Mycorrhizal associations relate to stable convergence in plant–microbial competition for nitrogen absorption under high nitrogen conditions
Abstract
Nitrogen (N) immobilization (Nim, including microbial N assimilation) and plant N uptake (PNU) are the two most important pathways of N retention in soils. The ratio of Nim to PNU (hereafter Nim:PNU ratio) generally reflects the degree of N limitation for plant growth in terrestrial ecosystems. However, the key factors driving the pattern of Nim:PNU ratio across global ecosystems remain unclear. Here, using a global data set of 1018 observations from 184 studies, we examined the relative importance of mycorrhizal associations, climate, plant, and soil properties on the Nim:PNU ratio across terrestrial ecosystems. Our results show that mycorrhizal fungi type (arbuscular mycorrhizal (AM) or ectomycorrhizal (EM) fungi) in combination with soil inorganic N mainly explain the global variation in the Nim:PNU ratio in terrestrial ecosystems. In AM fungi-associated ecosystems, the relationship between Nim and PNU displays a weaker negative correlation (r = −.06, p < .001), whereas there is a stronger positive correlation (r = .25, p < .001) in EM fungi-associated ecosystems. Our meta-analysis thus suggests that the AM-associated plants display a weak interaction with soil microorganisms for N absorption, while EM-associated plants cooperate with soil microorganisms. Furthermore, we find that the Nim:PNU ratio for both AM- and EM-associated ecosystems gradually converge around a stable value (13.8 ± 0.5 for AM- and 12.1 ± 1.2 for EM-associated ecosystems) under high soil inorganic N conditions. Our findings highlight the dependence of plant–microbial interaction for N absorption on both plant mycorrhizal association and soil inorganic N, with the stable convergence of the Nim:PNU ratio under high soil N conditions.
1 INTRODUCTION
It is well-known that plants and microorganisms compete for the limited N in the soil (Averill et al., 2014; Hodge et al., 2001; Kaye & Hart, 1997). This competition takes place through plant N uptake (PNU) and microbial N immobilization (Nim, including microbial N assimilation), which are among the most important processes of the N cycling in terrestrial ecosystems and have been estimated to be 330 and 1400 Tg N year−1, respectively (Chapin et al., 2011; Kuypers et al., 2018). As a plant physiological process, PNU largely determines ecosystem N-use efficiency and productivity, whereas Nim is mainly controlled by microbial activity and determines soil N retention to prevent leaching to water or emission into the atmosphere (Du et al., 2018; He et al., 2023). These two processes have been studied largely independently in the past (Fellbaum et al., 2012; Li et al., 2021), despite the importance of their interactions in determining the N cycling in terrestrial ecosystems (Deng et al., 2018; Zheng et al., 2022).
Traditionally, microorganisms are assumed to be the superior competitors over plants because of microbial-mediated soil mineralization of organic matter (Pastor et al., 1984; Schimel & Bennett, 2004). In line with this, a recent global synthesis revealed that soil microbes played a vital role in driving N mineralization in terrestrial ecosystems (Li et al., 2021). Ongoing rise in atmospheric CO2 concentrations may increase litter C:N ratios, augmenting microbial demand for inorganic N, and potentially intensifying the plant–microbial competition (Norby et al., 2010; Vitousek & Howarth, 1991). Most plants can overcome such competitive disadvantage by being able to consume organic N compounds directly via their roots or when in association with mycorrhizal symbionts, which partially bypasses the plant dependency on microbial-mediated inorganic N availability (Chalot & Brun, 1998; Näsholm et al., 1998). However, as mycorrhizal fungi can also compete with plants for soil organic N and contribute another pathway for N cycling in the soil, their association with plants makes it difficult to assess direct competition between plants and other soil microorganisms. Although increased external input (e.g., N deposition) and accelerated mineralization rates under climate warming may also enhance soil N availability and could partly relax the competition for soil N between plants and microorganisms (Du et al., 2021; Pold et al., 2017), it remains unclear what extent of N supply is required for such plant–microbial interactions.
