A circumpolar study unveils a positive non-linear effect of temperature on arctic arthropod availability that may reduce the risk of warming-induced trophic mismatch for breeding shorebirds
Abstract
Seasonally abundant arthropods are a crucial food source for many migratory birds that breed in the Arctic. In cold environments, the growth and emergence of arthropods are particularly tied to temperature. Thus, the phenology of arthropods is anticipated to undergo a rapid change in response to a warming climate, potentially leading to a trophic mismatch between migratory insectivorous birds and their prey. Using data from 19 sites spanning a wide temperature gradient from the Subarctic to the High Arctic, we investigated the effects of temperature on the phenology and biomass of arthropods available to shorebirds during their short breeding season at high latitudes. We hypothesized that prolonged exposure to warmer summer temperatures would generate earlier peaks in arthropod biomass, as well as higher peak and seasonal biomass. Across the temperature gradient encompassed by our study sites (>10°C in average summer temperatures), we found a 3-day shift in average peak date for every increment of 80 cumulative thawing degree-days. Interestingly, we found a linear relationship between temperature and arthropod biomass only below temperature thresholds. Higher temperatures were associated with higher peak and seasonal biomass below 106 and 177 cumulative thawing degree-days, respectively, between June 5 and July 15. Beyond these thresholds, no relationship was observed between temperature and arthropod biomass. Our results suggest that prolonged exposure to elevated temperatures can positively influence prey availability for some arctic birds. This positive effect could, in part, stem from changes in arthropod assemblages and may reduce the risk of trophic mismatch.
1 INTRODUCTION
Organismal responses to climatic warming often include phenological shifts in major life-history events, which can lead to heterogeneous responses among different functional groups within food webs (Thackeray et al., 2016). Small-bodied ectothermic organisms at lower trophic levels often respond with stronger phenological adjustments than large endothermic organisms at higher trophic levels, which can lead to a trophic mismatch between consumers and their food sources (Both et al., 2009; Cohen et al., 2018; Kharouba & Wolkovich, 2023). Arctic ecosystems are currently warming two to four times faster than the rest of the earth (IPCC, 2021; Rantanen et al., 2022). Hence, arctic food webs are especially likely to be subject to trophic mismatches between consumers and their resources (Post et al., 2009; Schmidt et al., 2017).
Insectivorous migratory birds breeding in the Arctic are expected to be especially prone to warming-induced mismatch (McKinnon et al., 2012; Miller-Rushing et al., 2010; Saalfeld et al., 2019; but see Corkery et al., 2019; McKinnon et al., 2013). Arthropods are resident ectotherms, and their phenology is strongly affected by local environmental conditions (Culler et al., 2015; Høye & Forchhammer, 2008a; Shaftel et al., 2021). By contrast, the migration and breeding phenology of some arctic birds can be affected by a wide range of cues and environmental conditions encountered en route to and at breeding sites (Liebezeit et al., 2014; Smith et al., 2010; Ward et al., 2016; Winkler et al., 2014). Arctic birds also have a relatively short window of time for reproduction, and food availability during the chick rearing period is especially critical as it may affect fitness through juvenile growth and survival (McKinnon et al., 2012; Reneerkens et al., 2016; Saalfeld et al., 2019, 2021). Chicks of insectivorous birds mostly feed on active and visible arthropods (Richards & Gaston, 2018; Schekkerman et al., 1998) and their availability is typically characterized by a relatively short summer pulse (Bolduc et al., 2013; Danks, 2004; Tulp & Schekkerman, 2008). Consequently, the response of arctic arthropods to warming can strongly affect the likelihood of trophic mismatch.
Studies based on time series are often used to predict long-term effects of warming on wildlife species and trophic interactions (Kharouba et al., 2018; Parmesan, 2007). However, empirical studies of the phenological responses of arctic arthropods to a prolonged increase in summer temperatures are rare and typically limited to single-site assessments (e.g., >25 years time series; Høye et al., 2021). Such studies showed that arctic arthropod assemblages can change relatively quickly when exposed to warmer temperatures for several years (e.g., changes in the relative abundance of functional groups; Koltz et al., 2018), which can cause cascading effects on arthropod availability for consumers (Schmidt et al., 2017).
Assuming that the variation of a parameter through space can also be used to predict its variation through time, a space-for-time substitution approach has the potential to improve our assessment of the long-term effects of warming on ecological communities (Blois et al., 2013; Elmendorf et al., 2015; Pickett, 1989). Although the approach has some important caveats, it can nonetheless be useful when the drivers that control biological processes are the same drivers that operate in space and if community dynamics and assemblages can respond relatively quickly to persistent environmental changes (Damgaard, 2019; Wogan & Wang, 2018).
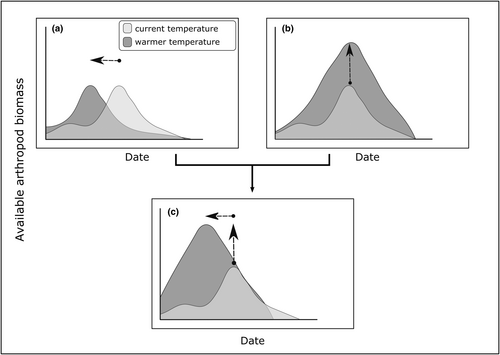
Here, we apply a space-for-time substitution approach to improve our ability to predict the general response of arctic arthropods to warming, and hence the potential consequences on consumers like migratory birds. Our study is based on a pan-arctic dataset of surface-active and low-flying arthropod biomass collected from the Subarctic to the extreme High Arctic. It spans a large temperature gradient (>10°C in June and July average daily air temperature) overlapping the expected temperature increase in the Arctic and allows comparisons between species assemblages shaped by long-term exposure to warmer temperatures. Temperature is known to affect arthropod emergence phenology (e.g., date of peak biomass; Figure 1a) but also the magnitude of peak arthropod biomass (Figure 1b; e.g., Bolduc et al., 2013; Tulp & Schekkerman, 2008). The consequences of warming on the availability of arthropods for Arctic-nesting birds are likely to depend on the strength of such combined effects (Figure 1c). Despite potential differences in temperature responses among arthropod species, we hypothesized that higher summer temperatures would be associated with earlier peak arthropod biomass and higher arthropod biomass available to Arctic-nesting insectivorous birds across the temperature gradient covered by our study sites.
