Migratory Birds Advance Spring Arrival and Egg-Laying in the Arctic, Mostly by Travelling Faster
Funding: Thomas K. Lameris, Michiel P. Boom and Mo A. Verhoeven were supported by the project ‘ArcticMigrants’ funded by the Dutch Research Council (ALWPP.2019.002). Margje E. de Jong and Isabella B. R. Scheiber were funded by a grant from the Austrian Science Fund (FWF P 32216). Jeroen Reneerkens was supported by two grants from the Netherlands Polar Programme (851.40.072 and 866.15.207) of the Netherlands Organisation for Scientific research (NWO) and from the Metawad project awarded by Waddenfonds (WF209925) to Jeroen Reneerkens and Theunis Piersma. Jeroen Reneerkens and Tom S. L. Versluijs also received INTERACT grants for Transnational Access from the European Community's Seventh Framework Programme (grant agreement No. 262693). Peter M. Glazov and Julia A. Loshchagina were supported by the Russian Ministry of Science and Higher Education (Project No. FMWS-2024-0007) and grant RSCF No. 22-17-00168. Alexander Kondratyev and Olga Kulikova were funded by theme No. 123032000020-7 (IBPN FEB RAS). Tracking studies were funded by: Netherlands Organisation for Scientific Research, Netherlands Polar Programme (grants NPP 866.13.005, NPP 866.13.010, NPP 866.15.206 and ALWPP.2016.024); the province of Fryslân (grant 01443719); the University of Amsterdam; the LUVRE project (www.luvre.org); the Vogelschutz-Komitee e.V. (VsK), Germany; the German Federal Ministry for the Environment, Nature Conservation, Nuclear Safety and Consumer Protection (BMUV; FKZ 3520685021) with support from Lower Saxonian Minister for Food, Agriculture and Consumer Protection (goose research 406-04321/1-1502/1) and the Ministries of Environment of Schleswig-Holstein and Lower Saxony, Naturschutzstiftung Emsland and NABU (bewick's); the Max-Planck Institute of Animal Behavior and the German Aerospace Center (DLR) through the ICARUS program, European Space Agency (ESA) as part of the Integrated Applications Promotion Program, the Fram Center flagship ‘Climate Change in Fjord and Coast’ grants nr 232019 and 2019147470 1152018 and the County Governor of Finnmark, Aarhus University, Denmark, GREA-Arctic Ecology Research Group; Austrian Science Fund (P 32216); Arctic Field grant in collaboration with NINA (ES676286); KNAW Ecology Fund, http://iap.esa.int/flysafe.
ABSTRACT
In the current warming climate, many organisms in seasonal environments advance their timing of reproduction to benefit from resource peaks earlier in spring. For migrants, the potential to advance reproduction may be constrained by their migration strategies, notably their ability to advance arrival at the breeding grounds. Recent studies show various changes in migration strategies, including wintering closer to the breeding grounds, earlier departure from the wintering grounds or faster travels by spending less time at stopover sites. However, whether such changes lead to earlier arrival or earlier breeding remains an open question. We studied changes in migration and reproduction timing in 12 populations of nine migratory birds, including seabirds, shorebirds, birds of prey and waterfowl breeding at Arctic sites bordering the Greenland and Barents Sea, a region undergoing rapid climate warming. The timing of migration and reproduction was derived from tracking and field data and analysed to study (1) how timing has changed in response to the changing moment of snowmelt at the breeding grounds and (2) what adjustments in migration strategies this involved. We found that in years with early snowmelt, egg-laying in multiple populations advanced, but only two waterfowl populations also advanced arrival in the Arctic. In contrast, arrival in the Arctic generally advanced with time, even when snowmelt or egg-laying dates did not advance. Earlier arrival with time was mostly explained by populations traveling to the Arctic faster, likely spending less time at stopover sites. Inability to forecast conditions in the Arctic may limit birds to adjust migration timing to annually varying snowmelt, but we show that several species, particularly waterfowl, are able to travel faster and advance the timing of migration over the years. The question remains whether this reflects adaptations to Arctic climate change or other factors, for example, environmental changes along the migratory route.
1 Introduction
The earth's climate is warming rapidly, resulting in strong environmental changes for all living organisms. One of the most important mechanisms by which climate warming is impacting animal populations, particularly in seasonal environments, is via changes in biotic interactions (Ockendon et al. 2014). Under increasing temperatures, organisms at lower trophic levels, such as plants and arthropods, can strongly advance their phenology (Thackeray et al. 2010). This creates an earlier seasonal ‘resource peak’ for their consumers (Kharouba and Wolkovich 2020) and, as a result, resources may become severely limited late in the season (but see: Reneerkens et al. 2016; Zhemchuzhnikov et al. 2021). Since limited resources during early life can lead to reduced growth or survival of offspring (Boom et al. 2021; Lameris et al. 2018), consumers could be expected to advance their timing of reproduction accordingly. However, large variation exists in the extent to which avian consumers adjust their timing of reproduction to earlier availability of their food (Tavera et al. 2024; Zhemchuzhnikov et al. 2021). Some species appear able to synchronise reproduction with food availability (Rakhimberdiev et al. 2018), while others show little or even no adjustment in timing (Keogan et al. 2018; Lameris et al. 2022; Reneerkens et al. 2016). For migratory species, advancements in the timing of reproduction are often considered to be constrained by the timing of migration (Both 2010; Both and Visser 2001), yet there are several examples of migratory species for which laying dates are to some extent independent of arrival dates (Lameris et al. 2018; Lourenço et al. 2011). For many species, it thus remains an open question as to whether advancements in laying dates are limited by the potential for earlier migratory arrival on the breeding grounds.
In theory, earlier migratory arrival is achievable by three, not mutually exclusive, mechanisms: (1) shortening migration distances by spending the non-breeding period closer to the breeding grounds (Visser et al. 2009), (2) earlier departure from the non-breeding grounds (Conklin et al. 2021) and (3) faster travel between non-breeding and breeding sites (Lameris et al. 2018; Morbey and Hedenström 2020). These strategies require responses at different instances during the migratory journey and may be limited by different constraints.
The location where migrants choose to spend the non-breeding period generally depends on local weather conditions and food availability, which ensure survival. As such, the first mechanism (spending the non-breeding period closer to the breeding grounds) is often associated with temperature increase (Linssen et al. 2023; Maclean et al. 2008; Visser et al. 2009) and increases in food availability (Clausen et al. 2018), enabling birds to survive in non-breeding areas closer to the breeding grounds. It has been hypothesised that from these areas, birds may be better able to predict the onset of spring in breeding sites, enabling earlier arrival (Visser et al. 2009).
The second mechanism, earlier departures from non-breeding grounds, is generally considered a response to changing environmental conditions, either at non-breeding grounds or at stopover sites further along the flyway (Conklin et al. 2021; Lisovski et al. 2024), and therefore is limited by the extent to which changing conditions are known from experience or can be predicted from afar (Kölzsch et al. 2015; Lameris, Scholten, et al., 2017; Tombre et al. 2008). Earlier departure will also be constrained by the ability to build up sufficient energy stores prior to departure, which need to be built up faster (Lindström et al. 2019) or initiated earlier (Ouwehand et al. 2023), as well as by the completion of plumage moult in some species (Newton 2009). Hence, earlier departures will importantly depend on local benign conditions for fuelling and moulting in the non-breeding area.
