Positive effects of tree species diversity on productivity switch to negative after severe drought mortality in a temperate forest experiment
Abstract
The synthesis of a large body of evidence from field experiments suggests more diverse plant communities are more productive as well as more resistant to the effects of climatic extremes like drought. However, this view is strongly based on data from grasslands due to the limited empirical evidence from tree diversity experiments. Here we report on the relationship between tree diversity and productivity over 10 years in a field experiment established in 2005 that was then affected by the 2018 mega-drought in central Europe. Across a number of years, tree species diversity and productivity were significantly positively related; however, the slope switched to negative in the year of the drought. Net diversity effects increased through time, with complementarity effects making greater contributions to the net diversity effect than selection effects. Complementarity effects were clearly positive in three- and five-species mixtures before the drought (2012–2016) but were found to decrease in the year of the drought. Selection effects were clearly positive in 2016 and remained positive in the drought year 2018 in two-, three-, and five-species mixtures. The survival of Norway spruce (Picea abies) plummeted in response to the drought, and a negative relationship between species diversity and spruce survival was found. Taken together, our findings suggest that tree diversity per se may not buffer communities against the impacts of extreme drought and that tree species composition and the drought tolerance of tree species (i.e., species identity) will be important determinants of community productivity as the prevalence of drought increases.
1 INTRODUCTION
Species loss driven by human activities triggered diversity and ecosystem productivity studies in both forests and grasslands over the last >30 years (Ammer, 2019). There is a consensus that biodiversity has a positive effect on ecosystem productivity (Grossiord, 2020; Kunert et al., 2012; Piotto, 2008; Reich et al., 2012; but see Jacob et al., 2010; Kirby & Potvin, 2007; Shovon et al., 2022), and the positive effect gets stronger over time (Jucker et al., 2020; Tilman et al., 2001; Urgoiti et al., 2022). Our understanding of the underlying mechanisms has deepened over the last three decades; however, we have a very limited understanding of how climatic extremes, for example, drought, can affect the relationship and its underlying mechanisms (Ammer, 2019; Grossiord, 2020). This is particularly important because, due to the ongoing climate change, an increasing frequency and severity of climatic extremes are expected (Trenberth et al., 2014).
Three mechanisms are mainly reported for the positive effect of diversity on productivity (hereafter diversity–productivity relationship or DPR): niche partitioning (Jucker et al., 2015), facilitation (Kothari et al., 2021), and selection effects (Tobner et al., 2016). Resource partitioning is an important part of niche partitioning and implies dissimilarities in resource acquisition mechanisms among species, reducing competition (Hooper, 1998). For example, trees with different rooting depths avoid competition for soil water uptake during a summer drought in a forest community (Silvertown et al., 2015). Facilitation can be defined as the positive effects of a species on its neighbor(s), for instance, when tree species with deep roots lift water from deep within soils to the soil surface and make it accessible to shallow-rooted species (Caldwell et al., 1998). Positive selection effects occur when species with particularly high monoculture yields are also dominant in mixtures (Hooper et al., 2005). Loreau and Hector (2001) statistically partitioned the net biodiversity effect into the complementarity effect (which includes both niche partitioning and facilitation) and the selection effect. Diverse plant communities, because of one or all the above mechanisms, commonly acquire more biomass compared to less diverse communities, resulting in a positive relationship between diversity and productivity. In addition, diverse communities have been shown to provide greater resistance to the negative effects of climatic extremes (Isbell et al., 2015).
According to the insurance hypothesis, biodiversity will help maintain ecosystem functioning during climatic stresses like droughts (Mulder et al., 2001; Yachi & Loreau, 1999). A recent meta-analysis on experiments manipulating both plant species diversity and environmental conditions found that biodiversity increases ecosystem functioning under both ambient and manipulated environmental conditions (i.e., warming, drought, nutrient addition, or CO2 enrichment), indicating that communities with high diversity are more resistant to environmental changes (Hong et al., 2022). These results are also in line with the stress gradient hypothesis (Bertness & Callaway, 1994), which postulates that species interactions can switch from competition in a benign environment to facilitation in a stressful environment. Accordingly, we can expect a stronger net biodiversity effect and, in particular, a higher complementarity among species in a stressful environment (e.g., Mulder et al., 2001; Steudel et al., 2012; but see Baert et al., 2018). In addition, environmental stress like drought can also favor more stress-tolerant species in diverse communities. Thus, increases in both complementarity and selection effects can potentially lead to a positive and stronger DPR in drought years (Ammer, 2019). An increased resistance of diverse communities to climate extremes, including drought, has been reported for experiments in grassland ecosystems (Isbell et al., 2015), but comparatively few experimental data exist for forest ecosystems (Schnabel et al., 2019, 2021). Importantly, grassland and forest ecosystems exhibit manifold differences in physiology and dynamics, including the temporal dynamics of the DPR (Guerrero-Ramírez et al., 2017). It is thus likely that grasslands and forests show important differences in how drought influences the DPR.
Here we report results from a long-term temperate tree diversity experiment, which in 2018 was exposed to the extreme pan-European drought (Figure 1a,b; also see Schuldt et al., 2020). Although the main objective of the experiment was to experimentally test the effects of tree species diversity on multiple aspects of forest ecosystem function (e.g., Gottschall et al., 2022), including productivity, the 2018 drought provided the opportunity to evaluate the effects of drought on the diversity–productivity relationship. Specifically, our long-term experiment allowed us to investigate how the effects of tree species diversity (in terms of species richness) on biomass production evolve through time across years, how these relationships across years are influenced by complementarity and selection effects, and how diversity effects on plot-level tree survival change across years. We expected (1) a positive effect of tree species diversity on productivity, (2) increasing complementarity along the diversity gradient, and (3) positive effects of diversity on tree survival across years. Given predictions from theory and empirical evidence that grasslands with higher plant diversity exhibit greater resistance to climatic extremes (including drought; Isbell et al., 2015), we furthermore expected that (4) the negative impacts of drought on productivity and tree survival are buffered by greater tree species diversity.