2 METHODS
2.1 Arthropod data
2.1.1 Field sampling
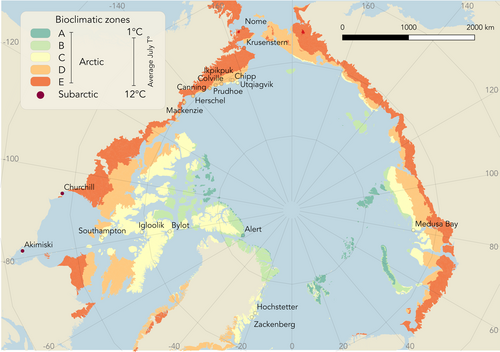
Arthropods were sampled during the breeding season of insectivorous birds (approximately June to August) at 19 field sites distributed across most Arctic and Subarctic bioclimatic zones (Figure 2; Leemans, 1992; Walker et al., 2005). Each site was sampled for 1–19 years, and over a period of 26–143 days per year (medians = 3 years and 56 days, respectively; Table 1), covering the estimated period of peak arthropod biomass. At each site, 6–20 traps were deployed and typically emptied every 1–3 days, except for Zackenberg, Hochstetter Forland, and Chipp River, where sampling was performed once a week. The biomass estimates were then converted to daily values as described below.
| Site name | Mean summer, T (°C) | Coordinates | Sampling years (n) |
|---|---|---|---|
| Utqiaġvik | 1.2 | 71°18′ N, 156°45′ W | 2010–2016 (7) |
| Alert | 1.5 | 82°30′ N, 62°21′ W | 2007–2008 (2) |
| Zackenberg | 1.6 | 74°28′ N, 20°34′ W | 1998–2016 (19) |
| Hochstetter Forland | 2.0 | 75°09′ N, 19°42′ W | 2011–2014, 2016–2017 (6) |
| Ikpikpuk | 2.2 | 70°33′ N, 154°43′ W | 2010–2012 (3) |
| Medusa Bay | 2.4 | 73°20′ N, 80°32′′ E | 1996, 2000–2002 (4) |
| Prudhoe Bay | 2.6 | 70°12′ N, 148°27′ W | 2010 (1) |
| Bylot Island | 3.1 | 73°80′ N, 79°58′ W | 2005–2017 (13) |
| Chipp River | 3.2 | 70°41′ N, 155°18′ W | 2013 (1) |
| Canning River | 3.5 | 70°26′ N, 145°51′ W | 2010–2012 (3) |
| Igloolik | 3.7 | 69°24′ N, 81°48′ W | 2014, 2017 (2) |
| Colville | 3.8 | 70°26′ N, 150°41′ W | 2011–2012, 2014–2017 (6) |
| Herschel Island | 4.1 | 69°35′ N, 138°55′ W | 2007–2008 (2) |
| Mackenzie Delta | 4.9 | 69°22′ N, 134°53′ W | 2011–2012 (2) |
| Southampton Island | 5.0 | 63°59′ N, 81°40′ W | 2006–2008, 2010–2012 (6) |
| Nome | 8.3 | 64°27′ N, 164°58′ W | 2010–2012 (3) |
| Churchill | 8.4 | 58°45′ N, 94°04′ W | 2010–2011 (2) |
| Cape Krusenstern | 9.7 | 67°06′ N, 163°29 W | 2011–2012 (2) |
| Akimiski Island | 11.7 | 53°00′ N, 81°20′ W | 2009 (1) |
At each study site, arthropods were sampled in dry uplands and low wetlands, which were the two main habitats used by the common species of shorebirds, passerines, and other insectivorous birds during their chick rearing period. Arthropods were collected in open areas within each habitat using modified Malaise traps with rectangular white pitfall traps (38 cm × 5 cm) at most sites (Bolduc et al., 2013; Brown et al., 2014), except Medusa Bay, Hochstetter Forland, and Zackenberg, where round white or yellow pitfall traps were used (10–11 cm in diameter; Schmidt et al., 2016; Tulp & Schekkerman, 2008). Therefore, we compared results obtained from these two trapping techniques conducted simultaneously at the same site before combining datasets (see below). Both techniques are passive traps and thus measure a combination of the abundance and the activity of surface-active and low-flying arthropods (Southwood & Henderson, 2000). Variation in biomass was used as a proxy of arthropod availability for surface-feeding insectivorous birds such as shorebirds (Bolduc et al., 2013; Kwon et al., 2019; McKinnon et al., 2012).
Arctic-breeding birds are likely to be gape-limited and restricted to prey that they can swallow whole. We excluded bumblebees and butterflies, as they were likely too large to be consumed by chicks (Kwon et al., 2019; Saalfeld et al., 2019; Schekkerman & Boele, 2009). We also excluded springtails (Collembola) and mites because they were a negligible part of the sampled biomass and were considered too small to be important prey for chicks (Bolduc et al., 2013; Ridley, 1980; Tulp & Schekkerman, 2008). The biomass of all remaining arthropods was pooled, as arctic insectivorous birds typically consume a broad diversity of species during the breeding season (Flemming et al., 2022; Wirta et al., 2015). The dry biomass for each trap was measured directly or estimated with equations using the length of the specimen to convert abundance of individuals to dry mass (see Appendix S1 for details). Wet and dry habitats were pooled to obtain a general index of arthropod availability (McKinnon et al., 2012). A standardized daily arthropod availability index (mg/trap) was calculated for each year and site, achieved by dividing the overall arthropod biomass by the number of sorted traps and the days elapsed between sampling events (Bolduc et al., 2013). Our index of arthropod abundance and activity accounted for the variable number of traps and the differences in sampling frequency across sites.
