). This shift suggests that higher trophic levels may play a critical role in regulating microbial necromass formation under no-till conditions with reduced soil disturbance.
The positive correlation between soil nematodes and microbial necromass has been attributed to microbial decomposition of nematode corpses, which stimulates microbial necromass formation (Kou et al. 2023). However, our study indicates that omnivores-predators influence microbial necromass through a downward trophic cascade along the omnivores-predators—fungivores—fungi food chain. In the no-till soil, there was an inverse relationship between omnivores-predators and fungivores, where an increase in omnivores-predators decreased the 13C content and enrichment rate of fungivores (Figure 5A and Figure 6A). This apparent reduction of fungivore activity disrupted the direct link from fungivores to fungi (Figures 5A and 6B) and was accompanied by an increase in the 13C content and enrichment rate of fungi (Figure S3). This cascade effect contributed to a superficial positive correlation between omnivores-predators and fungi (Figures 5A and 6B), which was crucial for the oscillations within the food chain (Hastings and Powell 1991; Terborgh and Estes 2013). The beneficial influence of omnivores-predators on fungi was further reflected in the fungal-to-bacterial necromass ratio, with fungi being the dominant contributor to this ratio in the no-till soil, surpassing other microbial groups (Figure 5B). By limiting fungivores, omnivores-predators indirectly accelerate fungal growth, leading to a greater contribution of fungi to the fungal-to-bacterial necromass ratio in the no-till soil compared to the till soil (Figure 6B).
Conversely, in the till soil, the inverse relationship between omnivores-predators and fungivores was absent. Instead, a direct trophic relationship between omnivores-predators and fungi was common, similar to the predator–prey relationship between fungivores and fungi. This relationship is supported by the feeding preferences of the omnivores-predators, with fungi being their second most important food resource in the till soil (Figure 4A,C). This is also reflected in the coordinated changes in 13C enrichment rates, where both omnivores-predators and fungivores increased in parallel with the fungal 13C enrichment rate (Figure 5A). In this case, omnivores-predators feed directly on fungi, shortening the trophic cascade and reducing the contribution of fungi to the fungal-to-bacterial necromass ratio.
Overall, omnivores-predators shaped fungi-fungal necromass dynamics through trophic cascades in the no-till soil. However, the causal interpretation derived from the observational data and the SEM model requires caution, as the direction of these relationships depends on model assumptions (Shipley 2016). To robustly disentangle trophic cascades within the complex soil food web, targeted manipulative experiments (e.g., quantifying fungal necromass formation across three or more trophic levels) are needed (Cao et al. 2024).
4.2 Bottom-Up and Top-Down Forces Jointly Trigger the Trophic Cascade in No-Till Food Web
A classic top-down cascade effect was evident in the no-till soil, where top-level omnivores-predators limited the activity of intermediate-level fungivores, indirectly promoting fungal growth and their necromass formation at the lower trophic level. According to trophic cascade theory, the bottom-up force, driven by resource availability, and the top-down force, driven by the traits of top predators (such as abundance and feeding strategy), are the primary factors triggering the occurrence of trophic cascades (Baum and Worm 2009; Pace et al. 1999; Terborgh and Estes 2013). By integrating a functional group network that reflected bottom-up responses with 13C-based predator–prey data, we constructed a framework of how these two forces interact to amplify the trophic cascade in the no-till soil (Figure 7).
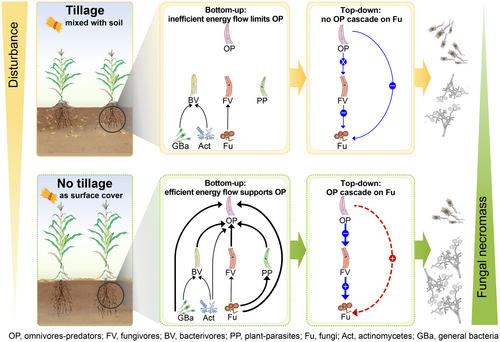
Bottom-up resource availability plays a fundamental role in shaping the trophic cascade (Osakpolor et al. 2023; Ripple et al. 2016; Terborgh and Estes 2013; Zhou et al. 2025), providing the necessary energy for omnivores-predators to exert their top-down control. Following the transition from till to no-till practices, bottom-up forces were markedly strengthened (Figures 2 and 7). Omnivores-predators formed stronger connectivity with a range of functional groups, from primary consumers (e.g., fungi and bacteria) to secondary consumers (e.g., fungivores and bacterivores), facilitating a more efficient transfer of energy from the basal resources through the food web (Pascual and Dunne 2006; Rooney and McCann 2012). This intensified energy flow supported the growth and reproductive success of omnivores-predators, as reflected in their increased 13C content and enrichment rate in the no-till soil (Figure S3).
