Drivers of habitat availability for terrestrial mammals: Unravelling the role of livestock, land conversion and intrinsic traits in the past 50 years
Abstract
The global decline of terrestrial species is largely due to the degradation, loss and fragmentation of their habitats. The conversion of natural ecosystems for cropland, rangeland, forest products and human infrastructure are the primary causes of habitat deterioration. Due to the paucity of data on the past distribution of species and the scarcity of fine-scale habitat conversion maps, however, accurate assessment of the recent effects of habitat degradation, loss and fragmentation on the range of mammals has been near impossible. We aim to assess the proportions of available habitat within the lost and retained parts of mammals' distribution ranges, and to identify the drivers of habitat availability. We produced distribution maps for 475 terrestrial mammals for the range they occupied 50 years ago and compared them to current range maps. We then calculated the differences in the percentage of ‘area of habitat’ (habitat available to a species within its range) between the lost and retained range areas. Finally, we ran generalized linear mixed models to identify which variables were more influential in determining habitat availability in the lost and retained parts of the distribution ranges. We found that 59% of species had a lower proportion of available habitat in the lost range compared to the retained range, thus hypothesizing that habitat loss could have contributed to range declines. The most important factors negatively affecting habitat availability were the conversion of land to rangeland and high density of livestock. Significant intrinsic traits were those related to reproductive timing and output, habitat breadth and medium body size. Our findings emphasize the importance of implementing conservation strategies to mitigate the impacts caused by human activities on the habitats of mammals, and offer evidence indicating which species have the potential to reoccupy portions of their former range if other threats cease to occur.
1 INTRODUCTION
Habitat loss and degradation are among the main causes of decline of terrestrial species globally (Goncalves-Souza et al., 2020; Hoffmann et al., 2010; IPBES, 2019; Maxwell et al., 2016).The main factors contributing to these threats are the conversion of natural ecosystems into croplands and rangelands for livestock, the destruction of forests for timber and infrastructure development (Mair et al., 2021), primarily driven by a growing human population and demand for natural resources (Godfray et al., 2010; Williams et al., 2021). Total global cropland in 2019 was estimated at 1244 Mha (Potapov et al., 2022), much of which would have replaced natural ecosystems at some stage. 137 Mha of forests, shrublands and grasslands were cleared for agricultural purposes between 1992 and 2015 alone (van Vliet, 2019), with an additional expansion between 193 and 317 Mha estimated for 2050 (Schmitz et al., 2014). Agricultural expansion in tropical regions often comes at the expense of both intact and degraded forests, with consequent loss of associated ecosystem services, damage to biodiversity, emissions of large amounts of greenhouse gases and alteration of hydrological regulation (Raven & Wagner, 2021).
Globally, nearly a quarter of the Earth's terrestrial surface is dedicated to livestock farming, and a significant portion of this land consists of protected areas and remaining natural habitats (Bar-On et al., 2018; Filazzola et al., 2020). Worldwide, about 33 billion chickens, 1.5 billion cattle and 1 billion goats, sheep and pigs are reared for meat and dairy production (FAOSTAT, 2022), most commonly in industrial-scale livestock production systems. These require high density of livestock in relatively small areas where large amounts of excretory products are released, resulting in habitat degradation (Li et al., 2016; Vigiak et al., 2019). Livestock production potentially affects terrestrial biodiversity, which is usually low in intensively managed grassland and arable land used to grow livestock and their feeds (Petz et al., 2014). The main impacts of livestock production on biodiversity occur through land use changes, ammonia emissions and deposition and direct contribution to climate change via greenhouse gas emissions. In addition, habitat changes and fragmentation linked to livestock farming can lead to the interruption of gene flow and migratory routes, the replacement of native species with invasives and the emergence of infectious diseases (Fuller et al., 2012; Leip et al., 2015; Reid et al., 2010).
