Microbiomes as Modulators of Human and Planetary Health: A Relational and Cross-Scale Perspective
Anna Handte-Reinecker and Mallika Sardeshpande should be considered joint first authors.
ABSTRACT
The various human microbiomes play critical roles in maintaining health and well-being, and they are continuously shaped by a complex web of internal and external factors. Research on human and environmental microbiomes is generally discrete within disciplinary areas such as medicine, microbiology, molecular ecology, etc. This paper presents a perspective based on a scoping review of the literature, aiming to explore how these interconnected microbiomes shape human health and well-being and, in turn, planetary health. We explore the working of human microbiomes from cellular mechanisms to population outcomes, and the role of intrinsic and extrinsic factors influencing these microbiomes. We argue that global trends such as the homogenization of diets, environments, and medical practices are driving shifts in microbial diversity, with far-reaching implications for human health and well-being as well as planetary health. Disruptions to microbial feedback mechanisms at individual, community, and ecosystem levels are often interconnected and exacerbated by biodiversity loss and environmental change. We underscore the need for holistic public health interventions that account for microbiome stewardship across scales. By examining these connections, we aim to highlight the importance of a systems-level understanding of the microbiome in public health.
1 Introduction
The human microbiome, an intricate community of trillions of microorganisms residing within and on our bodies, along with the molecules they produce, plays a crucial role in influencing our health and well-being. From bacteria, archaea, protists, fungi, and viruses, the microorganisms that make up our microbiomes have been linked to essential bodily functions in our digestion, immune systems, development, and mental and physical health. The active role of microbiota in the bodies of humans and animals is the result of co-evolution, co-development, co-metabolism, and co-regulation (Ma et al. 2023). Humans host distinct microbiomes, each tailored to the unique environments of different body sites (Reynoso-García et al. 2022). The gut microbiome is perhaps the most well-known microbiome site, playing a vital role in digestion, nutrient absorption, and immune function. However, many other microbial communities are equally important to human health, including those on the skin, in the oral cavity, and in the vaginal environment. Disruptions in microbiome communities cause changes associated with disease and lead to dysbiosis. Dysbiosis is associated with physiological disorders, as well as cardiometabolic diseases, type 2 diabetes, and gastrointestinal diseases (Reynoso-García et al. 2022; Shekarabi et al. 2024; Yarbrough et al. 2024).
Despite the growing interest in linking microbiomes to health, deciphering the complex internal and external factors that influence our microbiome and, consequently, our overall well-being remains a challenging and evolving field. This article explores internal and external factors that shape the human microbiome, emphasizing their impact on health and well-being. We conducted a scoping review of the literature using the terms “microbiome” and “environment,” and “health.” We report the most recent and comprehensive literature to develop our arguments, which in turn shape our perspective.
The microbiome can be considered an ecosystem that hosts a diversity of bacterial, fungal, viral, and other microbes in equilibrium. In the wider environment, microbiomes can regulate biophysical processes, such as carbon and nitrogen cycles (Zhu and Penuelas 2020; Silverstein et al. 2023). Microbiomes associated with plants and animals facilitate important biological functions such as their nutrient uptake. In the human body, microbiomes mediate a host of processes, culminating in the complex outcomes of health and well-being. The literature lists a wide variety of microbes whose under- and over-abundance characterizes disease in various organs (Chaudhary et al. 2024; Madhogaria et al. 2022). We argue that this internal biodiversity within organs of the human body is inherently influenced by the external biodiversity in the proximal and distal environment of the human body and can be a component of biodiversity stewardship (Svenning et al. 2024; McIntyre-Mills 2021). Building on this assertion, we investigate the state of the knowledge on the linkages between the human microbiome and health and well-being outcomes and the micro- and macro-environmental conditions that mediate these outcomes. Recent discourse has begun to link soil health to One Health and Planetary Health paradigms. There is growing acknowledgement of the role soil microbes play in regulating pathogens and nutrients, thereby impacting agricultural safety and efficiency (Montgomery et al. 2024); of the importance of integrative approaches to maintaining animal health in productive ecosystems (Bornbusch et al. 2024); and of the potential of stewardship of ecosystem health using microbial treatments (Peixoto et al. 2022). Indeed, exposure to and ingestion of soil bacteria have also been shown to improve immune response in humans and animals (Roslund et al. 2024). On the other hand, reduced exposure to nature and biodiversity has been linked to increased instances of allergies (Prescott 2020). Many of these approaches tend to focus on components of the external environment or human health and well-being outcomes without addressing human agency across scales in the manifestation and management of these outcomes (e.g., Ma et al. 2023; Robinson et al. 2024).
