Distinct responses to warming within picoplankton communities across an environmental gradient
Abstract
Picophytoplankton are a ubiquitous component of marine plankton communities and are expected to be favored by global increases in seawater temperature and stratification associated with climate change. Eukaryotic and prokaryotic picophytoplankton have distinct ecology, and global models predict that the two groups will respond differently to future climate scenarios. At a nearshore observatory on the Northeast US Shelf, however, decades of year-round monitoring have shown these two groups to be highly synchronized in their responses to environmental variability. To reconcile the differences between regional and global predictions for picophytoplankton dynamics, we here investigate the picophytoplankton community across the continental shelf gradient from the nearshore observatory to the continental slope. We analyze flow cytometry data from 22 research cruises, comparing the response of picoeukaryote and Synechococcus communities to environmental variability across time and space. We find that the mechanisms controlling picophytoplankton abundance differ across taxa, season, and distance from shore. Like the prokaryote, Synechococcus, picoeukaryote division rates are limited nearshore by low temperatures in winter and spring, and higher temperatures offshore lead to an earlier spring bloom. Unlike Synechococcus, picoeukaryote concentration in summer decreases dramatically in offshore surface waters and exhibits deeper subsurface maxima. The offshore picoeukaryote community appears to be nutrient limited in the summer and subject to much greater loss rates than Synechococcus. This work both produces and demonstrates the necessity of taxon- and site-specific knowledge for accurately predicting the responses of picophytoplankton to ongoing environmental change.
1 INTRODUCTION
Marine picophytoplankton are an abundant and diverse group of photosynthetic cells that are estimated to contribute up to 25% of global marine primary production (Flombaum et al., 2013; Fogg, 1986). Relative to larger phytoplankton, these smaller cells dominate in the warm, oligotrophic regions that make up most of the surface ocean. Increases in temperature and stratification associated with climate change are expected to favor the picophytoplankton over larger cells (Flombaum et al., 2020; Henson et al., 2021). The subsequent compositional changes in the plankton community are expected to have largely negative ecological and economic consequences, contributing to food web “degradation” and reducing yield from marine fisheries (Cheung et al., 2011; Schmidt et al., 1998, 2020). Global predictions about picophytoplankton are, however, largely derived from patterns in the open ocean, whereas the majority of the world's fisheries rely on coastal waters (Watson et al., 2016). Detailed knowledge of coastal picophytoplankton ecology is therefore critical for evaluating these predictions as they relate to ecosystems of economic interest.
The Northeast US Shelf (NES) is one example of a rapidly changing coastal ecosystem that supports a diversity of economically and culturally important fisheries (Balch et al., 2022; Chen et al., 2020; Mills et al., 2013; Shearman & Lentz, 2010). Since 2003, the picophytoplankton community within this ecosystem has been monitored at the Martha's Vineyard Coastal Observatory (MVCO) via autonomous in situ flow cytometry (Olson et al., 2003). This community can be divided into Synechococcus and picoeukaryotes, with Prochlorococcus only present offshore. Of these groups, picoeukaryotes are less abundant but reproduce and die more rapidly, playing a distinct and important role within the ecosystem (Fowler et al., 2020). The ecological consequences of increasing picophytoplankton abundance likely depend on any compositional changes that occur within the picophytoplankton community (Massana & Logares, 2013).
At MVCO, Synechococcus and the photosynthetic picoeukaryotes are highly synchronized in their responses to seasonal environmental variability. While different in magnitude, the annual cycles in cell concentration for these two groups are very similar in shape (Fowler et al., 2020). Daily cell division rate for both groups increases with temperature in the spring and decreases with light availability in the fall (Fowler et al., 2020; Hunter-Cevera et al., 2014, 2019) and both communities exhibit a positive relationship between temperature and cell concentration (Figure 1a,b). These findings at the nearshore site might lead us to predict that the two groups of picophytoplankton will have synchronized responses to other sources of environmental variability.
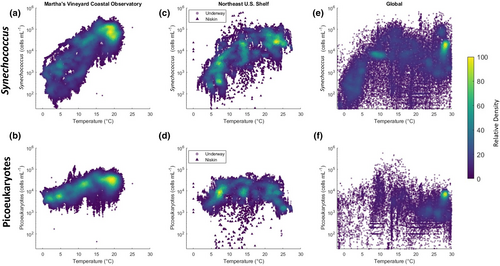
Global analyses, in contrast, suggest that these two groups will have divergent responses to climate change. Niche correlation models fit to a global dataset predict that Synechococcus will be supported at higher abundances under climate change scenarios, while the abundance of picoeukaryotes will decrease (Flombaum et al., 2020; Flombaum & Martiny, 2021). Why are the nearshore communities of Synechococcus and photosynthetic picoeukaryotes synchronized, when global data indicate the opposite? Answering this question will reveal the conditions under which the predicted global changes in picophytoplankton community composition may or may not extend to coastal ecosystems.
