Extreme Rainfall Amplified the Stimulatory Effects of Soil Carbon Availability on N2O Emissions
Zengming Chen and Nan Zhang contributed equally to this work and should be considered joint first author.
Funding: This research was financially supported by the National Key Research and Development Program of China (2022YFD1500303), the Natural Science Foundation of Jiangsu Province (BK20211610), the Natural Science Foundation of China (42077029, 42307386), Frontier Project from the Institute of Soil Science, Chinese Academy of Sciences (ISSASIP2212), Youth Innovation Promotion Association of Chinese Academy of Sciences (2022313), and IAEA coordinated research project (D15020).
ABSTRACT
Ongoing climate change is predicted to increase the frequency and intensity of extreme rainfall, which will dramatically alter soil nitrous oxide (N2O) emissions, especially changes in soil organic carbon (SOC) due to anthropogenic management. However, our ability to predict this effect is limited owing to a dearth of research. Therefore, we selected two croplands in Northeast China with the same quantity but contrasting availability of SOC to explore the in situ dynamics of N2O fluxes and N-cycling microbes through 2-year field experiment and N2O production pathways by laboratory 15N-tracing experiment. In a normal rainfall year, the croplands with high (HCA) and low (LCA) SOC availability emitted 0.66 and 0.25 kg N2O-N ha−1 without N-fertilization and 2.03 and 1.51 kg N2O-N ha−1 with N-fertilization, respectively. In a record-breaking wet year, multiple heavy rainfall events caused water supersaturation in the low-lying HCA cropland over 2 months. Consequently, the background N2O emissions increased by 508% compared with the normal rainfall year, and the N-induced N2O emission factor increased from 0.77% to 2.24%. Soil dissolved organic carbon (DOC) was identified as the primary driver of larger N2O fluxes from HCA cropland which facilitated denitrification by fueling nirS- and nirK-denitrifiers metabolism. Furthermore, a greater N substrate supply via a faster mineralization-nitrification coupling process promoted the contribution of autotrophic nitrification to N2O in HCA cropland. The N2O pulses from HCA soils during the waterlogging period were derived from stimulated denitrification, which dominated N2O production (> 90%). Simultaneously, C availability enhanced and nitrate was produced via archaeal nitrification, leading to an increased nirS/nosZII ratio that fostered N2O production through incomplete denitrification. Overall, our findings highlight the importance of avoiding the amendment of exogenous organic materials with high C lability, particularly under climate extremes, to eliminate the potential positive feedback of SOC management on climate change by inducing N2O emissions.
1 Introduction
Climate change is exacerbating climate extremes at an alarming rate, as evidenced by dramatic increases in the frequency, intensity, and duration of extreme weather events worldwide, especially in the middle-high latitude regions (Lesk et al. 2022; Ombadi et al. 2023). Extreme rainfall will dramatically alter the hydrological cycle of terrestrial ecosystems, thereby affecting soil carbon (C) and nitrogen (N) biogeochemical processes (Vereecken et al. 2022) and greenhouse gas (GHG) emissions (Hutchins et al. 2019). Nitrous oxide (N2O) is a potent GHG, and its atmospheric concentration has risen from 270 ppb before the preindustrial era to 336 ppb in 2022, primarily driven by soil N2O emissions (World Meteorological Organization 2023). Global soil N2O emissions have increased from 0.3 to 3.3 Tg N year−1 over the same period, and cropland contributed to 82% of the total increase (Tian et al. 2019). However, the estimation of cropland emissions remains highly uncertain, ranging from 1.5 to 5.0 Tg N year−1 (Tian et al. 2024; Wang et al. 2020). This large uncertainty is mainly due to the complex factors controlling N2O production. In addition to previously recognized management practices such as cropping systems, soil properties were demonstrated to be the key points driving the variation in the N2O emission factor (EF), especially soil organic C (SOC) (Cui et al. 2021; Chiesa et al. 2024; Ge et al. 2024).
Net soil emissions of N2O are the result of various microbially mediated processes, of which denitrification is considered the predominant pathway, as its capacity to produce N2O is much greater than nitrification (Liang and Robertson 2021). Therefore, the significance of SOC as an electron donor is evident in N2O production by denitrification, which is a respiratory process of heterotrophic microbes (Hu et al. 2015). It has been found that there were significantly positive correlations between SOC content and N2O emissions on the global scale (Bouwman et al. 2002; Cui et al. 2021; Li, Chen, et al. 2024). Similarly, Ding et al. (2019) reported that the net N2O production rate increased with increasing SOC concentrations as a consequence of promoted denitrification. Nevertheless, Jungkunst et al. (2006) did not observe a relationship between SOC content and N2O fluxes based on data synthesized from German croplands. Unexpectedly, higher N2O emissions were measured in situ from croplands with lower SOC content under conservation tillage (de Figueiredo et al. 2018), in which N2O exhibited a negative correlation with SOC. These inconsistent results could be attributed to the fact that the energy supply of SOC to microbes depends not only on its quantity but also on its availability (Baker et al. 2015; Gunina and Kuzyakov 2022).
The availability of SOC is determined by the proportions of the labile versus inert fractions (Pare and Bedard-Haughn 2013). Labile fractions can indirectly promote N2O production via nitrification as a result of more ammonium () being released from its easier biodegradation process, although ammonia-oxidizing microorganisms do not use organic C as an energy source (Pratscher et al. 2011; Xiao et al. 2019). More importantly, labile organic C can directly stimulate N2O production via denitrification. Dissolved organic carbon (DOC) is considered the most readily available C fraction in soils (Roth et al. 2019) and has been reported to stimulate N2O production by promoting the respiration of heterotrophic denitrifying bacteria, which consumes oxygen (O2) and turns to create anaerobic hotspots in favor of denitrification (Sennett et al. 2024). Nevertheless, others have found that the molecular composition, rather than the DOC content, plays a central role in regulating denitrification to produce N2O, as DOC is a complex mixture that contains various compounds (Saari et al. 2009). Generally, DOC with a higher proportion of oxygen-containing functional groups, such as amino acids and proteins, could provide more electron donors and induce higher activity of nitrite reductase (nirS and nirK) and N2O production compared to that containing more compounds of glucose (Curtright and Tiemann 2023; Hou et al. 2018). On the other hand, highly labile fractions can be quickly and easily consumed by soil microbes, which can effectively reduce the mineral N content in the short term by promoting N immobilization, thus reducing N2O emissions (Xia et al. 2018). Moreover, the supply of labile C substrates could also suppress N2O emissions, especially under high moisture conditions, owing to a shift towards complete denitrification, that is, N2O reduction to N2 (Qin et al. 2017; Surey, Lippold, et al. 2020; Surey, Schimpf, et al. 2020). Therefore, the effect of SOC energy on regulating N2O production also depends on its interaction with redox conditions as affected by soil moisture, which has not been fully elucidated, particularly under in situ conditions (Highton et al. 2020; Jerray et al. 2024).