In this article, we aim to document the pathways in which the human microbiome influences human health and well-being at cellular and body levels, and at the scales of built and natural environments. We then ascribe the degree of human agency over these pathways and make recommendations to better support the internal biodiversity of the human microbiome by enhancing the quality and access of external biodiversity in the human environment. Unlike other literature that makes recommendations for research and praxis in specific fields, we summarize broad implications for science, policy, and practice and also advocate for improved public education and awareness of the ways in which the microbiome functions across scales and systems. In doing so, we endeavour to expand the general perception of the microbiome as a diagnostic or therapeutic entity, and to demonstrate ways in which it could be considered an ecosystem to be responsively stewarded. We expect that this provides a novel contribution to the discourse on the role of biodiversity in human well-being and a crucial link between the One Health and Planetary Health discourse.
2 Microbiome and Health
Exploring the microbiome's importance for bodily functions is essential to understanding its impact on human health and well-being. The relationship between the human microbiome and human health and well-being is understood to be symbiotic. For example, the gut microbiome can modulate nutrient uptake in the primary incidence, which can have impacts on physiological and mental health. The microbiome is also capable of biosynthesis of active molecules contributing to the immune response and inter-organ communication (Filardo et al. 2024). The evidence on modalities through which this relationship manifests is limited to co-occurrence, and in some cases, correlation, rather than causality. For example, the composition and diversity of the microbiome can be an indicator of an imbalance associated with certain chronic conditions (Versalovic et al. 2017). Metabolites produced by microbiomes may also be used as proxies to detect disease and its severity. However, the lack of a deeper understanding of these pathways limits the potential for proactive interventions (Federici et al. 2020). For instance, interactions between microbial species within a community can influence metabolite production, obfuscating the presence or role of species or metabolites (Chevrette et al. 2022). Moreover, greater diversity may not necessarily imply greater abundance or greater health of a microbial community (Chattopadhyay et al. 2024). Whole genome sequencing, a resource-intensive technique, has nevertheless presented opportunities to delve deeper into the characteristics of the microbiome, such as virulence factors and drug resistance (Filardo et al. 2024). Multi-omics, an approach combining meta-genomics, meta-transcriptomics, meta-proteomics, and metabolomics, can help reconstruct the pathways through which the microbiome regulates physiological outcomes at the molecular level. While meta-genomics identifies microbial species, meta-transcriptomics quantifies microbial transcription activity, and meta-proteomics and metabolomics quantify protein synthesis and molecular composition within the environment (Filardo et al. 2024). Combined with existing genomic information and machine learning, this approach can help greater understanding of the cellular pathways through which the microbiome affects inter-organ communication, body response, and overall health and well-being.