2 MATERIALS AND METHODS
2.1 Study site and experimental design
The Kreinitz experiment site is located near the village of Kreinitz-Zeithain, Saxony, Germany (51°23′08″ N, 13°15′41″ E; 115 m a.s.l.). The local climate is characterized by a mean annual precipitation of 578 mm and a mean annual temperature of 9.4°C (30-year average 1981–2010, Oschatz station of the German Weather Service DWD, ~15 km from the site). Maximum precipitation is normally observed during the growing season (Table S1), which usually starts in late March and ends in late October/early November (Scheuner, 2004). The soil is a humic cambisol, developed on a base of sand and gravel (93% sand, 6% silt, 1% clay) with a pH of 5.5 (Schwarz et al., 2015).
Kreinitz was planted in a field that was cultivated until 1990. In summer 2005, the site was plowed and harrowed, and two experimental blocks were set up, each of them containing 49 plots of 5 m × 5 m area with distances of 2.5 m between plots and of c. 10 m between blocks. In November of 2005, 2-year-old tree saplings, obtained from a local nursery, were planted on each plot in five staggered rows with six individuals in each row (Figure S1; 30 trees per plot). The distance between rows was 1.0 m, and trees within rows were separated by 0.8 m, resulting in a density of 1.2 individuals m−2 within a plot.
To construct the gradient in tree diversity, six native tree species that are abundant in Central European forests were selected, including four broadleaved and two coniferous species: Fagus sylvatica L. (European beech), Fraxinus excelsior L. (common ash), Quercus petraea (Matt.) Liebl. (sessile oak), Tilia cordata Mill. (small-leaved linden), Picea abies (L.) H. Karst. (Norway spruce), and Pinus sylvestris L. (Scots pine). The 49 plots within each block were randomly assigned to six different diversity levels and to different species combinations within each diversity level: bare soil (not further considered in this paper), monocultures (six “combinations”), two-species mixtures (15 combinations), three-species mixtures (20 combinations), five-species mixtures (six combinations), as well as the six-species mixture (one combination), resulting in 49 treatments (48 excluding the bare soil). The 30 planting positions within each plot were randomly, but in equal proportions, assigned to the respective species. For the first 2 years (2006, 2007), plots were regularly weeded to facilitate the establishment of saplings, and dead saplings were replaced by individuals of the same age and same seed source. Leaf litter (c. 600 g m−2) was also added to each plot to facilitate tree establishment from planting until 2009, with litter identity and amount representing the planted tree species composition.
2.2 Data collection
After each of the growing periods of 2017 (year prior to the extreme drought), 2018, and 2019, tree survival was noted. Height and diameter for all living trees were recorded in 2008–2013, 2016, and 2018. Owing to the small stature of saplings during the first years, we determined the basal diameter (10 cm above soil surface) of individual trees using a digital caliper, and their height using a ruler. In later years and for larger trees, we used either telescoping height poles or Haglöf Vertex IV-GS hypsometers (Haglöf Sweden AB, Långsele, Sweden) to measure tree height, and diameter tapes or tree calipers to measure basal diameter. For multi-stemmed individuals (a small proportion of trees, primarily Tilia), we measured the height and diameter of up to three largest stems that were later included in the tree biomass calculation. Diameter measurements for 2016 included both basal diameter and diameter at breast height (DBH) to calibrate the two different measurements; for 2018, only DBH was measured. In 2017 and 2019, tree survival was quantified, but due to limited human resources, we were unable to measure height or diameter.
2.3 Estimation of tree biomass and total plot aboveground biomass
Individual tree biomass was estimated by calculating individual tree volume using field-measured tree diameter and height, and then multiplying volume by wood density, where the wood density of each species was obtained from the literature (Zanne et al., 2009). For consistency, we used basal diameters for all years. In 2018, only DBH was measured, so we used measurements of both DBH and basal diameter from 2016 and a generalized linear model incorporating species identity (R2 = .93) to predict the basal diameters in 2018 (see Figure S2; Table S2).
We converted all the total plot biomass values and plot biomass increment to plot biomass per unit area (Mg ha−1). This reflects the effect of biomass gains due to cumulative growth and biomass loss due to individual tree mortality, which results in losses of live biomass (Huang et al., 2018; Tobner et al., 2016).
2.4 Bark beetle effects
For the growing periods of 2018 and 2019, we visually surveyed each tree for infestation by bark beetles, including those trees that died during the respective season: We noted the presence or absence of beetles and considered trees to be infested if we observed feeding galleries below peeling bark patches. Such signs of bark beetle incidence were visible on P. abies (most likely produced by larvae of European spruce bark beetle, Ips typographus L.), P. sylvestris (undetermined bark beetle species), and F. excelsior (most likely larvae of lesser ash bark beetle, Hylesinus fraxini Panzer). Incidences of very low infestation may not have been detected, but our consistent approach allows reliable comparisons of bark beetle incidence among years, tree species, and diversity levels.