Generally, increased N2O fluxes are found immediately after rainfall as a result of facilitated denitrification by higher moisture content (Montoya et al. 2022; Snider et al. 2015). Some studies have attributed this increment in originally dry soils to the induced release of labile C and N from organic matter decomposition and accompanying increased microbial activity (Li et al. 2023). However, soil anaerobic conditions caused by excessive moisture after extreme rainfall events might enhance complete denitrification and thus reduce N2O emissions, particularly in the presence of an adequate supply of labile C (Baker et al. 2015; Pan et al. 2022). This may be due to the enhanced activity of N2O reductase (nosZ) with low oxygen tolerance (Domeignoz-Horta et al. 2016), as observed by Wei et al. (2022), who observed a lower ratio of N2O/(N2O + N2) under conditions of higher moisture and lower O2 levels. Additionally, soil water saturation can create an anaerobic environment that constrains ammonia oxidation, reduces production, and limits denitrification, thereby decreasing N2O emissions following extreme rainfall (Kuypers et al. 2018). However, Chen et al. (2016) found that extreme rainfall events increased N2O emissions by 1.7 times in a record wet year compared with the normal rainfall year. This was mainly due to the N2O plusses induced by optimum DOC/ ratio during the soil drying period following waterlogging that facilitate N2O production by denitrification rather than reduction to N2. These inconsistent results could be attributed to the distinct supply of soil C substrates and its interaction with moisture, while the involved mechanisms and responses of functional microbes remain obscure (Banerjee et al. 2016; Highton et al. 2020). Further empirical evidence is therefore urgently needed to improve our understanding of the underlying drivers of N2O production under intense extreme rainfall conditions.
Black soils contain 30% of the global cropland SOC stock and are recognized for their high fertility and productivity, playing a paramount role in food security and climate change mitigation (FAO 2022). However, owing to unsustainable practices, such as high-intensity cultivation and low input of organic materials, the SOC content in global black soils has lost by 20%–50%, and their quality is also gradually degrading (Li et al. 2022; Meng et al. 2024). This may have induced greater leakage in soil N cycling and nitrogenous gas losses (Elrys et al. 2023). Black soils are primarily distributed in the mid-high latitude regions of the Northern Hemisphere where they are highly sensitive to climate extremes. The intensifying extreme rainfall events and the dynamics of SOC availability will inevitably affect N2O emissions and might lead to black soil regions becoming a “hot spot” for global N2O emissions. We therefore hypothesized that (1) declined C availability induced lower N2O emissions from N-fertilized croplands; (2) extreme rainfall events increased N2O emissions by promoting denitrification; (3) and the interaction between higher labile C supply and moisture conditions could amplify N2O emissions. Confirmation of these hypotheses will enable us to elucidate the response of N2O emissions to extreme rainfall from soils with various C availability, thereby facilitating the integration of this knowledge into climate change and mitigation strategies to specify more effective practices for SOC sequestration while avoiding inducing high N2O emissions.
2 Materials and Methods
2.1 Study Site
The study was conducted in Baoqing County, Heilongjiang Province, Northeast China (46°41′ N, 132°01′ E). The region is a typical area distributed with black soils, located in the center of the Sanjiang Plain. It has a cold temperate continental monsoon climate. The 30-year (1989–2019) mean annual air temperature is 4.6°C, and the annual rainfall is 550–600 mm, two-thirds of which falls between May and September.
This area was originally occupied by wetlands dominated by Phragmites australis and Deyeuxia angustifolia. Agricultural utilization at this site dates back to approximately 100 years, which generated croplands with a clear sequence of cultivation gradients spanning from 5 to 100 years and divergent quantity and availability of SOC (Li et al. 2022; Li, Hong, et al. 2024). Croplands with the same quantity but different availability of SOC were selected in the current study (Chen 2025). The selected fields have been continuously cultivated with maize for 30 years (High carbon availability, HCA) and 85 years (Low carbon availability, LCA) under typical and consistent practices. These management practices involve a traditional up-down ridge tillage pattern, with ploughing performed annually, a single application of synthetic fertilizers, and removal of all crop residues post-harvest. The crops are maize (Zea mays L.), which is traditionally sown in early May and harvested in October under rain-fed conditions.
2.2 Field Experiment Design
Four treatments were arranged in a complete randomized block design with four replicates in HCA and LCA croplands, and each of the plots measured 3.9 m × 4.5 m. The treatments included no N or phosphorus (P) fertilization as control (CK), N (NF), and P (PF) fertilizers applied singly and together (NP). Urea was applied at a rate of 160 kg N ha−1, split between starter fertilizer and side-dressing at a ratio of 1:1. P fertilizer was applied as calcium superphosphate (75 kg P2O5 ha−1). Potassium (K) sulfate (60 kg K2O ha−1) was applied as the basal fertilizer for each treatment.
The experimental period encompassed two maize-cropping seasons, from May 2018 to October 2019 and May 2019 to October 2019. Following local practices, the field plots were split into ridges and furrows at a distance of 65 cm by rotary tillage after harvest in autumn. On 11 May 2018 and 9 May 2019, starter fertilizers were evenly applied to the ridges at a depth of 10 cm, and maize seeds were sown at a 20-cm plant spacing. Side-dressing with urea occurred on 7 July 2018 and 10 July 2019 at the maize V8 growth stage. Mature maize was harvested on 4 October 2018 and 11 October 2019. All crop residues were removed from the plots followed by manual tillage. Other field management practices, such as weed and pest control, were conducted according to the local agronomic practices.
2.3 Determination of Soil N2O Fluxes
The static chamber method was used to measure soil N2O, nitric oxide (NO), and carbon dioxide (CO2) fluxes across 2 years from 14 May to 14 November in 2018 and from 10 May to 2 November in 2019. A stainless steel base frame (65 cm × 20 cm × 20 cm) was inserted 10 cm into the soil. During gas collection, a stainless-steel sampling chamber (65 cm × 20 cm × 15 cm) was placed on the base sink of the frame and filled with water to ensure airtightness.