Inter-organ communication occurs primarily through the transmission of bioactive molecules through the blood, or neuroimmune signals through nerves, between organs. The bioactive molecules may be amino acids or fatty acids metabolized by organ cells, or hormones secreted by the body in response to internal or external conditions (Castillo-Armengol et al. 2019). The composition of the human microbiome and intra-community interactions therein can influence the metabolism of bioactive molecules, thus playing a significant role in inter-organ communication and the regulation of bodily functions. Across the body, the composition of microbiota in one organ can also have a bearing on the microbiota in another organ. For example, along the gut-lung axis, change in bacterial and fungal communities in one system, either through infection or over time, effects change in the communities in the other system (Enaud et al. 2020). These effects are at times the result of overlapping anatomical pathways such as the convergence of the gut and lung passages at the pharyngeal interface. However, in some cases, these effects are also indicative of long-range host immune response. For instance, the gut microbiome plays a crucial but understudied role in recruiting immune cells and directing them to extra-intestinal sites of disease (Zundler et al. 2023). The aetiology of disease may hold clues to our understanding of the causal chains and cellular pathways through which the microbiome affects or effects human health and well-being. Inherited diseases like cystic fibrosis have well-established links with congenital microbiome imbalances that are exacerbated over time (Frayman et al. 2024). Congenital diseases like autism and degenerative diseases like Parkinson's are correlated with significant deviations from a healthy microbiome; but the cause-effect relationship is as yet unclear, as is our understanding of disease development and symptom progression in these diseases (Madhogaria et al. 2022; Nuzum et al. 2022). Infectious diseases are perhaps the best-studied and well-defined instances of microbiome disturbances impacting human health and well-being. Acquired non-communicable diseases such as cancer, diabetes, and cardiovascular diseases are typically linked to lifestyle and exposure to certain conditions, either at cellular or environmental level. The micro- and macro-environment therefore becomes the next most useful framing to attribute agency in the link between the human microbiome and health and well-being.
Studies have shown that the gut microbiome influences local and general immune systems (Salvadori and Rosso 2024). The links between the gut microbiome and various organ systems have been established, with dysbiosis causing diseases. Organ systems include the gut-brain axis, gut-brain endocrine axis, gut-heart axis, gut-lung axis, gut-liver axis, gut-pancreas axis, gut-bone axis, gut-muscle axis, gut-skin axis, gut-reproductive axis, gut-kidney axis, and gut-bladder axis (Salvadori and Rosso 2024). An intricate gut mucosal immune network creates active immune responses against infections but not commensal microbiota to maintain healthy homeostasis (Tian et al. 2024).
The bidirectional communication between the gut microbiota and the brain influences neurological functions and behaviors. This interaction has implications for psychological disorders, such as anxiety, depression, and schizophrenia (Salvadori and Rosso 2024; Shobeiri et al. 2022). In its connection to the endocrine system, scientists have linked the gut microbiome to ovarian dysfunction regulation and insulin resistance in polycystic ovary syndrome (PCOS; Qi et al. 2019). Furthermore, the microbiome has been linked to neuroendocrine regulation in relation to obesity and depression (Milaneschi et al. 2019). Obesity is associated with a disrupted balance between Bacteroidetes and Firmicutes bacteria, and modifying the composition of the gut microbiota experimentally can influence the development of obesity (Turnbaugh et al. 2006; Cani et al. 2008). These changes are also linked to indicators of local inflammation, which may raise gut permeability to bacteria. This increased permeability can contribute to the onset and progression of systemic inflammation, known as metabolic endotoxemia. The resulting inflammatory response can activate inflammasomes and affect brain processes related to depression, forming a gut-brain-microbiota axis that may influence mood states (Cani et al. 2008).
For various non-communicable diseases, the role of the microbiome has been recognized. For instance, changes in the microbiome correlate with conditions such as type 2 diabetes and cardiovascular diseases (de Vries et al. 2024; Reynoso-García et al. 2022). Furthermore, the microbiome has been found to influence drug metabolism, impacting the efficacy of treatments and potentially leading to varied health outcomes (Reynoso-García et al. 2022). One study found that 76 gut microorganisms could contain enzymes that can metabolize or chemically modify 271 oral drugs (Zimmermann et al. 2019). It further found that interpersonal microbiome variation could influence why some patients had more severe side effects than others. A study also suggests that the composition of gut bacteria can influence the response to immunotherapy, with higher diversity and certain bacterial groups linked to better outcomes in metastatic melanoma treatment (Gopalakrishnan et al. 2018).
However, studies have produced inconsistent results regarding the links between bacterial abundances and well-being, highlighting the underlying complexities (de Vries et al. 2024). External and internal factors, such as diet, lifestyle, and environmental exposures, can influence the microbiome, affecting overall health and quality of life.