The third mechanism, increasing travel speed between non-breeding and breeding sites, can be achieved by spending less time at stopover sites and is probably a response to changing conditions en route (Lameris et al. 2018). Species can increase their travel speed by spending less time on stopover sites (Lameris et al. 2018; Rakhimberdiev et al. 2018), achieved either by building up energy stores at stopover sites faster (Lindström et al. 2019) or by departing with fewer stores, leading to arrival on their breeding site with a lower body mass (Lameris et al. 2018). Therefore, earlier departure from non-breeding sites or higher travel speed by spending less time on stopovers represent different choices on where to fuel body stores: in non-breeding sites before departure, on stopover sites during migration (Evans and Bearhop 2022) or at the breeding site after migration to recover before reproduction (Lameris et al. 2018). While those choices may result in earlier arrival timing, they may not necessarily allow for earlier breeding as this will also depend on energy stores (Bêty et al. 2003).
Whether birds can advance spring migration timing by shortening migration distances, earlier departures or faster travels will be linked to the environmental and internal constraints they face, which may vary among populations. For example, shifts in the non-breeding range may only be possible following changes in environmental conditions in the non-breeding range (Nuijten et al. 2020); advancements in the timing of departure may only be possible when conditions for fuelling at non-breeding sites improve (Ouwehand et al. 2023), as well as conditions on subsequent stopover sites; and an increase in migration speed by decreasing stopover time can likely only be achieved by birds fuelling faster on stopovers or travelling with less internal energy stores. To better understand what limits the advancement of migratory arrival and egg-laying, we here study changes in migration and reproduction timing in a range of arctic-breeding migratory bird populations. These populations collectively face warming conditions on their breeding grounds yet differ in migration strategy and likely vary in environmental and internal constraints during migration.
Arctic migratory birds form an ideal system to study variation in migratory advancements. The Arctic is a highly seasonal environment, which is warming four times faster than elsewhere (Rantanen et al. 2022). As such, the effects of warming are more easily detectable and are expected to have a large impact on species breeding here. The Arctic is home to many migratory breeding birds, ranging from small passerines to large waterfowl, with migration distances ranging between 1,400 (e.g., rough-legged buzzard Buteo lagopus, Pokrovsky et al. 2024) and 25,000 km (Arctic tern Sterna paradisaea, Egevang et al. 2010). Arctic climate warming is resulting in earlier snowmelt in spring (Box et al. 2019) which is a major determinant of the time suitable for the reproduction of migratory birds. Snowmelt both frees up available nesting sites and triggers forage plant growth (Cooper et al. 2011; Semenchuk et al. 2016) and arthropod emergence (Chagnon-Lafortune et al. 2024; Leingärtner et al. 2014), which are key resources for herbivorous and insectivorous birds. Earlier snowmelt is therefore expected to have major effects on the phenology of resources for birds (Lameris, Jochems, et al., 2017; Shaftel et al. 2021; Tulp and Schekkerman 2008). Facing rapid environmental changes, arctic migratory birds would be predicted to make similarly rapid adjustments in the timing of migration and reproduction in response. However, not all species seem to do so (Keogan et al. 2018; Post et al. 2018; Zhemchuzhnikov et al. 2021). Therefore, it is likely that some species are encountering constraints that limit their ability to advance migration timing.
We investigate the annual and long-term variation in the timing of migratory arrival and egg-laying on the breeding grounds in response to local changes in snowmelt conditions in a suite of nine migratory bird species breeding in the Barents and Greenland Sea region. We expect changes in timing with earlier snowmelt to vary between populations due to varying constraints, with the largest flexibility in timing for larger species of waterfowl, which bring additional 'capital' energy stores on migration (Klaassen et al. 2006) and have already shifted their non-breeding grounds further northward (Nuijten et al. 2020). Over time, we expect changes in migration timing to follow changes in snowmelt. We further analyse the mechanisms behind the variations and trends in arrival, examining whether these are explained by shifts in non-breeding ranges, departure timing, or travel speed. Finally, we consider whether the timing of arrival potentially limits advancements in the timing of egg laying. The variation among species and populations is discussed in the light of environmental and internal constraints, capitalising on differences in migration and reproduction strategies among species and populations, as well as on other existing case studies with approaches similar to ours.
2 Methods
We gathered data on the timing of spring migration and the timing of egg-laying in relation to the local timing of snowmelt for a suite of arctic-breeding bird species, using a large set of tracking data supplemented by field data on egg-laying dates. Rather than using tracking data of individuals tracked from the non-breeding grounds and breeding across the entire breeding range of a species, we focussed on tracking data of individuals breeding at specific Arctic breeding sites. In this way, we aimed to reduce variation in the timing of arrival in the Arctic caused by variations in breeding location (e.g., Conklin et al. 2010). All data and scripts for analyses can be found in Lameris et al. (2025).
2.1 Study Species and Sites
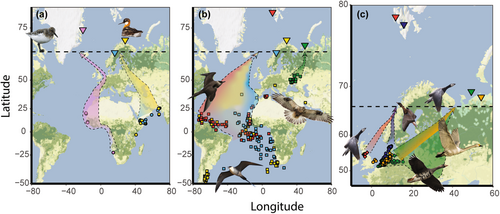
Our dataset contained tracking data (519 spring migrations of 274 individual birds) from 12 populations and nine migratory bird species, breeding at seven Arctic study sites in the Barents and Greenland Sea region (Figure 1; Table 1) from the period 2007–2023. For this paper, we treat each species at a separate study site as a separate population. The species and study sites included greater white-fronted goose Anser albifrons (Kolguev Island, north-western Russia), pink-footed goose Anser brachyrhynchus (Adventdalen, Svalbard), barnacle goose Branta leucopsis (Kolguev Island, north-western Russia and Kongsfjorden, Svalbard), tundra swan Cygnus colombianus (Malozemelskaya tundra, north-western Russia), red-necked phalarope Phalaropus lobatus (Ammarnäs, northern Sweden and Slettnes, northern Norway), sanderling Calidris alba (Zackenberg, north-eastern Greenland), Arctic skua Stercorarius parasiticus (Slettnes, northern Norway and Kongsfjorden, Svalbard), long-tailed skua Stercorarius longicaudus (Ammarnäs, northern Sweden) and rough-legged buzzard (Kolguev Island, north-western Russia). Birds were tracked using GPS loggers, GPS-GSM-transmitters, satellite transmitters (GPS-PTT) or geolocators (Global Location Sensing, GLS, Table 1). We tracked only females of the three goose species and both females and males of the other species. Most birds were captured and fitted with tracking devices on their breeding grounds (Table 1), and the study sites of these populations, defined as the area in which birds were captured, were relatively small (8–56 km2, Table S1). Only greater white-fronted geese, barnacle geese (north-western Russian population) and tundra swans were captured and tracked from their non-breeding grounds in the Netherlands and Germany. For these populations, we selected larger areas as study sites (6,061–15,132 km2) known to host major concentrations of these species (Glazov et al. 2021; Kondratyev et al. 2013; Rees 2010), namely Kolguev Island (greater white-fronted geese, barnacle geese) and the Malozemelskaya tundra region, west of the Pechora Sea (tundra swans). Only individuals who initiated a nest in these study sites were included (see methods section ‘Date of egg laying’). For tundra swans, nesting behaviour was more difficult to detect from tracking data, and we developed a more comprehensive method to evaluate this making additional use of acceleration data (supplemental materials). To increase the sample size for this species, we also included male individual swans that stayed in the Malozemelskaya tundra study site for the entire month of June. Rough-legged buzzards were captured and tracked from various locations on Kolguev Island (Pokrovsky et al. 2024), and we therefore used the study area of Kolguev Island also for this population. Detailed information on tracking methods can be found in the species-specific studies (for references and sample sizes, see Table 1).