To focus on the impact of extreme summer rainfall, the period following ice formation during winter was not included in this study. Gas samples were collected twice a week during the maize growing season and every 5 days after maize harvest until the soil froze. This sampling frequency has been proven to adequately depict the pattern of N2O fluxes in the study region, including peaks after N fertilization and rainfall (Chen et al. 2016). Multiple heavy rainfall events in August 2019 caused soil water supersaturation in the low-lying HCA cropland over 2 months, preventing access to the experimental field and sample collection. Gas sampling was conducted from 9:00 am to 12:00 pm to minimize diurnal variations. Four samples were withdrawn using syringes from the chamber headspace at 0, 10, 20, and 30 min after chamber closure and injected into a pre-evacuated 22-mL glass bottle. N2O and CO2 concentrations were measured using gas chromatograph (GC) (Agilent 7890, Agilent Technologies). Daily calibrations were performed with standard gases obtained from the National Institute of Metrology, China. The NO concentration was determined using an NO-NO2-NOX analyzer (model 42i, Thermo Fisher Scientific), as described in the Supporting Information.
The gas fluxes were calculated using linear fitting models of the gas concentration against the sampling time. Cumulative N2O emissions were calculated by sequentially summing the fluxes using linear interpolation between interval sampling dates. The N-fertilizer-induced N2O emission factors (EF) were calculated by dividing the difference in N2O emissions between the fertilized treatment and the control (CK) by the total amount of applied fertilizer N.
2.4 Measurement of Environmental and Soil Parameters
Climatic parameters, including the daily air temperature (AT) and precipitation, were recorded at a meteorological station during the experimental period. Synchronized with gas sampling, the soil temperature (ST) and volumetric soil water content (SWC) at a depth of 5 cm were measured using a geothermometer and a time domain reflectometry probe, respectively. As soil bulk density (BD) did not vary significantly among treatments during the experimental period, soil moisture content was expressed as water-filled pore space (WFPS) with SWC and average BD.
Surface soil (0–20 cm) was collected once every two gas sampling campaigns. Three soil samples in each plot were combined into one composite sample and then were immediately returned to the laboratory and stored at 4°C for the subsequent analyses. Soil and were extracted by 2-M KCl in a 1:5 soil-solution ratio and analyzed using a continuous-flow autoanalyzer (San++ System, Skalar Analytical BV). Extractable inorganic nitrogen (EIN) was calculated by the sum of and . DOC was extracted by deionized water in a 1:10 soil-water ratio and determined by a TOC analyzer (vario TOC Cube, Elementar Analysensystemes GmbH). The absorbance of the DOC solution at 254 nm was determined and divided by the DOC concentration and recorded as the specific ultraviolet absorption (SUVA), which was used as an indicator of the aromatic degree of DOC (Weishaar et al. 2003). The seasonal dynamics of soil , , and DOC concentrations and SUVA of DOC are presented in Figure S1. Additionally, according to the method described by Xiao et al. (2018), we calculated the fluorescence index (FI, representing the relative contribution of microbial and plant sources to dissolved organic matter) and humification index (HIX, a descriptor of the degree of humification) to further characterize the variation in the chemical composition of DOC during the waterlogging periods in the wet year.
Before the commencement of the experiment, soil sampling (0–20 cm) was collected to determine the main physicochemical properties. Soil pH, BD, particle size, and total P and K content were presented in Table S1. The SOC and total nitrogen (TN) were analyzed with a CN analyzer (Vario Max CN, Elementar). Soil carbohydrates, as readily utilized fractions to microbes, were measured using anthrone colorimetric procedures. The mineral-associated organic matter (MAOM) and particulate organic matter (POM) were separated from bulk soil by density fractionation. The molecular composition of SOC was further assessed by solid-state 13C nuclear magnetic resonance (NMR) spectroscopy, as detailed in the Supporting Information, and the results are shown in Figure S2.
Although HCA and LCA croplands had the same quantity of SOC, the former contained more available components, as indicated by greater DOC and carbohydrates content, higher POM/MAOM ratios, and lower SUVA values (Table 1). In addition, 13C NMR spectroscopy indicated that the SOC of HCA cropland had a larger abundance of labile groups, including O-alkyl C and di-O-alkyl C but less aromatic C and phenolic C (Figure S2). The calculated indices of lability and aromaticity of SOC further verified the greater availability and biodegradability of SOC for the HCA cropland (Bahadori et al. 2021; Luan et al. 2020).
| SOC | DOC | SUVA | Carbohydrate | POM/MAOM | LIC | Aromaticity | |||
|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | mg N kg−1 | g kg−1 | L mg−1 m−1 | mg kg−1 | |||||
| HCA | 20.1a | 6.1a | 22.9a | 58.4a | 7.7b | 124a | 0.23a | 1.7a | 21b |
| LCA | 20.7a | 4.4b | 15.5b | 41.8b | 13.8a | 87b | 0.12b | 1.3b | 30a |
- Note: Different letters following values within the same column indicate significant differences between HCA and LCA soil at p < 0.05.
- Abbreviations: DOC, dissolved organic carbon; LIC, lability index of SOC; MAOM, mineral-associated organic matter; , ammonium nitrogen; , nitrate nitrogen; POM, particulate organic matter; SOC, soil organic carbon; SUVA, specific ultraviolet absorption at 254 nm of dissolved organic carbon.
2.5 Quantification of N-Cycling Functional Gene Abundance
In order to explore the microbial driving mechanism of N2O emissions as affected by SOC availability and extreme rainfall events, we selected soil samples collected from two croplands during the summer when N2O fluxes peaked in both years and from HCA cropland before and after waterlogging in the wet year. The specific sampling date and results of each measurement can be found in the Supporting Information (Figure S3). To compare the differences between the two fields with distinct SOC availability levels more clearly, the results of each plot were represented by the averages across the 2-year period.
DNA was extracted from 1 g of freeze-dried soil using the FastDNA Spin Kit for Soil (MP Biomedicals, CA), and its concentration was determined using a NanoDrop spectrophotometer (ND-2000, Thermo Fisher Scientific). To estimate the abundance of microbes in the nitrification and denitrification processes, archaeal amoA (AOA), bacterial amoA (AOB), nirK, nirS, nosZI, and nosZII were determined by quantitative PCR (qPCR). The 16S rDNA and ITS genes were also quantified as proxies for the total bacterial and fungal communities using taxon-specific primers. Quantifications were conducted in a total reaction volume of 20 μL, including 10 μL of SYBR Green Master Mix (Q112-02, Vazyme), 1 μL of DNA template, 0.4 μL of each primer, and 8.2 μL of ddH2O on real-time PCR system (LightCycle480II,384, Roche). The primer sequences and qPCR protocols are listed in Table S2. The gene copy numbers of the target genes per gram of dry soil were calculated according to standard curves constructed using serial plasmid dilutions of known amounts of plasmid DNA-containing standard samples.