2.5 Calculation of net diversity effects, selection effects, and complementarity effects
2.6 Statistical analysis
We applied a Bayesian approach to fit generalized linear mixed effect models (GLM). For total aboveground biomass, we used a Gamma distribution with a log link function to estimate the main and interacting effects of species diversity and year. We fit the same model, but used Student's t-distribution, to model the annual biomass increment. To estimate how NE, SE, and CE responded to tree diversity across years and species diversity levels, we also fit a GLM with Student's t-distribution. We chose Student's t-distribution rather than normal distribution when the latter resulted in a lack of fit in the tails of the distribution owing to outliers. Models with Student's t-distribution outperformed the models with normal distribution in four cases (biomass increment, CE, SE, NE; see Table S3). The full model with Student's t-distribution had 71%, 92%, 94%, and 78% support for the biomass increment, CE, SE, and NE models, respectively. We did not find any evidence for a linear relationship between species richness and partitioned diversity effects when considering diversity as a continuous variable (Table S4). Therefore, we considered diversity as a discrete variable to determine if and how CE, SE, and NE differed among the diversity levels. In addition, this approach allowed us to test whether CE, SE, and NE were different from zero.
For analyzing survival until 2017, the last year before the extreme drought, we used 2008 as the “previous year of measurement” and calculated survival by relating the total number of trees that survived in 2017 to the total number of trees alive in 2008. The predrought mortality of most of the species was very low, so we considered 2008 as the “previous year of measurement”.
We analyzed plot-level data on bark beetle infestation in 2017 and 2018 using generalized linear mixed effect models with a binomial distribution. Similar to the survival data, we analyzed each tree species separately by relating the number of infested individuals to the total number of individuals present on the respective plot.
For all models mentioned previously, species diversity, year, and the interaction of species diversity and year were included as fixed effects, while the variables plot, block, the interaction between block and year, the interaction between combination and diversity, and the three-way interaction among year, combination, and diversity were treated as random effects. The full model in this study was defined as that which included the main effects of species diversity, year, and interaction between species diversity and year. To account for potential temporal autocorrelation, we included a first-order autoregressive covariance structure (AR) in all the abovementioned models. We found evidence of temporal autocorrelation in all models except for the survival model and the model for biomass increment, so we did not include an AR term in these two models (Tables 1–3). We used the R package BRMS 2.16.1 for data analysis within a Bayesian framework. Uninformative flat priors were assigned to model parameters to minimize their influence on posterior estimates. We fit all models using four Markov chain Monte Carlo chains, each of which had 2500 warm-up iterations (which were discarded) and 2500 sampling iterations. All parameters were checked for convergence among chains (Rhat <1.01). We did not perform any traditional significant tests in this study. However, the estimated mean values and slopes of the linear models were reported with their 95% credible intervals. Based on this, we evaluated the results of our statistical analyses in terms of statistical “clarity” rather than “significance” (Dushoff et al., 2019). All statistical analyses were performed in the R version 4.0.1 (R Core Team, 2022). Further details for the models are given in Box S1. All data used in this study will be found in Shovon et al., 2024.
| Parameters | Estimates (lower CI, upper CI) | |
|---|---|---|
| Total biomass | Total biomass increment | |
| Intercept | 3.9 (3.0, 4.9) | 27.3 (18.3, 36.7) |
| year2008 | 1.7 (0.4, 3.2) | |
| year2009 | 2.8 (1.4, 4.2) | 11.6 (−0.9, 23.6) |
| year2010 | 3.5 (2.3, 5.0) | 18.2 (5.5, 30.2) |
| year2011 | 4.0 (2.6, 5.4) | 20.3 (7.4, 32.1) |
| year2012 | 4.5 (3.1, 5.9) | 39.7 (26.9, 51.3) |
| year2013 | 4.9 (3.6, 6.4) | 45.0 (33.3, 57.8) |
| year2016 | 5.7 (4.3, 7.1) | 51.1 (38.7, 63.0) |
| year2018 | 5.6 (4.3, 7.1) | 6.8 (−5.1, 19.7) |
| Diversity | 0.08 (0.0, 0.1) | 2.7 (15.2, 37.3) |
| year2008:Diversity | 0.1 (0.0, 0.2) | |
| year2009:Diversity | 0.1 (0.0, 0.2) | 0.5 (−2.0, 3.2) |
| year2010:Diversity | 0.1 (0.0, 0.2) | 1.2 (−1.4, 3.9) |
| year2011:Diversity | 0.1 (0.0, 0.2) | 1.5 (−1.1, 4.2) |
| year2012:Diversity | 0.1 (0.0, 0.2) | 2.5 (−0.2, 5.3) |
| year2013:Diversity | 0.1 (0.0, 0.2) | 3.2 (0.2, 6.0) |
| year2016:Diversity | 0.1 (−0.0, 0.1) | 2.6 (−0.2, 5.4) |
| year2018:Diversity | 0.0 (−0.0, 0.1) | −11.0 (−14.4, −7.7) |
| R 2 | .35 (0.35, 0.36) | .55 (0.51, 0.58) |
| AR | 0.89 (0.84, 0.92) | |
| Family specific parameters | ||
| Shape | 413.5 (219.0, 729.1) | |
| Sigma | 4.2 (3.3, 5.4) | |
| Nu | 1.3 (1.0, 1.6) | |
| Group-level effects | ||
| Block | ||
| SD (intercept) | 0.7 (0.0, 3.5) | 7.3 (0.4, 26.0) |
| Block:combination | ||
| SD (intercept) | 0.0 (0.0, 0.1) | 1.8 (0.3, 3.1) |
| Diversity:combination | ||
| SD (intercept) | 0.1 (0.0, 0.3) | 6.5 (4.8, 8.7) |
| Diversity:year:combination | ||
| SD (intercept) | 0.1 (0.0, 0.1) | 8.0 (6.7, 9.3) |
| Year:block | ||
| SD (intercept) | 0.0 (0.0, 0.1) | 3.5 (0.9, 8.0) |
3 RESULTS
3.1 Variation in temperature and precipitation during the experiment
The climate at the experimental site is humid and, on average, characterized by slight maxima during the summer months (Table S1). In the summer of 2018, that is, in the 13th growing period after the establishment of the experiment, the strong pan-European drought resulted in no precipitation being recorded in the area for three consecutive months from August to October (Figure 1a,b). The total growing-season precipitation for 2018 was 102 mm and thus only 28.3% of the long-term average (April–October; see Table S1). This drought event was accompanied by higher than average temperatures: the growing-season temperature as measured from April to October was 16.9°C in 2018 and thus 24% higher than the long-term average of 13.6°C. The following year, 2019, was also drier and hotter compared to the long-term average but was not as intense as 2018. The 2019 growing season received 66.8% of the long-term average precipitation, and the temperature was 18.7% higher than the long-term average. Prior to the drought years, from 2008 to 2017, the average growing-season precipitation was 371.04 mm and the temperature was 14.9°C, which were 3% and 9% higher than the long-term average.