2.1.2 Data standardization
We carried out a calibration experiment because two different trapping techniques were used across our study sites (i.e., different colors, shapes, and sizes of the pitfall traps). We used both types of traps simultaneously at Bylot Island in 2018 to develop a linear regression linking the variation in the biomass of arthropods captured with the two different types of traps (see Appendix S2).
2.1.3 Arthropod phenology and biomass
Based on seasonal changes in the index of arthropod daily availability, we calculated three parameters for each year and field site: peak biomass, date of peak biomass (hereafter peak date), and seasonal biomass. Peak biomass was defined as the single highest recorded value of daily arthropod availability (mg/trap/day) and peak date was the rounded median date of the sampling period during which the peak biomass was observed. We explored alternative methods (generalized additive models and moving averages) and found similar results. Zackenberg, Hochstetter Forland, and Chipp River data were excluded from peak date analyses, because we considered that the weekly sampling frequency would strongly reduce the precision of the estimates compared to other sites that were sampled every 1–3 days. The daily index of arthropod availability can vary substantially before and after the peak date, and the period characterized by relatively high biomass can be relatively narrow or wide, depending on study years and sites (Bolduc et al., 2013; Saalfeld et al., 2019). To better describe the arthropod biomass available to birds, we also calculated the seasonal biomass, which is the cumulative biomass sampled during a 3-week period centered on the peak date (i.e., sum of daily biomass recorded during the period corresponding to the peak date ±10 days). Shorebird chicks are more vulnerable to starvation or marked reduction in growth rate during their first 9 days (McKinnon et al., 2012; Pearce-Higgins & Yalden, 2003). Our seasonal biomass estimates provided a proxy of food availability just before and after peak date, over a biologically significant period. Estimating seasonal biomass over a longer period generated similar results and the correlation between seasonal biomass estimates using a 21-day or 31-day window was high (r = .99, p < .001, n = 57). As the total sampling season was shorter at some study sites, the use of a 21-day window increased our sample size. Nevertheless, in nine out of 84 site-years, we were unable to estimate a seasonal biomass value using a 21-day window, as the period of sampling did not fully cover the minimum time window around the peak date. For 4 of the 9 cases where only 3 days were missing, the window was shifted up to 3 days earlier or later to include a full 21-day window of cumulative biomass (site and year: Canning in 2011, Mackenzie in 2011, Southampton in 2007, and Utqiaġvik in 2010).
2.2 Weather data
In addition to temperature, other environmental parameters such as timing of snowmelt, precipitation, and solar radiation can affect the phenology and availability of arctic arthropods. (Asmus et al., 2018; Bolduc et al., 2013; Høye & Forchhammer, 2008a, 2008b). Thus, we added these three variables as covariates in our statistical models. Although it would have improved our ability to explain the variation in arthropod phenology and availability, local weather data acquired using standardized protocols were not available for most sites and years. The weather data used for our models were thus extracted from the dataset produced by the global atmospheric reanalysis ERA-Interim (Dee et al., 2011). A reanalysis model continuously integrates data from satellites, weather stations, and other sources and validates the model predictions with every update. The model outputs are global data grids of daily weather conditions since 1979 with a spatial resolution of 79 km2 (Berrisford et al., 2009; Dee et al., 2011). This approach allowed us to fill temporal and spatial gaps in weather datasets for all study sites and to avoid systematic biases caused by the use of different field methods. Despite some errors inherent to this type of data, the surface air temperature estimate derived from ERA-Interim aligns well with the data collected from Arctic land stations, indicating a strong overall agreement (Simmons & Poli, 2015). Compared to other comparable reanalysis models, ERA-Interim is also noteworthy for the consistency of the model predictions for the Arctic region (Lindsay et al., 2014).
We employed ERA-Interim data for daily mean air temperature, daily cumulative precipitation, and daily surface solar radiation in order to derive relevant parameters for our models. We extracted values over the same period (June 5 – July 15) for all sites and years. The three variables included: (1) cumulative thawing degree-days (DD), which was calculated as the cumulative daily mean air temperature above 0°C; (2) the cumulative daily precipitation (PR); and (3) average surface solar radiation (RAD). The selected time period fully or partly overlaps the environmental conditions recorded before the peak date of arthropod biomass at all sites. Hence, data extracted over this period provided a proxy of the local environmental conditions that should have a strong effect on summer arthropod availability during the chick-rearing period (Meltofte et al., 2007; Tulp & Schekkerman, 2008). The use of the same 41-day period at all sites is likely not the best approach to explain intra-site variation in annual peak date and biomass (e.g., the period could be adjusted relative to site-specific annual snowmelt date; Asmus et al., 2018; Shaftel et al., 2021). However, the use of the same period for all sites allowed us to compare the relative weather conditions across the gradients covered by our study sites. Further, values extracted over slightly different time periods were highly correlated (see Appendix S3). Running analyses using these values did not change any of our main conclusions (e.g., in linear regression analyses linking environmental conditions and arthropod phenology or biomass, the use of slightly different time periods partially shifted some data points along the X-axis with limited effect on their rank; results not shown). Finally, we included the snow-free date (SN; O'Leary et al., 2017) as a proxy of the relative snowmelt phenology, which was extracted from an 8-day composite satellite dataset (MOD10A2; Hall et al., 2018). This dataset provided a consistent set of measurements across years and allowed us to have a standardized and almost complete dataset for all study sites. A few missing values were caused by excessive cloud cover (nine cases out of 84 site-years). These data points were excluded from models that included snow cover.