The difference between the trends in the coastal and global datasets seems to lie within the picoeukaryote dynamics. For Synechococcus, the positive relationship between temperature and cell concentration on the NES agrees well with global and open ocean observations (Flombaum et al., 2013; Stevens, Crockford, et al., 2023) while for photosynthetic picoeukaryotes, this relationship is more complicated (Figure 1). Thus, we here focus on the photosynthetic picoeukaryotes and explore the extent to which nearshore dynamics are representative of the broader continental shelf. We make use of flow cytometry data from 22 cruises on the NES and compare the responses of picoeukaryote and Synechococcus communities to environmental variability across seasons, latitude, and depth. We identify the conditions under which the two groups have either synchronized or divergent responses to changes in their environment. Lastly, we compare the realized niche of photosynthetic picoeukaryotes on the NES to that predicted by the niche correlation model of Flombaum et al. (2020) trained on a compilation of global observations. This work improves our understanding of the mechanisms that control picophytoplankton abundance across the gradient from coast to open ocean, and helps to reconcile the differences between regional and global predictions for picophytoplankton responses to ongoing environmental change.
2 METHODS
We analyzed data collected from 22 NES Long-Term Ecological Research (LTER) cruises between 2018 and 2022 (Stevens, Sosik, et al., 2023; Table S1). Cruises occurred in all seasons and transited from the coast to the continental slope primarily along the 150-km NES-LTER focal transect on the 70° 53′W longitude line. Surface water was sampled automatically from the underway science seawater flow every 2 min, and live picophytoplankton cells were counted by an Attune NxT Flow Cytometer (ThermoFisher) as described in Stevens, Crockford, et al. (2023). All particles within the samples were automatically classified according to their fluorescence and scatter characteristics (Sosik et al., 2003). Cells within these samples can be identified as eukaryotic phytoplankton, which have a red fluorescence signal (chlorophyll a), or Synechococcus, which have an orange fluorescence signal (phycoerythrin) as well as a red fluorescence signal. The picoplankton can be distinguished from larger cells on the basis of their scatter signature (Sosik et al., 2003). For this work, we focus our analysis on the red fluorescing picoplankton, which we will refer to as photosynthetic picoeukaryotes or simply picoeukaryotes. We include in our analysis 61,113 0.4-mL samples within the study region (Figure 2a). The corresponding underway measurements of Synechococcus have been described by Stevens, Crockford, et al. (2023). Comparable flow cytometry observations are also available from nearby MVCO (41°19.500′ N, 70°34.0′ W), where the dynamics of Synechococcus and picoeukaryotes have been described by Hunter-Cevera, Neubert, et al. (2016), Hunter-Cevera et al. (2019) and Fowler et al. (2020). Throughout our analysis, we will compare to and reference these previous works in order to provide necessary context.
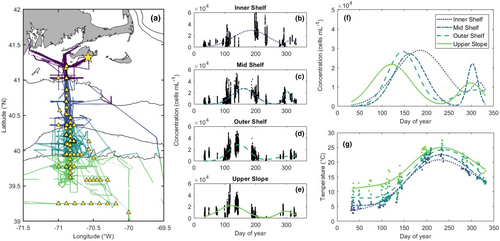
On 12 of the cruises, at designated NES-LTER stations, we analyzed additional subsurface flow cytometry samples from Niskin bottles on a CTD rosette (Figure 2a; Table S1). These samples were collected into 2-mL cryovials and preserved with glutaraldehyde at a final concentration of 0.125%. Starting in May 2019, Kolliphor-188 (previously Pluronic F68) was also added to samples at a final concentration of 0.01% following Marie et al. (2014). Samples were frozen in liquid nitrogen until they were returned to the shore-based laboratory for processing on the same flow cytometer that measures the underway samples while at sea. Again, cells were identified based on their scattering, phycoerythrin, and chlorophyll fluorescence signatures. More details on the instrument configuration and discrete sample processing are described in the metadata to the publicly available data package (Peacock et al., 2024).
Environmental and meteorological data, including seawater temperature, salinity, and incident solar radiation, were collected by each vessel while underway and from the CTD casts. Dissolved inorganic nutrients (nitrate plus nitrite, ammonium, silicate, and phosphate) were also measured from Niskin bottles as described in Sosik et al. (2021) and Marrec et al. (2021). For cruises with both underway and cast data, underway flow cytometry samples were associated with estimates of surface nutrient concentration by linearly interpolating across longitude and latitude from nutrient measurements made at depths less than 10 m.
We estimated picophytoplankton division rate by analyzing daily cycles in cell size within the underway flow cytometry data. Scattering signals were converted into cell volume estimates according to an empirically derived relationship from independently sized phytoplankton cultures (Archibald, 2021). Picophytoplankton cells generally grow while sunlight is available and decrease in size through division. We detected these changes in size and used them to estimate picoeukaryote division rate via a matrix model as described in Fowler et al. (2020). This method has previously been applied to flow cytometry data from both stationary (Dugenne et al., 2014; Hunter-Cevera et al., 2019; Sosik et al., 2003) and underway (Ribalet et al., 2015; Stevens, Crockford, et al., 2023) observations. As in Stevens, Crockford, et al. (2023), we report the division rates for 24-h periods beginning at dawn, and use a sliding window to quantify the sensitivity of each estimate to any discontinuities that result from patchiness or the movement of the vessel. Daily division rates are understood to be a summary of the activity of what is likely to be a diverse assemblage of picoeukaryote species.
