Canopy cover and soil moisture influence forest understory plant responses to experimental summer drought
Abstract
Extreme droughts are globally increasing in frequency and severity. Most research on drought in forests focuses on the response of trees, while less is known about the impacts of drought on forest understory species and how these effects are moderated by the local environment. We assessed the impacts of a 45-day experimental summer drought on the performance of six boreal forest understory plants, using a transplant experiment with rainout shelters replicated across 25 sites. We recorded growth, vitality and reproduction immediately, 2 months, and 1 year after the simulated drought, and examined how differences in ambient soil moisture and canopy cover among sites influenced the effects of drought on the performance of each species. Drought negatively affected the growth and/or vitality of all species, but the effects were stronger and more persistent in the bryophytes than in the vascular plants. The two species associated with older forests, the moss Hylocomiastrum umbratum and the orchid Goodyera repens, suffered larger effects than the more generalist species included in the experiment. The drought reduced reproductive output in the moss Hylocomium splendens in the next growing season, but increased reproduction in the graminoid Luzula pilosa. Higher ambient soil moisture reduced some negative effects of drought on vascular plants. Both denser canopy cover and higher soil moisture alleviated drought effects on bryophytes, likely through alleviating cellular damage. Our experiment shows that boreal understory species can be adversely affected by drought and that effects might be stronger for bryophytes and species associated with older forests. Our results indicate that the effects of drought can vary over small spatial scales and that forest landscapes can be actively managed to alleviate drought effects on boreal forest biodiversity. For example, by managing the tree canopy and protecting hydrological networks.
1 INTRODUCTION
Extreme events such as drought spells and heatwaves can have large impacts on ecosystems (Murray & Ebi, 2012; Schuldt & Ruehr, 2022) and are becoming more prevalent in many parts of the world (IPCC, 2021; Thiery et al., 2021). Climate change scenarios predict that many boreal ecosystems will shift from cool and short summers to long and warm summers with reduced soil water availability (Grossiord et al., 2014). Boreal forest species might be poorly adapted to drier conditions and increased frequencies of dry spells. Advancing our knowledge on how understory plants are affected by increased drought stress, and to what extent local vegetation and soil properties influence drought stress, is crucial for developing strategies to mitigate the impact of climate change on boreal forest biodiversity (Hylander et al., 2022).
The boreal forest understory is dominated by bryophytes and vascular plants, which employ different physiological mechanisms to cope with droughts. Vascular plants have adaptations to reduce desiccation, such as root browsing, symbiosis with mycorrhizal fungi, water loss regulation through stomatal conductance, and usage of stored resources (Koch et al., 2019; Osakabe et al., 2014). Still, extended periods of drought can result in reduced growth, pre-mature leaf shedding, as well as wilting and discoloration of leaves and shoots (due to chlorosis and necrosis; Chaves et al., 2003; Farooq et al., 2009; Oraee & Tehranifar, 2020; Xu et al., 2010). These negative drought effects are primarily caused by reduced resource availability and photosynthesis as a result of stomatal closure and nutrient immobility in the soil (Koch et al., 2019; Osakabe et al., 2014), and by damage of plant tissue through several mechanistic pathways including oxidative stress and impaired enzyme activities, as well as reduced cell expansion and division due to lack of turgor (Farooq et al., 2009). In contrast, bryophytes take up and lose water directly through their thin leaves, entering a temporary inactive metabolic state when they are dry (Busby et al., 1978; Oliver, 2005). While periodic desiccation is common in many bryophytes, extended periods of drought can reduce energy acquisition and damage cells (Proctor et al., 2007). Droughts can thereby negatively impact bryophyte performance in terms of reduced growth, vitality, survival, and reproduction (Koelemeijer et al., 2023; Merinero et al., 2020; Rydgren et al., 2006; Schmalholz & Hylander, 2011).
While the immediate effects of droughts are relatively well understood, less is known about the longer-term effects and to what extent plants can recover after drought events. Replacing damaged plant structures and replenishing storage takes time, and recent research suggests that climate-driven effects might often persist or appear after one or several years (Evers et al., 2021; Hacket-Pain et al., 2018; Tenhumberg et al., 2018). In some species, reproduction requires large amounts of resources, which, at least in some perennial vascular plants, are accumulated over several seasons (e.g. Tenhumberg et al., 2018). When resources are limited, as after drought events, growth and survival are often prioritized at the expense of reproduction (Eziz et al., 2017). Investigating multiple responses over an extended period after events of drought stress is therefore necessary to fully understand how organisms are affected.