Assuming that all present anthropogenic areas were natural habitats in historical times, terrestrial ecoregions have lost, on average, 37% of vertebrate habitat. Estimates suggest that approximately 14% of mammals endemic to a region might be threatened with extinction due to habitat loss and degradation (Goncalves-Souza et al., 2020). Indeed, these are the main drivers of mammal decline and affect 88% of the species classified as threatened in the IUCN Red List (IUCN, 2022). In the last five decades, mammal populations have experienced marked declines, with many iconic species now surviving almost exclusively in protected areas (Pacifici et al., 2020), where the rate of human impact has been generally less severe (Leberger et al., 2020). Halting the destruction of natural habitats is pivotal to ensure the long-term survival of animal populations, but detecting fine-scale environmental changes has proved to be challenging. Increasingly accurate land cover detection systems have been developed in recent years, which helped convert land cover/use categories into habitat classes (Jung et al., 2020; Lumbierres et al., 2022). However, large-scale assessments of the role that habitat loss played in the recent range declines of animal populations have been relatively rare due to the paucity of reliable data on both the distribution of species and habitat available in the past.
Here, we analysed the extent of distribution range that has been retained and lost by 475 mammal species since the 1970s–1980s, looking for differences in the proportion of habitat available between the lost and retained parts. By habitat we mean here the suitable areas in which species occur (Kearney, 2006), as identified by IUCN categories of habitat types (IUCN, 2022), while retained range refers to the portion of the species' range where it lived in the 1970s–1980s and where it continues to exist. Our goal is to assess whether species' ranges have not just contracted, but whether there has been a proportionally greater reduction in habitat availability compared to the remaining range. Then, we evaluated the factors possibly influencing habitat availability in both the retained and lost parts of the species' ranges. Our hypothesis was that the lost portions of the range have less habitat available today compared to the retained portions, due to extensive negative impacts of extrinsic factors such as greater livestock density and more intense changes in land use that degraded the habitat. We also hypothesized that intrinsic traits play a minor role in determining the amount of habitat available for a species, and the most relevant ones would be those related to generalist attitudes and ability to occupy larger territories.
2 MATERIALS AND METHODS
2.1 Data collection and elaboration
The workflow of the analyses is summarized in Figure 1.
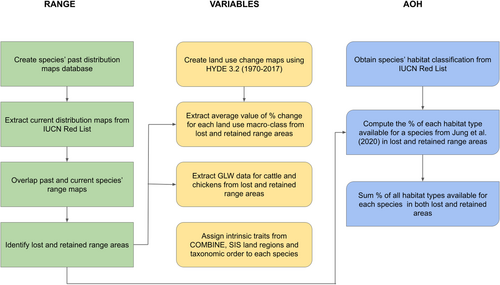
We expanded the Pacifici et al. (2019) database containing distribution ranges of 205 mammal species for the period 1970s–1980s at the global scale. Following their approach, we first conducted a literature search of the studies reporting past distributions of mammals for the two decades of interest. Then, we retained only those maps produced following current IUCN mapping standards and protocols and for which the 1970s–1980s distribution was compatible with textual descriptions of the past range found in the recent literature. As an additional step, all the new maps produced were reviewed by species' experts, who were often also involved in the current IUCN Red List assessments. This ensured not only that the maps produced were as accurate and reliable as possible, but also that they were built consistently with those used to create current IUCN Red List range maps. The current Red List range maps (IUCN, 2022) were used to compare the past and present distributions of each species and obtain the portions of the range that have been lost, retained and gained (if any) in the past 50 years. Comparisons of ranges were performed in GRASS GIS version 7.8.6 (GRASS Development Team, 2020).
All the predictive variables we used are reported and described in Table S1. In order to test the role of land use changes in the past 50 years in determining habitat availability, we extracted land use classes from the HYDE 3.2 History Database of the Global Environment (Klein Goldewijk et al., 2017). HYDE combines information on historical human population estimates and land use classes for the period 10,000 BCE–2017 CE. Those classes are defined as anthromes or anthropogenic biomes, which represent biomes that have been created and sustained by direct human interactions with ecosystems (Ellis & Ramankutty, 2008). The anthromes are divided into 20 classes of urban areas, villages, croplands, rangelands, semi-natural and wilderness areas (Ellis & Ramankutty, 2008). We extracted anthromes raster maps at a resolution of 5 arc minutes (ca. 10 km) for the years 1970 and 2017, reflecting the baseline periods for the past and current distribution maps. We reclassified the rasters by grouping the 20 classes of land use into four macro-classes: settlements (classes 11–24), croplands (classes 31–34), rangelands (classes 41–43) and natural areas (classes 51–63; see Table S2), to reduce the number of variables for statistical modelling. Then, we computed the differences in the proportion of each macro-class of land use between 1970 and 2017. In the extraction of the values for the environmental variables, we decided to use the original polygons for the species' ranges as masks, instead of converting them to rasters, to reduce the errors associated with the arbitrary choice of the range resolution.