3 Internal Factors Affecting Human Microbiomes
Internal factors that impact our microbiome include genetics, biological sex, age, and lifestyle choices such as diet, drugs, medications, smoking, and exercise, which significantly influence the composition and function of the human microbiome (Adak and Khan 2019; Kim et al. 2020; Salvadori and Rosso 2024; Shobeiri et al. 2022). These factors interact in multifaceted ways to determine the diversity and stability of microbial communities within the body. Due to these complexities, dysbiosis related to internal factors may be difficult to discern from one another. The composition of gut microbiota can vary based on geography and diet (de Vries et al. 2022). Western diets, characterized by high fat and sugar intake, disrupt circadian rhythms and lower microbial diversity, favoring harmful bacterial growth (Moreira Gobis et al. 2024). Moreover, throughout the human lifespan, the microbiome evolves, from infancy through old age (Adak and Khan 2019). Early microbial colonization shapes immune system development, while aging alters microbial diversity and function, influencing susceptibility to age-related diseases.
Furthermore, biological sex and other biological factors show that diet-driven microbiota changes can be sex-dependent (Shekarabi et al. 2024; Bridgewater et al. 2017). Hormonal differences between biological sexes may shape microbial communities, as alpha diversity in gut microbiota appears to significantly differ during puberty between female and male mice (Yurkovetskiy et al. 2013). Sex differences in gut microbiota may also affect the development of various diseases. For example, irritable bowel syndrome (IBS) occurs twice as often in females (Kim and Kim 2018). Additionally, most immune cells express sex hormone receptors, which can affect immune response (Elderman et al. 2018). Responses to treatments may also differ based on sex. In a study on Wistar rats, scientists found differing inflammatory responses triggered by probiotics between males and females (Lee et al. 2017).
This topic can be extended to discuss the vaginal microbiome, highlighting its crucial role in reproductive health. Throughout a female's reproductive cycle and post-reproductive stages, distinct diversity and vaginal microbial signatures have been identified (Kaur et al. 2020). Observed changes are likely to be related to varying progesterone and estrogen levels in the vaginal epithelium influencing glycogen availability, ultimately contributing to bacterial diversity changes throughout the different stages. When hormone levels are unbalanced, it can result in vaginal microbiome dysbiosis, such as bacterial vaginosis (Kaur et al. 2020).
Medical interventions, particularly the use of antibiotics, also have a profound impact on microbiomes. While antibiotic use can be crucial in treating infections, they do not exclusively target harmful pathogens but also affect key beneficial bacteria. For example, a commonly prescribed course of antibiotics can result in a sustained reduction of microbiota diversity, potentially impacting health (Abeles et al. 2016).
Furthermore, the excessive use of antibiotics can give rise to strains that become antibiotic-resistant and lead to a reduction in microbial diversity connected to obesity, allergies, asthma, and changes in metabolism (Lathakumari et al. 2024). Antibiotic-induced gut dysbiosis can affect humans from infancy to adulthood (Lathakumari et al. 2024). Population-level antibiotic consumption is particularly problematic as it can lead to widespread dysbiosis, reduced microbial resilience, and increased susceptibility to infections and chronic diseases (Lee et al. 2023). Internal factors intricately interact to shape the human microbiome, forming the foundation for its composition and influence on health. However, human microbiomes exist in a state of constant interplay with the external environment, resulting in dynamic shifts that can have consequences for health and well-being.
4 External Factors Influencing Human Microbiomes
From the moment humans enter the world, they encounter microorganisms that will populate their microbiomes. Vitally important is the vertical transmission of microbiota that occurs during childbirth from birthing parent to child (Ma et al. 2023). Delivery of a newborn either vaginally or by cesarean section has been shown to influence the microbial seeding of their gastrointestinal tract (Dominguez-Bello et al. 2019). This is a pivotal moment as the initial microbial composition can impact the development of the immune system, potentially affecting the risk of conditions such as allergic rhinitis, asthma, celiac disease, diabetes mellitus, and gastroenteritis (Trinh et al. 2018; Neu and Rushing 2011). Continuing throughout human life, the environment plays a critical role in determining the diversity and composition of the microbiomes. Emerging evidence suggests that the environments in which we live greatly drive microbiome composition and health. Research has shown that the external environment, including the microbiomes of animals that humans come in contact with, impacts human microbiomes and health outcomes (Trinh et al. 2018).