The intensity of drought that forest understory organisms actually experience depend on the local environment and can vary over short horizontal distances of just a few meters. Two factors that play a key role for differences in drought impacts in forests are the forest structure (e.g., dense vs. open forests) and the ambient soil moisture (e.g., on dry soils vs. in wetter habitats) (Aguirre et al., 2021; De Frenne et al., 2021; Wolf, 2023). The higher relative humidity under a closed canopy reduces transpiration from leaves and evaporation from the soil, and delays understory plant desiccation, which may particularly be beneficial for bryophytes whose thin leaves dry out rapidly (Man et al., 2022; Oliver, 2005; Stewart & Mallik, 2006). Soil moisture primarily affects plants via water uptake through the roots but also through evaporative cooling of the understory environment (Davis et al., 2019; Luan & Vico, 2021; von Arx et al., 2013). Both canopy cover and soil moisture are strongly influenced by forest management (De Frenne et al., 2021; Greiser et al., 2018). However, we still know little about the extent to which spatial variation in these variables can buffer the effects of summer droughts on understory plants.
-
How does summer drought affect the performance of understory plants and for how long do these effects persist?
Hypothesis 1.—We expected that all species would be negatively affected by drought, but that the effects would vary in magnitude and duration between species, for example, bryophytes would suffer larger immediate effects, due to their ecophysiology and morphology.
-
Do canopy cover and soil moisture levels influence the response of understory species to drought?
Hypothesis 2.—We expected that the negative effects of drought are buffered in sites below dense canopies and with high ambient soil moisture. We also expected that canopy cover would be relatively more important in bryophytes and soil moisture relatively more important in vascular plants.
2 METHODS
2.1 Study system
2.1.1 Study area
The study was conducted in a managed forest landscape in central Sweden (Ekopark Färna, Västmanland, Sweden; Figure 1). Forests in the area are dominated by Norway spruce (Picea abies L.) in different seral stages, including recently clear-cut areas and even-aged stands of different ages. The area also contains older spruce forest patches mixed with broadleaved trees, influenced by natural disturbances (e.g., following bark beetle outbreaks, wind damage). The climate is seasonal with summer (June, July, and August) temperatures around 16°C and winter (December, January, and February) temperatures around −4°C, with most of the precipitation occurring during the summer (SMHI, 2022). Although predictions regarding future droughts for central Sweden are uncertain, soil water availability is predicted to decrease during summers (Sjökvist et al., 2019), due to increased evaporation with warming and more variable precipitation.
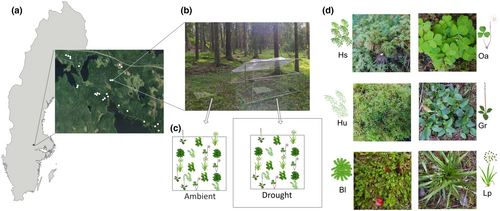
2.1.2 Study species
We selected three species of vascular plants and three species of bryophytes that typically grow in the boreal forest understory. We included species that we expected to vary in terms of drought sensitivity (Figure 1d, Table S1). The orchid Goodyera repens (L.) R. Br. (Orchidaceae) is an indicator species in older forests (Swedish Forest Agency, unpublished; Nitare & Norén, 1992) and declines towards forest edges (Koelemeijer et al., 2022), probably due to its sensitivity to harsh microclimates. The forb Oxalis acetosella L. (Oxalidaceae) is a widespread species, but has been shown to be sensitive to drought (Govaert et al., 2021). In contrast, the graminoid Luzula pilosa (L.) Willd. (Juncaceae) is common in both forests and on clear-cut areas. For the bryophytes, we selected the common moss Hylocomium splendens (Hedw.) Schimp. (Hylocomiaceae) as a representative of a more drought-tolerant species, and the moss Hylocomiastrum umbratum (Hedw.) Fleisch. (Hylocomiaceae) and the liverwort Barbilophozia lycopodioides (Wallr.) Loeske (Anastrophyliaceae), as more sensitive species, based on that H. umbratum is an indicator species of older forest (Swedish Forest Agency, unpublished; Nitare & Norén 1992) and that liverworts are often relatively sensitive to drought (Proctor et al., 2007). The moss H. splendens regularly reproduces sexually by producing sporophytes. For successful fertilization, the antherozoid (sperm equivalent) has to swim from the antheridia to the archegonia, a process that cannot occur without liquid water. Following fertilization, usually the year after, the archegonia develop into sporophytes producing spores (Proctor et al., 2007; Rydgren et al., 2006). Sexual reproduction is rarer for the other transplanted bryophytes. A detailed description of each study species can be found in Table S2.