Since livestock grazing is one of the most important factors contributing to habitat degradation and loss (Mair et al., 2021), we considered the Gridded Livestock of the World version 3 (GLW 3) database from FAO, which includes the global distribution of the major types of livestock (cattle, sheep, chickens, goats, etc.) in 2010, expressed as total number of heads per pixel at a resolution of 0.083 decimal degrees—roughly corresponding to 5 min of arc. We considered the densities of cattle (Gilbert, Nicolas, et al., 2018), chickens in intensive and extensive systems (Gilbert, Conchedda, et al., 2018) and sheep (Gilbert, Conchedda, et al., 2018) for our analysis because they are the most widespread types of livestock. As we did for the changes in land use, we extracted the average number of heads per pixel in the polygons of range retained and lost.
Assuming that some groups might be more sensitive than others to habitat changes, (e.g. ungulates directly feeding on the plants that are damaged or extirpated), we used the taxonomic order as an additional variable in our models. Since the areas where the species live can also be differently affected by habitat changes (e.g. countries in some regions have a higher relative loss of forest cover compared to the rest of the World; Global Forest Watch, 2022), we used the Land Regions identified by the IUCN Red List (e.g. South America, Europe, Northern Africa; IUCN, 2022) to test for differences in habitat availability in different parts of the world. Each species was assigned to the Land Region in which most of its range lost or retained fell. Finally, to assess whether species with different intrinsic traits differed in the amount of habitat available (inferring possible associations with sensitivity to habitat loss), we selected a set of 15 traits associated with biogeography (e.g. elevation range), behaviour (e.g. fossoriality, type of activity pattern, etc.), reproduction (e.g. litter size, weaning age, generation length, etc.), morphology (adult body mass) and specialization (altitude and habitat breadth) from the COMBINE database (Soria et al., 2021; Table S1). This database contains complete information for all mammal species for 21 traits, obtained by combining both raw and imputed data.
2.2 Area of habitat calculation
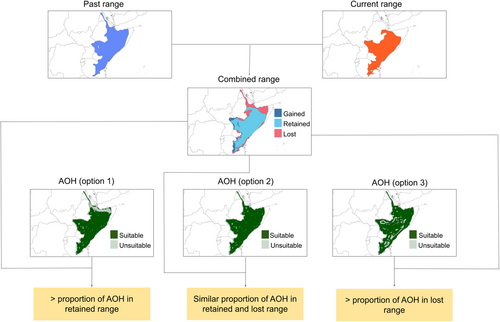
We calculated the amount of area of habitat (AOH, Brooks et al., 2019) using current terrestrial habitat types provided by Jung et al. (2020), which rely on the same habitat classification scheme used by the IUCN Red List (IUCN, 2022). ‘AOH’ is the habitat available to a species, usually considered within its current range (Brooks et al., 2019). This differentiates range—‘limits of distribution of a species, accounting for all known, inferred or projected sites of occurrence’ from habitat—‘the area, characterised by its abiotic and biotic properties, that is habitable by a particular species’ (Brooks et al., 2019). In order to assess if the amount of habitat available was different in the lost and retained portions of the species' ranges, we computed the amount of AOH for each species within the boundaries of the union between past and current ranges. We first downloaded the map of terrestrial habitat types version 4 from Jung et al. (2020), which contains 47 terrestrial habitat types as defined in the IUCN habitat classification scheme level 2 (more specific compared to level 1 broader classes). This map used the year 2015 as a reference and combined land cover, climate and land use data, reflecting level 2 IUCN habitat classes at a ~1 km spatial resolution. Each IUCN level 2 habitat class is assigned a unique value, which corresponds to the values of their final raster map. The main advantage of using the map from Jung et al. (2020) is that it makes the association with species' habitats very intuitive. We obtained the list of habitats for each species, as reported in the IUCN Red List, using the rredlist package in R (Gearty & Chamberlain, 2022). We extracted the list of all habitat classes from the Jung et al. (2020) map and then calculated the proportion of each class in both the retained and lost portions of the species' ranges using GRASS GIS. We then considered only those classes that were considered as habitat and summed the percentages. In this way, we obtained the proportion of habitat available for each species in the retained and lost parts of its range (see the three different types of options in Figure 2).