| Species | Breeding location of population | Study years | # tracks with arrival in Arctic (# individuals) | # laying dates from tracked birds | # laying dates from other birds | Tracking device type | Movebank ID's | References |
|---|---|---|---|---|---|---|---|---|
| Barnacle goose | Svalbard (Kongsfjorden) | 2021–2023 | 33 (19) | 33 (19) | — | GPS-GSM | 1,176,499,696 | de Jong et al. (2024) |
| North-western Russia (Kolguev Island) |
2008–2011, 2017–2023 |
84 (49) | 84 (49) | — | Satellite transmitter, GPS-GSM |
29,799,425, 1,114,583,459, 137,654,491 |
van der Jeugd et al. (2014) and Boom, Lameris, et al. (2023) | |
| Greater white-fronted goose | North-western Russia (Kolguev Island) | 2014, 2016 – 2023 | 38 (12) | 38 (12) | — | GPS-logger, GPS-GSM | 12,135,743, 180,156,318, 9,589,196, 44,083,081 | van Wijk et al. (2012) and Kölzsch et al. (2016) |
| Pink-footed goose | Svalbard (Adventdalen) | 2019–2023 | 26 (11) | 26 (11) | — | GPS-GSM | 1,239,582,803 | Schreven et al. 2021 |
| Tundra swan | North-western Russia (Malozemelskaya tundra) | 2017–2023 | 41 (23) | 12 (9) | — | GPS-GSM | 181,958,207, 1,881,288,761, 1,812,016,959 | Nuijten and Nolet (2020) and Linssen et al. (2023) |
| Red-necked phalarope | Central Sweden (Ammarnäs) | 2014–2017 | 12 (11) | — | 65 | Geolocator | 99,570,338 | van Bemmelen et al. (2019) |
| Northern Norway (Slettnes) | 2015–2017 | 10 (7) | — | 83 | Geolocator | 968,983,708 | van Bemmelen et al. (2019) | |
| Sanderling | Northeast Greenland (Zackenberg) | 2014, 2016, 2017 | 9 (8) | 5 | 92 | Geolocator | Reneerkens et al. (2019 ) |
|
| Arctic skua | Svalbard (Kongsfjorden) | 2010–2017 | 71 (35) | 67 (34) | — | Geolocator | 2,746,281,685 | van Bemmelen et al. (2024) |
| Northern Norway (Slettnes) | 2015–2018 | 60 (41) | 53 (36) | — | Geolocator | 968,984,187 | van Bemmelen et al. (2024) | |
| Long-tailed skua | Central Sweden (Ammarnäs) | 2012–2015 | 38 (21) | — | 89 | Geolocator | 968,980,842 | van Bemmelen et al. 2017 |
| Rough-legged buzzard | North-western Russia (Kolguev Island) | 2014–2017 | 14 (7) | 7 | — | GPS-GSM | 9,493,874 | Pokrovsky et al. (2021) |
2.2 Tracking Data Preprocessing
Tracking data were acquired from Movebank (Kays et al. 2022) and datasets from authors, with all data sources described in Table 1. For Arctic skuas, long-tailed skuas, red-necked phalaropes and sanderlings, we used previously obtained tracks from GLS data as described in (van Bemmelen et al. 2017, 2019, 2024; Reneerkens et al. 2019). All studies used a light intensity threshold of 2.0 to determine twilight events. To avoid large errors around the equinox, latitude data were deleted from 14 days prior to 18 days after the spring equinox. For greater white-fronted geese (Kölzsch et al. 2016), barnacle geese (Boom, Lameris, et al., 2023; Kölzsch et al. 2015), pink-footed geese (Schreven et al. 2021), tundra swans (Linssen et al. 2023; Nuijten and Nolet 2020) and rough-legged buzzards (Pokrovsky et al. 2021), previously published GPS tracks were downloaded from Movebank. GPS tracks were filtered to exclude outliers, identified as positions where the speed required to travel from that to the next position was larger than 120 km/h [which is 10 km/h faster than recorded flight speeds for these species, (Alerstam et al. 2007; Miller et al. 2005)]. We only included GPS- and geolocator tracks that contained at least one position per day from January until arrival at the Arctic Circle.
2.3 Migration Timing, Migration Distance and Travel Speed
From tracking data, we extracted individual data on spring migration timing including departure from the non-breeding area and arrival in the breeding area, as well as total migration distance and travel speed. First, individual non-breeding sites were calculated as mid-winter geographical centroids: the median position during January and February, a period during which all populations are considered to be present in their non-breeding regions. The calculation of the departure date from the non-breeding site differed between geolocation- and GPS-based tracks. For GPS tracks, we defined the departure date as the date with the last position in spring within 200 km of the mid-winter centroid. For GLS tracks, which suffer from a larger positional error, especially in latitude during the period around the equinox (Lisovski et al. 2012), we closely inspected raw position estimates to find the first date with consistent directional movement away from the non-breeding site.
The arrival date in the Arctic was determined as the date at which birds crossed the Arctic Circle (latitude 66.33° N). For GPS data, this was defined as the first date above 66.33° N. GLS tracks are calculated based on sunrise and sunset events and therefore cannot be calculated for positions above the Arctic Circle during the polar day with 24 h of continuous light (Lisovski 2018). For these tracks, we defined crossing of the Arctic Circle as the first day after the date with the last dark night (which usually occurred at c. 60° N latitude). An earlier study found that the crossing from these last positions to the Arctic is generally rapid without stops (van Bemmelen et al. 2024).
Total migration distance was calculated as the cumulative sum of great circle distances between daily averaged positions, from departure from the non-breeding site to arrival in the Arctic (at the Arctic Circle). Travel speed was calculated as the total migration distance divided by the time between departure and arrival in the Arctic in km/day. Due to high flight speeds, the actual time spent flying is relatively short; therefore, variation in migration speed mostly represents variation in the amount of time spent on stopover sites along the migratory routes (Alerstam and Bäckman 2018). We emphasise that our measure of travel speed is not the same as migration speed, which includes the time spent fuelling at non-breeding sites before departure (Alerstam 2003).