2.2 Experimental set-up
2.2.1 Site selection and species transplantations
We selected 25 sites along gradients of both canopy cover and soil moisture, aiming for an even bivariate distribution of these two factors (Table S2, Figure S1). While canopy cover and soil moisture might be correlated in the field, we deliberately selected sites with different combinations of soil moisture and canopy cover. To accomplish this, we selected sites ranging from very open to very closed canopy based on visually estimated canopy cover, along a gradient of soil moisture. Soil moisture was assessed based on the species composition of the vegetation, which changes markedly from dry, via mesic to moist and wet conditions, and is largely governed by topography (Påhlsson, 1994). While visual cues were used to select sites, the estimates of canopy cover and soil moisture used in the statistical analyses were based on subsequent measurements (see below). At each of the 25 sites, we established two plots of 1 × 1 m (one treatment and one control plot), in which we transplanted three patches of each of the six species (Figure 1c). Patches were placed in a randomized order along four rows (Figure 2c), with the same order both in treatment and control plots at each site. In total, this resulted in 150 transplants of each species. We transplanted the species in the autumn of 2020, the year before the drought experiment started, to give the transplants time to establish. Details regarding transplantation is provided in Supplementary Methods S1.
2.2.2 Rainout shelter design and experimental drought
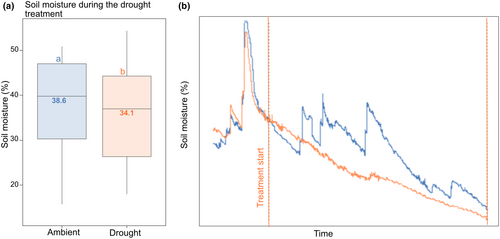
We experimentally induced a 45-day drought during the summer 2021 (June 2, 2021–July 16, 2021), by installing rainout shelters that completely intercepted all rainfall during this period over one of the plots at each site. The rainout shelters were 2 × 2 m, to allow for a 0.5 m buffer zone on each side of the drought-treated plots (a detailed description of the rainout shelters is provided in Supplementary Methods S2, Figure S2). There is no reason to believe that surface water after rains would flow across any of the plots, since rainfall normally directly infiltrates through the moss cover and litter on boreal forest floors. The drought period was chosen to mimic a recent extreme summer drought in the area in 2018, which had a length of 36 days (Koelemeijer et al., 2022). We extended it to 45 days to increase drought stress in duration, since we could not experimentally replicate high air temperatures and vapor pressure deficits (VPD). With this set-up, we aimed to induce a scenario relevant for future conditions with increased soil drought and dry spells during the summer (Grossiord et al., 2014; IPCC, 2021; Sjökvist et al., 2019). The control plots received ambient rainfall, which added up to 87 mm (although throughfall that reaches the forest understory will be less) distributed over 13 days during the duration of the experiment (Schuldt & Ruehr, 2022, weather station Skinskatteberg, Västmanland). In all treatment and control plots, we tracked soil moisture levels using TMS-4 loggers (TOMST, installed according to Wild et al., 2019). These loggers recorded soil moisture of the topsoil in the middle of each plot every 15 min. Raw soil moisture measures, which are based on conductivity pulses, were calibrated to percentage soil moisture volume. Soil moisture was significantly reduced under the rainout shelters compared to in the control plots (p = 0.006, Supplementary Methods S3, Figure 2b), mainly driven by the absence of peaks in soil moisture after rainfall events (Figure 2a).
2.3 Data collection
2.3.1 Local conditions
We calculated site-level measures of soil moisture and canopy cover, in order to test how among-site variation in these factors interacted with the drought. For soil moisture, we used the average value of moisture in May 2021, the month before the drought from both control and treatment plots (drought plots were at that time still untreated). This soil moisture value was thus used to characterize the ambient site conditions (wet vs. dry sites). To calculate canopy cover for each site, we used hemispherical photographs taken with a Lumix Panasonic camera (DMC-G80M) and a circular fisheye lens (MFT 4 mm F2.8 210°). We used the Hemispherical 2.0 package in the software ImageJ (version 1.53 a) to process the photos into binary images. Canopy cover ranged from 47.5 to 93.8%, with two sites having very low cover (47.5 and 56%), due to logging in the winter after the transplantation. The distribution and correlation of canopy cover and soil moisture levels across all sites can be found in Figure S1.