Most of terrestrial plants form a symbiosis with either arbuscular mycorrhizal (AM) or ectomycorrhizal (EM) fungi to enhance their access to nutrients, and to overcome abiotic and biotic stresses (Averill et al., 2019; van der Heijden et al., 2015; Zhou, Zhou, Eldridge, et al., 2022; Zhou, Zhou, He, et al., 2022). Arbuscular mycorrhizal fungi are monophyletic, species-poor groups (0.8%–3.2% of estimated mycorrhiza species) but associate with more than 71% of plant species, with their hyphae capable of penetrating the cell wall and cell membrane within the host plant roots (Brundrett, 2009; Smith & Read, 2008). Ectomycorrhizal fungi are relatively species-rich groups (40%–50% of total estimated mycorrhiza species) while associated with less than 2% of plant species, with their hyphae unable to penetrate individual root cells (Averill & Hawkes, 2016; Brundrett, 2009; Cheng et al., 2016). Plants associating with AM fungi (hereafter AM plants for brevity) acquire N primarily through their roots and through hyphal foraging of N that is released by the microbial mineralization of foliage litter and other organic matter (Carrara et al., 2021; Phillips et al., 2013). Given that most AM fungi have limited saprotrophic abilities and inorganic N forms are relatively mobile in soils, it is suggested that AM plants primarily utilize inorganic N forms (Smith & Smith, 2011). Conversely, EM-associated plants (hereafter EM plants) acquire N by allocating C belowground to both free-living and symbiotic rhizosphere microbes to stimulate the production of extracellular enzymes, which are used to mine organic N from organic matter from soil and litter (Brzostek et al., 2015; Phillips et al., 2013).
The AM- and EM-dominated ecosystems vary in how they optimize N acquisition to maintain N retention (Carrara et al., 2021; Guo et al., 2022; Henriksson et al., 2021). Under the limited N conditions, EM plants typically allocate more C to free-living and symbiotic rhizosphere microorganisms in the soil to support PNU than AM plants, which allows these fungi to immobilize a greater proportion of N from their surroundings and may subsequently deprive plants with access to soil N- at least in the short term (Alberton et al., 2007; Corrêa et al., 2008; Franklin et al., 2014). Moreover, EM fungi also degrade organic N compounds more efficiently than AM fungi by producing extracellular enzymes and are therefore able to supply host plants with more inorganic N (Argiroff et al., 2022; Li et al., 2024; Terrer et al., 2016). On the contrary, both AM and EM plants tend to reduce belowground C investment in soil microorganisms under enhanced N availability (Carrara et al., 2018; Janssens et al., 2010; Zhang et al., 2023). Specifically, EM plants reduce the C investment of N acquisition from organic N mining toward root foraging of inorganic N, whereas AM plants tend to shift their mycorrhizal-based to root-based inorganic N foraging at enhanced N supply (Carrara et al., 2021; Lambers et al., 2008; Phillips et al., 2013).
In this study, we assembled a global database of the Nim:PNU ratio through compiling the available data of Nim and PNU across terrestrial ecosystems dominated either by AM or EM plants (1018 observations from 184 studies, Figure S1). We further collected climatic, soil properties, and plant trait information (including mycorrhizal associations) from respective studies to explain the variation in the Nim:PNU ratio. We specifically examine the relative importance of mycorrhizal associations, climate, plant, and soil properties on the Nim:PNU ratio in terrestrial ecosystems. Using our database, we construct a global map of the Nim:PNU ratio in terrestrial ecosystems to understand geographic patterns of plant–microbial interactions for acquiring soil N. We also hypothesized that (1) we expect negative correlations between PNU and Nim in AM fungi-associated ecosystems while positive correlations in EM fungi-associated ecosystems, and (2) we expect that the Nim:PNU ratio would gradually decrease at low soil N content and stabilize at high soil N content for both AM- and EM-dominated ecosystems.