2.6 Laboratory 15N Tracing Experiment
A paired 15N-labelled laboratory assay was conducted to quantify the N2O production pathways affected by C availability and moisture regime. Soil samples were collected from two croplands that had not received N fertilizer prior to the commencement of the experiment. We designed 60% and 120% WFPS treatments to simulate normal and waterlogged moisture conditions, respectively, based on the variation in soil WFPS as observed in situ over the 2-year period. Two 15N treatments were designed in three replicates, of which either or was labeled with 15N at 10 atom%. For each replicate, fresh soil (20 g on an oven-dried basis) was sieved to < 2 mm, packed into incubation bottles, and compacted to the target height approximating the field soil BD. After a 24-h pre-incubation period at 25°C, a solution of 15NH4NO3 or NH415NO3 was added to the 250 mL bottles at a rate of 50 mg N kg−1. This rate is equivalent to 120 kg N ha−1, similar to the fertilization rate in the field, so as to simulate the mineral N concentrations when the N2O fluxes peaked in the normal rainfall year as well as under waterlogged conditions. Soils were adjusted to the target moisture content by weighing based on the mass water content calculated from the WFPS and incubated at 25°C for 3 days. Five groups of soil were incubated for destructive soil sampling and N2O emission measurements. The soil moisture content was maintained constant by weighing during the incubation. Gas and soil samples were collected immediately (0 day) and 1, 2, and 3 days after NH4NO3 addition. Soil and were extracted to determine the concentrations and 15N abundances by a continuous-flow autoanalyzer and elemental analyzer-isotope ratio mass spectrometry system (2000 Delta V Advantage, Thermo Fisher) (EA-IRMS), respectively. After extraction, soils were washed with deionized water three times, oven-dried at 55°C, and sieved through 150 μm to determine the 15N abundances of organic N by EA-IRMS. Gas samples were simultaneously collected from the incubation bottles to measure N2O flux rates. Before the first gas sample collection, an equal volume of fresh air was added to maintain the headspace pressure. At each sampling event, 40 mL of headspace gas was collected and injected into two pre-evacuated vials at 0 and 6 h post-bottle sealing for the analyses of N2O concentration and isotopic composition by GC and IRMS (MAT 253, Thermo Fisher), respectively.
2.7 Calculations and Statistical Analyses
Significant differences in inorganic N content, DOC content, SUVA value, cumulative N2O emissions, and microbial abundances among treatments and between the two croplands over 2 years were assessed using one-way analysis of variance (ANOVA) in SPSS 20.0. The significance level was set at p < 0.05. Data normality (Kolmogorov–Smirnov test) and homogeneity of variance (Levene's test) were checked, and the ln transformation was used, if necessary. The random forest analysis was utilized with the ‘rfPermute’ package in R 4.0.3 software to evaluate the main factors affecting N2O fluxes and the significance of each predictor variable. Redundancy analyses (RDA) were performed to verify the relationship between response variables (N2O fluxes and abundance of target genes) and explanatory variables (soil properties) using Canoco5 software. Structural equation models (SEM) were developed to evaluate the direct and indirect impacts of edaphic conditions on N2O fluxes during the waterlogging period using AMOS 22.0. Explanatory factors were selected for analysis in the SEM model based on Pearson, random forest, and RDA results. Path models were deemed acceptable if they met the criteria of goodness-fit-index (GFI) > 0.90, χ2/df < 3 and chi-squared-test (p) > 0.05.
3 Results
3.1 Environmental Conditions
The ST varied following the trend of AT (Figure 1a) and exhibited no difference between the HCA and LCA croplands. Annual precipitation was 672 mm in 2018 and reached 899 mm in 2019, with the latter representing a historical maximum and a 77% increase compared with the long-term mean. Notably, the monthly precipitation in August 2019 reached a record-breaking high of 388 mm due to five rainstorm events (daily rainfall > 50 mm) (Figure 1b), resulting in supersaturated moisture conditions for the low-lying HCA cropland over 2 months. During the normal rainfall periods, WFPS varied from 27% to 77% until August 2019, when the soil was waterlogged, with WFPS remaining at 97%–120%.

3.2 Soil Mineral N and DOC
High concentration was observed during the waterlogging period, even comparable to the peaks after side-dressing; while the concentration was relatively low (Figure S1a,b). Mean and concentrations over the whole experimental period were significantly higher in HCA than LCA cropland without N fertilization while HCA had significantly lower but higher concentration when N was applied (Figure S4a,b). Moreover, the / ratio was higher in HCA than LCA cropland (averaged at 5.8 vs. 3.4), potentially indicating a greater capacity of nitrification of HCA versus LCA (Figure S5).
DOC concentration over 2 years was significantly higher in HCA than the LCA cropland, averaged at 53.9 and 40.1 mg C kg−1, respectively (Figure S4c), while the opposite was true for the SUVA value, indicating the greater biodegradability of DOC in the HCA soils (Figure S4d). In addition, significant stimulating effects of N and P fertilization on DOC concentrations were found in HCA soils rather than in LCA soils (Figure S4c).
3.3 Soil N2O Emissions
During the normal rainfall year, seasonal dynamics of N2O fluxes in HCA cropland were similar to LCA, and the peak value of 372 μg N m−2 h−1 was observed in the NF treatment of HCA (Figure 2). During the wet year, N2O fluxes in HCA cropland peaked at 521 μg N m−2 h−1 during the waterlogging period, which were greatly higher than that in LCA (211 μg N m−2 h−1). It should be noted that the NO fluxes and N2O/NO flux ratios remained at rather low levels under the waterlogging conditions (Figures S6 and S7).