2.3 Data analysis
We analyzed the effect of cumulative thawing degree-days and other environmental parameters on the date of peak arthropod biomass, the peak value for biomass per day, and the seasonal biomass (i.e., cumulative biomass for the peak date ±10 days). Based on previous studies (Bolduc et al., 2013; Høye & Forchhammer, 2008b; Tulp & Schekkerman, 2008), we created candidate models that included DD and combinations of other environmental parameters (for a full list, see Appendix S5). We calculated the correlation between all variables using Pearson's correlation test. Snow-free dates and DD were only moderately correlated (r = −.45, p < .001, n = 75 site-years) so they were included in the same model. Cumulative precipitation and radiation were more strongly correlated (r = −.68, p < .001, n = 84) and hence were not included in the same statistical model. The correlations were low among all other covariables (r ≤ .28). Our candidate models also included a segmented linear regression, or “broken-stick” regression, to test for break points in the relationships between temperature and arthropod parameters (Muggeo, 2003). We chose segmented regression over other non-linear regressions because the simplicity of its structure facilitates the interpretation of results, and both polynomial and segmented models yielded similar results. Segmented regression with two segments was performed with the R package segmented (Muggeo, 2008), which iteratively fits linear regressions with varying breakpoints, searching for the smallest “gap” between regression lines (based on minimizing the residual standard error).
Our models were weighted to account for unequal sampling in our dataset because the number of years of observations per site varied from 1 to 19 years. Each site had a total weight of one, and thus each observation for annual peak biomass and other variables was weighted by 1/number of years of data included for a given study site. This approach allowed us to run segmented regressions using the same structure for all models, and to test for the presence of abrupt changes (breakpoints) in the relationships using model selection based on Akaike information criterion corrected for small sample sizes (AICc; Burnham & Anderson, 2002). In the absence of breakpoints, weighted linear regressions and mixed models using the within-group centering method (van de Pol & Wright, 2009) yielded similar outcomes such that DD always appeared in the top models, and parameter estimates were virtually identical (see Appendix S6). We checked for collinearity using the variation inflation factor (VIF), which was low (≤3) for all covariates included in the candidate models (Zuur et al., 2010). The model with the lowest AICc was considered the best fitting, and models with a ΔAICc <4 are presented for comparison (Burnham & Anderson, 2002; see Table 2). The effect of a parameter was illustrated using the 95% confidence interval of its estimate. All analyses were performed using functions of Program R (ver. 3.5.2) and model selection was conducted using the MuMIn package (Bartoń, 2018; R Core Team, 2018).
| df | logLik | AICc | ΔAICc | Weight | |
|---|---|---|---|---|---|
| (a) Peak date | |||||
| *DD + SN | 4 | −192.70 | 394.25 | 0.00 | 0.43 |
| DD + PR | 4 | −193.35 | 395.55 | 1.30 | 0.22 |
| DD (seg) + SN | 6 | −191.18 | 396.23 | 1.98 | 0.16 |
| DD (seg) + PR | 6 | −191.49 | 396.85 | 2.60 | 0.12 |
| Null model | 2 | −208.61 | 421.47 | 27.22 | 0.00 |
| (b) Peak biomass | |||||
| *DD (seg) | 5 | −460.52 | 931.92 | 0.00 | 0.31 |
| DD (seg) + RAD | 6 | −459.51 | 932.25 | 0.33 | 0.26 |
| DD (seg) + PR | 6 | −460.00 | 933.24 | 1.32 | 0.16 |
| DD (seg) + SN | 6 | −460.39 | 934.01 | 2.09 | 0.11 |
| DD | 3 | −464.70 | 935.74 | 3.82 | 0.05 |
| Null model | 2 | −465.95 | 936.07 | 4.15 | 0.04 |
| (c) Seasonal biomass | |||||
| *DD (seg) | 5 | −554.58 | 1120.16 | 0.00 | 0.41 |
| DD (seg) + RAD | 6 | −553.98 | 1121.38 | 1.22 | 0.22 |
| DD (seg) + SN | 6 | −554.51 | 1122.44 | 2.28 | 0.13 |
| DD (seg) + PR | 6 | −554.52 | 1122.46 | 2.30 | 0.13 |
| Null model | 2 | −562.04 | 1128.27 | 8.11 | 0.0 |
- Note: DD = cumulative thawing degree-days between June 5 and July 15 for 19 sites distributed across most Arctic and Subarctic bioclimatic zones, with between 1 and 19 years of sampling. The models presented here have a ∆AICc ≤4, while only the models with a (*) were retained for further analyses and discussions. The AICc weight represents the relative weight attributed to the model. For full model selection and summary of all models, see Appendix S5.
- Abbreviations: PR, cumulative precipitation between June 5 and July 15; RAD, average solar radiation between June 5 and July 15; seg, segmented regression with two segments; SN, first snow-free day.