2 MATERIALS AND METHODS
2.1 Data collection
Meanwhile, we also collected data for site location (longitude and latitude), soil properties (pH, texture, bulk density, organic C content, total N, and C: N ratio), plant properties (vegetation type, leaf area index [LAI], and NPP), and microbial biomass C, N, and C: N (MBC, MBN, and MCN). Mean values were taken from tables or extracted from figures using the GetData software (version 2.22). We extracted those variables based on the empirical reports of the reference studies with the highest priority. The rest missing data were filled from the public database based on location. That is, vegetation type, LAI, and NPP were extracted from MODIS at 1 ◊ 1 km resolution, whereas soil pH, total N, soil textures, soil organic C, and bulk density were extracted from SoilGrids at 250 ◊ 250 m resolution and at 0–30 cm depth, and finally, MBC and MBN were extracted from Gao et al. (2022) at 1 ◊ 1 km resolution and also at 0–30 cm depth. We also extracted mean annual temperature (MAT) and mean annual precipitation (MAP) from the WorldClim database (www.worldclim.org/data/bioclim.html) using the geographical coordinates of the study sites. We assigned mycorrhizal association of an ecosystem based on: (1) the empirical reports in the reference studies; (2) the proportion of dominant plant species >70%, which were assigned to mycorrhizal type using a FungalRoot database of Wang and Qiu (2006) and Soudzilovskaia et al. (2020); and (3) for those tree species that may be dually colonized by both AM and EM fungi, we relied on expert opinions and checked the dominant mycorrhizal types in the FungalRoot database, to classify them to AM or EM.
In this study, we classified four ecosystems: cropland, forest, grassland, and ‘other’ ecosystems. AM types include 638, 153, 96, and 12 data sets for cropland, forest, grassland, and other ecosystems, respectively. EM types include 82, 21, and 16 data sets for forest, grassland, and other ecosystems, respectively; there were no data for cropland. As studies of dominant plants associated with non-mycorrhizal fungi were too few among the searched studies to conduct any meaningful analysis, we only focused on AM and EM ecosystems.
To predict global patterns of the Nim:PNU ratio, we collected the global mycorrhizal plant distribution, which were obtained from Soudzilovskaia et al. (2020). Meteorological data included gridded MAT and MAP, which were obtained from WorldClim 2.0 (Fick & Hijmans, 2017). The soil properties, including texture (clay, silt, and sand contents), soil organic carbon (SOC), soil pH, total nitrogen (TN), the ratio between soil carbon and nitrogen (soil C:N), and bulk density (BD), were obtained from the SoilGrids (Hengl et al., 2017). All soil properties were averaged over a soil depth of 1–30 cm. Growing-season gridded leaf area index (LAI), net primary productivity (NPP), normalized difference vegetation index (NDVI), and vegetation type were from the NASA's Earth Observatory Team (MOD15 product). LAI, NPP, and NDVI were averaged into annual means. Global MBC and MBN were obtained from a global database (Gao et al., 2022). Global SIN data were taken from the mean value of eight models from CMIP6 (ACCESS-ESM, AWI-ESM, CESM2, CMCC-CM2-SR5, CMCC-ESM2, MPI-ESM-1-2-HAM, MPI-ESM1-2-LR, NorESM2-MM), since there was no data from SoilGrids. We selected the model outputs from the period of 2000–2015, which were then averaged into annual means. All the gridded data were aggregated to 1 km × 1 km spatial resolution (Table S1).
2.2 Data analysis
To prepare the datasets for statistical analysis, we averaged the values of Nim and PNU of the dominant vegetation type according to mycorrhizal type (i.e., AM or EM) for each site if more than one vegetation type were reported. If measurements for the same site were reported for more than 1 year, the Nim and PNU were also averaged across the years. Site-level Nim:PNU ratio was calculated as an indicator of N competition between plants and soil microorganisms in the ecosystem. The best situation is that both Nim and PNU data occurred at the same sites with the similar N condition. However, the original observations of PNU and Nim presented in the database did not meet this best condition. We therefore supplemented the Nim and PNU estimates as follows: (i) Variable selection: a generalized variance-inflation factor (GVIF) and a linear model were used to select the best-explaining variables from the possible variables (i.e., climate, plant, and soil properties) for Nim and PNU, respectively. (ii) Model selection: five machine learning approaches (i.e., multiple linear regression [MLR], k-nearest neighbors [KNN], support vector regression [SVR], random forest [RF], and artificial neural networks [ANN]) were used to separately estimate Nim and PNU, with randomly select 70% of observations for training and the rest 30% observation for testing (Deng et al., 2018; Du et al., 2020). By comparison of cross-validated R2 and root mean square error (RMSE) of the five model, we selected RF model as the best model for Nim and PNU estimation. (iii) Model validation: based on the selected RF model, we estimated the relative importance of the predictor variables by 1000 parallel runs of RF model for PNU and Nim, respectively, and selected the RF model with both the highest R2 (0.90 and 0.88) and lowest RMSE (0.084 and 31.7) to validate data for estimation of Nim and PNU (Figure S4). (iv) Upscaling: the selected RF model was used to predict the global Nim and PNU, and then calculated the Nim:PNU ratio at a 1 km × 1 km resolution. The coefficient of variation (CV) of the predicted Nim:PNU ratio was calculated by 100 parallel runs of RF model.