2.4 Date of Egg-Laying
The date of egg-laying, defined as the date at which the first egg was laid, was determined from (i) field observations, (ii) patterns in light measurements, and (iii) GPS tracking data. (i) In field observations, the date of egg-laying for individuals with and without tracking devices was either observed directly, back-calculated from incomplete clutches in geese (van der Jeugd et al. 2009) and shorebirds (Liebezeit et al. 2014; Reneerkens et al. 2016), back-calculated from observed hatching dates [by subtracting the period required for incubation, based on (Cramp and Perrins 1988) or assessed by egg floatation (Liebezeit et al. 2007)]. (ii) For GLS data, laying dates were determined based on regular periods of darkness of at least 1 h while the bird was in an area with continuous daylight (Verhoeven et al. 2020). (iii) For GPS data of geese (of which only females were tagged), the timing and position of nesting were determined from GPS locations after arrival on the breeding site, where the nest location was determined as a position where the daily standard deviation in latitude was less than 25.4 m (Schreven et al. 2021) for at least three consecutive days. The first of these 3 days was then determined as the date of egg-laying. For greater white-fronted geese and barnacle geese from Kolguev Island, for which both population-average laying dates from field data as well as extracted from tracking data were available, yearly averaged laying dates correlated strongly between both methods (Pearson's correlation = 0.83, t = 4.38, p = 0.002, n = 9 years). In contrast to geese, where only the female incubates the eggs, in tundra swans, females and males take turns to sit on the nest. This makes it more difficult to distinguish nest positions, and we therefore used a modified method for which also accelerometer data were used (for details see Supporting Information Methods). For GPS data of rough-legged buzzards, we determined the start of nesting as the first day in a period for which a bird stayed within one position, that is, a difference between GPS positions of less than 3 m, for more than 1 day. This method was verified using accelerometer data (for details, see Curk et al. 2022). For long-tailed skuas, red-necked phalaropes and sanderlings, laying dates could not (with a few exceptions) be determined for tracked individuals, as geolocators stopped functioning prior to breeding or birds did not breed. To analyse trends in laying dates for these populations, we supplemented our dataset with laying dates determined from field data for the same study sites and years (Table 1).
2.5 Date of Snowmelt
Snow cover for all study sites was estimated using satellite images of the 500 m resolution MODIS Terra Surface Reflectance Daily Global product [MOD09GA, v6.1, (Vermote and Wolfe 2021)]. The analysis was conducted in Google Earth Engine (Gorelick et al. 2017), using the R-package RGEE (Aybar et al. 2020) and the automated workflows for the quantification of snowmelt by Versluijs (2024).
For each satellite image and within each study site, we then calculated the percentage of snow cover as the fraction of pixels with NDSI values larger than 0.4 (Dozier 1989; Hall et al. 1995). We fit a general additive model (GAM; Wood 2023) to these annual time series of snow cover and extracted the moment the GAM first dropped below 50% snow cover, which we used in subsequent analyses as the date of snowmelt.
2.6 Statistical Analyses
For analyses, we used generalised linear mixed models [GLMMs, using r-package ‘lme4’ (Bates et al. 2012)] including a random intercept for individual birds in models with year as the independent variable and random intercepts for individual birds and year in all other models. The year and date of the snowmelt were centred by subtracting population-specific means. As strong interannual variation in environmental conditions (incl. date of snowmelt) could distort temporal trends, we excluded short time series (less than 5 years) from analyses of trends over time (i.e., over the years). Below, the steps of the full analysis are described in detail:
- date of snowmelt (Dsm) = intercept (intrcpt) + year (Y) + population (P) + P × Y
- arrival date (Da) = Dsm + P + Dsm × P + (1|ID) + (1|Y)
- Da = Y + P + Y × P + (1|ID)
- laying date (Dl) = Dsm + P + Dsm × P + (1|ID) + (1|Y)
- Dl = Y + P + Y × P + (1|ID)
- Da/departure date (Dd)/migration distance (MD)/travel speed (V) = Dsm + (1|ID) + (1|Y)
- slope arrival date ~ date of snowmelt (Dasm) = intrcpt + slope departure date (Ddsm) + slope migration distance (MDsm) + slope travel speed (Vsm)
- Da/Dd/MD/V = Y + (1|ID)
- slope arrival date–year (Day) = intrcpt + Ddy + MDy + Vy.
Finally, we analysed whether variation in laying dates between individuals and among years could be explained either by local date of snowmelt or individual arrival date, using GLMMs (x) including date of egg laying as the dependent variable, and date of snowmelt, arrival date and population, as well as interactions date of snowmelt × population and arrival date × population, as independent variables, along with random intercepts described above. In these analyses, laying and arrival dates were centred by subtracting population-specific means.
(x) Dl = Dsm + Da + P + Dsm × P + Da × P + (1|Y) + (1|P)
We used AICc values to compare the performance of models including all possible combinations of independent variables, including an intercept-only model. For most analyses, this meant comparing the performance of models including either the date of snowmelt or the year as independent variables with intercept-only models. We selected the model with the lowest AICc, but models within 2 ΔAICc of the top supported model were also considered informative, except when containing additional parameters (Arnold 2010). All analyses were conducted using R (version 4.3.1).
2.7 Data From Literature
We collated papers which reported on arrival and laying date of arctic migratory birds relative to the date of snowmelt in the Web of Science database, conducted on 7 February 2024. We used the search term: bird AND Arctic AND (snowmelt OR snow cover OR snow) AND (migration timing OR arrival OR laying date OR nest initiation OR egg) using the option ‘all fields’. This resulted in 213 papers, which were scanned for statistics and data on the timing of migratory arrival (on northern stopover sites or breeding sites), measured for at least 3 years in relation to any metric of snow cover. We omitted studies where it was not possible to extract slopes, arrival, laying, or snowmelt dates from either the text, tables, or graphs. Data were found in six papers (Table S2). Three papers (which were not identified in the search) known to contain similar data were added (Ely et al. 2018; Hupp et al. 2018; Lameris et al. 2018). Unpublished data for three additional species were supplemented by S. Volkov, with the data provided in the dataset linked to this paper (Lameris et al. 2025) and data collection following methods described in Volkov and Pozdnyakov (2021). Among all considered studies, the snow cover fraction used or extracted as a measure of ‘date of snowmelt’ varied between 60% and 0%. In general different fractions of snow cover will be highly correlated (e.g., 0.99 Pearson's correlation between 0.25 and 0.75 fraction snow cover in our own dataset), although the moment of 0% snow cover may show less of a correlation with mid-point snow cover. In total, the dataset from the literature contained 18 species (five species of waterfowl, 10 shorebirds, two passerines and one gull) from six different locations in the Holarctic (Table S2). We extracted slopes of the change in arrival dates, laying dates and date of snowmelt over time, and slopes of the change in arrival dates and laying dates in relation to date of snowmelt. We then used t-tests to analyse whether the slope in the change in arrival date and laying date, with time as well as in relation to the date of snowmelt, differed from zero. In addition, we used LMs to analyse whether changes in arrival and laying date over time correlated with changes in the date of snowmelt over time, comparing models with either slope of arrival or laying date as the dependent variable and the slope of date of snowmelt as an independent variable, and performing model selection as described above.
3 Results
3.1 Trends in Date of Snowmelt
The date of snowmelt generally advanced over the years, although the null model was competitive (−0.82 ± 0.43 mean slope coefficient ± standard deviation, units days per year, models without year = 1.4 ΔAICc compared to model with year). This trend did not differ between populations (models with interaction term population x year = 44.1 ΔAICc compared to the model without).