2.3 Statistical analysis
All analyses were performed using R Statistical Software (v4.1.2; R Core Team, 2021). To determine whether there was a difference in the total available habitat across species in the lost and retained parts of the range, we ran a two-sample Kolmogorov–Smirnov test (α = .05). The null hypothesis of the test assumes that the two samples belong to the same distribution. We ran two statistical models, one for the variables affecting habitat availability in the lost portions of the range, and another for the variables influencing habitat availability in the retained range. We applied generalized linear mixed models (package lme4 in R; Bates et al., 2015) to test these relationships, using chicken, sheep and cattle densities, change in anthromes and the intrinsic traits extracted from the COMBINE database (Soria et al., 2021) as fixed effects, and using the species' taxonomic order, and the Land Region where the species' range mostly occurs as random effect variables (Table S1). The percentages of habitat available, in both models and range area (lost or retained), were our response variables. We performed a variable selection based on the akaike information criterion (AIC) to identify the most parsimonious model.
Finally, to check whether there was a statistically significant difference in the percentage difference of habitat available between the lost and retained areas among taxonomic groups and Land Regions, we conducted a Kruskal–Wallis test (α = .05). This is a non-parametric method for testing whether samples originate from the same distribution. If the p-value was below the .05 threshold, we also performed a post-hoc Conover–Iman test with the R package conover.test (Dinno, 2017) to determine which taxonomic orders or Land Regions differed. This test shows an adjusted p-value for each pair of levels of these two categorical variables.
3 RESULTS
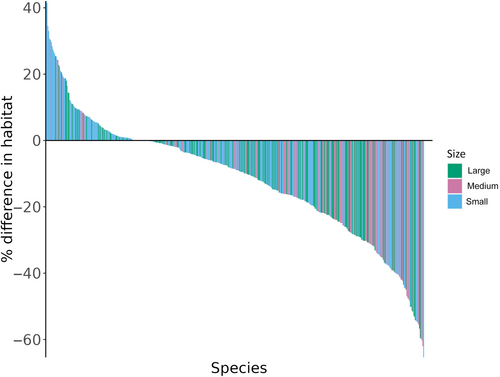
We obtained best guesses past distribution data for a total of 475 species of mammals, thus increasing the original Pacifici et al. (2019) sample size by 133%. Out of 475 species, 450 (Table S3) had both lost and retained areas in their range and, of those, 265 species (59%) had a lower proportion of habitat (>5% difference) in the lost range compared to the retained range. Only in 63 species (14%) the percentage of habitat available in the lost range was higher than that in the retained range (>5% difference), while for 122 (25%) species percentages were comparable (between −5% and +5%). The percentages of habitat available in the lost and retained range were significantly different (Kolmogorov–Smirnov p-value <.0001), as less habitat is available in the lost portions of the species' ranges compared to the retained portion for most of the species (Figure 3). This trend was similar in all of the taxonomic groups and Land Regions with the majority of species of our sample (Figures S1 and S2). The species with less habitat available in the retained range compared to the lost range were mostly small-bodied species (Figure 3).
The differences in habitat availability between lost and retained areas were significant among taxonomic groups (Kruskal–Wallis chi-squared = 34.443, p-value .011) but not among Land Regions (Kruskal–Wallis chi-squared = 16.167, p-value .14). However, when analysing each pair separately with the Conover–Iman test, some significant differences arose in both tests (Land regions and taxonomic groups, see Tables S4 and S5). All the median differences in the percentages of habitat available between the lost and retained areas showed negative values for all taxonomic orders (Table S6), with the exception of the Dasyuromorphia (0.98). The orders Tubulidentata (−59.6), Pholidota (−34) and Peramelemorphia (−22.1) were those in which this discrepancy was more evident, indicating that the percentage of habitat available in the lost range was much smaller compared to that in the retained range (Table S6).