To describe cross-shelf patterns in the cruise data, we grouped our measurements into four subregions according to bottom depth: “Inner Shelf” (<50 m), “Mid Shelf” (50–100 m), “Outer Shelf” (100–500 m), and “Upper Slope” (>500 m). We also divided our observations into four seasons following Hunter-Cevera et al. (2019) (winter, January 1–Mar 31; spring, April 1–June 14; summer, June 15–September 14; and fall, September 15–December 31).
We compared our underway observations on the NES to the concentrations of picoeukaryote cells predicted by a niche correlation model trained on globally distributed observations (Flombaum et al., 2020). The model takes as input temperature, photosynthetically active radiation, and nitrate concentration and predicts the concentration of photosynthetic picoeukaryotes that will be present under the given conditions. Flombaum et al. (2020) trained 100 realizations of the model on observations of picoeukaryotes from 39 cruises from around the world. We provided these trained models with the environmental conditions we observed on the NES-LTER cruises as input and compared the predicted and observed picoeukaryote concentrations. Specifically, we used the temperature measurements of each underway sample, nitrate plus nitrite values interpolated from cast samples, and monthly surface photosynthetically active radiation measurements for the study region obtained from MODIS (NASA, 2023). To test for the effect of nutrient availability specifically, we also compared the predicted concentration values to those that would be predicted if nitrate concentrations were higher. That is, we altered the environmental inputs to the model such that nitrate values did not fall below 1 μM. This value is roughly where picoeukaryote concentrations peak for selected fixed PAR and temperature values presented in Flombaum et al. (2020). Any nitrite plus nitrate measurements below 1 μM in our dataset were replaced with 1 μM for this test. Higher values were unchanged.
3 RESULTS
Picoeukaryote concentration in surface waters ranged from 80 to 70,000 cells mL−1. Averaged across space, surface concentration was highest in the spring (mean 17,000 cells mL−1) and lowest in the winter (mean 7000 cell mL−1), but many cruises exhibited strong cross-shelf gradients in surface abundance (Figure 3; Figure S1).
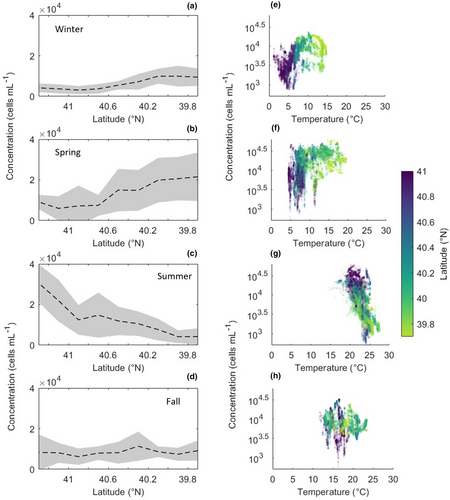
The direction of the cross-shelf gradients in surface abundance changed regularly over the annual cycle (Figure 3). In winter and spring, picoeukaryotes in surface waters were more abundant offshore than nearshore. This pattern was evident in 8 of 10 cruises in these seasons (Figure S1). In summer, surface concentration decreased with distance from shore, from an average of 25,000 cells mL−1 on the Inner Shelf to 3700 cells mL−1 on the Upper Slope. Notably, this gradient is opposite in direction but equal in range to that seen in the spring, such that when underway data from all cruises are pooled, concentration across the four subregions shows no spatial trend. Five of six fall cruises sampled a roughly even distribution of picoeukaryotes in surface waters across the shelf (Figure S1). The average fall surface concentration was 8900 cells mL−1, which corresponds to an increase relative to the late summer values (Figure 2b–e).
Discrete flow cytometry samples from Niskin bottles reveal seasonal differences in the depth distribution of the picoeukaryotes. In winter, picoeukaryote concentration peaks at roughly 10,000 cells mL−1 at the surface and decreases gradually with depth (Figure 4). In summer, subsurface maxima are regularly observed (49 out of 76 summer casts) with maximum concentrations approaching 40,000 cells mL−1. The depth of these maxima increases with distance from shore. That is, summer picoeukaryote abundances peak near the surface on the Inner Shelf and this peak deepens to around 50 m depth on the Upper Slope (Figure 4). Late in the fall, the depth profiles in cell concentration become more uniform for both picoeukaryotes (Figure S2) and Synechococcus (Figure S3).