The top-down control, in turn, is driven by the increased abundance and activity of omnivores-predators, which regulate lower trophic levels by predation (Figure 7). As predators reduce the abundance of intermediate prey, a top-down cascade is triggered (Baum and Worm 2009; Ripple et al. 2016; Terborgh and Estes 2013). The transition from till to no-till practices resulted in distinct community-level changes for omnivores-predators, characterized by increased richness and abundance (Figure S1 and Table S4), and a shift in the dietary preferences, in particular an increase in consumption of fungivores to 51% (Figure 4C). These changes led to a typical top-down control by omnivores-predators on fungivores, as evidenced by their opposite temporal changes in the 13C content over the 1-year tracing period (Figure 6A).
Fungivores were represented by specialist fungivorous nematodes, whose activity was limited by omnivores-predators (Figures 5A and 6A,B). This limitation may increase the likelihood of fungal predation by other fungivore consumers, such as mites and collembolans (Potapov et al. 2022). Mites and collembolans, as typically facultative fungivores, are more mobile than nematodes and can move in and out of the food web more freely (Coleman et al. 2024; Potapov et al. 2022). Consequently, their interactions with fungi are likely to be less stable and more incidental, in contrast to the sustained relationship between fungivorous nematodes and fungi. This is particularly true in the soil food web based on soil clods, which harbor more nematodes but have fewer mites and collembolans (Kladivko 2001; Zhang et al. 2021). If mites and collembolans benefited from competition with fungivorous nematodes, we would expect changes in fungal 13C content over time, rather than the smooth temporal trend observed (Figure S3). Additionally, the reduced activity of fungivorous nematodes decreased their feeding pressure on fungi (Figure S5). Thus, the role of these mobile and facultative fungivore consumers is less important in the context of the stable and sustained interactions between fungivorous nematodes and fungi (Duffy et al. 2007; Filip et al. 2014). In this way, fungi benefit from the top-down trophic cascades triggered by omnivores-predators and dominate the transfer of straw-C to fungal necromass in the no-till soil (Figure 7).
In this framework, the bidirectional driving forces of bottom-up resource availability and top-down control increase the complexity and stability of the no-till soil food web structure. By providing a continuous flow of energy through the food web, bottom-up forces reinforce top-down cascading effects exerted by omnivores-predators, ultimately promoting fungal biomass and necromass accumulation in the no-till soil (Figure 7).
Soil microenvironment inhabited by organisms can also influence the occurrence of trophic cascades (Kravchenko et al. 2020; Pausch et al. 2018). For example, soils with high water-holding capacity and good pore connectivity increase the probability of encounters between consumers/predators and food sources/prey (especially invertebrates living in the water film), thereby creating trophic cascades (Erktan et al. 2020a). In our study, no-till over a five-year period allowed the soil environment to stabilize (Six et al. 2004), enabling soil organisms to adapt to the higher water holding capacity and increased inter-pore connectivity (Lavelle and Spain 2001). Such microenvironmental changes in the no-till soil, compared to the till soil, have been well demonstrated (Blanco-Canqui and Ruis 2018; Guo et al. 2020; Zhang et al. 2015). Thus, the feeding strategy of omnivores-predators and their limitation to fungivores most likely reflects inherent trophic relationships shaped by the microenvironment. While microenvironmental influences on trophic cascades were not the focus of this study, future research investigating these effects will further our understanding of soil ecosystem functioning.