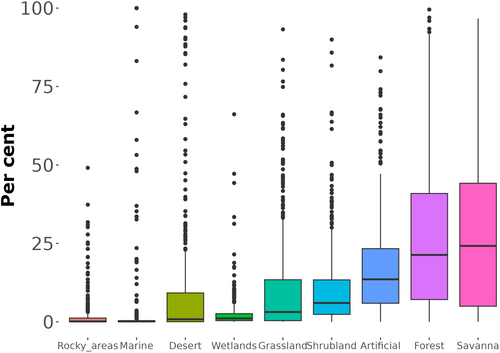
After grouping level 2 habitat types into nine level 1 IUCN habitat classes (rocky areas, marine, desert, wetlands, grassland, shrubland, savanna, forest and artificial), ‘savanna’ and ‘forest’ were the habitat types with greater proportions of area in the lost range (median 24.2% and 21.3% respectively; Figure 4). The ‘artificial’ macro-class was in third place (median 13.6%; Figure 4).
In the most parsimonious models, only taxonomic order was retained as random effect for both the lost (AIC 4166 compared to AIC 4060 of the full model) and retained portions of the range (AIC 4233 compared to AIC 4077 of the full model; Table S7). The amount of cropland that has not been changed into any other habitat type, chicken density in extensive systems, species size, habitat breadth, weaning age, litter size and litters per year were the significant fixed predictors of the lost model. For the retained model, the fixed significant predictors were cattle density, habitat breadth, litter size, litters per year, gestation length, weaning age, species average density (expressed as number of individuals/km2), altitude breadth and the conversion of natural land and cropland to rangeland (Table 1).
| Variable | Estimate | Standard error | p-Value |
|---|---|---|---|
| Lost range | |||
| Medium size | −12.89 | 4.10 | .0006 |
| Weaning age | 0.042 | 0.001 | .00004 |
| Habitat breadth | 4.69 | 0.04 | .000005 |
| Litters per year | 4.113 | 1.72 | .018 |
| Litter size | 2.055 | 1.05 | .053 |
| Chicken ext density | −0.003 | 0.0001 | .067 |
| Cropland | −0.272 | 0.06 | .0003 |
| Retained range | |||
| Cattle density | −0.003 | 0.001 | .007 |
| Natural to rangeland | −1.412 | 0.71 | .0482 |
| Cropland to rangeland | −2.644 | 1.27 | .0385 |
| Habitat breadth | 3.091 | 0.99 | .0021 |
| Litter size | 2.645 | 1.17 | .025 |
| Litters per year | 3.038 | 1.77 | .0883 |
| Gestation length | 0.053 | 0.023 | .0234 |
| Weaning age | 0.029 | 0.011 | .009 |
| Altitude breadth | 0.0037 | 0.001 | .0117 |
- Note: ‘Chicken ext density’ represents the density of chicken in extensive systems.
Livestock density was negatively associated with habitat availability in both models. In particular, high chicken density correlated with less habitat available in the lost parts of the range, and high cattle density correlated with less habitat available in the retained range. The latter is also supported by the fact that both conversions from natural land and cropland to rangelands were negatively associated with habitat availability. Concerning the effects of livestock grazing in each Land Region, several large mammals in South America (i.e. those above 15 kg) showed a higher cattle density in the lost range compared to the retained range while in some regions, like North America and Oceania, there is no evident variation (Figure S3).
Among the variety of traits considered, both proxies of reproductive timing (weaning age) and output (litter size, litters per year, gestation length) positively correlated with the amount of habitat available. Medium-size (compared to large) and habitat generalist species (compared to habitat specialists) conserved more habitat. Finally, mammals living at lower densities had more habitat available in the retained range compared to those living at higher densities (Table 1).