2.3.2 Species response measurements
Growth and vitality of individual transplants were recorded on three occasions during the growing season in 2021 and on one occasion in 2022. Pre-drought measurements were taken April 6–9, 2021 or June 1–4, 2021, depending on species (Figure S3). For all species, we recorded responses directly after the drought (July 15–19, 2021), 2 months after the drought (September 15–19, 2021), and 1 year after the drought (July 15–19, 2022). Data on reproduction were collected the year after the drought in June 2022. An overview of all measurements taken can be found in Figure S3.
For the vascular plants, we calculated growth by taking the log-ratio of the difference in number of leaves (L) at t + 1 and t, using the formula log(Lt + 1)–log(Lt). A value below zero indicates a decrease in number of leaves and a value above zero indicates an increase. We calculated growth over the total period (from pre-drought until 1 year after the drought), as well as over all the time intervals (immediately after drought, after 2 months, and after 1 year). We also calculated the specific leaf area (SLA) for a few leaves (if possible, three for each transplant, nine per plot for each species) that were produced during the drought period, by dividing the leaf area (cm2) by its dry-mass (g) (Wellstein et al., 2017). Leaf area was measured using ImageJ using photographs of leaves together with a scale-bar and leaf dry-mass was measured after oven-drying at 60°C for 72 h (Wellstein et al., 2017). Since we had to harvest leaves for this, we only measured SLA immediately after the drought. We subtracted the number of harvested leaves from the initial pre-drought leaf number and assume that effects of this on the effect sizes are small and similar for drought and control plots.
For the mosses H. splendens and H. umbratum, we marked five shoots per transplant (15 per plot, 750 shoots in total for the experiment). These mosses produce a distinct growth segment each year, on top of the previous years' segment (Figure S4). We calculated growth by measuring the difference in length of the marked segment (from the start of the segment until the apical tip) over sampling intervals (t and t + 1). We also measured the segment that was produced during the growing season 1 year after the drought (Figure S4). In addition, we recorded number of new segments produced in 2022 on the marked shoot as a measure of branching, since branching could be a response to drought (e.g., tight mats are the slowest to dry out, Proctor et al., 2007). For the liverwort B. lycopodioides, which has a creeping growth pattern, we measured growth as the relative increase in patch size (St + 1/St). We photographed patches together with a scale bar and measured patch size by manually tracing the patches in ImageJ.
Vitality was assessed for vascular plants and bryophytes using a scale from 1 to 7 developed by Dynesius et al. (2008) to describe vitality after exposure to drought: (1) dead, (2) some leaves green, (3) some shoots green, (4) half of the shoots alive, (5) alive but affected, (6) most of the shoots vigorous, and (7) the entire plant fresh and growing (Dynesius et al., 2008; Greiser et al., 2021). See Figure S5 for description of each vitality level using this scale. Note that there had been no rain the days before the end of the drought treatment. Thus, we believe that the risk of a biased vitality assessments between treatment and control due to temporary desiccation should be minimal.
We assessed reproductive output only for L. pilosa and H. splendens. The bryophytes B. lycopodioides and H. umbratum seldom reproduce sexually, and the vascular plants O. acetosella and G. repens had very low flowering frequency in the experiment. For L. pilosa, we measured the number of inflorescences per transplant, mean number of fruits per inflorescence (based on fruit count of two inflorescences), the number of developed seeds per fruit, and the average weight of developed seeds. Developed seeds were distinguished from non-developed based on appearance (Figure S6). For H. splendens, we counted the number of sporophytes per transplant, the average number of spores per capsule, and the proportion of spores that were aborted (Supplementary Methods S4, Figure S6).
2.4 Statistical analyses
All statistical analyses were performed in R (Version 2022.7.1, R core team).
We examined the effects of the drought on growth, vitality and reproduction using generalized mixed effect linear models, using the package lme4 (Bates et al., 2015). To investigate whether ambient soil moisture and canopy cover influenced responses to the drought, we modeled their interactions with the drought. For models where the interaction with canopy cover was significant, we also conducted the analyses omitting the two very open sites, to examine to what extent these two sites were driving the pattern. We ran separate models for each time-interval (Figure S3) and for each species separately. For the models of vitality, we included vitality in the previous time-interval (t-1) as a covariate in order to test the change in vitality. Since we only recorded SLA directly after the drought, we only had one model per vascular plant species for this variable.