4.3 Implication for Harnessing Top-Down Control to Improve Soil C Sequestration
The bottom-up resource availability and top-down predation jointly shape fungi-fungal necromass dynamics in the no-till system, leading to a 14% increase in the contribution of fungi to the ratio of fungal to bacterial necromass relative to the till system (Figures 6B). Notably, despite the same amount of maize straw input, continuous tillage resulted in the direct consumption of the fungi by omnivores-predators (Figure 4A,C). This direct consumption interrupted the trophic cascade and dampened the positive effects of omnivores-predators on fungal biomass and necromass formation (Figure 7). Consequently, the impact of tillage systems on the soil food web extends beyond bottom-up resource availability, and top-down predation regulation seems to play an outsized role in shaping energy flow and C cycling of the entire ecosystem (Coleman et al. 2024; Pascual and Dunne 2006).
Microbial necromass, particularly fungal necromass, is the most important contributor to soil C persistence because its components are slowly decomposable compared to bacterial necromass and can be adsorbed on minerals and form the most stable soil C pool—mineral-associated organic C (MAOC) (Klink et al. 2022; Sokol et al. 2022; Whalen et al. 2022). The 13C in situ tracing experiment revealed a strong linear correlation (R2 = 0.73) between the fungal-to-bacterial necromass ratio and MAOC-13C (Figure S6), confirming the dominant contribution of fungal necromass to soil C stabilization. Given the longer turnover time of MAOC (mean 129 years) compared to other soil C pools (Zhou et al. 2024), the beneficial influence of omnivores-predators on fungi-fungal necromass dynamics may disproportionately increase long-term soil C sequestration by reinforcing fungal-derived C stabilization.
Overall, top-down predation regulation, rather than bottom-up resource availability, appears to be crucial in determining the efficiency of soil C sequestration. These findings provide a novel perspective for agricultural management, highlighting the importance of protecting and maintaining top predators within the soil food web. By doing so, it is possible to boost fungal necromass accumulation, thereby strengthening and stabilizing the long-term soil C pool, contributing to sustainable agricultural practices and climate change mitigation efforts.
5 Conclusions
Our findings provide field-based support for a potential top-down cascading mechanism, by which top predators influence microbial necromass accumulation. In the no-till soil, abundant and diverse omnivores-predators amplified the fungi-fungal necromass dynamics by increasing predation pressure on fungivores. This trophic interaction was associated with increased fungal biomass and benefited the conversion of straw-C into fungal necromass. Conversely, continuous tillage disrupted these trophic interactions via direct consumption of fungi by omnivores-predators, which interrupted the trophic cascades and dampened the fungal necromass accumulation. These results highlight the sensitivity of predator–prey interactions to agricultural practices and their possible role in shaping soil C cycling. To advance these insights, manipulative experiments across three or four trophic levels are needed to establish causality between trophic cascades and necromass formation. Nevertheless, our study offers valuable insights to develop management strategies that exploit top-down cascading effects of top predators to reinforce soil C sequestration in agricultural ecosystems.
Author Contributions
Elly Morriën: writing – original draft, writing – review and editing. Aizhen Liang: funding acquisition, resources, supervision. Edith Bai: writing – original draft, writing – review and editing. Yakov Kuzyakov: writing – original draft, writing – review and editing. Zhongjun Jia: writing – original draft, writing – review and editing. Shixiu Zhang: conceptualization, data curation, formal analysis, funding acquisition, investigation, writing – original draft, writing – review and editing.
Acknowledgments
We sincerely thank the anonymous editor and reviewers for their valuable feedback and constructive comments, which greatly improved the quality of this manuscript. We also extend our heartfelt gratitude to Prof. Neil B. McLaughlin (Ottawa Research and Development Centre, Canada) for his language editing, Prof. Hongbo He (Shenyang Institute of Applied Ecology, CAS) for her guidance on amino sugar determination and calculation, and Prof. Dima Chen (Inner Mongolia University) for his valuable suggestions. This study was funded by the National Natural Science Foundation of China (42077046), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA28020401 and XDA28080000), the Youth Innovation Promotion Association of the Chinese Academy of Sciences (2021228), the Young Scientist Group Project of the Northeast Institute of Geography and Agroecology (2022QNXZ04), and the RUDN University Strategic Academic Leadership Program.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in Dryad at https://doi.org/10.5061/dryad.nvx0k6f3s.