4.1 Microbial Transfer From Animals
Microbial transfer, whether pathogenic or non-pathogenic, can occur through contact with a variety of animals, including livestock, pets, wild animals, and even other humans. For example, in the homes of pig farmers, a larger diversity and abundance of microbes have been found when compared to that of suburban homes (Vestergaard et al. 2018). A further study found that pig farming impacts the nasal microbiome (Kraemer et al. 2018). Living with pets or other humans can also impact human microbiomes. For example, the close contact of people living together can create microbial sharing on the skin microbiota, and owning a pet shows higher skin microbiome diversity (Ross et al. 2017; Song et al. 2013). It has also been found that people with pets have more similarities in nasal and skin microbiomes when compared to those without (Misic et al. 2015).
Microorganisms can also be the cause of infections in zoonotic diseases. These organisms often originate in wild animals (Ma et al. 2023) but have also been found to come from livestock. One review suggests that people living in proximity to livestock farms show the presence of zoonotic bacteria in their microflora and may have the potential to act as vectors for zoonotic diseases (Kozajda et al. 2024). Infected individuals could act as vectors for zoonotic diseases, facilitating the spread of pathogens to the wider population.
Concerningly, pathogens can mutate, giving rise to new variants and posing a risk to public health. Human-animal interactions have played a significant role in the emergence of several major infectious diseases, such as the COVID-19 pandemic. In the case of SARS-CoV-2, the virus responsible for the COVID-19 pandemic and believed to have originated in bats, rapid mutations led to the emergence of new variants with differing levels of transmissibility and severity (Zhou et al. 2020). The interactions between humans, animals, and their shared environments significantly shape the human microbiome and affect health.
4.2 Microbial Transfer From Environments
Just as interactions with animals can significantly shape the human microbiome, the built environments where we live and work also play a role in determining the diversity and composition of our microbial communities. Through what is known as microbiome transfer, microorganisms in our environment, such as those in households and workplaces, are transferred to inhabitants (Lai et al. 2017; Mosites et al. 2017). Studies suggest that living in closed environments compared to more open natural spaces, for example, animals in captivity or humans in urban built environments, may lead to extensive host-environmental microbial sharing (Hyde et al. 2016). This may be the result of circular microbial transfer between host and environment and environment and host with little exposure to external microbes (Hyde et al. 2016).
Built environments, characterized by altered microbial exposure compared to natural settings, can lead to decreased microbial diversity (Bosch et al. 2024). In fact, the urbanization of built environments has been found to change environmental microbiomes (Trinh et al. 2018). Urban areas are dominated by human-associated microbes which, in turn, significantly influence the composition of human microbiomes (Zampolli et al. 2024).
Moreover, lifestyle changes associated with urbanization, such as high levels of hygiene, diets with increased consumption of refined sugars, and antibiotic use, have led to adverse health outcomes such as colorectal cancer, metabolic conditions, and antibiotic resistance (Reynoso-García et al. 2022).
Thus, urbanization influences microbial composition through its impacts on microbial exposure and lifestyle changes. This is seen in the fact that the microbiomes of people living in rural areas differ notably from those in urban areas (Kisuse et al. 2018; Lokmer et al. 2020).
4.3 Life History and Socioeconomic Factors
Social stress, particularly among migrants, further influences the microbiome. Stress can lead to changes in gut microbiota composition, which may contribute to health issues such as depression and sleep disorders (Salvadori and Rosso 2024). Migration often brings significant changes to the microbiome due to new environmental exposures and lifestyle modifications. Research indicates that immigrants from non-Western countries moving to the United States tend to experience a decline in gut microbiome diversity and functionality as indigenous strains are replaced by those associated with the United States (Vangay et al. 2018). This transformation is linked to a rise in obesity and related health concerns. Socioeconomic factors play a role in shaping the microbiome directly and indirectly, with changes in diet, physical activity, and acculturation affecting microbial diversity and health outcomes (Fanfan et al. 2024). Migrants often face stress and lifestyle changes that alter their microbiomes, influencing their risk of developing cardiometabolic, immune-related, and mental health disorders (Figure 1).