4 DISCUSSION
Our results show that the amount of habitat available in the lost parts of mammal species' ranges today is considerably lower than that in the retained portions of their ranges. Although we cannot demonstrate that the current amount of non-habitat in lost areas is proportional to the amount of habitat lost in the past 50 years, the trend we identified supports the hypothesis that there is a strong correlation between the two. As a result of the procedure followed to generate and define them, IUCN species ranges may include non-suitable habitat within their boundaries (Red List Technical Working Group, 2021). Given that we followed the IUCN Red List mapping standards for producing past distribution ranges, and that many of the experts who reviewed the maps were often involved in producing the current maps for a given species, we assume that the proportion of non-habitat included in the ranges in both time periods is comparable. Therefore, the difference found when using current habitats only should be due to recent habitat loss.
We found the largest differences in habitat availability in taxonomic orders apparently more subject to threats different from habitat loss. For example, both the pangolins (Pholidota) and the aardvark (Orycteropus afer) are heavily hunted for local and international markets (IUCN, 2022). Despite poaching being the major threat to these species, especially pangolins, it is important to consider that the areas they occupy have been seriously impacted by habitat loss in recent years (Romero-Muñoz et al., 2020). For instance, since 2017, Ghana and Cote d'Ivoire, countries where these taxa occur, ranked first and second in terms of their per cent increase in primary forest loss (60% and 26% respectively), while in the period 2010–2020 the Democratic Republic of the Congo had the second highest loss of tropical primary forest globally (−1,101,000 ha/year; FAO, 2020a).
Additionally, sample species living in sub-Saharan Africa showed more significant differences in habitat availability between lost and retained areas compared to those in North America. From the second half of the 1800s–1920s, temperate forests were the habitat type with the greatest losses, as human population growth was higher in today's rich countries and that generated a greater need for natural resources and land for agriculture (Mather & Needle, 2000). Afterwards, deforestation rates accelerated worldwide, mostly driven by losses in tropical areas, such as central Africa, South-East Asia and the Amazon basin (Hosonuma et al., 2012). The extensive growth of agriculture in sub-Saharan Africa that followed deforestation is strongly and positively correlated with increased extinction risk to endemic species (Perrings & Halkos, 2015).
In those cases where we did not observe substantial differences in habitat availability between lost and retained portions of the species' range, or a higher proportion of habitat in lost areas, we could assume that range losses of the species under consideration were not related to habitat decline. In Oceania for example, invasive alien species have been the main driver of loss (Doherty et al., 2016). In addition to that, most of the species in which we did not observe substantial differences occupy more than a single broad IUCN level 1 habitat class (e.g. forest, savanna, shrubland, etc.), meaning they are not habitat specialists and typically more tolerant to land cover changes. Range losses, in these cases, are usually modest and might be due to hunting and persecution, as in the case of small medium-sized carnivores (e.g. mongooses, felids) and widespread African ungulates. The fact that natural habitat classes are still well represented in lost areas supports this hypothesis and provides hope for future species recovery. Habitat restoration is one of the most complex conservation interventions as many factors have to be taken into account such as habitat requirements of a species, processes that maintain an ecologically functioning habitat over time (George & Zack, 2001), as well as the timing needed by natural vegetation to recover. However, if the habitat of a species is still available in a recently lost part of the range, conservation actions can promote the recolonization of those areas in relatively short times.
We also recognize that the presence of a relevant amount of habitat in the lost range can be partially influenced by the limitations associated with the AOH models. AOHs have two main issues, the first one is related to the low resolution of land use satellite data required for their construction (global satellite data exist at 100 m resolution, which is still not enough detail to capture some vegetation types), and the second one is linked to the scarcity of occurrence data available for some species to establish species–habitat relationships. For example, artificial land cover classes are challenging to distinguish from natural ecosystems (e.g. natural forests vs. plantations, cultivated fields vs. grasslands; Álvarez-Martínez et al., 2018) and we might have underestimated the true impact of land conversion to agriculture. We suggest considering the use of more sophisticated AOH models (e.g. with a spatial resolution of less than 50 m) that may become available in the future for spatial analyses of this type. However, we are confident that the result of low habitat availability we have obtained in the lost range may only be exacerbated when using finer resolution data.