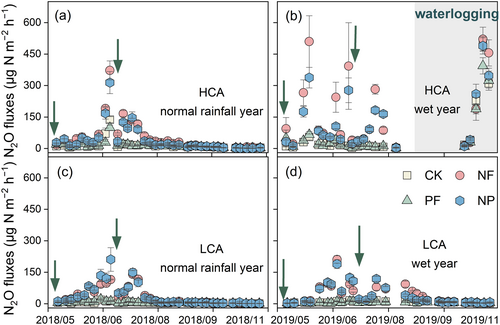
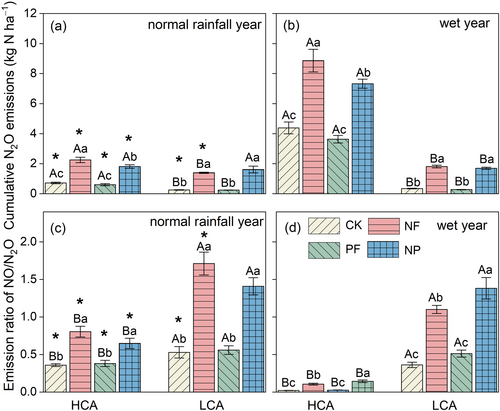
During the normal rainfall year, the cumulative background N2O emissions were significantly higher in HCA than LCA (0.72 vs. 0.26 kg N ha−1) (Figure 3a). P fertilization showed no significant effect on N2O emissions, while N fertilization from HCA and LCA croplands significantly increased them to 2.25 and 1.40 kg N ha−1, respectively. During the record wet year, cumulative N2O emissions became greatly higher in HCA than LCA, with background emissions of 4.38 and 0.35 kg N ha−1, respectively, and 8.86 and 1.81 kg N ha−1 for NF treatment (Figure 3b). Compared with the normal rainfall year, the N2O-EF in the NF treatment significantly increased in the wet year, rising from 0.77% to 2.24% in the HCA cropland and from 0.57% to 0.73% in the LCA (Figure S8). Notably, the disparity in N2O emissions between the two croplands was amplified by extreme rainfall events. The emission ratio of NO/N2O in LCA was higher than that in HCA, especially in the wet year, which was no more than 0.15 for all treatments in HCA (Figure 3c,d).
3.4 N-Cycling Functional Genes
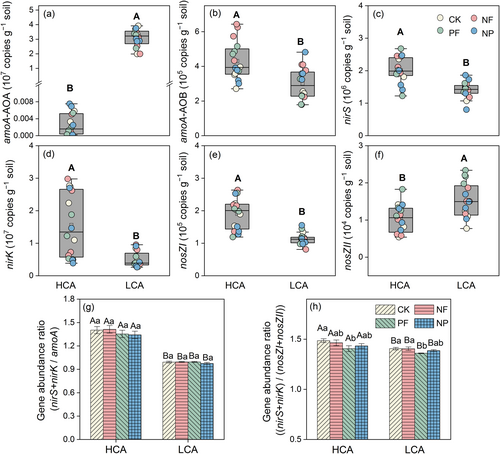
The abundance of AOA in the LCA croplands was greatly higher than that in the HCA croplands (Figure 4a), while that of AOB was higher in the HCA croplands, especially with N fertilization (Figure 4b). The nitrite-reducing community was dominated by nirK- rather than nirS-harbouring bacteria, and the abundance of nirK and nirS was significantly higher in the HCA cropland compared to the LCA cropland (Figure 4c,d). For the N2O reducing community, nosZI-harbouring bacteria were approximately 100-fold more abundant than nosZII. Interestingly, the abundance of nosZI was higher in HCA croplands than in LCA croplands (Figure 4e), whereas nosZII, with stronger N2O reduction potential, was lower in HCA cropland (Figure 4f). Additionally, the ratios of (nirS + nirK)/amoA and (nirS + nirK)/(nosZI + nosZII) were higher in the HCA croplands than in the LCA croplands (Figure 4g,h).
The abundance of N-cycling genes in the HCA cropland before and during the waterlogging period was determined to explore the microbial mechanisms underlying explosive N2O fluxes (Figure S9). No significant changes were observed in the abundance of AOA, whereas AOB abundance decreased after the soil was waterlogged. The abundance of nirS was increased, whereas nirK decreased due to waterlogging conditions. The abundances of nosZI and nosZII decreased, particularly after N-fertilization treatment. Additionally, the abundance of nrfA was decreased. Changes in the abundance of 16S rDNA genes were minor, whereas ITS increased significantly after the soil was waterlogged.
3.5 Factors Driving N2O Emissions
Random forest analysis revealed that the impacts of available C and N substrates on N2O fluxes were greater than those of micro-environmental indicators such as moisture and temperature (Figure 5a–c). During the normal rainfall year, EIN and DOC were the most important variables driving N2O fluxes. In the wet year, the primary drivers shifted towards DOC, EIN, and WFPS. An analysis which combined all data over the 2 years revealed that DOC remained the most critical factor in regulating N2O fluxes, indicating that increased moisture reinforced the stimulatory effect of DOC on N2O fluxes.
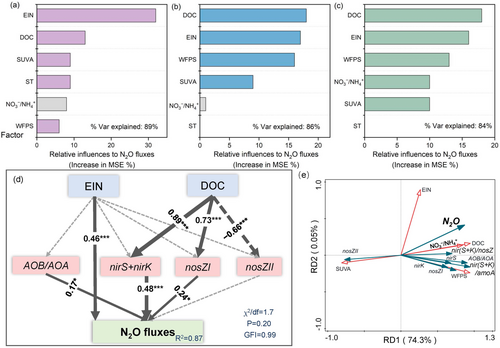
SEM explained 87% of the variability in N2O fluxes (Figure 5d), which were directly related to the abundances of nirS, nirK, nosZI, and AOB/AOA ratios, indirectly regulated by DOC concentrations as well as directly related to the supply of EIN.
RDA analysis revealed that N2O fluxes were positively correlated with DOC concentrations and AOB/AOA, (nirS + nirK)/amoA, and (nirS + nirK)/(nosZI + nosZII) ratios but negatively correlated with SUVA and nosZII. Furthermore, the abundances of nirS and nirK were positively linked to DOC concentrations (Figure 5e). Overall, the first axis explained 74.3% of the variability in total N2O fluxes and microbial communities.
During the waterlogging period, there was a significantly positive correlation between the N2O and CO2 fluxes (Figure 6a). Furthermore, N2O fluxes increased with increasing concentration but decreased logarithmically with increasing the HIX of DOC (Figure 6b,c).