2.4 Model illustration
To contextualize and better assess the implications of our findings, we used top-ranked models for each of our three availability parameters to illustrate the potential effect of a prolonged temperature increase on the availability of arthropods to birds. To do so, we used our models to predict peak date, peak biomass, and seasonal biomass for hypothetical sites, based on the range of temperature values observed in our data. We then used these values to create illustrative symmetrical bell-shaped curves representing daily arthropod availability, comparable to seasonal patterns observed at our study sites (Bolduc et al., 2013; Høye & Forchhammer, 2008a). These curves illustrate the seasonal variation in arthropod availability under a given temperature scenario. We then calculated the changes in arthropod peak date and biomass for a warmer temperature scenario (a temperature increase of 80 DD, equivalent to an increase of 2°C in average in the daily temperature between June 5 and July 15). Using the predicted values (arthropod peak date and biomass), we also generated a new bell-shaped curve that represents the expected seasonal availability of arthropods. The use of slightly different curve shapes did not affect our main conclusions (results not shown). To illustrate the magnitude of the expected changes in arthropod phenology relative to the current shorebird breeding phenology, we then superimposed the current hatching periods recorded for the most common arctic shorebird species found at various monitoring sites located along the temperature gradient covered by our study. The range of the current hatching period at a given site was obtained by averaging the annual earliest and latest hatch dates recorded between 2010 and 2016, after excluding upper and lower 5%, for each year (Lanctot et al., 2016). In our model illustration, we opted to represent bird phenology as static to keep the focus on arthropod data. However, bird phenology may also advance with climate warming (Liebezeit et al., 2014; Ward et al., 2016), although generally slower than the food resource (Reneerkens et al., 2016; Saalfeld & Lanctot, 2017; Zhemchuzhnikov et al., 2021). The strength of such potential adjustments is unknown, but it would contribute to reduce the likelihood of trophic mismatch.
3 RESULTS
A total of 16,134 arthropod traps were sampled at 19 different study sites for 1–19 years at each site, for a total of 84 site-years. Weather conditions varied substantially between sites (Figure 3; Appendix S4). The cumulative thawing degree-days between June 5 and July 15 varied from 15 DD to 426 DD.
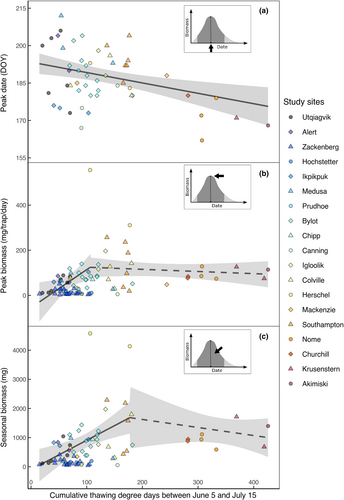
The peak biomass value per site, averaged over years, ranged from 6 to 433 mg/trap/day, while the average seasonal biomass (assessed over the 21-day period centered on the peak date) varied from 77 to 4354 mg per site. Peak biomass and seasonal biomass were highly correlated (r = .96, p = .001, n = 75 site-years). Arthropod peak dates varied substantially within and among sites (50 days range; Figure 3a), but site-averaged peak dates occurred within the 1-month period between June 17 and July 18 across sites. In some cases, the intra-site range in peak dates was almost as large as inter-site variation (Figure 3a). For instance, peak dates were spread over a 24-day period at Bylot Island, a High Arctic site with a 13-year time series. This period included the average peak dates observed at 10 out of the 16 study sites where peak dates were estimated. Hence, in some years, birds nesting at Bylot Island experienced a range of arthropod phenology that was normally observed in much warmer or colder breeding sites.
Variation in arthropod peak dates was best explained by linear effects of DD and snowmelt (SN; Table 2). The top model indicated that an increase of 25 DD advanced the peak date by 1 day on average (b = −0.04, 95% CI = −0.07 to −0.02; Figure 3a; Table 2a). Peak date was 1 day earlier, when snow-free date was 4 days earlier, on average (b = 0.25, 95% CI = 0.06–0.43). Unlike the linear effect of DD found for peak date, the variation in peak biomass and seasonal biomass were best explained by a segmented effect of DD (Table 2). We found that DD had a positive effect on peak biomass but only below a threshold of 106 DD (95% CI = 64–148 DD; Figure 3b). Below this threshold, an increase in 25 DD generated on average a 43.5 mg/trap/day increase of peak biomass (b = 1.74, 95% CI = 0.24–3.24). Above 106 DD, the effect was not significant (b = −0.07, 95% CI = −0.35–0.22). Similarly, the positive effect of DD on seasonal biomass was detected only below a threshold of 177 DD (95% CI 89–266 DD; Figure 3c). Below this threshold, a rise of 25 DD increased seasonal biomass by 270 mg on average (b = 10.8, 95% CI = 4.22–17.38) while the relationship was not significant above the threshold (b = −2.28, 95% CI = −8.20–3.64). Excluding extreme values observed at one site (Herschel) had a marked influence on the parameter estimate but did not affect the main patterns (peak biomass: b = 0.75, 95% CI = 0.25–1.26, threshold = 133 DD, 95% CI = 73–194 DD; 2nd segment: b = 0.04, 95% CI = −0.14–0.22; seasonal biomass: b = 7.77, 95% CI = 2.28–13.25, threshold = 134, 95%CI = 59–209; 2nd segment: b = 1.46, 95% CI = −0.49–3.41).