3.2 Effect of tree diversity on total biomass and biomass increment
We found clear and positive effects of planted tree species diversity on aboveground plot-level biomass from 2008 to 2018 (see Figures 2a and 3a; Table 1). In addition, the slopes remained clearly positive from 2008 to 2016, but they did not increase from year to year (Figure 3a). At the last measurement (2016), prior to the extreme drought, the biomass of six-species mixtures was 25% greater than the average monoculture biomass (predicted value; 95% credible interval: lower limit 25%, upper limit 27%). In the year of the drought, in 2018, the effect of diversity on plot biomass was still positive but became much weaker and not clearly different from zero, as the biomass in the six-species mixture was only 25% (24%, 25%) greater than monoculture biomass in the same year.
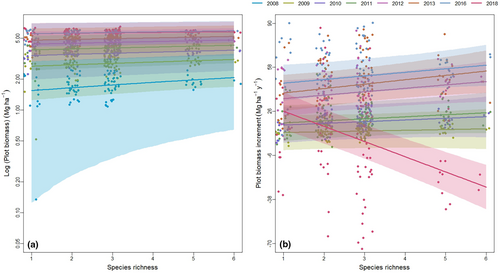
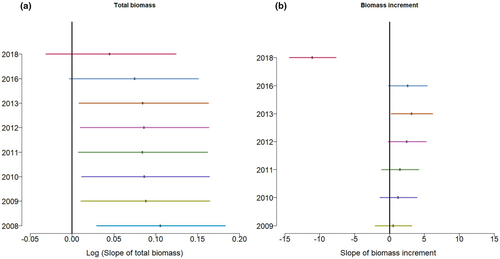
The effect of species diversity on plot-level biomass increment was positive from 2009 to 2016, and the slope increased with time until 2013, although it was clearly different from zero only in 2013. However, with the onset of drought in 2018, the slope switched to negative (see Figure 3b; Table 1). With the addition of one tree species, annual biomass increment increased on average by 3.1 Mg ha−1 year−1 (0.2, 6.2) during the 2012–2013 inventory period. However, from 2016 to 2018, annual biomass increment decreased on average by 11.1 (7.7, 14.3) Mg ha−1year−1 with the addition of one species in a plot.
3.3 Complementarity, selection, and net biodiversity effect
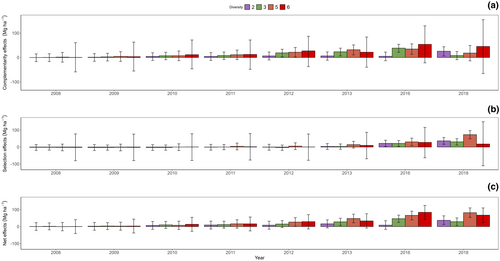
The net biodiversity effects, complementarity, and selection effects were not different from zero during the first four inventories from 2008 to 2011. In 2012, the complementarity effects were clearly positive in plots with three or five species. Complementarity effects in diverse plots (except the six-species mixture) continued to be greater than zero until the 2018 drought. During the drought year, the complementarity effects were again not different from zero, except in the two-species mixtures. Selection effects turned positive in 2016, and remained positive in 2018, the drought year, across three diversity levels: two-, three-, and five-species plots. The net diversity effects in 2018 were also positive across plots with all diversity levels. Plots with six species had complementarity and selection effects not different from zero during the whole study period (Figure 4a–c; Table 2).