To select predictors in explaining the variation of Nim:PNU ratio, we adopted a generalized variance factor (GVIF) to check the multicollinearity among the potential predictors (Table S2a). We also examined the correlation between the Nim:PNU ratio and the potential parameters to select the predictors (Table S2b). A random-forest approach in the context of meta-analysis (meta-forest; R package: meta-forest) was used to identify the importance of selected predictors (Terrer et al., 2021; Zhou, Zhou, Eldridge, et al., 2022; Zhou, Zhou, He, et al., 2022). Specifically, the meta-forest model was trained with preselected predictors- and then calculated the importance of the variable in explaining the variation in the Nim:PNU ratio. We further quantified how the importance of these predictors of the Nim:PNU ratio varied at low and high N conditions. The low and high N categorization of a study site was based on soil characteristics and occasional fertilizer treatments, following the approach by Vicca et al. (2012) and Terrer et al. (2021). Specifically, we used the five most important indicators (soil inorganic N, vegetation type, site history, and fertilizer's intensity) to assign high N condition and the low N condition (including low and medium). In our manuscript, we combined “low and medium” into low N condition, since medium N condition is difficult to assign. We also added an indication of the certainty level of the classification of each data set and only when the certainty level >1 which was assigned as high N condition. Ultimately, 204 AM- and 19 EM-observations were assigned to high N condition, and the rest is assigned to low N condition (Tables S3 and S4). To examine the differences of the Nim:PNU ratio between AM- and EM-dominated ecosystems for total data, low and high N nitrogen conditions, we also checked the residual distribution and selected both Wilcoxon rank-sum test and t-test based on normal Q-Q plots (Figure S5). The results showed a significant difference in the Nim:PNU ratio between AM- and EM-associated ecosystems for total data and low N conditions (p > .001), while there was no statistical difference under high N conditions (p < .05; Figure S6; Table S3).
Structural equation model (SEM) was used to evaluate the direct and indirect relationships of the Nim:PNU ratio with SIN, climate, plant, soil, and microbial properties. Owing to the strong correlations among the factors within each group, we conducted principal component analysis (PCA) to create a multivariate functional index before implementing the model structure for the SEM (Chen, Liu, et al., 2019; Chen, Swenson, et al., 2019). The first component (PC1) of the five combined group properties (i.e., climate, microbe, soil, plant, and SIN, Figure S7) were then introduced as a new variable to the subsequent analysis. The goodness of fit of the final SEM model was evaluated using the chi-squared test and the root mean squared error of approximation. The SEM analyses were conducted using AMOS 21.0 (Amos Development Corporation, Chicago, IL, USA).
3 RESULTS
3.1 Factors driving the spatial variability of the global Nim:PNU ratio
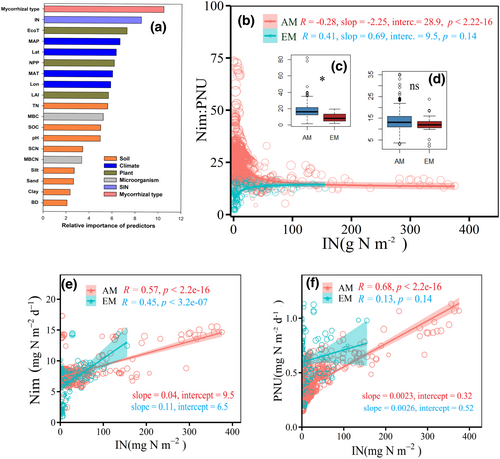
Across a broad range of factors considered in this synthesis, the mycorrhizal type was the most important factor in determining the variance in the Nim:PNU ratio (relative importance = 21.8%; Figure 1a). We found that the Nim:PNU ratio significantly decreased from 16.8 ± 0.5 to 13.8 ± 0.7 from low soil inorganic nitrogen (SIN) to high SIN conditions by an exponential decay function (slope = −2.25, intercept = 25.5, p < .001) in AM-dominated ecosystems, whereas this ratio increased from 9.22 ± 1.0 to 12.1 ± 1.2 by an exponential function (slope = 0.69, intercept = 9.5, p < .001) in EM-dominated ecosystems (Figure 1; Figure S6).This contrasting trend in the Nim:PNU ratio was likely due to a lower rate of increase in Nim in AM-dominated ecosystems (slope = 0.04, p < .001) compared with EM-dominated ecosystems (slope = 0.11, p < .001), while there was no significant difference in PNU (slope = 0.0023 vs. 0.0026, p > .05) between in EM- and AM-dominated ecosystems (Figure 1e,f).