We scaled growth and vitality within species by for each datapoint subtracting the mean and dividing by the standard deviation using the scale() function. We included site ID (n = 25) as a random effect for all models. For the models of the two moss-species' growth, we added transplant ID as a nested random effect, since we followed five shoots per transplant. We assumed a Gaussian distribution for all models, except those with count data (e.g., branching and reproduction) where we fitted negative binomial or Poisson models. We verified that the model assumptions were met, using the packages performance and Dharma (Lüdecke et al., 2021).
3 RESULTS
3.1 Effects of drought on boreal understory plants over different time intervals
The drought had immediate negative impacts on growth or vitality of all species, but the effects were more persistent for bryophytes and the old-growth forest associated orchid Goodyera repens. Among the vascular plants, G. repens experienced the strongest immediate negative effects on vitality and growth (+3.6% growth in T, +61% in C, where T is drought treatment and C is the ambient control, Figures 3 and 4), and produced smaller but thicker leaves during the drought (lower SLA, Figure S7). G. repens experienced a reduction in the number of leaves 2 months after the drought (−18% in T, + 3% in C, Figures 3 and 4), and also a reduction in vitality (Figure 3). One year after the drought, G. repens had not recovered fully from the drought (−8.4% in T, +68% in C over the pre-drought to post-drought period), despite a higher growth-rate during the time-interval 2 months until 1 year after the drought (+19% in T, −3% in C, Figure 3, Figure 4). Luzula pilosa and Oxalis acetosella experienced immediate negative effects on vitality, and L. pilosa also on growth (+48% in T, +59% in C). Neither L. pilosa nor O. acetosella experienced effects during subsequent intervals after the drought treatment and no negative effects remained visible 1 year after the drought (Figure 3). Surprisingly, L. pilosa produced a higher number of seeds per fruit found in the drought plots the year after (Figure 5a, Figure S8).
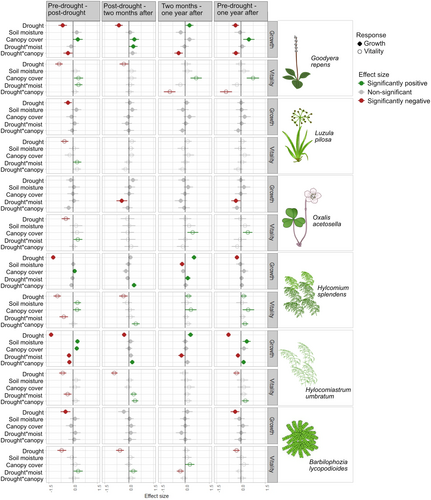
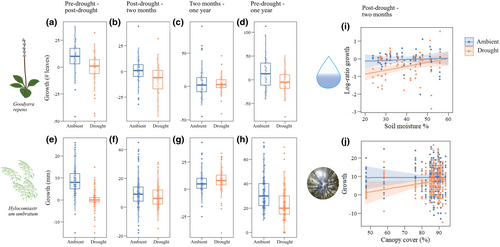
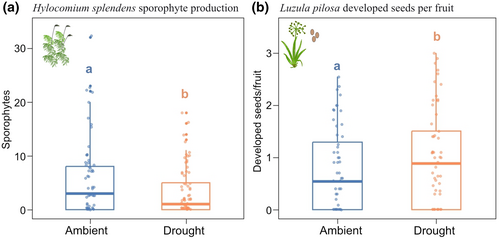
Drought responses were larger for bryophytes compared to vascular plants (Figure 3). The mosses H. splendens and H. umbratum barely grew during the 45 days that the drought treatment lasted, leading to a 98% growth reduction compared to the control (mean growth 0.2 and 0.16 mm in T, and 8.2 and 8.4 mm in C, respectively for these species, Figure 4). Also the liverwort B. lycopodioides was largely negatively affected during the 45-day drought, both in terms of growth (−15% in T, +5.6% in C) and vitality. Growth of H. umbratum during the 2 months after the drought was reduced by 25% (6.9 mm in T, 9.3 mm in C). However, growth rates of H. splendens and B. lycopodioides was similar in drought and control plots 2 months post-drought, even though their vitality remained lower after 2 months. Surprisingly, during the period from 2 months to 1 year after the drought, growth segments of H. umbratum and H. splendens elongated twice as much in the drought plots compared to the control (H. splendens: 8.8 mm in T, 4 mm in C and H. umbratum: 8.5 mm in T, 5.5 mm in C, Figure 4). Still, they did not reach the same total size as in the control (H. splendens 34 mm in T, 36 mm in C and H. umbratum: 22 mm in T, 32 mm in C, Figure 3, Figure S1). The new years' segment (i.e., that grew from the marked segment that was exposed to the drought) was shorter in the drought-treated plots compared to the control for both species (H. splendens: 18 mm in T, 19 mm in C. H. umbratum: 9.5 mm in T, 13 mm in C, Figure S9), contrasting the higher growth rates of the marked segment during the year after the drought. Drought did not affect the branching patterns of the two mosses (Figure S9). The drought negatively affected sporophyte production in H. splendens the year after the drought (Figure 5b,c; Figure S8).