When moving from extrinsic to intrinsic drivers, if a habitat offers abundant resources and favourable conditions for reproduction and survival, it can support the prolonged reproductive processes associated with longer gestation length and weaning age. Studies have indicated the influence of habitat availability on the reproductive success of terrestrial mammals beyond litter size and litters per year. For instance, a study by Clutton-Brock et al. (1982) investigated the relationship between habitat quality and reproductive parameters in red deer (Cervus elaphus). This study found that deer in high-quality habitats with abundant forage resources exhibited longer gestation periods and later weaning ages, suggesting a link between habitat quality and reproductive investment. Similarly, a study by Cameron et al. (2016) examined the reproductive traits of African elephants (Loxodonta africana) across different habitats and reported longer gestation lengths and extended lactation periods for elephants in habitats with higher resource availability and reduced competition. These extended reproductive processes allow the species to capitalize on the available resources, ensuring the successful development and survival of their offspring. Thus, these findings highlight the impact of habitat quality and resource availability on the reproductive strategies of terrestrial mammals.
Species that occupy areas suitable for agriculture, contain trees for timber production or are strategically located in terms of human infrastructure development, are usually more subject to habitat conversion. Being a habitat generalist reduces the likelihood that habitat loss affects the majority of the species' range simply because variety of habitats directly translates to greater availability of resources. Our results are in agreement with previous studies on other taxa that show that the number of generalist species increases regionally when the amount of the more natural habitat types declines, and the amount of other habitat types and edges increase within the landscape. This is likely primarily driven by the fact that competition is reduced in less natural habitats, and generalist species are the ones that predominantly occur (Banks-Leite et al., 2014; Jonsen & Fahrig, 1997; Stuart-Smith et al., 2021).
Our modelling of the variables associated with habitat availability in both lost and retained parts of the species' ranges supports an impact of livestock density on species' habitat availability. Livestock grazing can affect native species by reducing food available for herbivorous and granivorous mammals (Crowley & Garnett, 2001; Steen et al., 2005). Prolonged grazing can lead to irreversible changes to vegetation dynamics and nutrient cycling (van de Koppel et al., 1997), functionally altering the landscape by reducing its overall productivity. Small mammals can be impacted through the simplification of the vegetation structure (Ferreira et al., 2011; Mortelliti et al., 2010), which increases the risk of predation, and through trampling by introduced herbivores which may cause soil compaction, thus affecting the habitat quality for burrowing and fossorial species (Smit et al., 2001). The negative effects of livestock can spread across the food webs and indirectly affect predators (Huaranca et al., 2022; Vial et al., 2011). For example, the grazing of livestock in the Bale Mountains National Park (Ethiopia), with its influence on herbivorous rodents, is believed to have a detrimental impact on the endangered Ethiopian wolf Canis simensis, which specializes in preying on rodents (Vial et al., 2011).
A significant decline in the biomass of native large herbivores has been noted globally, largely due to growing livestock production, especially cattle (Ripple et al., 2015). Our results showed that large mammals in South America have significantly less habitat in the lost range compared to the retained range. Cattle grazing is expected to rise in the coming years in South America, which is home to two major beef producers, Brazil and Argentina (FAO, 2020b), and where natural vegetation such as in the Cerrado and in the Amazon is being cleared for this purpose (Alkemade et al., 2013). Large native herbivores such as camelids, cervids and the capybara (Hydrochoerus hydrochaeris) have already been negatively affected by the massive presence of cattle and sheep, mainly due to competition and habitat alteration (Di Bitetti et al., 2020). In fact, native herbivores were found to use foraging areas less often when cattle were present, and they also avoided being active at the same time as grazing cattle. In addition to persecuting wild herbivores, ranchers often engage in grassland burning to stimulate pasture regrowth, but this practice, which is favourable for cattle, decreases the complexity of vegetation and negatively impacts the occupancy probability of some species (Di Bella et al., 2011). These habitat alterations may have caused a contraction of the past species ranges and explain the net difference in habitat availability between lost and retained areas.
Chicken density in extensive farm systems can affect habitat availability in different ways. Extensive farm systems require a significant amount of land to accommodate the chickens. For instance, when comparing the land area required to produce 1 kg of protein from vegetables or legumes (e.g. soybeans) to the average land area needed for producing common cuts of meat, it is found that chicken requires three times more area (Röös et al., 2013). This can result in the conversion of natural habitats, such as forests or grasslands, into agricultural land. As a result, wild species that rely on these habitats for shelter, foraging or reproduction may lose their habitat and be displaced or unable to survive.