| Parameters | Estimates (lower CI, upper CI) | ||
|---|---|---|---|
| Net effect | Complementarity effect | Selection effect | |
| Intercept | 22.4 (7.6, 35.2) | 14.8 (−2.2, 31.2) | 8.5 (−8.7, 25.2) |
| year2008 | 0.6 (−22.0, 18.3) | 0.8 (−18.2, 20.6) | −1.9 (−24.7, 20.6) |
| year2009 | 2.9 (−16.1, 22.8) | 3.3 (−15.6, 23.2) | −2.5 (−25.9, 20.3) |
| year2010 | 9.6 (−10.6, 28.8) | 7.7 (−11.8, 26.4) | −1.3 (−24.1, 22.3) |
| year2011 | 13.0 (−6.7, 31.7) | 9.3 (−8.9, 30.4) | 1.4 (−21.9, 24.1) |
| year2012 | 19.4 (−1.6, 37.2) | 18.9 (−1.9, 37.2) | 0.6 (−23.14, 22.5) |
| year2013 | 31.2 (12.0, 49.8) | 21.0 (1.4, 41.6) | 7.8 (−14.9, 31.3) |
| year2016 | 50.8 (30.0, 69.2) | 33.2 (10.9, 56.1) | 25.0 (−1.7, 50.7) |
| year2018 | 53.7 (33.7, 74.2) | 24.7 (−7.0, 56.8) | 39.8 (3.8, 76.1) |
| Diversity2 | 10.7 (−8.2, 30.1) | 6.9 (−7.9, 20.0) | 6.6 (−9.7, 22.6) |
| Diversity3 | 18.3 (−0.3, 33.2) | 13.7 (−0.6, 26.2) | 5.5 (−9.7, 19.6) |
| Diversity5 | 30.9 (9.5, 49.2) | 16.7 (−0.1, 32.8) | 15.5 (−2.9, 33.2) |
| Diversity6 | 30.8 (−6.3, 66.5) | 22.8 (−28.2, 70.7) | 6.9 (−62.3, 75.2) |
| Year2008:Diversity2 | 1.0 (−21.2, 22.0) | 0 (−15.8, 15.4) | −1.7 (−19.7, 15.4) |
| Year2009:Diversity2 | 2.0 (−20.2, 24.0) | 1.2 (−14.6, 16.7) | −2.8 (−21.3, 13.6) |
| Year2010:Diversity2 | 6.8 (−15.4, 29.0) | 4.8 (−11.5, 19.5) | −2.6 (−20.3, 15.2) |
| Year2011:diversity2 | 8.8 (−14.8, 30.0) | 4.9 (−11.3, 19.6) | −0.8 (−19.4, 16.1) |
| Year2012:Diversity2 | 7.7 (−16.5, 29.6) | 6.7 (−8.9, 22.9) | −0.8 (−19.7, 16.6) |
| Year2013:Diversity2 | 15.3 (−7.9, 37.5) | 6.6 (−9.1, 22.8) | 3.6 (−14.7, 21.4) |
| Year2016:Diversity2 | 7.9 (−16.7, 32.6) | 5.0 (−11.7, 22.0) | 21.5 (1.9, 40.3) |
| Year2018:Diversity2 | 36.6 (12.4, 61.4) | 26.0 (7.0, 43.3) | 36.2 (14.7, 55.8) |
| Year2008:Diversity3 | 1.3 (−18.3, 22.8) | 0.8 (−15.0, 15.2) | −1.6 (−18.5, 13.1) |
| year2009:Diversity3 | 3.1 (−17.0, 21.5) | 2.9 (−12.7, 17.3) | −3.0 (−18.9, 12.7) |
| year2010:Diversity3 | 10.2 (−9.6, 29.8) | 7.3 (−7.6, 22.1) | −2.8 (−19.4, 12.0) |
| year2011:Diversity3 | 11.8 (−7.8, 30.3) | 8.1 (−7.6, 22.5) | 0.0 (−16.1, 15.5) |
| year2012:Diversity3 | 15.0 (−5.1, 33.1) | 19.3 (4.0, 34.1) | −3.5 (−19.7, 12.3) |
| year2013:Diversity3 | 28.1 (8.8, 46.8) | 23.5 (7.2, 38.0) | 3.1 (−14.1, 18.0) |
| year2016:Diversity3 | 46.4 (26.2, 66.7) | 38.5 (7.2, 38.0) | 21.2 (3.4, 39.0) |
| year2018:Diversity3 | 28.9 (5.2, 49.5) | 8.4 (−8.9, 24.3) | 30.6 (11.4, 49.6) |
| year2008:Diversity5 | −0.9 (−23.2, 22.2) | 1.9 (−18.2, 21.4) | −2.0 (−22.2, 17.7) |
| year2009:Diversity5 | 2.0 (−21.6, 24.5) | 4.8 (−15.5, 24.0) | −2.0 (−22.2, 18.0) |
| year2010:Diversity5 | 7.3 (−15.4, 29.9) | 7.4 (−12.4, 26.9) | 0.1 (−20.1, 19.8) |
| year2011:Diversity5 | 15.6 (−6.7, 38.0) | 11.6 (−9.1, 30.3) | 4.2 (−14.9, 25.5) |
| year2012:Diversity5 | 26.3 (3.4, 48.9) | 22.0 (1.3, 40.6) | 5.6 (−15.5, 25.0) |
| year2013:Diversity5 | 47.4 (24.9, 70.9) | 32.0 (12.5, 52.7) | 14.3 (−5.9, 34.0) |
| year2016:Diversity5 | 65.7 (41.2, 88.9) | 35.0 (13.6, 56.4) | 30.4 (7.6, 52.1) |
| year2018:Diversity5 | 82.1 (55.2, 107.2) | 18.0 (−12.7, 48.6) | 73.1 (49.4, 97.9) |
| year2008:Diversity6 | 0.7 (−45.0, 41.3) | 0.8 (−58.6, 58.9) | −2.7 (−77.2, 76.0) |
| year2009:Diversity6 | 4.3 (−40.3, 46.6) | 4.4 (−56.5, 62.3) | −2.5 (−79.5, 74.6) |
| year2010:Diversity6 | 13.7 (−27.9, 56.1) | 11.6 (−48.7, 71.8) | −0.1 (−81.9, 76.1) |
| year2011:Diversity6 | 16.0 (−27.4, 59.9) | 12.7 (−47.4, 76.5) | 1.6 (−71.7, 84.9) |
| year2012:Diversity6 | 28.3 (−15.3, 71.7) | 27.4 (−36.3, 89.1) | 0.5 (−74.8, 81.0) |
| year2013:Diversity6 | 33.1 (−6.8, 78.1) | 21.8 (−39.9, 85.0) | 10.0 (−62.8 94.2) |
| year2016:Diversity6 | 83.3 (37.4, 125.5) | 54.3 (−21.2, 129.7) | 27.7 (−63.7, 118.1) |
| year2018:Diversity6 | 67.4 (37.4, 125.5) | 46.1 (−66.1, 154.9) | 19.5 (−109.0, 151.7) |
| R 2 | .15 (0.10, 0.21) | .10 (0.06, 0.15) | .11 (0.07, 0.16) |
| AR | 0.74 (0.66, 0.81) | 0.7 (0.62, 0.77) | 0.84 (0.79, 0.89) |
| Family specific parameters | |||
| Sigma | 21.0 (17.3, 24.7) | 19.7 (16.7, 22.6) | 17.2 (14.3, 201) |
| Nu | 2.0 (1.4, 2.9) | 2.1 (1.5, 2.9) | 1.9 (1.3, 2.6) |
| Group-level effects | |||
| Block | 9.6 (0.3, 35.5) | 7.8 (0.2, 28.8) | 7.2 (0.2, 25.9) |
| SD (intercept) | |||
| Block:combination | 4.2 (0.1, 12.0) | 4.1 (0.1, 10.9) | 3.9 (0.1, 11.2) |