3.2 Divergent N acquisition strategies between AM- and EM-dominated ecosystems regulated by soil inorganic N
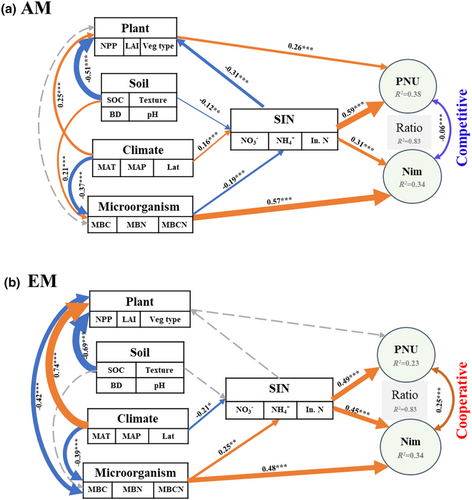
For both AM- and EM-dominated ecosystems, structural equation model (SEM) analysis revealed that SIN was the most important factor in driving the variation in the Nmin:PNU ratio through its direct effects on both PNU and Nim (Figure 2). In AM-dominated ecosystems, both plant (r = −0.31, p < .001) and microbial variables (r = −0.19, p < .001) used in SEM were negatively correlated with soil inorganic N, whereas the relation between PNU and Nim displayed a slight negative correlation (r = −0.06, p < .001). These correlations are indicative of a weak competition for soil N between plants and microorganisms in AM-dominated ecosystems (Figure 2a). Conversely, we found a positive correlation (r = 0.25, p < .001) between PNU and Nim in EM-dominated ecosystems (Figure 2b) indicative of cooperative interactions between plants and microorganisms for soil N. Sensitivity analysis showed that the Nim:PNU ratios were positively correlated with microbial C:N ratio in AM-dominated ecosystems, while negatively related with microbial C and microbial C:N ratio in EM-dominated ones (Figure S7).
The Nim:PNU ratio changed from low to high SIN conditions in both AM- and EM-dominated ecosystems, albeit variably (Figures 1 and 3). Specifically, the greater decrease in the Nim:PNU ratio along the soil N gradient was mainly due to the faster increase in PNU (+76%) than Nim (+26%) in AM fungal-dominated ecosystems (Figure 3; Figures S10 and S11). In EM fungal-dominated ecosystems, a relatively smaller decrease in the Nim:PNU ratio was due to a faster increase in Nim (+40%) than PNU (+5%) with increasing SIN (Figure 3; Figures S10 and S11).
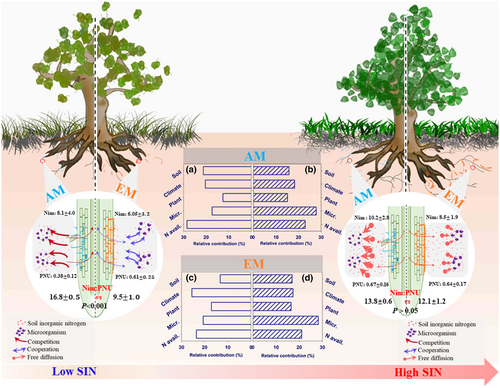
With the increase in soil N, the relative contributions of microbial properties (namely MBC, MBN, and MBCN) on the Nim:PNU ratio increased (+37% and +60%), and the contribution of climatic variables decreased (−32% and −10%) in both AM and EM fungal-dominated ecosystems (Figure 3; Figure S12). The effects of plant properties (namely NPP, LAI, and Vegetation type) on the Nim:PNU ratio increased in AM-dominated ecosystems (+20%) but decreased in EM-dominated ecosystems (−3.4%). By contrast, the effects of soil properties (namely SOC, BD, texture, and pH) on the Nim:PNU ratio decreased in AM-dominated ecosystems (−25%), whereas it increased in EM-dominated ecosystems (+27%).