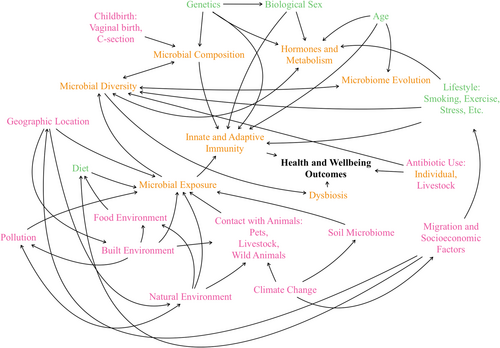
5 Discussion
Our synthesis of the literature indicates that the human microbiome is an intrinsic part of human health and well-being, and this manifests at different scales, from cellular and inter-organ communication to landscape and macroecological feedback loops. A key theme that emerges is that homogenization and disruption of feedback mechanisms across these scales are reducing adaptive capacity and increasing the prevalence and transmission of disease (Figure 2). In this section, we first expand on these emergent themes. Then, we outline some of the co-benefits associated with reversing these trends of homogenization and feedback disruption, and how these tie into one another across the scales of external and internal biodiversity, as well as host-habitat interactions. Finally, we recommend some ways forward for science, policy, practice, and public engagement.
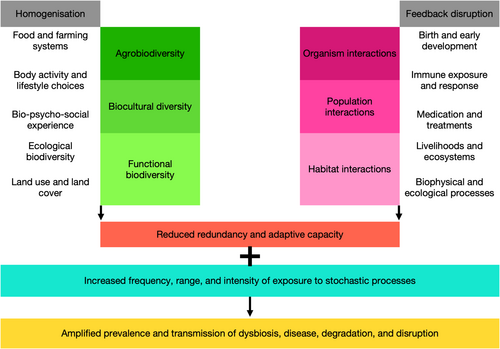
The homogenization of agrobiodiversity occurs concurrently with reduced dietary and nutritional diversity, resulting in poorer outcomes for human health. For example, reduced consumption of fibrous foods impacts the microbiome and is correlated with certain NCDs (Avery et al. 2021); consumption of fresh produce grown in healthy soil can encourage exchange between the soil and human microbiome, boosting immunity (Roslund et al. 2024); conversely, consumption of food produced using antibiotics is likely to transmit antimicrobial resistance (Ashwini et al. 2023). Homogenized diets are accompanied by increasingly homogenized lifestyles that are urbanized, sedentary, and include concomitant choices of convenient processed foods and regular consumption of alcohol, caffeine, sugar, tobacco, and other stimulants. These lifestyle choices are well known to precipitate non-communicable diseases. Exposure to poorer quality of built (e.g., industrial, residential) and natural (e.g., agricultural, urban) environments can have negative impacts on physical and mental health. Manifestations of this include nature deficit disorder (Alvarez et al. 2022), extinction of experience (Prescott 2020), and biophobia (Soga et al. 2023), all of which constitute vicious cycles of deprivation of quality natural environments and a subsequent loss of motivation to keep healthy. Urbanizing lifestyles are resulting in significant homogenization of biocultural diversity, undervaluing the role of biodiversity in socio-cultural processes that contribute to human health and well-being (McMillen et al. 2020). The homogenization of ecological biodiversity in agricultural, industrial, and urban landscapes exacerbates this effect. The attendant loss of other ecosystem functions and redundancy in these land uses has cascading effects on long-range biodiversity characteristics such as habitat connectivity and overall resilience (Peixoto et al. 2022).