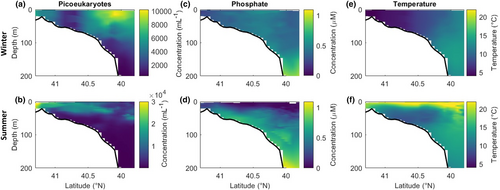
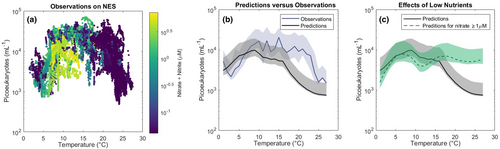
Picoeukaryote concentration peaks at intermediate temperatures (Figure 1d). The temperature measurements of our underway samples ranged from 1 to 28°C, with waters tending to be warmer both in summer and further from shore (Figure 2g). Below 22°C, the relationship between temperature and underway concentration along the latitudinal transect resembles that seen at MVCO over the same range of temperatures. However, in the summer, offshore waters are at or above the maximum temperatures observed near the coast. On summer cruises, the relationship between surface concentration and temperature becomes strongly negative (Pearson's correlation coefficient −0.58; p < .001; Figure 3g). Subsurface samples in the summer likewise reveal a peak in concentration at temperatures just below 20°C (Figure S4a).
In stratified conditions, warm surface waters are extremely nutrient poor (Figure 4d). Nutrient samples from summer cruises regularly approach our detection limits of 0.04 μM for nitrate plus nitrite (95% of samples from <10 m depth) and 0.009 μM for phosphate (27% of samples <10 m depth). While picophytoplankton are generally successful in oligotrophic conditions, we find a positive correlation between photosynthetic picoeukaryotes and phosphate when phosphate falls below 0.1 μM (Pearson's correlation coefficient 0.48; p < .001; Figure S5a). We find no such relationship for Synechococcus or for the picoeukaryotes and nitrite plus nitrate (Figure S5). It is possible that limitation may occur in these cases below our nutrient detection limits. Because we can detect phosphate at levels that are evidently relevant to picoeukaryote ecology, we focus our nutrient analysis on phosphate rather than nitrate. However, we note that phosphate and nitrite plus nitrate are positively correlated with each other and negatively correlated with temperature (Figure S4).
The niche correlation model trained on global data predicts the surface concentration of picoeukaryotes on the NES with reasonable accuracy. The model reproduces the overall shape of the relationship between temperature and cell concentration, including both the positive relationship in winter and the sharply negative relationship in summer (Figure 5a,b). The model would not predict a decline in picoeukaryote abundance in summer if our warmest observations did not coincide with low nutrients (Figure 5c). The predictions and observations differ most noticeably in the 15–23°C range, when nutrients are scarce but observed cell concentrations are higher than the model predicts. These samples correspond primarily to nearshore, summer conditions.
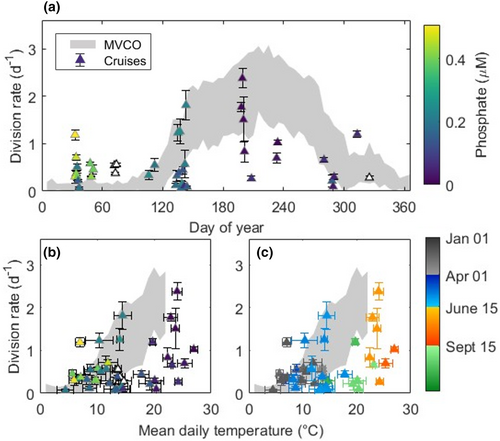
Picoeukaryote division rates estimated from the matrix model fit to flow cytometry data ranged from 0.06 to 2.38 day−1. Division rates were lowest in fall and winter (means both 0.46 day−1; SD 0.41 and 0.27 day−1, respectively) and highest in summer (mean 1.21 day−1; SD 0.72 day−1; Figure 6). Compared with division rates at MVCO, offshore values were particularly high in winter: The average (0.46 day−1) was greater than 99% of all winter division rate measurements made at the observatory. When accounting for temperature, however, winter division rates offshore are mostly within the range of values expected from the relationship at MVCO (Figure 6b). Fall division rates were low considering water temperature (Figure 6c), as has been seen at MVCO (Fowler et al., 2020), and comparable in value to division rates at MVCO at the same time of year (Figure 6a). Division rates from summer cruises were somewhat lower than the division rates measured at MVCO. The average summer division rate offshore was below 85% of all summer division rates at MVCO (mean 2.0 day−1), and the warmest temperatures do not correspond to the highest division rates in the cruise data set.
4 DISCUSSION
We will now describe in detail the cross-shelf patterns in picoeukaryote dynamics and discuss the likely drivers of the dynamics observed. Because the cross-shelf gradients in concentration vary dramatically and predictably with the time of year, we will separate our discussion by season. We will compare our observations of the picoeukaryotes across the continental shelf both to the picoeukaryote community at MVCO and to Synechococcus throughout the region, each of which we have analyzed independently in our earlier work (Fowler et al., 2020; Stevens, Crockford, et al., 2023). By identifying controls on the realized niche of picoeukaryotes across a coast-to-open ocean gradient, we test the extent to which current understanding of global picoplankton communities applies to nearshore systems.