3.2 Population-Level Trends in Timing of Arrival and Laying
Arrival date in the Arctic was related to the date of snowmelt on the breeding grounds (model with population only = 26.8 ΔAICc compared to the model with date of snowmelt, population and interaction, Table S3) and differed between populations (model without interaction = 30.5 ΔAICc compared to a model with, Table S3). The confidence intervals of most population-specific trends overlapped with zero (Table 2; Figure 2), with the exception of four populations. Greater white-fronted geese and tundra swans arrived earlier in years with early snowmelt at their breeding grounds (greater white-fronted geese: 0.50 ± 0.11, 95% CI [0.28–0.72], tundra swans: 0.38 ± 0.08, 95% CI [0.22–0.54]). Arctic skuas breeding in Slettnes and long-tailed skuas arrived later in years with earlier snowmelt (Arctic skua: −0.27 ± 0.08, 95% CI [−0.43 to −0.12]; long-tailed skua: −0.36 ± 0.08, 95% CI [−0.53 to −0.21]).
| Population | Arrival–snowmelt | Laying date–snowmelt | Snowmelt–time | Arrival–time | Laying date–time |
|---|---|---|---|---|---|
| Barnacle goose (Kolguev Island) | 0.14 ± 0.10 [−0.05, 0.33] | 0.31 ± 0.10 [0.12, 0.50] | −1.01 ± 0.54 [−2.11, 0.09] | −0.32 ± 0.18 [−0.68, 0.04] | − 0.41 ± 0.19 [−0.78, −0.04] |
| Barnacle goose (Kongsfjorden) | 0.25 ± 0.21 [−0.17, 0.67] | 0.26 ± 0.18 [−0.10, 0.61] | −1.37 ± 6.47 [−14.45, 11.71] | ||
| Greater white-fronted goose | 0.50 ± 0.11 [0.27, 0.73] | 0.57 ± 0.10 [0.37, 0.77] | −1.89 ± 1.11 [−4.12, 0.34] | −0.73 ± 0.40 [−1.54, 0.01] | −1.71 ± 0.36 [−2.42, −1.00] |
| Pink-footed goose | −0.10 ± 0.15 [−0.40, 0.21] | 0.34 ± 0.13 [0.07, 0.60] | −1.02 ± 2.89 [−6.87, 4.83] | −1.04 ± 0.83 [−2.64, 0.55] | 0.59 ± 0.70 [−0.80, 1.98] |
| Tundra swan | 0.38 ± 0.08 [0.22, 0.54] | 0.35 ± 0.13 [0.10, 0.61] | −3.61 ± 1.73 [−7.11, −0.12] | −1.01 ± 0.39 [−1.79, −0.23] | −1.84 ± 1.08 [−3.96, 0.29] |
| Red-necked phalarope (Ammarnäs) | −0.25 ± 0.19 [−0.63, 0.12] | 0.62 ± 0.11 [0.40, 0.84] | −0.58 ± 4.09 [−8.85, 7.70] | ||
| Red-necked phalarope (Slettnes) | 0.27 ± 0.18 [−0.09, 0.63] | 0.24 ± 0.07 [0.09, 0.39] | 14.26 ± 6.47 [1.18, 27.34] | ||
| Sanderling | −0.54 ± 0.39 [−1.30, 0.22] | 0.80 ± 0.14 [0.53, 1.07] | −2.85 ± 4.24 [−11.41, 5.71] | ||
| Arctic skua (Kongsfjorden) | 0.09 ± 0.10 [−0.10, 0.28] | 0.00 ± 0.09 [−0.18, 0.17] | 0.40 ± 1.41 [−2.46, 3.25] | −0.52 ± 0.30 [−1.12, 0.07] | −0.47 ± 0.40 [−1.27, 0.32] |
| Arctic skua (Slettnes) | −0.27 ± 0.08 [−0.43, −0.12] | −0.04 ± 0.08 [−0.20, 0.11] | 10.91 ± 4.09 [2.64, 19.18] | ||
| Long-tailed skua | −0.37 ± 0.08 [−0.53, −0.21] | 0.08 ± 0.09 [−0.11, 0.27] | 15.03 ± 6.47 [1.95, 28.11] | ||
| Rough-legged buzzard | −0.15 ± 0.18 [−0.50, 0.20] | 0.13 ± 0.18 [−0.22, 0.48] | 1.57 ± 2.89 [−4.28, 7.42] | −1.72 ± 1.13 [−3.98, 0.53] | 0.33 ± 1.30 [−2.24, 2.98] |
- Note: Bird trends over time are only analysed for populations for which more than 5 consecutive years are available. Trends marked in bold indicate when 95% confidence intervals did not overlap with 0. Trends in snowmelt date and arrival date–time are all marked in bold as the best model showed a common trend for all populations.
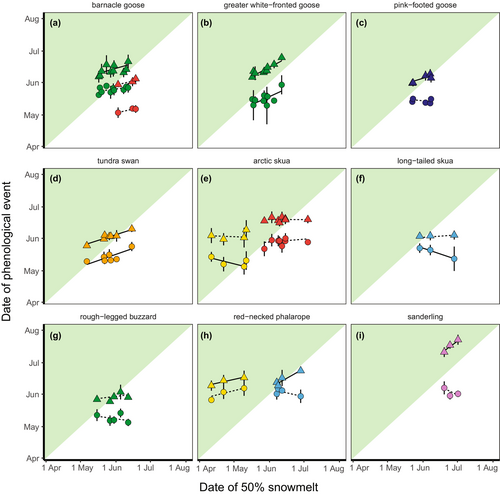
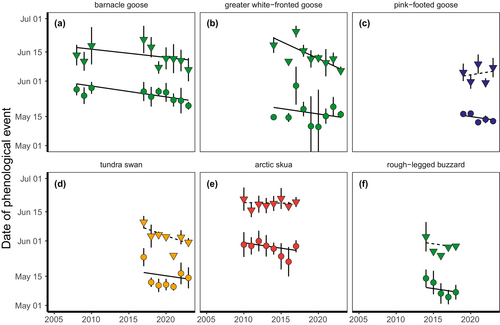
Arrival date in the Arctic advanced with time (0.52 ± 0.13 days earlier arrival per year, a model with a population only = 11.0 ΔAICc compared to a model with year and population, Figure 3) which did not vary between populations (model with interaction = 2.7 ΔAICc compared to model without, Table S3).
Egg-laying dates advanced with earlier snowmelt (model with a population only = 83.0 ΔAICc compared to model with population, date of snowmelt and their interaction; Figure 2, Table S3). Again, the effect of snowmelt differed between populations (model without interaction = 25.4 ΔAICc compared to model with). Confidence intervals did not overlap with 0 for several populations, showing earlier laying dates with earlier snowmelt for red-necked phalaropes (Ammarnäs: 0.62 ± 0.11, 95% CI [0.40–0.84]; Slettnes: 0.24 ± 0.08, 95% CI [0.09–0.39]), sanderlings (0.80 ± 0.14, 95% CI [0.53–1.07]), barnacle geese (Kolguev Island, 0.31 ± 0.10, 95% CI [0.12–0.50]), greater white-fronted geese (0.57 ± 0.10, 95% CI [0.38–0.77]), pink-footed geese (0.34 ± 0.13, 95% CI [0.07–0.60]) and tundra swans (0.35 ± 0.13, 95% CI [0.10–0.60], Table 2).