| SD (intercept) | |||
| Diversity:Combination | 9.2 (0.6, 19.8) | 3.7 (0.2, 10.1) | 5.0 (0.2, 14.1) |
| SD | |||
| Diversity:year:Combination:year | 3.6 (0.2, 7.8) | 1.6 (0.0, 4.6) | 1.3 (0.0, 3.6) |
| SD (Intercept) | |||
| Year:block | 4.3 (0.7, 9.9) | 1.6 (0.1, 5.3) | 1.6 (0.1, 3.6) |
| SD (Intercept) | |||
3.4 Effect of tree species diversity on survival
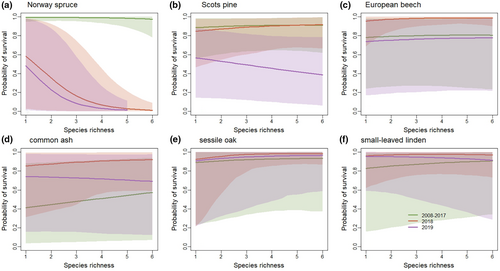
Survival rates of Norway spruce, Scots pine, sessile oak, small-leaved linden, and European beech trees were high and varied from 78% to 99% (relative to the number of planted trees) during 2008 to 2017 (see Figure 5a; Table 3). However, the survival of common ash trees was lower compared to other tree species and varied from 41% to 57% during the same period. Survival rates of none of these tree species were related to species diversity from 2008 to 2017. During the drought year in 2018, the survival of Norway spruce trees (relative to the number of alive trees in 2017) plummeted and was negatively related to species diversity: survival was 58% (3%, 98%) in monocultures, 35% (0.9%, 92%) in two-species mixture, 16% (0.3%, 74%) in three-species mixtures, 2% (0.0%, 21%) in five-species mixtures, and 0% (0.0%, 0.1%) in the six-species mixture. A similar trend was also found in 2019: survival was 48% (1%, 98%) in monocultures, 23% (0.3%, 87%) in two-species mixtures, 8% (0.0%, to 58%) in three-species mixtures, and 8% (0.0%, to 58%) in five-species mixtures (Figure 5a). Unlike Norway spruce, survival (relative to the number of alive trees in 2017) of Scots pine, sessile oak, and small-leaved linden were not affected by the 2018 drought year (Figure 5b,e,f). The survival of European beech and common ash rather increased during the 2018 mega-drought compared to their survival from 2008 to 2017 (Figure 5c,d). However, the survival of Scots pine, common ash, and European beech decreased during 2019 (Figure 5b–d), the second year of drought in a sequence (Figure 1a,b), compared to the survival rates of 2018. In contrast, the survival of sessile oak and small-leaved linden remained high even in 2019.
| Parameters | Estimates (lower CI, upper CI) | |||||
|---|---|---|---|---|---|---|
| Norway spruce | Beech | Ash | Pine | Oak | Linden | |
| Intercept | 3.62 (0.9, 6.4) | 2.1 (−0.1, 3.9) | 0.9 (−0.9, 2.5) | 1.5 (−0.2, 2.8) | 3.2 (0.0, 5.6) | 3.7 (1.3, 5.6) |
| Diversity | −1.1 (−1.8, −0.5) | 0.2 (−0.2, 0.6) | 0.1 (−0.1, 0.3) | 0.0 (−0.2, 0.3) | 0.4 (−0.1, 0.9) | 0.1 (−.3, 0.5) |
| Year 2017 | 6.6 (3.6, 10.1) | 1.7 (−0.6, 3.4) | −0.1 (−2.2, 1.6) | 2.5 (0.9, 3.8) | 3.6 (0.6, 5.7) | 2.6 (0.3, 4.2) |
| year2018 | −2.4 (−5.5, 0.3) | 5.2 (2.7, 7.4) | 2.7 (0.6, 4.4) | 2.3 (0.6, 3.6) | 5.3 (2.2, 7.8) | 5.2 (2.8, 7.4) |
| year2019 | −3.7 (−6.9, −7.4) | 1.4 (−1.0, 3.1) | 1.2 (−0.8, 3.0) | −0.1 (−1.8, 1.1) | 4.5 (1.4, 6.8) | 4.0 (1.7, 5.7) |
| Diversity:year2017 | −0.3 (−1.3, 0.8) | 0.0 (−0.4, 0.5) | 0.1 (−0.1, 0.4) | 0.1 (−0.4, 0.5) | 0.2 (−0.5, 0.9) | 0.2 (−0.3, 0.7) |
| Diversity:year2018 | −1.3 (−2.3, −0.6) | 0.4 (−0.7, 1.7) | 0.2 (−0.4, 0.7) | 0.2 (−0.3, 0.7) | 0.5 (−0.7, 2.0) | 0.1 (−0.9, 1.4) |
| Diversity:year2019 | −1.6 (−2.9, −0.5) | 0.1 (−0.4, 0.5) | −0.1 (−0.4, 0.3) | −0.2 (−0.5, 0.2) | 0.4 (−0.5, 1.4) | −0.2 (−0.8, 0.6) |
| R 2 | .98 (0.97, 0.99) | .96 (0.94, 0.97) | .87 (0.82, 0.91) | .94 (0.92, 0.95) | .99 (0.98, 0.99) | .99 (0.98, 0.99) |
4 DISCUSSION
In our long-term forest biodiversity experiment, we found that the positive relationship between species diversity and productivity (Figures 2a,b and 3a,b) was partly explained by a positive complementarity effect (Figure 4a; Table 2) in the first 11 years of the experiment. However, the persistent positive effects of tree diversity on productivity we observed shifted to negative in response to the extreme drought of 2018, and at the same time, complementarity decreased while the selection effect remained positive (Figure 4a,b). This shift occurred because of the decreased survival of dominant Norway spruce trees (in terms of biomass; see Figure S3a–d) in diverse plots. Contrary to our expectation, greater tree species diversity per se may not make forest communities resistant to climatic extremes, due to the strong effects of declines in productive species.