3.3 Global pattern of Nim:PNU ratio for AM- and EM-dominated ecosystems
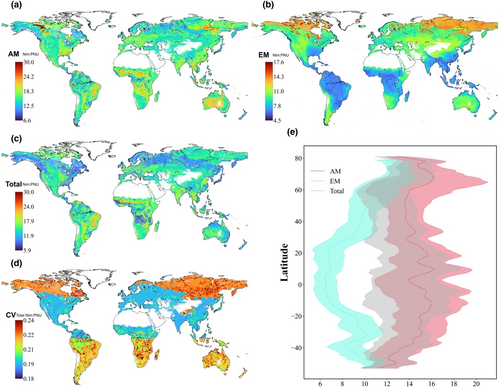
The mean Nim:PNU ratio across terrestrial ecosystems is 13.2 ± 2.8, with 14.7 ± 3.2 for AM fungal-dominated ecosystems and 9.8 ± 2.6 for EM fungal-dominated ecosystems (Figure 4). Specifically, the EM-dominated ecosystems from low-latitude regions showed a lower Nim:PNU ratio compared with AM-dominated ecosystems. There is more uncertainty for those area of South America, Africa, Northern Canada, and most of Russia, where there were only few (or no) data sites in the global map (Figure 4e).
4 DISCUSSION
4.1 Different patterns of Nim:PNU ratio in low and high SIN conditions between AM- and EM-dominated ecosystems
The Nim:PNU ratio was significantly different between AM- and EM-dominated ecosystems under low SIN conditions, but converged under high SIN conditions. Several underlying mechanisms may contribute to this opposite pattern under low N content and to the convergence under high N content. First, in EM-dominated ecosystems, most microbes (including EM fungi) may rely on plants supplying belowground C to meet their stoichiometric requirements for their growth (Franklin et al., 2014; Näsholm et al., 2013). In limited N condition, host plants often supply their microbial partner with C, and even increase the C investment to maintain N demand for plants (Näsholm et al., 2013; Zak et al., 2019). This cooperative interaction may explain why EM fungal-associated plants are able to sustain a higher PNU rate compared with AM fungal-associated plants under low SIN conditions (Figure 1j). Furthermore, the relatively low contribution of plant properties in explaining the variation in the Nim:PNU ratios compared with soil inorganic N (Figure 3; Figures S10–S12) indicates that the nutrient acquisition strategies in EM-dominated ecosystems (including plants and microorganisms) may shift from organic N mining toward the foraging of inorganic N by soil microorganisms as increasing SIN (Carrara et al., 2021). In comparison with EM-dominated ecosystems, the relative importance of plant properties in explaining the variation in the Nim:PNU ratio was higher at high N concentration in AM-dominated ecosystems (Figure 3), implying that nutrient acquisition strategies from the inorganic N foraging through mycorrhizae turn to direct foraging by roots from low to high soil N conditions (Phillips et al., 2013; Tedersoo & Bahram, 2019).
Second, differences in litter quality between AM and EM fungal-associated plants may also have influenced how the Nim:PNU ratio responded to soil N enrichment (Hodge et al., 2001; Lin et al., 2017). Several studies have reported that AM fungal-associated plants produced litters that decomposed faster than those of EM fungal-associated plants (Barceló et al., 2022; Carrara et al., 2021; Smith & Wan, 2019). Such fast-decaying plant litters are known to promote the formation of stable mineral-associated organic matter (Cotrufo et al., 2013; Terrer et al., 2021). However, much of the organic N may be difficult to be absorbed by both AM fungi and fungal-associated plants, whereas it should be accessible to EM fungal-associated plants and microorganisms including EM fungal species, most likely due to their ability to produce specialized enzymes for N acquisition (Sokol & Bradford, 2019; Terrer et al., 2021).