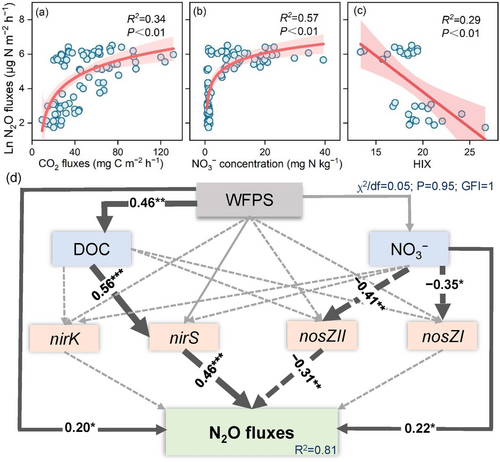
SEM was further constructed to investigate the cascading effects of soil moisture on N-related functional genes and N2O fluxes as induced by extreme hydrological events (Figure 6d). The SEM explained 81% of the variability in N2O fluxes, which were directly related to the abundances of nirS and nosZII, as well as WFPS and supply. The N2O fluxes, which were evidenced to be mainly influenced by nirS, were directly related to nirS and indirectly related to the DOC concentration. N2O fluxes were directly negatively correlated with nosZII, which was mainly regulated by directly.
3.6 N2O Production Processes
Total N2O emissions over the whole incubation period were 10.4 and 2.84 μg N kg−1, respectively, from the HCA and LCA soils under 60%WFPS (Figure 7a). Elevated soil N2O emissions were measured in both soils at higher moisture condition of 120%WFPS, with the emissions measured at 4971 and 1056 μg N kg−1, respectively. The disparity in N2O emissions between the two croplands was amplified under higher moisture conditions, which was consistent with the results of the in situ experiments.
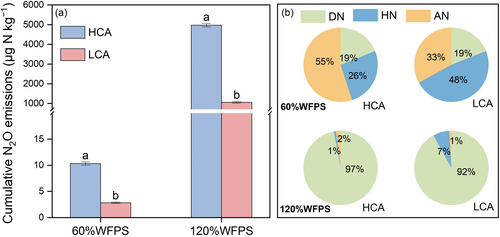
Autotrophic nitrification was the primary process of N2O production in HCA soils, with a higher contribution compared to LCA soils (55% vs. 33%), while the fraction of N2O derived from heterotrophic nitrification was higher in LCA soils (26% vs. 48%) (Figure 7b). The contribution of denitrification to N2O production was relatively low and insignificant between the two soils at 60%WFPS. On the contrary, the contribution of denitrification in HCA was significantly higher under 120%WFPS (97% vs. 92%), which was the predominant process of N2O production. In addition, almost no N2O was produced via autotrophic nitrification under extremely high moisture conditions.
4 Discussion
4.1 Microbial Mechanism Driving N2O Emissions as Regulated by Carbon Availability
N2O emissions from HCA cropland were larger than those from LCA croplands during the normal rainfall year, with a significantly higher EF of 0.77% versus 0.57%. These values were lower than the IPCC default value of 1.00% and the global mean of 1.02% for maize croplands (Cui et al. 2021). Consistently, Chen et al. (2014) reported a value of 0.34% also in black soils under maize cultivation in Northeast China. We further synthesized the literature results of in situ N2O emissions over at least the entire growing season from the black soil area located in Northeast China (Table S3, Figure S10). Out of expectation, cumulative N2O emissions decreased with increasing SOC content, which was consistent with the negative correlation between them under conservation tillage (de Figueiredo et al. 2018) but contrary to the well-acknowledged positive relationships observed on regional or global scales (Bouwman et al. 2002; Cui et al. 2021; Ge et al. 2024). Moreover, the ratio of SOC to TN (C/N) exhibited a stronger relationship with N2O emissions than SOC content, indicating that N2O emissions were more tightly linked to the availability of SOC than its quantity (Yao et al. 2022). Although black soils have the highest SOC content among croplands worldwide, they are less enriched in active components (e.g., DOC and carbohydrates) (Li et al. 2022). Generally, a higher C/N ratio was associated with a larger proportion of recalcitrant organic C (e.g., aryl and phenolic groups; Figure S2), exhibiting slower decomposition and turnover rates (Soldatova et al. 2024), leading to a lower available substrate supply for denitrification and N2O production (Chen, Li, et al. 2021). POM comprised of plant-derived materials has been shown to be positively associated with hotspots of microbial activity in the soils, including denitrification, despite MAOM being the main portion of bulk soil organic matter (Stuchiner et al. 2024). Greater soluble organic C could be derived from POM-fueled microbial processes and may create favorable condition for denitrification (Chen, Li, et al. 2021).
Indeed, the N2O fluxes were observed to increase with higher DOC content, alongside a negative relationship with the aromaticity of DOC (Figure 5e). Similarly, Saari et al. (2009) also identified DOC concentration and decomposability as important controllers for N2O release from forest soils. Previous research had indicated that DOC regulated N2O emissions through directly affecting dominant microbial production pathways (Wieder et al. 2011; Yin et al. 2017). As shown in Figure 3 and Figure S7, the NO/N2O ratio in the HCA cropland was well lower than 1, which indicated that more in situ N2O was produced from heterotrophic denitrification, especially after rainfall and N-fertilization (Chen, Tu, et al. 2021; Ji et al. 2020). The DOC with high bioavailability serves as an electron donor and promotes denitrification to produce N2O under lower O2 conditions (Stuchiner and Von Fischer 2022). The nir(S + K)/amoA ratio, regarded as a valuable proxy for coupling the microbial genetic potential of denitrification versus nitrification (Liao et al. 2021), was positively related to N2O fluxes and was significantly higher in HCA cropland (Figures 4g and 5e). Moreover, the SEM results confirmed that DOC stimulated N2O production by facilitating the nirS- and nirK-type denitrifying bacteria (Figure 5d). It has been reported that these microbes prefer to inhabit soils rich in DOC and exhibit positive responses to labile C amendments (Florio et al. 2019). Therefore, the larger N2O emissions in the HCA cropland were attributed to greater C availability, which promoted denitrification by fueling nirS- and nirK- denitrifiers.