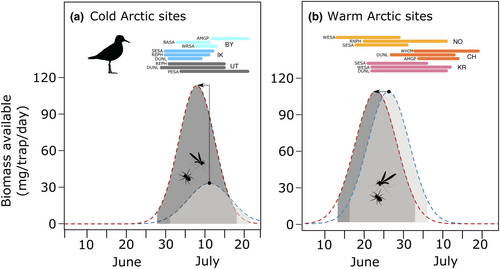
3.1 Model illustration
Based on our empirical results, we created two alternative scenarios to visualize the potential effects of a temperature increase on arthropod availability for consumers, one above and one below the temperature threshold identified by our segmented biomass models (Figure 4). We created scenarios for (i) a relatively cold, generic Arctic site (current average of 50 DD; Figure 4a) versus (ii) a relatively warm, generic Low Arctic site (current average of 320 DD; Figure 4b). In both cases, we illustrated a temperature increase of 80 DD (equivalent to a 2°C increase in average daily air temperature between June 5 and July 15). A change of 80 DD allowed us to stay within the temperature range covered by each segment of the regression (below and above the breakpoint). For the generic cold site, we superimposed hatching periods of the three most common species at three of our cold study sites as points of reference (Bylot, Ikpikpuk, and Utqiaġvik; Lanctot et al., 2016). We repeated the same steps for the generic warm site, using data from three of our warm sites (Churchill, Cape Krusenstern, and Nome; Lanctot et al., 2016). In both scenarios, the model predicted a 3.2-day shift in average arthropod peak date (Figure 4a,b). Peak dates would occur during the current shorebird hatching periods recorded at representative study sites, except for the latest breeding species (especially for American Golden-plovers, Pluvialis dominica, and Whimbrels, Numenius phaeopus; Figure 4a,b). While peak biomass is predicted to remain similar for the generic warm site, the model predicted an increase of peak arthropod biomass (80 mg/trap/day, from 30 to 110 mg/trap/day) for the generic cold site (Figure 4a). Such a marked increase in arthropod availability induced by warming would occur during the hatching periods currently observed for most shorebird species breeding in relatively cold Arctic sites (Figure 4a).
4 DISCUSSION
Our results, based on pan-Arctic arthropod monitoring spanning a gradient exceeding 10°C in average daily air temperatures during June and July, provide support to our hypothesis that extended exposure to elevated temperatures can advance arthropod phenology while increasing their potential biomass accessible to consumers like Arctic-nesting shorebirds. Our investigation revealed a strong positive correlation between temperature and arthropod availability (peak or seasonal biomass) below specific temperature thresholds. Moreover, the correlation between temperature and arthropod phenology, as expressed by the date of peak biomass available to birds, was relatively moderate across our large temperature gradient, with an average of a 1-day shift for an increase of 25 DD (roughly equivalent to a 0.6°C difference in average summer temperature). This shift is small when considering the broad spectrum of annual arthropod peak dates encountered by birds breeding at several Arctic sites (intra-site variation reaching >15 days; Kwon et al., 2019; this study). Overall, our findings suggest that prolonged exposure to elevated temperatures can have a positive effect on arctic arthropod availability that may help counteract warming-induced phenological shifts of arthropods and reduce the risk of trophic mismatch for shorebirds.
The non-linear relationship between temperature and biomass of arctic arthropods outlined in our study is a novel finding. The physiological functions of arthropods such as metabolism and growth rates, are largely constrained by temperature, and some arctic arthropods could benefit from a temperature increase in northern regions (Barrio et al., 2017; Bolduc et al., 2013; Shaftel et al., 2021). Although positive responses may be the case for some arthropod groups and during specific timeframes, recent studies have also shown negative or heterogeneous responses of some arctic arthropods to rising temperatures (Bowden et al., 2018; Høye et al., 2021; Loboda et al., 2017). Above a temperature threshold, the positive effects of temperature on biomass and activity rate of some surface-active and low-flying arthropods could thus be counterbalanced by the negative effects on other taxa, leading to the observed null effect of temperature increase on arthropod availability (see also Høye et al., 2021; Koltz et al., 2018).
A wide array of mechanisms can lead to a negative effect of temperature on arctic arthropods (Høye, 2020). For example, higher summer temperatures can lead to a decrease in arthropod body size, possibly due to metabolic costs (Bowden et al., 2015), a reduction of arthropod activity rates above a certain temperature threshold (Asmus et al., 2018) and reduced vegetation nutritive quality (Welti et al., 2020). Warmer winter conditions can also have a negative impact on summer arthropod abundance through a higher frequency of freeze–thaw events or reductions in insulation due to less snow cover during winter (Ávila-Jiménez et al., 2010; Everatt et al., 2015; Høye et al., 2021). Changes in water availability induced by changes in patterns of precipitation, melting of permafrost, or higher evaporation rates, can in turn negatively affect the abundance of some arthropod species (Ávila-Jiménez et al., 2010; Bowden et al., 2018).
After controlling for cumulative thawing degree-days (DD), the relationships we documented between arctic arthropod phenology and other weather variables are generally consistent with previous findings. For example, we found that later snowmelt was associated with later peak date, which is consistent with previous studies that identified snow dynamics as a major predictor of arctic arthropod phenology (Høye, 2020; Høye & Forchhammer, 2008a). On the other hand, Kwon et al. (2019) reported a strong negative effect of timing of snowmelt on peak arthropod biomass available to birds. We did not detect such an effect, likely due to our reliance on less precise estimates of snowmelt date. Finally, summer precipitation is known to negatively affect daily availability of arthropods (Asmus et al., 2018; Shaftel et al., 2021; Tulp & Schekkerman, 2008), but few studies investigated the effect of summer precipitation on arthropod phenology (negligible effects reported in Saalfeld et al., 2019). Use of high quality local weather data could improve the understanding of the positive effect of summer precipitation that we found on arthropod peak date (see Appendix S5).
The performance of space-for-time substitution to predict the response of ecological systems to global warming can be highly variable (Damgaard, 2019). Spatial variation often results from long-term processes that can lead to misestimating short-term responses to warming (Elmendorf et al., 2015). The main assumptions of our approach were that (1) arthropod communities assemblages sampled across our broad temperature gradient would respond to a given temperature increase in a similar way or (2) arthropod assemblages would change relatively quickly following warming, and the new assemblage would respond to a temperature increase in a similar way to assemblages currently found at warmer sites. Although the same dominant taxa are usually present in arctic arthropod assemblages (Bolduc et al., 2013; Shaftel et al., 2021) and rapid climate-induced changes in the composition of these assemblages have been observed (Koltz et al., 2018; Loboda et al., 2017), the validity of these assumptions remains uncertain. Moreover, some ecological processes indirectly affecting the biomass of arthropods available to consumers, such as vegetation changes, may operate over longer timescales. The effect of warming on arthropod availability could be assessed by integrating longer time series data from several sites encompassing diverse environmental conditions and experiencing temperature increases over time (Damgaard, 2019).