4.1 Diversity–productivity relationship during drought
The effect of biodiversity on productivity was positive in this temperate forest experiment from 2008 to 2016 (Figure 3a,b), consistent with the results of long-term experiments in grassland ecosystems and of a large tree diversity experiment in subtropical China (Huang et al., 2018; Reich et al., 2012). Thus, our results suggest that tree diversity can increase forest productivity and lead to more rapid biomass accumulation in mixed plots compared to average monocultures (Feng et al., 2022). Our results furthermore confirm that the positive effect of diversity on plot-level biomass increases with time, but in contrast to other studies on forest ecosystems, this was also true for the annual increment of biomass (see Guerrero-Ramírez et al., 2017). However, this relationship did only persist as long as weather conditions were close to historically experienced conditions. The mega-drought of 2018 strongly decreased the positive relationship between diversity and total aboveground biomass, and shifted the direction of the relationship between diversity and biomass increment from positive to clearly negative (Figures 2a,b and 3a,b). These results are contradictory to the insurance hypothesis (Yachi & Loreau, 1999), which postulates that biodiversity helps maintain ecosystem functioning during stress conditions, and differ from empirical findings from multi-year grassland studies where a positive diversity–productivity relationship persisted in drought years even though the total productivity was decreased (Hong et al., 2022; Isbell et al., 2015). Isbell et al. (2015) reported a moderate effect of diversity on the resistance of grassland communities during extreme droughts. Grossiord, Granier, Ratcliffe, et al. (2014) found in a pan-European study that the effect of diversity on drought resistance was positive in two forest types, but not significant in three others and suggested that impacts of drought on the diversity–productivity relationship are potentially more context dependent in forests than in grasslands. Furthermore, a simulation model as well as a meta-analysis across biodiversity experiments that manipulated abiotic conditions indicated a unimodal relationship between the slope of the biodiversity–ecosystem functioning relationship and stress intensity (Baert et al., 2018). We suggest that in this framework, our severe drought can be seen as located at the extreme end of the stress gradient, where the slope of the biodiversity–ecosystem functioning relationship decreases.
4.2 Mechanisms underlying the diversity effect
Consistent with the limited data from temperate North American and subtropical Chinese tree diversity experiments (Huang et al., 2018; Urgoiti et al., 2022), we also found complementarity and net diversity effects in mixed plots became clearly positive with time—at least prior to the mega-drought (Figure 4). An increase in niche complementarity with stand development significantly contributes to the strengthening of the positive diversity–productivity relationship in forest ecosystems. For example, different crown and root traits allow different species to fill crown and soil spaces, respectively, to acquire limiting resources (e.g., light and belowground resources) more efficiently, which in turn leads to higher biomass production in diverse forests (Ma & Chen, 2017; Williams et al., 2017). In addition to niche partitioning, facilitation can also become more important over time (Kothari et al., 2021). A greenhouse study using bryophytes further revealed that facilitative interactions were responsible for the increase in the biodiversity–productivity relationship under drought conditions (Mulder et al., 2001). In our experiment on tree diversity; however, complementarity effects disappeared and became close to zero in three and five-species mixtures during the drought in 2018 and remained positive in two-species mixtures only. This suggests certain combinations of two tree species can complement each other in terms of resource uptake during drought, however, an increase in species richness may increase the chance of having functionally redundant neighbors who have similar resource uptake mechanisms. Thus, two opposing mechanisms might have neutralized the complementarity effects in more diverse plots. Other studies reported positive, neutral, and negative complementarity effects in drought conditions (Grossiord, Granier, Gessler, et al., 2014; Grossiord, Granier, Ratcliffe, et al., 2014). Ammer (2019) argued that drought can shift the diversity effects in mixed forest communities—mixtures determined by complementarity effects may develop into mixtures characterized by selection effects. For example, in our experiment, the dominance (based on biomass) of both drought-tolerant species, Scots pine and sessile oak, increased in five and six-species mixtures during the drought year (see Figure S3a–d; Table S5). With the increase in dominance of drought-tolerant trees, competitively weak drought-intolerant trees (here, Norway spruce) lost their dominance in the drought year. An experiment in a temperate forest in Canada (Belluau et al., 2021) found a reduction in the slope of diversity–productivity relationship in rain exclusion plots relative to irrigated plots. However, they did not find negative diversity–productivity and diversity–complementarity relationships like this study, perhaps because of low tree mortality in their experiment.