Egg-laying dates advanced with time (model with a population only = 20.5 ΔAICc compared to a model with population, year and their interaction, Figure 3), and this response also differed between populations (model without interaction = 9.4 ΔAICc compared to model with, Table S3). Confidence intervals of most populations overlapped with 0, with the exception of barnacle geese (Kolguev Island, −0.41 ± 0.19, 95% CI [−0.78 to −0.04]) and greater white-fronted geese (−1.71 ± 0.36, 95% CI [−2.42 to −1.00]).
3.3 Factors Explaining Population-Level Trends in Arrival Timing
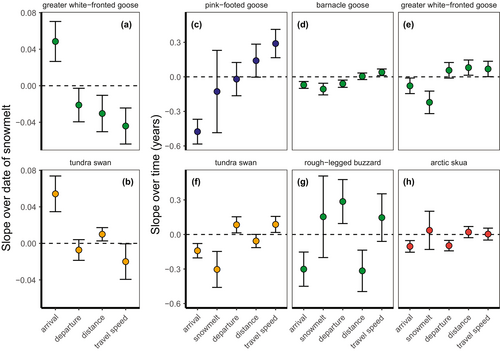
Slopes of arrival date over the date of snowmelt were not explained by slopes of departure, distance, or travel speed over snowmelt (model with travel speed = 0.6 ΔAICc compared to the intercept-only model, Table S4). For greater white-fronted geese and tundra swans, which both advanced arrival in years with earlier snowmelt (see above), earlier arrival appeared to be associated with later departure, longer distance and higher travel speeds (greater white-fronted goose, Figure 4a) and shorter migration distance and higher travel speeds (tundra swan, Figure 4b), respectively. Populations that showed earlier arrival date with time also showed higher travel speed with time (intercept-only model = 4.8 ΔAICc compared to model with travel speed, Table S4, Figure 4c–h), specifically in pink-footed geese, barnacle geese, white-fronted geese and tundra swans.
3.4 Timing of Egg-Laying in Relation to Arrival
Variation in individual egg-laying dates was explained both by date of snowmelt (0.22 ± 0.05 days earlier egg-laying per earlier day of snowmelt, model with arrival date and population = 15.1 ΔAICc compared to model with date of snowmelt, arrival date and population, Table S5) as well as arrival date (0.20 ± 0.05 days earlier egg-laying per earlier day of arrival, a model with the date of snowmelt and population = 8.6 ΔAICc compared to a model with arrival date, date of snowmelt and population). The date of snowmelt explained more variation compared to the arrival date (model with arrival date and population = 7.7 ΔAICc lower compared to a model with the date of snowmelt and population).
3.5 Data From Literature
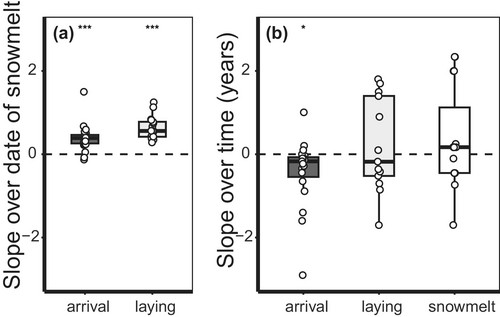
In studies retrieved from the literature, arrival and laying dates advanced with the date of snowmelt, although laying dates advanced on average faster (0.62 days advance per earlier day of snowmelt, t = 9.39, p < 0.001) than the arrival date (0.39 days advance, t = 6.03, p < 0.001, Figure 5). Arrival dates also advanced over time (0.47 days advance per year, t = −2.25, p = 0.037, Figure 5), but laying dates did not (t = 0.66, p = 0.52, Figure 5). On average, the date of snowmelt did not advance in these studies (t = 1.50, p = 0.15, Table S2) and earlier laying dates, but not arrival dates with time were explained by trends in the date of snowmelt (laying dates: intercept-only model = 15.5 ΔAICc compared to model with date of snowmelt; arrival dates: model with date of snowmelt = 1.4 ΔAICc compared to intercept-only model).
4 Discussion
Trends in the date of snowmelt were highly variable between populations (standard deviation of 0.39 for a slope of −0.83), yet in general, snowmelt appeared to advance with time. Arctic migratory bird populations showed varying responses in arrival timing with earlier snowmelt, and only two out of 12 populations, both waterfowl species (greater white-fronted geese and tundra swans), advanced their arrival with an earlier date of snowmelt. At the same time, most populations did advance egg-laying dates in years with earlier snowmelt (seven out of 12 populations). With time, populations advanced the timing of arrival, but not in all cases the date of egg-laying. To explain these patterns, we discuss responses in arrival and laying dates in the context of environmental and internal constraints.
4.1 Earlier Arrival in the Arctic
In years with early snowmelt, an advancement in laying dates is facilitated by a similar advancement in the timing of arrival (as we find that laying dates are partially explained by arrival timing) which shows that earlier arrival is an important prerequisite for earlier laying dates. As such, we would expect advancements in arrival in years with earlier snowmelt, which would aid individuals in advancing their laying dates. We found only two out of 12 populations, both waterfowl species, to advance arrival with earlier snowmelt. This suggests that for most populations, a flexible response in arrival timing to annually varying environmental conditions is either not possible or not beneficial, as birds face environmental or internal limitations. Environmental barriers, such as the large stretch of ocean between the European mainland and Svalbard (Geisler et al. 2022), may limit terrestrial birds to predict environmental conditions on their breeding grounds (Kölzsch et al. 2015). For comparison, the two waterfowl populations that did show adjustment of arrival timing to date of snowmelt (greater white-fronted geese and tundra swans) used relatively many stopovers along the way [on average per individual, 3.0 ± 1.6 for greater white-fronted geese and 3.4 ± 1.3 for tundra swans; see also (Nuijten and Nolet 2020; van Wijk et al. 2012)], which is more stopovers than other populations in our dataset (Table S3). The use of multiple stopovers will allow birds to gradually assess the progression of spring onset while at the same time offering more options to depart earlier.