Third, the economics of trading partnerships with the inherent stoichiometry may also partly explain the different patterns of the Nim:PNU ratio between AM- and EM-dominated ecosystems under low SIN conditions (Hodge et al., 2001; Kaye & Hart, 1997). In the N-limited environments, N exchanges are costly, which then force host plants to maximize their return of investment in the N supply by their partners, subsequently causing an adjustment in their nutrient acquisition strategies (Kiers et al., 2011). This may explain why the Nim:PNU ratio at low SIN differ between AM- and EM-dominated ecosystems, and the Nim:PNU ratio was negatively correlated with microbial C:N ratio (MBCN) in AM-associated ecosystems while positively correlated with MBCN in EM-associated ones (Figure 2; Figures S7 and S8). However, as soil N becomes more available, a stable and homogenized state for a multi-partner (e.g., plants and mycorrhizal fungi) is achieved when individuals allocate resources among symbionts in proportion to the relative benefits they receive from their partners (Averill et al., 2022; Henriksson et al., 2021). This means that the N absorption capacity with plant–microbial interaction divided by mycorrhizal association decreases in high SIN, and thereby alter N cycling through perhaps lower microbial diversity or more active N diffusion (Figure 3; Read, 1991; Hodge et al., 2000).
4.2 Different interactions between plants and microbes for N absorption between AM- and EM-dominated systems
Both plant and microbial properties were significantly associated with SIN in our analysis, but also led to contrasting plant–microbial interactions between AM and EM-dominated ecosystems (Figure 2). For the EM-dominated ecosystems, our results showed that the PNU efficiency positively correlated with Nim (Figure 2b), indicating a cooperative relationship between plants and soil microorganisms for the N absorption. It may be due to the C and N trade between plants and microorganisms to degrade organic matter in the rhizosphere (Deng et al., 2018). Plants need to invest a substantial amount of belowground C to produce extracellular enzymes to prime microbial decomposition of organic matter, thus increasing the N supply for both plants and microorganisms in EM-dominated ecosystems (Brzostek et al., 2015; Hobbie et al., 2006; Yin et al., 2014). This may also explain why soil organic matter significantly affects plant N uptake (Figure S5) and may accelerate SOC losses in EM-dominated ecosystems (Terrer et al., 2021). Although EM fungi can mobilize more stable soil organic N and thereby promote N cycling in ecosystems, a major fraction of absorbed N may be used for their own growth and transfer only marginal amounts of N to their hosts, especially under conditions of high C supply (Näsholm et al., 2013). In this case, soil microbes (including EM fungi) drive the observed increase in the Nim:PNU ratio across SIN concentrations that could be driven by efficient N immobilization (Figure 1c; Figure S4). However, as plants have long life spans relative to soil microorganisms, they would ultimately benefit from the high N fluxes due to microbial turnover. This may be the reason that microbial biomass positively correlates with the SIN content (Figure 2), and microbial N content plays the most important factor for both PNU and Nim under high SIN concentration in EM-dominated ecosystems (Figures S9 and S10).
In AM-dominated ecosystems, a weak negative correlation between PNU and Nim is indicative of a neutral interaction or potentially weak competition for N absorption between plants and soil microorganisms, especially for that with limited soil N conditions (Figure 2). The higher growth rates and litter quality of AM fungal-associated plants lead to faster litter decomposition and rapid N loss in AM-dominated ecosystems (e.g., N leaching; Nuccio et al., 2013; Lin et al., 2016). Thus, there are negative correlations of SIN with both plant properties (related to growth rate and litter quality) and soil microorganisms (related to litter decomposition) in AM fungal-dominated ecosystems (Figure 2). In addition, AM-dominated ecosystems are generally equipped with less specialized enzymes for N acquisition, and the organic N in stable mineral-associated organic matter is inaccessible to AM-associated plants or their associated mycorrhizae (N is tightly protected in organic forms; Carrara et al., 2021; Phillips et al., 2013; Smith & Smith, 2011). Those effects would presumably aggravate N limitation in low SIN conditions, which could increase competitive interactions between plants and soil microorganisms for N absorption in AM-dominated ecosystems.
4.3 Limitation and implications
Our study reveals different patterns of plant–microbial interactions for N absorption between AM- and EM-dominated ecosystems. Nevertheless, we acknowledge that analysis based on empirical data, especially when a proportion of data have undergone a gap-filling process, have biases associated with the sampling and upscaling processes, such as due to variability in spatial resolution of the data. First, we lacked the real matched Nim and PNU measurement data in natural conditions, which might introduce some uncertainties in mapping global Nim:PNU ratio. Uncertainty analysis as shown by the coefficient of variation (CV) illustrated the greater uncertainty in high latitude of the Northern Hemisphere as well as South America and Africa in the Southern Hemisphere, since few studies had been done there (Figure 4d; Figure S1). Second, although we verified that the unbalanced data did not influence the reliability of results (Figure S8), the sample sizes of PNU and SIN measurements for EM-dominated ecosystems were very limited (n = 119) compared with those for AM-dominated ones, which may impede our understanding of whether the pattern of Nim:PNU ratio in EM-dominated ecosystems are suitable in a wider range of soil N conditions. Future studies deserve to perform the matched measurements of Nim and PNU and to increase the experiments in EM-dominated ecosystems.