3.2 Effects of canopy cover and soil moisture on responses to drought
Local environmental conditions influenced how species responded to the drought. In line with our hypotheses, a higher soil moisture reduced the immediate negative drought effects on vitality for the vascular plants G. repens and O. acetosella, and reduced drought-induced die-off of leaves of G. repens 2 months after the drought (Figures 3, 4j). Some effects deviated from expectations, such as larger negative drought effects on O. acetosella in wetter sites, as well as larger negative effects on growth and vitality of G. repens in more closed sites (Figure S10). The interactive effects of canopy cover and drought for G. repens, disappeared when the two most open sites were omitted from the analysis, indicating that these sites disproportionally drove that pattern.
Interactive effects between the drought and local conditions were stronger for bryophytes than for vascular plants (Figure 3, Figure S11). The negative drought effects on growth and vitality in H. splendens and H. umbratum 2 months after the drought were smaller under higher canopy cover (Figures 3, 4k). In addition, negative drought effects on sporophyte production the year after the drought were significantly lower at sites with higher canopy cover. These patterns remained also when the two most open sites were omitted. Soil moisture reduced the negative effects of the drought on vitality 2 months after the drought in H. umbratum and B. lycopodioides (Figure 3, Figure S11). In contrast, the higher growth rates of segments of H. umbratum during the year after the drought, were larger in dryer sites in the landscape (Figure 3, Figure S11).
4 DISCUSSION
We assessed the immediate and delayed effects of a 45-day simulated summer drought on the performance of six boreal forest understory plants, using a transplant experiment with rainout shelters. We found immediate negative effects of the drought on all species. Bryophytes experienced stronger and more persistent effects than vascular plants, and species associated with older forest were most severely affected, presumably because they are adapted to stable and humid microclimates. Drought impacts were influenced by local environmental conditions. Higher soil moisture reduced some drought effects on vascular plants, and both higher canopy cover and higher soil moisture reduced drought effects for bryophytes. These results indicate that the effects of drought may vary over small spatial scales and that forest landscapes can be actively managed to alleviate drought effects on boreal forest biodiversity.
4.1 Effects of drought on boreal understory plants over different time intervals
The observed immediate adverse effects on vitality and growth of all vascular plants after the summer drought are not surprising, considering that water is a critical resource for metabolic processes and that desiccation can lead to internal cell damage through several pathways (Farooq et al., 2012). In addition, the older forest associated orchid G. repens altered its leaf morphology by producing thicker and smaller leaves during the time of the drought (reduced SLA). This is a common response to drought due to reduced cell expansion and/or thickening of cell walls, which can increase water use efficiency (Wellstein et al., 2017). During the 2 months after the drought, there was a sustained decrease in leaves in G. repens, presumably due to tissue damage resulting in early leaf senescence or by actively shedding leaves to conserve energy and reduce further water loss (Chaves et al., 2003). The negative drought effects in this species were still visible after 1 year, even though there was a slight increase in growth rates the year after the drought. Higher growth rates after drought have been demonstrated in previous experiments, where drought treated plants frequently approached the size of control plants (Seifarth et al., 2021; Xiao, 2001; Xu et al., 2010), but the mechanisms behind this remain to be investigated. We did not find long-term effects of the drought on O. acetosella and L. pilosa. These species seemed to have recovered growth rates and vitality within 2 months after the drought. However, it is important to bear in mind that we only measured above-ground structures in this study. This was motivated by our objective to follow individuals over a longer time period and harvesting their roots would have been destructive. Vascular plants reallocate resources between different structures when exposed to drought (Eziz et al., 2017; Seifarth et al., 2021). The observed recovery may thus partly be due to redistribution of below-ground stored resources to above-ground structures (Villar-Salvador et al., 2015). On the other hand, studies have shown that plants exposed to drought allocate more energy to root growth in order to increase water uptake (optimal resource partitioning; Chaves et al., 2003; Xu et al., 2010, but see Eziz et al., 2017).