Feedback disruptions caused by decoupling microbial communication from life history outcomes have not just health and well-being impacts, but may also involve compromises in social-ecological development. The pathway of birth and early development (including breastfeeding and nurturing) can profoundly alter the human microbiome, notably the development of immune regulation. The rise in instances of non-vaginal births and non-lactation in urban populations may induce a greater propensity for disease and disorders among infants. As is widely known, suppression of immune, hormonal, and nervous systems through synthetic medications and treatments can result in maladaptive outcomes such as allergies and drug dependencies (Prescott 2020). At a landscape scale, the disconnect between environmental microbiomes and livelihoods (e.g., through application of synthetic soil, plant, and animal treatments) can have long-term detrimental impacts on livelihoods, including reduced ecological yields, high dependency on chemical inputs, and greater susceptibility to disease and disruption (Montgomery et al. 2024). Ultimately, the reduction and exclusion of biodiversity—from microbial to megafaunal—from extractive and productive landscapes (e.g., plantations, watercourses) lowers the ecological metabolism and resilience, eventually rendering it unproductive and prone to collapse (Svenning et al. 2024). Land-based livelihoods like smallholder farming, agropastoralism, and forest-linked small enterprises are increasingly under threat from degradation induced by loss of biodiversity (Fromentin et al. 2022). Further, as climate change impacts the environmental microbiome, certain diseases may become more prevalent over time across rural and urban landscapes (Ahmed et al. 2019). Science, policy, and practice aiming to conserve biodiversity, improve human health and well-being, restore ecosystems, and mitigate or improve resilience to climate change would do well to acknowledge the microbiome as an essential modulator of these outcomes (Byers 2024).
Our work highlights the extension of two important concepts across microbial to macroecological scales, namely health and stewardship. Human health, once considered a primarily biological domain, human health has been expanded to include mental well-being. The One Health model emphasizes the interconnectedness of human, animal, and environmental health, recognizing that the health of humans is closely linked to the health of animals and the environment. Planetary Health is an integrative approach that considers the role of changing environmental conditions influencing environmental microbiomes, biophysical processes, and, subsequently, human microbial communities (Zhu and Penuelas 2020; Gunawan et al. 2023). We posit that using a microbiome lens across these health paradigms can yield constructive outcomes for multispecies well-being (McGreevy et al. 2022). We believe stewardship is an important pathway to operationalizing this approach. The term is used in the biological and medical sciences to indicate responsible and judicious use of intervention agents to achieve desirable outcomes (Imlay et al. 2024; Svenning et al. 2024). In the ecological and social sciences, the term implies responsible and judicious use of natural resources and processes (including biodiversity) to achieve human health and well-being (McMillen et al. 2020; McIntyre-Mills 2021). The importance of a microbiome-focused One Health model underscores the need to consider microbial interactions across different ecosystems (Tomasulo et al. 2024).
Fortunately, reversing homogenization and feedback disruption across scales comes with several concomitant and interlinked benefits for internal and external microbiomes, as well as human health and well-being. Improving biodiversity on farm and in urban environments often comes with significant socioeconomic and cultural gains for human beings, and also for the environment. Initiatives such as high value nature farming (e.g., Maskell et al. 2019) could include practices to enhance soil microbial composition (Singh et al. 2023). Urban greening for biodiversity could include design components that enable humans to better interface with the plant and microbial community (Mills et al. 2020). Some scientists have suggested that urban greening and increasing exposure to natural environments can enhance the urban microbiome and potentially improve public health (Matthews et al. 2024). Evolving human-animal relationships, particularly from farm animals to pets, call for further research on the co-evolution of these microbiomes, especially in light of urbanization (Ross et al. 2017; Vestergaard et al. 2018). Environments where food is produced, processed, and distributed could be routinely screened for microbial communities to better understand and anticipate zoonotic transmission through trophic chains (e.g., De Filippis et al. 2021). Efforts to enrich microbiomes for environmental outcomes such as carbon and microbial control (e.g., Silverstein et al. 2023; Peixoto et al. 2022) should recognize the free and frequent interface between human and environmental microbiomes.