4.1 Winter and spring
In winter and spring, picoeukaryote concentration increases with distance from shore in both surface and subsurface samples (Figures 3 and 4). This spatial pattern can largely be explained by the spatial variability in temperature. The offshore waters of the NES are generally warmer than the nearshore waters, and this spatial gradient is strongest early in the year before thermal stratification is established (Figure 2g). Surface seawater samples in winter and spring range from 1 to 20°C, and temperature is significantly positively correlated with picoeukaryote concentration (Pearson's correlation coefficient 0.53; p < .001). Note that this relationship describes the variability across both space (within cruises) and time (between cruises).
A similarly positive relationship between temperature and picoeukaryote concentration is observed at MVCO over time alone (Figure 1b). Analysis of the annual cycle at MVCO suggests that the nearshore picoeukaryote community is temperature limited in winter and spring (Fowler et al., 2020). Specifically, increases in temperature over the course of the spring lead to increases in division rate which outpace mortality, resulting in an annual spring bloom. This conclusion is further supported by the cruise data presented here. The division rates measured on winter cruises were significantly higher than those measured at MVCO at the same time of year (Figure 6a), but tend to be within the range of values seen at MVCO on days at the same temperatures (Figure 6c). The warmer offshore waters seem able to sustain higher picoeukaryote division rates through the winter.
4.1.1 Spring bloom phenology
The temperature variability across the shelf and the resulting difference in division rate evidently contribute to a cross-shelf difference in the timing of the picoeukaryote spring bloom (Figure 2b–f). We define a bloom as a local maximum in cell concentration that results from rapid population growth. Although we do not have complete annual coverage in the cross-shelf data, it seems that picoeukaryote concentration offshore increases before the concentration nearshore increases. In particular, the maximum concentrations we measured on the Upper Slope occurred on the 117th day of the year, which is 51 days prior to the average peak at MVCO and earlier in the year than 18 of the 19 annual blooms in the MVCO time series. Put briefly, bloom-level cell concentrations occurred on the Upper Slope more than a month earlier than the spring bloom would be expected at the nearshore observatory. Our data indicate a cross-shelf shift in the timing of the annual cycle.
The cross-shelf pattern in the timing of the spring bloom is similar to the pattern that has been described for Synechococcus on the NES (Stevens, Crockford, et al., 2023). Moreover, these results support the prediction that increased spring temperatures may lead to earlier spring picoplankton blooms (Hunter-Cevera, Neubert, et al. 2016). Such predictions presuppose, however, that top-down processes will not change so as to alter the observed patterns. The analyses of NES data have so far shown temperature in winter and spring to be correlated both with picoplankton concentration and with maximum division rate, suggesting that bottom-up processes are driving the spatial and temporal patterns in picoplankton abundance during these seasons.
4.2 Summer
While the winter and spring cruise data support and extend the hypotheses developed in previous work, our summer cruise data are not in line with our earlier expectations. On the basis of observations of picoeukaryotes at MVCO and of Synechococcus across the NES, we expected to see high abundances of picoeukaryotes in the summer throughout the region. At MVCO, picoeukaryotes are most abundant in the summer, and their dynamics are synchronized with those of Synechococcus (Fowler et al., 2020). Within underway cruise data, Synechococcus are abundant in the summer across all four subregions, from the Inner Shelf to the Upper Slope (Stevens, Crockford, et al., 2023). We find that the same is not true for the picoeukaryotes. Offshore surface waters have greatly reduced concentrations of picoeukaryotes during summer months (Figure 3c), both compared with earlier in the year and compared with the nearshore observations in summer. Something about the offshore conditions is not captured by the nearshore observatory and elicits a divergent response between the two picoplankton groups.
Note that while offshore surface concentrations are lowest in the summer, picoeukaryote division rate is highest at that time of year (Figure 6). High division rates in the spring and summer, paired with a drop in concentration, imply high picoeukaryote mortality. Our previous work emphasized that the picoeukaryotes at MVCO are subject to intense top-down control in the summer, regularly experiencing loss rates greater than 2.0 day−1 (Fowler et al., 2020). If grazer or viral activity is comparable across the shelf, only a slight reduction in reproduction offshore could lead to a stark decrease in picoeukaryote concentration. The results of Landry et al. (2023) suggest that grazing rates on picophytoplankton will be even higher under more oligotrophic conditions offshore, and a similar pattern was observed by Marrec et al. (2021) on one of our summer LTER cruises. Such a trend in grazing pressure could lead to the cross-shelf pattern in picoeukaryote abundance we observe. On the California coast, Landry et al. (2023) found this relationship to be even stronger for Synechococcus than picoeukaryotes, however, which is at odds with the summer patterns in abundance on the NES. We conclude that spatial differences in picoeukaryote division rate, exacerbated by their high loss rates relative to Synechococcus, lead to the distinct trend in surface picoeukaryote concentration that we see on summer cruises.