Earlier migrations may be further limited by internal constraints, as earlier departures from stopover sites will come at the cost of (re)fuelling. When birds travel with only enough energy stores to fuel a flight to the next stopover site, shorter stays will only be possible if birds can also fuel faster (Lindström et al. 2019), as they otherwise do not have the stores to reach their next destination. While the limited number of populations adjusting arrival to annually varying snowmelt could suggest that internal constraints play an important role in limiting earlier arrival, our result on advancements in arrival with time in populations with long time series shows otherwise. Apparently, many of our study populations are able to make advancements in arrival in the Arctic with time, which is further reinforced by similar trends in the data that we extracted from the literature. Earlier arrival with time is mainly explained by faster migrations, further showing that birds are not lacking in body stores to spend less time on stopover sites. Many of the populations in our long-term dataset are large waterfowl species that are, at least to some extent, capital breeders (Hahn et al. 2011; Klaassen et al. 2017; Nolet 2006), carrying body stores to not only sustain migratory flight but partially also reproduction (Kölzsch et al. 2016). Using these stores to sustain extended migratory flight instead, would allow them to increase travel speed by spending less time on stopover sites. At the same time, this will reduce body stores with which they arrive on the breeding grounds and which they can use for reproduction, as previously shown for barnacle geese (Lameris et al. 2018). Yet, our results show that even Arctic skuas and rough-legged buzzards, which are capital breeders to a lesser extent (Hobson et al. 2000), appear able to advance arrival with time, suggesting that other factors such as improved feeding conditions during migration may play an additional role. When faster migrations go hand in hand with earlier departure from stopover sites and therefore shorter fuelling times, it is important to consider that this may not be without consequences, as it can lead to reduced survival (Rakhimberdiev et al. 2018) or delays in breeding after arrival (Lameris et al. 2018).
4.2 Earlier Laying Dates
A remaining question is whether populations that do not adjust migration timing to local environmental conditions are, as a result, limited in advancing their laying dates. We find that all populations in our dataset, except one waterfowl and shorebird population, advanced laying dates with earlier snowmelt but not with time. Similarly, our analysis of data from the literature showed a similar pattern of laying dates strongly advancing with earlier snowmelt, and with time only when date of snowmelt also advanced. A response of laying dates to year-to-year variation in environmental conditions is found for many other similar populations, both in the Arctic (Lameris et al. 2019; Liebezeit et al. 2014; Schmidt et al. 2023; Smith et al. 2010; Tavera et al. 2024) as well as in many other seasonal environments (Crick et al. 1997; Visser et al. 2012; Zhemchuzhnikov et al. 2021). Populations that adjust laying dates to the date of snowmelt but not their arrival date show an increasing interval between arrival and laying in years with late snowmelt. This suggests that birds delay breeding when the snow melts late, waiting until breeding patches become snow-free or food becomes available (Meltofte et al. 2007; Prop and de Vries 1993; Smith et al. 2010); yet our results show that the arrival date may pose an additional limitation to earlier breeding. Furthermore, multiple populations in our study show similar advancements in arrival and laying dates, suggesting a strong link in timing between these processes. One would need to experimentally advance arrival timing in order to fully confirm whether timing in arrival constrains the laying date.
At the same time, the lack of a response in laying date to annually varying conditions in both skuas and in rough-legged buzzards may not be explained by a similar lack in response of arrival dates. Strong phenological adjustments are likely to be more profitable for organisms at lower trophic levels, feeding on primary producers and primary consumers that typically advance quickly in response to climate warming (Thackeray et al. 2010, 2016). In contrast, organisms at higher trophic levels may have less benefit of breeding early due to an already slower phenological response of their prey, which may explain a limited response. Rough-legged buzzards on Kolguev Island prey mostly on waterfowl offspring (Curk et al. 2022) and may not need to advance laying dates to have access to prey throughout their breeding season, even if waterfowl advance laying dates. Long-tailed skuas, which also did not adjust laying dates to the date of snowmelt, depend on their reproductive success and nesting on available rodent prey, which can vary significantly from year to year (Barraquand et al. 2014; Gilg et al. 2006), but may be less related to the timing of snowmelt or other temperature-driven processes (Grabowski et al. 2013). Finally, the studied population of Arctic skuas is mainly kleptoparasites of other seabirds, which generally do not advance their timing of breeding over time or in response to temperature (Keogan et al. 2018).
4.3 Long-Term Responses in Arrival
The analysis of tracking data as well as data from literature shows trends towards earlier arrival dates in the Arctic, even if this does not always coincide with earlier snowmelt. Furthermore, long-term advancements in laying dates do not appear to be a general pattern in our dataset or the data extracted from the literature. This suggests that birds respond to other factors or general trends in environmental conditions and could even imply a response to long-term climatic conditions. Birds may show a trend towards earlier arrival as they experience a lower risk of arriving too early, before snowmelt and while food is still not available (Prop and de Vries 1993). In addition, changes in environmental conditions along the migratory flyways might also enable earlier migrations (Ouwehand et al. 2023). Non-breeding sites as well as stopover sites may have become more benign for migrants in terms of climate and, especially for herbivorous waterfowl, food availability. This could facilitate earlier departures and spending the non-breeding period closer to the breeding grounds, but also more rapid energy deposition prior to migratory departure (Lindström et al. 2019), which would allow the skipping of subsequent stopover sites (Eichhorn et al. 2009). Such changes in the migratory strategies of individual birds may rapidly spread through the population if individuals learn from each other (Oudman et al. 2020; Tombre et al. 2019). Earlier arrival will benefit reproduction in general, even if birds do not advance laying dates, due to better chances for territory or mate acquisition (Drent et al. 2003; Kokko 1999), as well as gathering energy stores which could benefit reproduction (Boom, Schreven, et al., 2023; Hupp et al. 2018).
4.4 Additional Factors
Several additional factors might partially explain the trends and variations in our study. First of all, the variation in size of study sites and potentially resulting variation in snowmelt patterns could also affect the relationships found. Specifically for the smallest study sites, for example the breeding islands of barnacle geese and Arctic skuas in Kongsfjorden on Svalbard, a limited number of pixels are available to analyse snow cover (Table S1). This could lead to inaccuracies in the date of snowmelt and, as such, affect the likelihood of finding relationships with migration and reproduction timing.
We find that, although the arrival date in the Arctic correlated with the date of snowmelt, the arrival date significantly advanced only for two populations of waterfowl. Potentially, the location at which populations cross the Arctic Circle—our measure/proxy for arrival date—may still be too far from the eventual breeding locations to predict conditions at breeding sites. When we compare the timing of arrival at the actual breeding site in relation to the date of snowmelt, which was possible for six populations with GPS tracks (Figure S2; Table S3; Supporting Information Methods), we find only one additional population, barnacle geese breeding on Kolguev Island, that arrived earlier in years with early snowmelt. This is in accordance with earlier findings for this population (Lameris et al. 2018). Three other populations, the barnacle geese breeding in Kongsfjorden, rough-legged buzzards and pink-footed geese, do not show adjustments in arrival date at their respective breeding grounds with earlier snowmelt (Figure S1). In addition, long-tailed skuas arrived later in years when snow melted earlier. This counterintuitive result could perhaps be explained by a strong delay in the date of snowmelt over the 3 years over which this population was tracked (Figure S1), with birds thus also showing strong advancement of arrival date with time.
Concerning trends of arrival over time, we have so far not considered individual effects. Individual migrations may become more efficient with increasing age or selective disappearance of individuals with later migration phenology (Aikens et al. 2024; Sergio et al. 2014), as well as learned experience about optimal migration timing, that is, earlier arrival, to achieve high reproductive success. For most populations, with the exception of pink-footed geese and long-tailed skuas, new individuals were tagged in almost every year of the study. Therefore, while individual changes could explain results to some extent, such changes will also have been present at the population level.