Some denitrifiers carry the nosZ gene encoding N2O reductase, which is the only known biological sink of N2O. Thus, the determination of N2O emissions by denitrification depends on the extent of reduction of N2O to N2 by the denitrifying community. The microbial ratio of (nirS + nirK)/(nosZI + nosZII) in the HCA cropland was higher than that in the LCA (Figure 4h), indicating a greater potential for N2O production than further reduction (Liao et al. 2021). Indeed, the abundance of nosZI in HCA cropland was higher than that in LCA and had a positive correlation with N2O fluxes (Figure 5e). In contrast, the abundance of nosZII, which has a stronger N2O reduction ability (Linton et al. 2020), and the ratio of nosZII/(nirS + nirK) were lower in HCA than in LCA (Figure S11). Domeignoz-Horta et al. (2017) found that the diversity of nosZII but not of nosZI clade was the key determinant explaining the variation of in situ N2O emissions. Here, we observed that nosZI-bacteria was positively dependent on the DOC supply. Many of the alphaproteobacterial species with nosZ clade I are plant-associated, including Bradyrhizobium spp. and Paracoccus spp., suggesting that N2O-reducing organisms within these lineages are adapted to utilize a variety of labile C sources in root exudation, containing low molecular weight carbohydrates and organic acids (Abdelfattah et al. 2021; Maheshwari et al. 2023). In this study, the HCA cropland was characterized by higher crop yields with well-developed root systems (Table S4), which facilitated the exudation of easily decomposable substrates, thus enhancing the activity of nosZI rather than nosZII (Ai et al. 2020). Thus, the lower N2O reduction potential in the HCA cropland was due to the poor abundance of nosZII. It should be noted that the responses of nosZII abundance and physiological activity to C substrates are still unclear. A deeper insight into the mechanisms driving niche differentiation between microbes with clades I and II nosZ is needed, especially in response to agricultural C management and climate change, thereby ultimately favoring the development of N2O mitigation strategy (Hallin et al. 2018; Hiis et al. 2024; Xu et al. 2020).
Apart from the greater capacity for N2O production via denitrification in HCA soils, stronger nitrification could also contribute to larger emissions. Actually, nitrification plays a dual role in the production of N2O, by directly producing N2O and providing substrate for denitrification. A higher / ratio of HCA than LCA potentially indicated its greater capacity for nitrification (Figure S5). Identically, the 15N tracing experiment showed that the contribution of autotrophic nitrification to N2O production was higher in HCA soil (Figure 7b). Additionally, N2O fluxes were increased with a higher ratio of AOB/AOA, and AOB was more abundant in HCA, as facilitated by its higher supply compared to LCA (Figure S4a). Although ammonia-oxidizers do not use organic C as an energy source, higher-quality soil organic matter could promote microbial mining for N, (Liu et al. 2022) as evidenced by the higher gross rate of mineralization of HCA soils (Figure S12). This alleviated the limitation of substrate availability for nitrification by AOB and resulted in a more rapid and tight mineralization-nitrification coupling process (Figure S12) (Xiao et al. 2019), which in turn could provide more substrate to stimulate the denitrification rates and thus N2O production. Moreover, the N2O production capacity of AOB was twice that of AOA, where production is limited to presumably abiotic hybrid formation (Hink et al. 2018; Prosser et al. 2020). Furthermore, the ability to denitrify is a common trait among AOB, so N2O can be produced not only as a by-product of hydroxylamine metabolism but also likely through nitrifier denitrification as discussed above (Wrage-Mönnig et al. 2018).
In contrast, the fraction of N2O produced via heterotrophic nitrification in the LCA soil was 1.4 times greater than that of autotrophic nitrification (Figure 7b). It has been traditionally deemed that heterotrophic nitrification principally occurs in grassland and forest soils, and is generally negligible in croplands (Zhang et al. 2015). However, Chen et al. (2014) observed a distinct occurrence of heterotrophic nitrification in cultivated black soils, which contributed up to 25%–49% of N2O production. Fungi are considered the most efficient microbes performing heterotrophic nitrification (Martikainen 2022). Compared with bacteria, fungi are more acid-tolerant and require less N per unit biomass C accumulation, and ligninolytic fungal genera among Basidiomycetes and Ascomycetes could decompose recalcitrant compounds more efficiently (Wang and Kuzyakov 2024). Consequently, heterotrophic nitrification may play a vital role in N2O production in LCA soils with more aromatic components (Chen et al. 2015; Zhang et al. 2019). However, the ability of N2O production by fungal heterotrophic nitrification is much weaker compared to bacterial autotrophic nitrification (Zhang et al. 2015) and accordingly did not lead to high N2O emissions from LCA soils. To get a more reliable perspective on the contribution of heterotrophs to nitrification, the physiology of soil heterotrophic nitrifiers and the trade-off between autotrophic and heterotrophic nitrification, as controlled by the dynamics of SOC quality, require further study.
4.2 Processes Responsible for N2O Pulses Over the Waterlogging Period
During field monitoring, the study site experienced multiple heavy rainfall events in the summer of 2019, which became a record-breaking wet year with annual precipitation 1.8-fold higher than the long-term mean. Compared with the normal rainfall year, N2O emissions from the HCA and LCA increased by 84% and 27%, respectively. In particular, the N2O-EF of HCA cropland was greatly increased from 0.77% to 2.24%, which was three times greater than that of LCA and doubled the average global upland (1.05%) as estimated by Wang et al. (2020). The occurrence of climate extremes in the middle-high latitude regions is predicted to increase as accompanied by climate warming (Ombadi et al. 2023). Our results clearly indicated that extreme rainfall could potentially induce this region to become a “hotpot” of N2O emissions, particularly for fields characterized by high SOC availability.
Enhanced N2O emissions from HCA cropland during the extreme rainfall year were primarily due to explosive fluxes during the waterlogging period. Similar results were observed in cultivated black soils (Chen et al. 2016), semiarid grasslands (Li et al. 2023), and forests (Leitner et al. 2016). Nevertheless, the mechanisms responsible for emission pulses during the waterlogging period remain obscure because the transient and intense effects of extreme rainfall are highly challenging to capture (Fu et al. 2023; Knapp et al. 2017).
Montoya et al. (2022) supposed that in situ N2O pulses after irrigation were ascribed to stimulated denitrification. Our laboratory 15N tracing experiment evidently revealed that larger N2O emissions under supersaturated moisture conditions were almost exclusively produced by denitrification (Figure 7b). The flux ratio of NO/N2O was no more than 0.12 during the waterlogging period (Figure S7b). This also suggested that the peak N2O fluxes presented in the field could be predominantly attributed to denitrification when the soil moisture began to slowly decrease but remained saturated. It should be noted that the N2O production pathways were quantified using 15N tracing incubations, which may have biases due to the differences between laboratory and field conditions, although they are unlikely to affect the main conclusions. The in situ 15N labeling method is recommended to decipher the processes of N2O production in response to extreme hydrological events.