Our findings highlight the importance of mycorrhizal association and soil inorganic N on the Nim:PNU ratio, which may provide some important implications for model development and ecosystem management to assess plant–microbial interactions for N absorption in a N-enriched world. First, the relationships between plants and soil microorganisms for N absorption lead to the different patterns of Nim:PNU ratio along the SIN content in the two mycorrhizal-associated ecosystems. Such distinct findings among AM- and EM-dominated ecosystems can be valuable for the development of terrestrial C cycling models to accurately predict the nutrient limitation under global environmental changes. Second, we found the Nim:PNU ratio for both AM- and EM-associated ecosystems gradually converged around a stable value under high SIN conditions, suggesting that plant–microbial interaction for N absorption both equalizes and stabilizes at increased N supply. This may be important for tree species selection in forest restoration under different nutrient conditions. Third, the Nim:PNU ratio may offer a valuable index of N status for both AM- and EM-associated ecosystems, which could be incorporated into land surface model to improve the prediction of C cycle-climate feedback.
In summary, our study reveals a general pattern of weak competition for N between AM fungal-associated plants and microorganisms, whereas EM fungal-associated plants cooperate with microorganisms for N. Plant–microbial interactions measured as the Nim:PNU ratio further converge in both AM- and EM fungal-dominated ecosystems where SIN content is high. Moreover, divergent patterns of plant–microbial interactions in AM and EM-dominated ecosystems under low N conditions reflect the optimal nutrient absorption strategies between plants and soil microorganisms, which is likely to disappear in fertilized systems with potential consequences of soil N dynamics. Future research combined with gene sequencing and isotopic tracer technique (e.g., stable isotopic probing) can provide more information on nutrient transfer pathways between plants and soil microorganisms across different mycorrhizal-associated plants, which would be key to elucidating the relationship between plant and soil microorganisms in terrestrial ecosystems under climate change.
AUTHOR CONTRIBUTIONS
Zhenggang Du: Conceptualization; data curation; formal analysis; funding acquisition; methodology; project administration; writing – original draft; writing – review and editing. Lingyan Zhou: Formal analysis; methodology; writing – review and editing. Madhav P. Thakur: Validation; writing – review and editing. Guiyao Zhou: Methodology; validation; writing – review and editing. Yuling Fu: Validation; writing – review and editing. Nan Li: Data curation; methodology. Ruiqiang Liu: Validation; writing – review and editing. Yanghui He: Validation; writing – review and editing. Hongyang Chen: Validation; writing – review and editing. Jie Li: Validation; writing – review and editing. Huimin Zhou: Data curation; methodology. Ming Li: Data curation; methodology. Meng Lu: Data curation; methodology. Xuhui Zhou: Conceptualization; funding acquisition; project administration; validation; writing – original draft; writing – review and editing.
ACKNOWLEDGMENTS
The authors thank the three anonymous reviewers for their insightful comments and suggestions. We also thank all the researchers whose published research made this meta-analysis possible. This research was financially supported by the National Natural Science Foundation of China (grant nos. 32241032, 31930072, 42261144688, 32271713, 32071593, and 42203076), and the Fundamental Research Funds for the Central Universities (grant no. 2572022BA08). MPT acknowledges the support from the Swiss State Secretariat for Education, Research and Innovation (SERI) under contract number: M822.00029. G. Zhou acknowledges the support from Ramón y Cajal project (RYC2022-035226-I) funded by the Spanish Ministry of Science and Innovation, the NextGenerationEU program of the European Union (MICIU/AEI/10.13039/501100011033 y el FSE+), and AYUDAS DE EXCELENCIA RYC-MAX 2023 project from Spanish National Research Council.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in “figshare” at http://doi.org/10.6084/m9.figshare.25763862.