5 Conclusions
Our study shows that migratory birds advance their arrival in the Arctic. Earlier arrival in response to annually varying environmental conditions may only be possible for populations, such as some waterfowl populations, which make several stopovers on the way from which they can predict breeding site conditions. At the same time, a range of migratory species (incl. four waterfowl populations, Arctic skua and rough-legged buzzard) is able to advance arrival, explained mostly by faster migrations and thus less time spent on stopovers. Advancements in arrival timing may be facilitating simultaneous trends in earlier laying dates, both in years with earlier snowmelt and with time. These trends with time perhaps are responses to a general trend in earlier springs. An important consideration is that faster migrations by earlier departure from stopover sites may not be without consequences and can result in reduced survival (Rakhimberdiev et al. 2018) or delays in breeding after arrival (Lameris et al. 2018). Nevertheless, our results suggest considerable flexibility in migration timing behaviour which allows birds to rapidly respond to climate warming.
Author Contributions
Thomas K. Lameris and Rob S.A. van Bemmelen conceived the idea for this study; Thomas K. Lameris, Michiel P. Boom, Rascha J.M. Nuijten, Götz Eichhorn, Bruno J. Ens, Klaus-Michael Exo, Petr M. Glazov, Sveinn Are Hanssen, Philip Hunke, Henk P. van der Jeugd, Margje E. de Jong, Andrea Kölzsch, Alexander Kondratyev, Helmut Kruckenberg, Olga Kulikova, Hans Linssen, Julia A. Loshchagina, Maarten J.J.E. Loonen, Jesper Madsen, Børge Moe, Sander Moonen, Gerhard J.D.M. Müskens, Bart A. Nolet, Ivan Pokrovsky, Jeroen Reneerkens, Isabella B.R. Scheiber, Hans Schekkerman, Kees H.T. Schreven, Ingrid Tulp, Mo A. Verhoeven, Tom S.L. Versluijs, Sergey Volkov, Martin Wikelski and Rob S.A. van Bemmelen contributed to data collection; Helmut Kruckenberg, Bart A. Nolet, Jeroen Reneerkens, Isabella B.R. Scheiber and Martin Wikelski obtained funding; Thomas K. Lameris analysed the data; Thomas K. Lameris wrote the manuscript; and all other authors contributed to the writing and approved the final manuscript.
Acknowledgements
We are very grateful to all the people who helped in the field, especially Gerhard Nikolaus (†), Alexander Dmitriev, Heidi Schmid, Rolf Weinzierl, Liya Pokrovskaya, Annabel Slettenhaar, Pyter Bootsma and Anne Voorenkamp. For logistical support in the field, we thank Aarhus University. We thank Theo Gerritsen (†) for the development of specific tags (barnacle geese, greater white-fronted geese and Bewicks swans) and Mindaugas Dagys at Ornitela for logistical support of transmitter studies. We thank Charlie Simon for help with preliminary data analysis.
Ethics Statement
All research was conducted under permission from national committees on animal experimental work, following approved protocols by the Netherlands Institute of Ecology (NIOO13.14, NIOO 14.07), Centrale Commissie Dierproeven (permits 2016518, 20173788 and 202114712), Norwegian Food Safety Authority (FOTS id 2086, 23358, 29614, 3817, 5276, 6328, 6329, 7421, 8538, 15726) Jordbruksverket to Lund University, Sweden (permits M160-11, M470-12, M472-12) and Lower Saxony State Office for Consumer Protection and Food Safety. Tagging of tundra swans in Lower Saxony, Germany, was carried out under the permission of ‘Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit’ (LAVES; AZ 20/3603). Catching and tagging of Bewick's swans in the Netherlands was carried out under exemption of the Flora- en faunawet 75A, obtained through Dienst Regelingen (permit FF/75A/2016/044) and Wet natuurbescherming issued by Omgevingsdienst Brabant Noord (permits Z/046757 and Z/141920). No specific permissions were required for work on Rough-legged Buzzards and Greater White-fronted Geese on Kolguev Island from Federal Service for Supervision of Natural Resources (Rosprirodnadzor) according to §44 and §6 of the Federal Law of the Russian Federation No. 52 from 24.04.1995 (last update 08.08.2024) ‘On Wildlife’, as there were no Special Protected Natural Territories in our study area, and our activities did not include withdrawal of investigated species from nature. Capture and marking of pink-footed geese in Svalbard was permitted by Mattilsynet (Norwegian animal research authority) to Aarhus University (reference no. 17/210528) and by the Governor of Svalbard (reference no. 17/01420-4) and capture on Isdammen was permitted by Longyearbyen Lokalstyre (reference no. 2018/347-5-X70). Capture of barnacle geese was approved by the Governor of Svalbard (RIS ID 11237). For using GSM-GPS transmitters on the territory of Russia, IP applied for and obtained permit No. 77–18/0854/4388 from The General Radio Frequency Centre, permit No. RU/2018/406 from the Federal Service for Supervision of Communications, Information Technology and Mass Media (Roskomnadzor), and permit No. RU0000045099 from the Federal Security Service; no permissions were required from the Federal Service for Technical and Export Control (FSTEC/FSTEK) according to Russian Federation government decree No. 633 from 29.08.2001 and Letter from FSTEK No. 240/33/1373 from 06.04.2015.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Tracking data used in this study are available from sources described and referenced in Table 1, including de Jong et al. 2024 (https://doi.org/10.1101/2024.08.30.610510), van der Jeugd et al. 2014 (https://doi.org/10.5441/001/1.ps244r11), Boom, Lameris, et al. 2023 (https://doi.org/10.1007/s00442-023-05386-x), van Wijk et al. 2012 (https://doi.org/10.1111/j.1600-0706.2011.20083.x), Kölzsch et al. 2016 (https://doi.org/10.1111/oik.03121), Schreven et al. 2021 (https://doi.org/10.1186/s40317-021-00249-9), Nuijten and Nolet 2020 (https://doi.org/10.5751/ACE-01682-150214), Linssen et al. 2023 (https://doi.org/10.1111/gcb.16953), van Bemmelen et al. 2019 (https://doi.org/10.3389/fevo.2019.00086), Reneerkens et al. 2019 (https://doi.org/10.1111/1365-2656.13118), van Bemmelen et al. 2024 (https://doi.org/10.1186/s40462-024-00459-9), van Bemmelen et al. 2017 (https://doi.org/10.3354/meps12010) and Pokrovsky et al. 2021 (https://doi.org/10.1111/1365-2656.13484). A complete dataset with dates of migratory arrival in the Arctic, egg-laying dates and snowmelt for our study sites is available at Dryad Digital Repository in Lameris et al. 2025 (https://doi.org/10.5061/dryad.w0vt4b93d). Satellite images used to estimate snow cover were obtained from the NASA EOSDIS Land Processes Distributed Active Archive Center at https://doi.org/10.5067/MODIS/MOD09GA.061. Satellite images used to estimate cloudiness were obtained from the NASA EOSDIS Land Processes Distributed Active Archive Center at https://doi.org/10.5067/MODIS/MOD44W.006. Satellite images used to locate waterbodies were obtained from the NASA MODIS Adaptive Processing System, Goddard Space Flight Center at https://doi.org/10.5067/MODIS/MOD35_L2.006.