There are a few possible explanations for a cross-shelf trend in summer division rate. First, there may be spatial variability in the taxonomic identity of the picoeukaryotes across the NES. The picoeukaryotes are a diverse group (Díez et al., 2001; Moon-van Der Staay et al., 2001; Zeidner et al., 2003), and it is very likely that our flow cytometry measurements are summarizing multiple taxa. While we might expect a taxon to invade the offshore waters if it were capable of dividing more rapidly under the offshore conditions, we know that coastal microbial community composition can vary across short distances and may not reflect an ecologically stable state (Doré et al., 2022; Wang et al., 2019). We will therefore discuss two physiological explanations for the observed patterns, while recognizing that taxonomic differences may also be at play.
Picoeukaryote division offshore may be inhibited directly by the high temperatures of Slope Sea and Gulf Stream waters. The temperature response curves of picoeukaryotes in culture have been seen to peak anywhere between 8 and 30°C (Demory et al., 2019; Stawiarski et al., 2016), and certain strains fail to grow at all above their temperature optima (Stawiarski et al., 2016). At MVCO, temperatures rarely exceed 22°C. Maximum division rate increases with increasing temperature, but many daily division rates fall below the maximum at each temperature (Fowler et al., 2020). In the cruise data, the highest division rate (2.38 day−1) was measured from samples with an average temperature of 24°C (July 18, 2021). The only division rates measured at higher temperatures were 1.0 and 0.70 day−1 in August, 2019 (Figure 6). These lower division rates could indicate a strict temperature threshold, as observed in picoeukaryote cultures, but our limited sample size at temperatures above 24°C prevents us from saying so conclusively.
Alternatively, nutrient limitation may drive a cross-shelf difference in picoeukaryote division rate. Both picoeukaryotes and Synechococcus are generally successful in nutrient-poor waters, but Synechococcus may be more competitive during periods of intense nutrient starvation (Schmidt et al., 2020). Prochlorococcus is arguably even more successful in oligotrophic conditions and likely intensifies the competition offshore. High abundances of Prochlorococcus are evident in some offshore NES samples, consistent with previous descriptions of its distribution (Olson et al., 1990). In the Baltic Sea, Alegria Zufia et al. (2021) found that competition for nutrients among picophytoplankton was heightened at higher temperatures, reducing picoeukaryote growth rates. On the NES, cyanobacterial abundance is negatively correlated with all our nutrient measurements (Figures S5 and S6). In contrast, picoeukaryote concentration is positively correlated with phosphate at very low phosphate concentrations (Figure S5a). Under extreme phosphate scarcity and high temperatures, it seems the picoeukaryotes may be outcompeted by cyanobacteria, disrupting the otherwise synchronous environmental responses seen at MVCO.
Our division rates provide some additional support for the argument that nutrient availability limits picoeukaryote growth in offshore waters. Average phosphate concentration of the cruise samples is a reasonable predictor of the difference between each daily division rate and that of the same yearday at MVCO (Figure S7). It is very difficult, however, to distinguish between nutrient limitation and inhibition by high temperatures within our dataset, since nutrient concentrations on the NES are negatively correlated with temperature across season, latitude, and depth.
Instead, we can use global observations to consider the separate effects of increased temperatures and reduced nutrients on picoeukaryote abundance. When provided with the true environmental conditions on the NES as input, the niche correlation model of Flombaum et al. (2020) predicts the observed summer decline in picoeukaryote concentration at high temperatures (Figure 5b). If nutrients were more freely available, however, the model predicts that picoeukaryote concentration in the warmest waters would be as much as 10 times the observed values (Figure 5c). This analysis suggests that, based on global distributions, high temperatures alone are not limiting the standing stock size of the picoeukaryotes on the NES.
The distinction between inhibition by temperature or nutrient limitation has implications for the evolutionary pressures on the picoeukaryote community. For example, if the picoeukaryotes are in fact nutrient-limited offshore, mixotrophy may be increasingly advantageous since phagotrophy would increase a cell's access to limiting nutrients. Mixotrophy has been observed within picoeukaryotes both in the lab and in situ (Li et al., 2022; McKie-Krisberg & Sanders, 2014; Zubkov & Tarran, 2008). While the exact effects of mixotrophy on microbial community ecology are not well known, its importance for nutrient cycling in oligotrophic systems is increasingly recognized. Incubation experiments with nutrient augmentation treatments would be necessary to distinguish between nutrient limitation and temperature inhibition on picoeukaryote reproduction at our site.
4.2.1 Depth distribution
Water column profiles reveal that although offshore cell concentration is diminished in summer at the surface, relatively high picoeukaryote densities persist at depth (Figure 4). Subsurface maxima in phytoplankton biomass are typically considered to arise from a balance of light and nutrient limitation. During summer on the NES, the location of the maximum in picoeukaryote concentration is deeper than the peak in Synechococcus concentration (Figures S2 and S3). This depth partitioning is consistent with the hypothesis that picoeukaryotes at our site are more readily nutrient limited than Synechococcus. East of the Sargasso Sea, Veldhuis et al. (2001) observed the same pattern across depth, and found that pico- and nanoeukaryotes were more likely than Synechococcus to have reduced viability in surface waters. That is, large numbers of eukaryotic cells were unable to divide, contributing to their relatively low abundance at the surface.