Allocation of resources to reproductive structures is typically least prioritized during or after stress (Eziz et al., 2017). Contrary to this, we observed increased seed production in L. pilosa following the drought. Such higher allocation of energy to reproduction could be a mechanism to enhance dispersal to more suitable habitats under unfavorable conditions. Even if such responses are more rare than the opposite, it has been shown in some annual plants (Eziz et al., 2017) and after insect herbivory induced stress (Garcia & Eubanks, 2019).
Bryophytes were more strongly affected by the drought than vascular plants. The moss layer is often the first to dry out and periodic desiccation is a natural event for most bryophytes (Oliver, 2005; Proctor et al., 2007). However, their poikilohydric nature makes them also sensitive to environmental changes that can cause high mortality of individual shoots (Schmalholz & Hylander, 2011) or local extinctions of sensitive species, for example, following canopy clearing (e.g., Hylander et al., 2005). Bryophytes enter an inactive metabolic state during desiccation and it is not surprising that the experimental drought resulted in immediate halted growth. Contrary to the results for the vascular plants, we observed negative effects on the growth and vitality for all three bryophytes up to 1 year after the drought, which is not surprising considering their affiliation to forest understories (in particular H. umbratum and B. lycopodioides are affiliated with moist and shaded forests). Extended drought periods hamper energy acquisition for growth and reproduction, especially since bryophytes do not have storage structures like vascular plant roots. Furthermore, tissue damage occurs both during dehydration and upon rehydration, and cellular repair requires energy and could have contributed to the lagged effects in growth and vitality that we observed (Glime, 2017; Oliver, 2005; Proctor et al., 2007). It is known that H. splendens requires about 10 days to regain 50% of its photosynthetic potential after desiccation (Proctor et al., 2007), but numbers for H. umbratum and B. lycopodioides have not been reported. The persistent effects were most pronounced in the sensitive and older forest indicator moss H. umbratum, which is generally found in in moist and shaded environments.
Interestingly, during the interval from 2 months until 1 year after the drought, both mosses (H. splendens and H. umbratum) allocated energy and resources to elongate the drought-affected segment, while the segment of the following season was reduced. This is in line with previous research suggesting that new buds do not start to grow until the old segments of H. splendens have reached a certain size (Busby et al., 1978; Tamm, 1953). The resource allocation to different structures in bryophytes is less understood than in vascular plants, but a previous study has shown that internal transport of water and solutes occurs (Sokołowska et al., 2017). In addition, Busby et al. (1978) suggested that hormones inhibit growth of the new segment until the old segment has completed its growth. Finally, lower photosynthetic carbon assimilation, simply due to a smaller segment area, could also have contributed to less growth of the following seasons' segment.
The number of sporophytes of the moss H. splendens was reduced the year after the drought compared to the controls. Both energy limitation and lack of fertilization could have reduced sporophyte production. The process of sporophyte production generally takes up to 1 year (Proctor et al., 2007; Rydgren & Økland, 2003). Any resource or energy limitation during this period is likely to have lagged effects on reproduction, in line with previous research suggesting trade-offs between growth and reproduction in bryophytes (Bisang & Ehrlén, 2002; Rydgren & Økland, 2003) and that a certain segment size (i.e., resource availability) is necessary for reproduction (Rydgren & Økland, 2003), referred to as reproductive threshold in vascular plants. In addition, sporophyte abortion following drought and resource limitation has been shown in another species (Stark, 2002), but is something we did not investigate in this study. Moreover, drought may hinder fertilization, since this process can only occur with sufficient water (Rydgren et al., 2006). In agreement, fertilization in H. splendens takes place in July (Rydgren et al., 2006), coinciding with the second part of the drought treatment, and sporophytes mature in summer the year after (Rydgren & Økland, 2003). However, local moss population dynamics might be more dependent on vegetative growth and propagation than sexual reproduction.