The significant relationship between CO2 and N2O fluxes during the waterlogging period suggested that N2O emissions were associated with the activity of heterotrophic microbes, which were stimulated by soil extractable N and DOC (Figure 6a). The sudden increase in water content caused aggregates to disintegrate or organic–inorganic complexes to break down, leading to the dissolution of readily decomposable components to increase the DOC content (Védère et al. 2022). However, this increment did not immediately induce the peak of N2O fluxes. Interestingly, we found that N2O fluxes increased linearly with the HIX index, suggesting that the N2O production rate was more likely to be regulated by DOC quality. The decreased values of SUVA, HIX, and FI indices (Figure S13) implied a reduction in the high-molecular-weight humic substances but possibly an increment in the plant-derived components during the waterlogging period (Zhou et al. 2019). In general, plants enhanced their resistance to anaerobic stress by releasing root exudation (Chai and Schachtman 2022; Henry et al. 2007). Additionally, we observed an increase in fungal abundance during the flooding process (Figure S9h). Compared to bacteria, fungi could more efficiently promote water transport and acquire resources through their mycelial networks, thus tending to dominate more of the activity of microbial communities in waterlogged environments (Wang et al. 2023). Fungi have a greater ability to decompose recalcitrant humic substances into low-molecular-weight molecules (Yuste et al. 2011), which were more easily utilized by heterotrophic denitrifiers and contributed to the enhanced N2O emissions under extreme hydrological conditions.
The N2O fluxes were observed to increase logarithmically with increasing concentration during the waterlogging period (Figure 6b). However, we observed that an extremely lower concentration of initially after the soil was saturated with the successive rainstorm events (< 2 mg N kg−1), which was below the reported threshold of 3 mg N kg−1 for high N2O fluxes larger than 100 μg N m−2 h−1 (Chen et al. 2016). This was mainly because rapid flow paths might occur after the extreme rainfall, causing a “hot moment” for leaching (Castellano et al. 2010). Additionally, Chen et al. (2016) deduced that the low concentration after soil waterlogged might also be attributed to the occurrence of dissimilatory nitrate reduction to ammonium (DNRA), as supported by the simultaneous increase in concentration. However, in the current study, the functional gene of nrfA did not exhibit a significant increase (Figure S9i), which suggested that high might not be produced by the DNRA process but by the decomposition of organic matters under the waterlogging conditions (Qu et al. 2014). Subsequently, accompanied by the disappearance of soil surface water, nitrification gradually proceeded as soil aeration enhanced. A positive relationship between the abundance of AOA and content was observed (Figure S14) possibly due to the tolerance of AOA communities to low O2 concentrations and their preference for survival in stressful environments compared to AOB (Prosser et al. 2020). Therefore, the nitrification process driven by AOA in waterlogged soil might be the source of as the substrate for N2O production by denitrification. It is suggested that the dynamics of soil O2 concentrations should be included and linked to in situ N2O fluxes in future studies in order to elucidate the mechanisms of N2O emissions, especially in rainfed croplands, where redox conditions are sensitive to rainfall events.
Apart from the enhancement of C availability and supply, substantial post-rainfall N2O emissions could also be related to the increase in denitrifying microbes (Montoya et al. 2022). In the present study, the abundance of nirS increased significantly, while nirK and both clades of nosZ decreased after soil waterlogged (Figure S9). Compared with nirK, nirS denitrifiers are better adapted to soil conditions with high moisture and low O2 content (Qin et al. 2018; Shi et al. 2021). The nirS communities have been demonstrated to be more closely related to the supply of labile C substrates under sub-anoxic than aerobic conditions (Yuan et al. 2012), which implied distinct regulatory mechanisms of substrates on denitrifying microorganisms as affected by changing water conditions. Accordingly, enhanced C availability might have stimulated the activity of nirS denitrifiers during the waterlogging period in this study. As for the decreased abundance of nosZ gene, the inhibition of nosZ activity by increased content might be the reason (Friedl et al. 2020; Hallin et al. 2018). This could be partly supported by the lower abundance of nosZ gene in soils treated with N-fertilization as compared with the CK treatments (Figure S15). Furthermore, N2O fluxes during this period were observed to positively increased with the ratio of nirS/nosZII (Figure S16). Therefore, it could be concluded that the explosive N2O emissions as induced by the extreme hydrologic conditions were mostly derived from the nirS-driven incomplete denitrification as promoted by the increased substrates of available C and .
5 Conclusions
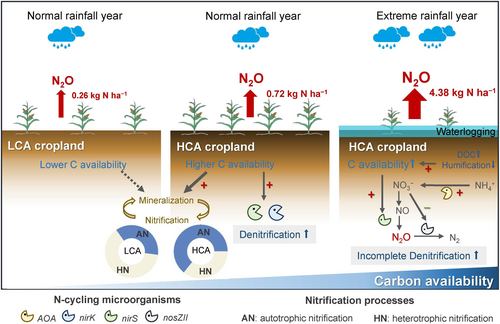
Overall, our study demonstrated the powerful roles of soil moisture, C availability, and their interactions in regulating soil N2O emissions (Figure 8). Higher C availability in soils could induce greater N2O emissions, with this stimulation amplified during the waterlogging period following extreme rainfall events, thereby triggering a surge in N2O emissions. The stimulated N2O pulses will have further consequences for greenhouse gas inventories. As the frequency and intensity of global extreme rainfall are expected to increase, the stimulation of N2O emissions by available C substrates would be further magnified, thus strengthening the positive feedback on climate change. Therefore, we suggest the necessity of avoiding the amendments to exogenous organic materials with high C lability in the formulation of soil C sequestration strategies for agricultural systems, considering their potential to stimulate N2O emissions. Otherwise, the benefits of increasing SOC stocks as GHG sinks and the improvement of fertility could be largely offset by the stimulation of additional N2O emissions, particularly in the context of frequent extreme rainfall events. Simultaneously, this study underscores the importance of considering multiple simultaneous pathways when addressing N2O emissions in response to environmental changes and field management, a crucial aspect for model development and mitigating strategy improvement.
Author Contributions
Zengming Chen: conceptualization, funding acquisition, investigation, methodology, writing – original draft, writing – review and editing. Nan Zhang: data curation, investigation, writing – original draft, writing – review and editing. Ye Li: investigation, methodology. Shiqi Xu: writing – review and editing. Yulian Liu: investigation, methodology. Shujie Miao: writing – review and editing. Weixin Ding: funding acquisition, supervision, validation, writing – review and editing.
Acknowledgements
This research was financially supported by the National Key Research and Development Program of China (2022YFD1500303), the Natural Science Foundation of Jiangsu Province (BK20211610), the Natural Science Foundation of China (42077029, 42307386), Frontier Project from the Institute of Soil Science, Chinese Academy of Sciences (ISSASIP2212), Youth Innovation Promotion Association of Chinese Academy of Sciences (2022313), and IAEA coordinated research project (D15020).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are openly available in Figshare at https://doi.org/10.6084/m9.figshare.28603286.