The same depth partitioning that we report here has been observed in the central North Atlantic (Buck et al., 1996; Glover et al., 1986) and central North Pacific (Campbell & Vaulot, 1993). In contrast, in a more coastal setting, the opposite pattern was observed (Schmidt et al., 2020). In that case, the success of Synechococcus at depth was attributed to its superior green light-harvesting efficiency (Schmidt et al., 2020). Synechococcus grows more efficiently under low green light conditions, while some picoeukaryotes perform better under blue-violet light (Glover et al., 1986). Interestingly, on the NES, we find the location of the subsurface maximum in picoeukaryote concentration deepens with distance from shore (Figure 4). Blue-violet light predominates in oceanic waters, so the gradient that we observe across the NES transect may arise from differences in light color at depth. We argue that the deepening of the peak in picoeukaryote abundance across the shelf is the combined effect of reduced nutrients offshore, deepening the upper limit of their depth distribution, and increased availability of blue light, deepening the lower limit.
It is worth repeating that any effect of stratification on picoeukaryote division rate is relatively small. Even when surface conditions are apparently inhibiting picoeukaryotes and ideal for cyanobacteria, the picoeukaryotes are dividing roughly 1 day−1 faster than Synechococcus. Still, Synechococcus are an order of magnitude more abundant, while the picoeukaryotes are driven to winter-level concentrations at the surface. This result reinforces the idea that picoeukaryotes on the NES are subject to extreme top-down control in summer months. Even slight reductions in division rate coincide with dramatic changes in standing stock.
4.3 Fall
The annual cycles in picoeukaryote concentration inferred from our underway data are all suggestive of a small fall bloom (Figure 2b–f). In the MVCO time series, a second annual peak in concentration is observed in some, but not all, years, and does not persist in the interannual average (Fowler et al., 2020). Interannual differences in the timing of the fall bloom may hide this signal in the MVCO average, while the cruise data, which includes fewer years, might simply reveal a stronger signal because it is not as smoothed. Alternatively, the lower offshore picoeukaryote concentrations in summer may make a slight increase appear more dramatic than at MVCO.
It is possible that the increase in offshore surface concentration in the fall is the result of physical processes only. As discussed in the previous section, concentrations of picoeukaryotes at the end of the summer are highest below the surface. In addition to reintroducing nutrients, increased mixing associated with wind events and surface cooling in the fall may transport cells directly toward the surface, distributing them more evenly throughout the water column.
One piece of evidence to suggest that the apparent fall surface bloom is not strictly the result of vertical redistribution comes from the division rate time series at MVCO. Even though the average annual cycle in cell concentration at MVCO reveals no fall peak, the average annual cycle in division rate does (Figure 6a). Over the course of the fall at MVCO, growth of the picoeukaryotes is limited by the decreasing light availability (Fowler et al., 2020). Since the reduction in light availability is monotonic, the local maximum in division rate around yearday 320 suggests a repeated transition either in the conditions affecting growth rate or in the picoeukaryote community composition itself. If at this time of year, there is a bloom in some picoeukaryote taxa across the entire NES, that signal may be evident offshore but masked nearshore by the already relatively high surface concentrations of other taxa. More years of observation and an analysis of metabarcoding data from the cruises will provide more insight into the regularity of the fall dynamics and links to community composition.
4.4 Is the nearshore NES exceptional?
The seasonal and spatial patterns in picoeukaryote abundance on the NES are consistent from year to year, but are at odds with some of our expectations both from global trends and from the dynamics of the picoplankton community at MVCO. Specifically, nearshore picoeukaryote concentration is positively correlated with temperature at all temperatures (Figure 1b). The cruise data follows this same positive trend at low temperatures, but at temperatures above those experienced at MVCO, the relationship becomes negative. If we had extrapolated from the observatory data, we would have made very poor predictions of offshore picoeukaryote abundance.
The cruise data also do not align with the temperature trends of global observations. At any fixed nutrient concentration, Flombaum et al. (2020) reported that picoeukaryote density will peak at roughly 8.5°C and reach a local minimum at 21°C. The picoeukaryote community on the NES follows almost the opposite pattern (Figure 1d–f). Picoeukaryotes in our dataset persist at high densities between 10 and 20°C and decline sharply at temperatures above 21°C. From further analysis of the model presented in Flombaum et al. (2020), we can attribute this difference to the low nutrient conditions that coincide with warm offshore waters on the NES. Given observed nutrient conditions as well as temperature, the niche correlation model trained on global observations is able to predict the overall shape of the relationship between temperature and cell concentration on the NES.
That said, the niche correlation model fails to predict the concentration of picoeukaryotes on the NES within the 15–23°C range (Figure 5b). Measurements within this range are predominantly from Inner and Mid Shelf samples on summer cruises, when nitrate and nitrite are scarce but cell concentrations are high. Comparing our observations to model predictions under simulated nutrient-enriched conditions, it is as if the nearshore picoeukaryote community is resistant to the effects of low nitrate.