Our results regarding the effects of drought on plant performance should be regarded as conservative estimates. Natural summer droughts often coincide with heatwaves and low air humidity, which were not manipulated in our experiment. Previous studies have shown that the combined effects of altered precipitation and temperature have more substantial effects than changes in each factor in isolation (Aguirre et al., 2021; Park Williams et al., 2013). Increased vapor pressure deficits (VPD) drive stomatal closure and can result in reduced photosynthesis and carbon starvation (Grossiord et al., 2020). In addition, plants exceed lethal temperatures faster, when respirational cooling is reduced after stomatal closure (Hussain et al., 2019; Lipiec et al., 2013) and temperature reduces recovery rates after desiccation in bryophytes (Oliver, 2005). Drought experiments with rainout shelters thus constitute a good way to investigate ecological responses to drought in a controlled way, but they do have limitations in that they do not mimic all effects of a natural drought and as a consequence might lead to underestimations of drought effects (Aguirre et al., 2021).
4.2 Effects of canopy cover and soil moisture on responses to drought
Soil moisture and canopy cover influenced plant responses to drought. In line with our hypothesis, vascular plants growing in wetter sites were less affected by the drought. Local conditions were more important for drought responses of bryophytes than for vascular plants, presumably due to their poikilohydric physiology (Man et al., 2022). Canopy cover and soil moisture reduced negative drought impacts on bryophytes during the 2 months after the drought. Bryophytes did not grow during the drought. Thus, the buffering of canopy cover for longer-term effects on growth and vitality did not act through reduced leaf transpiration and maintaining growth during the 45 day drought event, but rather through alleviating damage of internal structures. The mechanisms behind this damage alleviation are not fully understood, but high light radiation exacerbates damage on internal cell structures during desiccation (Gauslaa et al., 2012; Proctor et al., 2007) and recovery rates after desiccation are reduced under high temperatures (i.e., low canopy cover) conditions (Oliver, 2005; Proctor et al., 2007), potentially due to enhanced photodamage, oxidative stress, photoinhibition, and denaturation of proteins (Farooq et al., 2012; Heber & Lüttge, 2011 ; Proctor et al., 2007). In addition, the rate of drying affects cellular damage and this is likely mediated by both canopy cover and soil moisture. Cellular damage is larger when bryophytes dry out rapidly, while slower drying allows for preparation of protective and repairing mechanisms (e.g., protein synthesis, Proctor et al., 2007; Glime, 2017). The larger drought effects in wetter places for O. acetosella were surprising, since this species is affiliated to moister soils.
4.3 Conclusions and management implications
Boreal ecosystems are expected to shift from cool and short summers to long and warm summers with reduced soil water availability (Grossiord et al., 2014). We showed that boreal forest understory species, in particular bryophytes, were adversely affected by a simulated summer drought. Drought effects are likely to be even more important during real droughts that go paired with heatwaves. Our study indicates that landscape management may constitute an important tool to mitigate the effects of future droughts by preserving moist and shaded places in the forest, particularly in areas that harbor sensitive understory species (Hylander et al., 2022). Forest managers can also actively manipulate shading and soil moisture levels, by implementing continuous-cover forestry and by hydrological restoration of ditched areas and canalized streams (De Frenne et al., 2021; Greiser et al., 2018; Hylander et al., 2022).
AUTHOR CONTRIBUTIONS
Irena A. Koelemeijer: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; writing – original draft. Isabelle Severholt: Data curation; formal analysis; investigation; methodology; writing – review and editing. Johan Ehrlén: Conceptualization; methodology; writing – review and editing. Pieter De Frenne: Writing – review and editing. Mari Jönsson: Writing – review and editing. Kristoffer Hylander: Conceptualization; funding acquisition; methodology; writing – review and editing.
ACKNOWLEDGEMENTS
This work was supported by the following funding sources: Albert & Maria Bergströms stiftelse, Bolin center for climate research, Formas with Grant no. 2018-02829 to Kristoffer Hylander and Grant no. 2016-00461 to Mari Jönsson. The authors have no conflict of interest. We thank Malin Borg, Vanessa Väisänen, Chaima Naciri, Agnes Pierre, Henk Koelemeijer, and Ineke Geertjes for their help during field data collection and Malin Borg for tracing the B. lycopodioides patch sizes.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data is available on Dryad. https://doi.org/10.5061/dryad.pnvx0k6x4 https://datadryad.org/stash/share/ExwKbRQ54RpKcJHGS5tu01eadq5L3TdXY24HLJ_Poxo)