Although climate change has led to significant changes in the timing of critical life history events among interacting species (e.g. Schmidt et al., 2023), the prevalence of warming-induced mismatch remains low in terrestrial study systems linking the level of asynchrony to individual fitness (Kharouba & Wolkovich, 2023). Our results also suggest that some generalist insectivorous arctic bird populations may be less vulnerable to mismatch than expected due to a potential warming-induced increase in food availability. Moreover, some shorebirds (and other insectivorous birds) can advance their breeding dates under warmer conditions (Kwon et al., 2019; Liebezeit et al., 2014; Ruthrauff et al., 2021). Although this advancement may not perfectly track phenological shifts in environmental conditions (Saalfeld & Lanctot, 2017), it should also reduce the risk of trophic mismatch. Some arctic shorebird species, like Dunlin (Calidris alpina) and Sanderling (Calidris alba), are already breeding late relative to seasonal peaks in arthropod abundance (McKinnon et al., 2012, 2013; Reneerkens et al., 2016; see also Figure 4) and hence may not benefit from a potential positive effect of temperature on arthropod peak biomass. Birds having more specialized diets or those dependant on highly nutritional food resources could also be more vulnerable to warming-induced changes in prey phenology and quality (Arnold et al., 2010; Wilde et al., 2020; Zhemchuzhnikov et al., 2022). Hence, further investigations may be useful to fully quantify the risk of mismatch for arctic insectivorous birds, while considering that higher temperatures encountered by chicks could provide thermogenic relief that can compensate (or not) for their lack of synchrony (Lameris et al., 2022; McKinnon et al., 2013; Saalfeld et al., 2021).
Climate warming can lead to significant shifts in the timing of key life history events in Arctic ecosystems (Post et al., 2018). Based on a space-for-time substitution, our pan-arctic study indicates that the positive effects of prolonged exposure to elevated temperatures on food availability may help counteract warming-induced phenological shifts in peak food availability for some arctic birds. Incorporating time series data from Arctic sites where temperatures have been increasing over time and the inclusion of weather parameters outside the breeding season could strengthen our findings. Additionally, employing higher arthropod taxonomic resolutions may help pinpoint the specific ecological processes driving warming-induced changes in arthropod availability for birds.
AUTHOR CONTRIBUTIONS
Aurélie Chagnon-Lafortune: Data curation; formal analysis; investigation; methodology; project administration; software; visualization; writing – original draft. Éliane Duchesne: Software; visualization; writing – original draft. Pierre Legagneux: Conceptualization; writing – review and editing. Laura McKinnon: Investigation; methodology; writing – review and editing. Jeroen Reneerkens: Methodology; writing – review and editing. Nicolas Casajus: Software. Kenneth F. Abraham: Investigation; writing – review and editing. Élise Bolduc: Data curation; investigation; methodology. Glen S. Brown: Investigation; writing – review and editing. Stephen C. Brown: Investigation; writing – review and editing. H. River Gates: Investigation; writing – review and editing. Olivier Gilg: Investigation; supervision; writing – review and editing. Marie-Andrée Giroux: Investigation; writing – review and editing. Kirsty Gurney: Investigation; writing – review and editing. Steve Kendall: Investigation; writing – review and editing. Eunbi Kwon: Investigation; writing – review and editing. Richard B. Lanctot: Investigation; writing – review and editing. David B. Lank: Investigation; writing – review and editing. Nicolas Lecomte: Investigation; writing – review and editing. Maria Leung: Investigation; writing – review and editing. Joseph R. Liebezeit: Investigation; writing – review and editing. R. I. Guy Morrison: Investigation; writing – review and editing. Erica Nol: Investigation; writing – review and editing. David C. Payer: Investigation; writing – review and editing. Donald Reid: Investigation; writing – review and editing. Daniel Ruthrauff: Investigation; writing – review and editing. Sarah T. Saalfeld: Investigation; writing – review and editing. Brett K. Sandercock: Investigation; writing – review and editing. Paul A. Smith: Investigation; writing – review and editing. Niels Martin Schmidt: Investigation; writing – review and editing. Ingrid Tulp: Investigation; writing – review and editing. David H. Ward: Investigation; writing – review and editing. Toke T. Høye: Conceptualization; investigation; methodology; supervision; writing – review and editing. Dominique Berteaux: Supervision; writing – review and editing. Joël Bêty: Conceptualization; investigation; methodology; project administration; supervision; writing – original draft.
ACKNOWLEDGMENTS
We express profound gratitude for the dedication and hard work of the countless individuals who contributed to the data collection for this study; their tireless efforts form the very foundation of this publication. Our field study was supported by the Fonds de recherche du Québec-Nature et Technologies (FRQNT), NSERC, the Canada Research Chair program, the Canadian Innovation Fund, ArcticNET, the Garfield Weston Award for Northern Research, Computational Biodiversity Science and Services training program (BIOS2), and the Northern Scientific Training Program of Polar Knowledge Canada. Logistical support was also provided by Polar Continental Shelf Program, Parks Canada, the Qikiqtaruk-Herschel Island Territorial Park and the Igloolik Hunters and Trappers Organization. Funding sources for individual study sites are listed in table below. We thank Jennie Rausch, Paul Woodard and Hans Schekkerman for sharing their data with us. We also thank Greenland Ecosystem Monitoring for providing access to arthropod data from Zackenberg. We acknowledge the support of the Government of Nunavut and Parks Canada for wildlife research permits. We are indebted to the support from Arctic communities and their Hunter and Trapping Organizations. We also thank the Barrow Arctic Science Consortium, and Umiaq, LLC. The findings and conclusions in this article are those of the author(s) and do not necessarily represent the views of the U.S. Fish and Wildlife Service. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the US Government.