It is possible that nitrogen remains available to the picoeukaryotes in coastal waters either at concentrations that are below the detection limit of our measurements (<0.04 μM) or in a different form. Picophytoplankton have been shown to be efficient consumers of urea and to have a preference for ammonium over nitrate (Alegria Zufia et al., 2021; Balode et al., 1998; Domingues et al., 2011). Mixotrophic picoeukaryotes, as previously mentioned, may be able to meet their nitrogen needs through phagotrophy. It is also worth highlighting that the niche correlation model uses nitrate as the only nutrient input, while in our data, phosphate seems to be more well correlated with picoeukaryote abundance and division rate (Figures S5 and S6). Whether due to differences in the picoeukaryotes themselves or in the N:P ratios they experience, it seems the global data that was used to train the niche correlation model is not completely representative of the nearshore NES community.
This result contrasts with that for Synechococcus, for which the relationships between temperature, division rate, and cell concentration are consistent across the NES and agree well with global patterns (Stevens, Crockford, et al., 2023). Having documented synchronized responses of Synechococcus and picoeukaryotes to environmental variability at MVCO, we did not expect the cruise data to reveal a divergent response to offshore conditions. Yet we find that, for the picoeukaryotes, the offshore summer conditions are meaningfully different from those experienced at the observatory. This result highlights the limitations of making inferences about picoeukaryotes from geographically disparate observations. The spatial scope and resolution of our cruise data are evidently necessary to characterize the realized niche of the picoeukaryotes on the NES.
5 CONCLUSIONS
We have investigated the realized niche of photosynthetic picoeukaryotes across a coast-to-open ocean gradient. We find that the controls on picoeukaryote abundance vary across seasons and distance from shore and can be distinct from those on Synechococcus. Specifically, picoeukaryote growth is temperature limited in the winter and spring, but in summer, offshore nutrient limitation may contribute to depth partitioning between picophytoplankton groups. As we have seen at MVCO, the picoeukaryotes are subject to extreme top-down control relative to cyanobacteria, rendering their standing stock sensitive to even slight reductions in division rate. This result indicates that the picoeukaryotes are an active and important component of the phytoplankton community on the NES and underscores the necessity of taxon-specific research in picophytoplankton ecology.
Our work also highlights the importance of site-specific research, particularly for coastal ecosystems. On the NES, picoeukaryotes on the Inner Shelf do not exhibit signs of nutrient limitation in the summer. Their response to increased temperature and stratification is distinct from that seen on the Outer Shelf and in the open ocean. We expect increasing temperatures to lead to earlier picoeukaryote spring blooms across the region, but warmer summers and heatwaves may lead to earlier ends to the spring bloom offshore. We suspect that the diversity of the picoeukaryote assemblage contributes to the difficulty of generalizing across populations and of making predictions for the future.
AUTHOR CONTRIBUTIONS
Bethany L. F. Stevens: Conceptualization; data curation; formal analysis; investigation; methodology; software; visualization; writing – original draft; writing – review and editing. Emily E. Peacock: Data curation; investigation; methodology; project administration; validation; visualization; writing – review and editing. E. Taylor Crockford: Data curation; investigation; methodology; writing – review and editing. Alexi Shalapyonok: Investigation; methodology; writing – review and editing. Michael G. Neubert: Formal analysis; funding acquisition; methodology; supervision; writing – review and editing. Heidi M. Sosik: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; software; supervision; writing – review and editing.
ACKNOWLEDGMENTS
The authors are grateful to Adam Martiny for sharing with us the trained neural network model presented in Flombaum et al. (2020) and for granting us permission to use it in this analysis. We also thank Rob Olson for his contributions to generating and maintaining the ongoing MVCO flow cytometry time series, and the team members of the NES-LTER and the Shelfbreak Productivity Interdisciplinary Research Operation at the Pioneer Array for their support in collecting data. We thank the Ocean Observatories Initiative operations team for the opportunity to collect NES-LTER observations on Pioneer Array maintenance cruises. Data collection on AR43 was made possible by the Woods Hole Oceanographic Institution's Ocean Twilight Zone Project, funded as part of the Audacious Project housed at TED, and AR62 and AR38 were supported by the Woods Hole Oceanographic Institution's Access to the Sea Fund. This research was supported by the National Science Foundation (#OCE-1657803, #OCE-1655686, and #OCE-2322676) and the Simons Foundation (561126).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the Environmental Data Initiative Portal at: https://doi.org/10.6073/pasta/be91515864dafe7d539c0858280bdded (Preserved flow cytometry samples from Niskin bottles). https://doi.org/10.6073/pasta/127dd033e69d0e1a3f4900d47254d425 (Underway flow cytometry samples). https://doi.org/10.6073/pasta/ec6e5c76c7ad4e0da0a8d1cec84fa3f5 (Dissolved inorganic nutrients from Niskin bottles).