4.3 Tree survival and species diversity
A recent study found that diverse forests in North America experience higher tree mortality compared to monocultures by analyzing plot network data (Searle et al., 2022). However, we did not find any relationship between species diversity and tree survival or mortality in the years before the drought event occurred (Figure 4). Because of the drought in 2018, the survival of Norway spruce trees became negatively related to species diversity, which has not been reported in other studies on drought-induced mortality. Drought-induced mortality of Norway spruce has also been reported from non-experimental observations in Central Europe following the drought years 2018 and 2019 (Obladen et al., 2021). Arend et al. (2021) found fast dehydration and hydraulic collapse as the cause of drought-induced mortality of spruce trees during the extreme drought year. But why was there a higher mortality of Norway spruce in more diverse plots? We reviewed three possible reasons for high tree mortality in diverse communities: (1) stronger competition for water; (2) higher demand for soil moisture; and (3) higher pest infestation. First, higher productivity of mixed forest communities means higher biomass and basal area, which leads to increased competition for limiting resources. In consequence, interspecific competition for soil moisture may also increase during a drought year (Jucker et al., 2014; Shovon et al., 2021). Norway spruce has a shallow-root system compared to its neighboring tree species (Schume et al., 2004). We therefore suggest that Norway spruce, being a shallow-rooted species, lost the competition for soil water against competitively superior neighbors during drought, and consequently, its survival plummeted in diverse plots. In addition, we also observed a weak negative relationship between survival and diversity in the following year 2019 (Figure 5) for Scots pine. This might be a result of a time lag in drought-induced mortality (Bigler et al., 2007) in the following year, which was also hot and dry (Figure 1). Second, diverse forests have a generally higher transpiration rate because of higher biomass and thus require more soil moisture compared to monocultures (Kunert et al., 2012). This may have further narrowed the availability of soil water and, in turn, increased competition for that resource during the drought year. Higher mortality in mixed communities also reportedly ease pressure on soil moisture availability and lead to higher growth of the surviving trees (Brantley et al., 2013). In our experiment, the massive mortality of Norway spruce perhaps helped the other five tree species to survive well in the mixtures (Figure 5).
Lastly, drought-affected forests are more vulnerable to pest infestation (Netherer et al., 2019). In our experiment, three out of six species were infested by different species of bark beetle: Norway spruce, Scots pine, and common ash. We also found a weak positive trend of beetle infestation in live Norway spruce trees along the diversity gradient in 2018 (Figure S4a–c). There might be different underlying reasons for higher beetle infestation in more diverse plots: first, faster growth and a lack of variation in height of Norway spruce trees in diverse plots (Figure S5a–f) can make them more vulnerable to beetle infestation (Baier et al., 2002). Second, host trees experience associational susceptibility with a greater risk of infestation in the presence of other hosts in a mixture (Berthelot et al., 2021). Such spillover would only be possible if the same beetle species were present on the three tree species. On Norway spruce, it was almost certainly the European spruce bark beetle (I. typographus), which has occasionally been reported to infest Scots pine as well. However, for common ash, damaging beetles were most likely the lesser ash bark beetle (H. fraxini). Although we lack more precise data on beetle species among the different tree species in our experiment, it is less likely that associational susceptibility was responsible for greater pest infestation in diverse plots. Thus, greater demand for water, increased competition owing to drought, and perhaps also infestation by beetles might all have contributed to the greater tree mortality of Norway spruce in mixtures.
Our study sheds light on the critical role of species identity (Tobner et al., 2016) in shaping the responses of forest ecosystems to extreme drought. Notably, the vulnerability of dominant Norway spruce trees to the extreme drought highlights the complicated interactions between species identity, physiological traits, and stress tolerance. The contrasting response of the drought-tolerant species in this study, Scots pine and sessile oak, which increased in dominance during the drought, underscores the importance of species-specific adaptive strategies. These insights underscore the need to consider species identity when predicting the dynamics of biodiversity–ecosystem functioning relationships under changing climatic conditions.
Considering the increasing frequency and intensity of drought events as an important phenomenon associated with climate change, our study reveals that tree diversity is not a panacea in the context of global climate change effects on forests. Unlike grassland studies, the results of our experiment with temperate forest trees suggest that greater biodiversity does not generally provide greater resistance to drought. Though we did not measure resistance in our experiment directly (e.g., by experimentally manipulating water availability), we found negative effects of tree diversity on both productivity and tree survival during the drought year. The decreased survival of Norway spruce trees played a crucial role in the disruption of the diversity–productivity relationship in the drought year. Unanswered questions that might be addressed with larger more complex experimental designs in the future include how drought impacts may vary with community density, or the time since experiment establishment. Finally, additional research on the interaction between species diversity and species composition under benign and drought conditions is needed to guide the establishment of future forests that can conserve ecosystem services provided by the forest communities in the changing climate.
AUTHOR CONTRIBUTIONS
Tanvir Ahmed Shovon: Formal analysis; visualization; writing – original draft; writing – review and editing. Harald Auge: Conceptualization; data curation; funding acquisition; methodology; supervision; writing – original draft; writing – review and editing. Josephine Haase: Data curation; writing – review and editing. Charles A. Nock: Data curation; supervision; writing – original draft; writing – review and editing.
ACKNOWLEDGMENTS
The Kreinitz Experiment is a cooperative research project funded by the Helmholtz Centre for Environmental Research—UFZ. We especially thank Daniel Prati for his great commitment during the first years of the experiment, and the many other people who have assisted with the establishment and maintenance of the experiment and who are too numerous to be listed. We acknowledge the Departments of Community Ecology, Soil Ecology, Soil System Science, Environmental Microbiology, and Computational Landscape Ecology, and the team of the Bad Lauchstädt field station of the UFZ. In addition, we appreciate the support from the Experimental Interaction Ecology group of the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig in 2016–2018, Felix Gottschall and Ulrich Pruschitzki, as well as Kyle Kovach. For providing weather data, we thank Alexander Harpke (data of the experimental site 2010–2020) and the German Weather Service DWD (long-term averages 1981–2010, and monthly data 2008–2010). CAN recognizes funding from DFG Project no. 1225/2-1. CAN and TAS acknowledge the support of the Natural Sciences and Engineering Research Council of Canada (NSERC IRC; 550067-19).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Dryad at 10.5061/dryad.vt4b8gv08.