| Akimiski Island | Ontario Ministry of Natural Resources, Arctic Goose Joint Venture, Government of Canada Program for International Polar Year |
| Alert | Canadian Wildlife Service (CWS) and the Canadian Forces |
| Bylot Island | Fonds Québécois de recherche sur la nature et les technologies (FQRNT), Natural Sciences and Engineering Research Council of Canada (NSERC) Northern Internship Program and Discovery Grant, the Garfield Weston Award for Northern Research, ArcticNet, Northern Ecosystem Initiatives, and the International Polar Year Project ArcticWOLVES. Logistical support was provided by the Polar Continental Shelf Project and Parks Canada |
| Cape Krusenstern | U.S. Fish and Wildlife Service (Region 7 Migratory Bird Management Division), University of Alaska Fairbanks, Murie Science and Learning Center Research Grants (National Park Service) |
| Canning River | U.S. Fish and Wildlife Service (Arctic National Wildlife Refuge), donors to Manomet, Inc. |
| Chipp River | U.S. Geological Survey Changing Arctic Ecosystem Initiative and the Wildlife Program of the USGS Ecosystem Mission Area |
| Churchill | NSERC Canada, Environment and Climate Change Canada Science Horizons program. Northern Scientific Training Grant Program, Trent University, National Science Foundation (U.S. #DDIG-1110444), Faucett Family Foundation, David and Lucile Packard Foundation, American Museum of Natural History, Cornell University, Cornell Lab of Ornithology, and Ducks Unlimited Canada. U.S. Fish and Wildlife Service (Region 7 Migratory Bird Management Division), University of Alaska Fairbanks, Murie Science and Learning Center Research Grants (National Park Service), Manomet Center for Conservation Science |
| Colville | U.S. Geological Survey Changing Arctic Ecosystem Initiative and the Wildlife Program of the USGS Ecosystem Mission Area |
| Herschel Island | NSERC Canada, Indigenous and Northern Affairs Canada, Polar Continental Shelf Program, Qikiqtaruk-Herschel Island Territorial Park, Aurora Research Institute, The W. Garfield Weston Foundation |
| Hochstetter Forland | French Polar Institute (IPEV; program 1036 “Interactions”), Agence Nationale de la Recherche (project PACS: ANR-21-CE02-0024) |
| Igloolik | Canada Research Program, National Science and Engineering Research Council of Canada (NSERC), EnviroNord (NSERC CREATE Training Program in Northern Environmental Sciences), Canadian Foundation for Innovation, New Brunswick Innovation fund to NL, ArcticNet to NL, Indigenous and Northern Affairs Canada, Government of Nunavut – Department of Environment, and Université de Moncton, Polar Continental Shelf Program, Government of Nunavut, the Hunters and Trappers Organization of Igloolik. Logistical support was provided by the Polar Continental Shelf Project |
| Ikpikpuk and Prudhoe | Alaska Department of Fish and Game Partner Program, Bureau of Land Management, Disney Conservation Awards, Kresge Foundation, Liz Claiborne/Art Ortenberg Foundation, U.S. Fish and Wildlife Avian Influenza Surveillance grant, and individual donors to the Wildlife Conservation Society |
| Mackenzie Delta | Environment and Climate Change Canada, Canadian Wildlife Service, Indigenous and Northern Affairs Canada, Cumulative Impacts Monitoring Program and Arctic Research Infrastructure Fund, and Manomet Inc. |
| Medusa Bay | Dutch Ministry of Agriculture and Nature Management and Food Safety (division DWK), Netherlands Organization for Scientific Research, European Science Foundation |
| Nome | Alaska Department of Fish and Game (State Wildlife Grant T-16) and National Science Foundation Office of Polar Programs (Award OPP-1023396) |
| Southampton Island | Environment and Climate Change Canada and the Polar Continental Shelf Program |
| Utqiaġvik | U.S. Fish and Wildlife Service (Region 7 Migratory Bird Management Division, Arctic Landscape Conservation Cooperative, and Alaska Region and National Avian Health Programs), National Fish and Wildlife Foundation, Neotropical Migratory Bird Conservation Act, Manomet, Inc., Minnesota State University Moorhead, North Dakota State University, University of Alaska Fairbanks, University of Colorado Denver, Kansas State University, University of Missouri Columbia |
| Zackenberg | Greenland Ecosystem Monitoring program and Danish Environmental Protection Agency |
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data supporting the results in the paper and scripts for data standardization and analyses are openly available at https://zenodo.org/doi/10.5281/zenodo.11214202. Arthropod trapping data for Colville River are openly available in the Alaska Science Center Data Repository at https://doi.org/10.5066/P9AMSIEJ. Arthropod trapping data for Utqiaġvik, Nome, Cape Krusenstern, Canning River, Prudhoe Bay, Ikpikpuk and Mackenzie Delta are openly available online in the Arctic Data Center at https://arcticdata.io/catalog/view/doi:10.18739/A2CD5M. Arthropod trapping data for Zackenberg are available on the Zackenberg Research Station website (https://g-e-m.dk/gemlocalities/zackenberg/data). Arthropod trapping data for Akimiski, Alert, Herschel and Bylot (2005–2008) are openly available in the Polar Data Catalogue at https://www.polardata.ca/pdcsearch/, references numbers are respectively 1736, 744, 750 and 721. Arthropod trapping data for Bylot (2010–2017) are openly available in NordicanaD repository at https://nordicana.cen.ulaval.ca/dpage.aspx?doi=45579CE-2262D40C8DFE49F0.