At population level, dietary diversity and body activity have been shown to influence not only the human microbiome (Gupta et al. 2017), but also the quality of mothers' milk (Klein et al. 2018), and are found to be inversely proportional to the risk and incidence of atrial fibrillation (Rowan et al. 2021). Creating biophilic food environments (e.g., fresh produce markets, nutrition gardens) will enable human exposure to nutritional and microbial diversity. Fostering awareness about the accumulated effects of dietary choices on human health and the environment through these food environments will encourage better food choices. Medical treatments, including microbial and antimicrobial therapy, should be combined with adequate education of the role of good nutrition and health in caring for the human microbiome. This awareness about stewardship of internal biodiversity in the human microbiome (intra-organism interactions) may develop or extend into better understanding and care for external biodiversity in the ecological biome or macrobiome (habitat interactions; Kershaw 2023). Paediatric and geriatric care could incorporate more ecological approaches to ameliorate the effects of biophobia, solastalgia (Soga et al. 2023), and the dysbiosis and dysfunction that can manifest in physical and mental health.
Exposure to biodiversity across scales can have positive outcomes not only for physical and mental health, but also for cognitive development (Lee et al. 2021; McNamara and Wertz 2021). Ecological and ethnobiological knowledge can have a positive influence on bio-psycho-social experience, also playing into traditional medicine and spiritual pathways to healing and well-being (Engemann et al. 2019; Mollee et al. 2017). Together, these alternative therapeutics can manifest in supplemental systems to support modern medicine in achieving positive population health outcomes. Social prescription programmes can encourage such linking and learning. The role of functional biodiversity at the ecosystem scale and its interaction with livelihoods is increasingly recognized in the paradigms of ecosystem-based adaptation and nature-based solutions. Although these interventions focus primarily on agriculture and biodiversity conservation (Donatti et al. 2020), there is a growing acknowledgement of the role of biodiversity in supporting liveability and livelihoods across the rural–urban gradient (Lebrun et al. 2021). Policies, plans, and programs aimed at conserving biodiversity for ecosystem services should consider the specificity of and local appropriateness of interventions (Chausson et al. 2020). Mobilizing indigenous knowledge, public awareness, and relational values of biodiversity will reduce the likelihood of maladaptations, and better enable synergistic outcomes for all (Seddon et al. 2021).
6 Conclusion
This article sought to identify the complex relationships between the human microbiome and its external and internal influences, discerning factors that shape health and well-being. By recognizing these interactions, we can help raise awareness, inform interventions, and identify knowledge gaps in the field. This understanding of the relevance of microbial interactions from cellular to planetary health scales can inform perspectives and practice across scientific domains. Key messages from the literature emphasize the symbiotic relationship between our microbiomes and essential biological functions. Both internal and environmental factors shape human microbiomes and thus our health and well-being. Global trends of homogenization in our diets, environments, and medication use have driven changes in our microbiomes.
We advocate for awareness of current health trends related to microbiome health and urge policy makers to consider microbiome-informed public health interventions when trying to tackle them. As microbial transfer occurs between people, animals, and our environment, one can not be addressed without the others. Feedbacks between microbial communities ensure natural functioning and self-sustenance at the levels of organs, organisms, populations, communities, and ecosystems. Increasing disruptions in the form of biological, chemical, and physical interventions interrupt these feedback mechanisms. Policy and practice in environmental and human health should consider synergistic interventions to restore the functioning and feedback mechanisms of the microbiomes they engage with. Examples of operationalizing this are: including environmental microbiomes in ecological assessments and practices; designing safe living interfaces for environmental and human microbiomes; promoting routine microbiome stocktaking of industrial and informal food environments; integrating biophilic and medical interventions for physical, mental, and environmental health; and mobilizing learning around microbiome stewardship across biological, landscape, medical, and social practice. Future research should focus on understanding homogenization across microbial communities and disruption feedback, from individual to environmental levels, while simultaneously minimizing exposure to pathogenic microbes, including zoonotic diseases.
Author Contributions
Anna Handte-Reinecker: conceptualization, data curation, methodology, visualization, writing – original draft, writing – review and editing. Mallika Sardeshpande: conceptualization, data curation, methodology, visualization, writing – original draft, writing – review and editing.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