While sheep density was not a significant variable in our models, we recognize that the presence of sheep in several regions of the world negatively affected the distribution of many terrestrial mammals. In Patagonia, guanaco (Lama guanicoe) populations were extirpated from most of their range by the booming of sheep industry and recovered only when sheep ranching collapsed in the second half of the 20th century (Novaro & Walker, 2021). In Africa and Arabia, sheep ranching led to competition with wild herbivores, both through direct means such as food removal and indirectly through habitat degradation and loss (Keesing & Young, 2014). This competition becomes most apparent during periods of scarcity, such as severe droughts. However, it is important to acknowledge the limitations of the analyses conducted in this study. The reliance on livestock data, which is known to be little reliable for certain countries, presents a constraint on the accuracy of the findings. While these data represent the best available information at present, it is crucial to recognize the potential for improvement through further testing and the acquisition of better data. In future studies, it would be valuable to explore alternative data sources or conduct on-the-ground research to enhance our understanding of the complex dynamics between livestock density, habitat availability and the competitive interactions among terrestrial mammal species. We acknowledge that the livestock density estimates we used do not align precisely with the timeframe of current ranges, having 2010 as reference year. However, FAO's long-term consumption projections (FAO, 2018) indicate that total and per capita consumption of animal source foods in developing countries, excluding Africa, is expected to grow at a substantially slower annual rate than in the previous 40 years. Therefore, even though the growth rates of livestock production are projected to decrease, it will still be growth, albeit at a slower pace. Hence, we can assume that between 2010 and the present, there are unlikely to be substantial deviations from the densities we have used.
Our findings show that many species of mammals in our sample have a lower proportion of habitat in their lost range than in their retained range. Although we cannot directly attribute those differences to habitat loss, we found evidence that some factors were strongly associated with habitat availability. Lower habitat availability is likely influenced by changes in land use, increased livestock density and urbanization, all variables related to habitat deterioration and decline. Importantly, the datasets and approach we used can be easily applied to other taxa with well-known historical distributions. For instance, birds are typically extensively studied, and obtaining historical data for them can be relatively straightforward. Additionally, there is already available AOH data for this group (Lumbierres et al., 2022), and similar models for other taxa, such as amphibians and reptiles, are either under development or already accessible at the local scale (Nania et al., 2022). Our results not only highlight the need for effective conservation efforts to address the negative impacts of human activities on biodiversity and natural systems but also provide evidence on which species can potentially recolonize parts of their former range if other threats stop. In fact, for those mammals where a good amount of habitat has been conserved in their lost range, there is still hope for recovery if we identify and act on the other factors affecting their decline. It is essential not only for preserving mammals but also for conserving all those animal groups that interact with them through predator and competitor limitation, seed dispersal, etc. Targeted conservation actions are fundamental to mitigate and possibly reverse the negative trends some species have recently experienced in their population numbers and distribution ranges.
AUTHOR CONTRIBUTIONS
Michela Pacifici: Conceptualization; formal analysis; methodology; writing – original draft. Andrea Cristiano: Formal analysis; resources; writing – review and editing. Maria Lumbierres: Formal analysis; resources; writing – review and editing. Mauro Lucherini: Resources; writing – review and editing. David Mallon: Resources; writing – review and editing. Erik Meijaard: Resources; writing – review and editing. Sergio Solari: Resources; writing – review and editing. Marcelo F. Tognelli: Resources; writing – review and editing. Jerrold L. Belant: Resources. Thomas M. Butynski: Resources; writing – review and editing. Drew Cronin: Resources; writing – review and editing. Jean-Pierre d'Huart: Resources; writing – review and editing. Daniele Da Re: Resources; writing – review and editing. Yvonne A. de Jong: Resources; writing – review and editing. Arjun Dheer: Resources; writing – review and editing. Li Fei: Resources. Sonia Gallina: Resources; writing – review and editing. John M. Goodrich: Resources; writing – review and editing. Abishek Harihar: Resources; writing – review and editing. Carlos A. Lopez Gonzalez: Resources; writing – review and editing. Sarah R. B. King: Resources; writing – review and editing. Rebecca L. Lewison: Resources; writing – review and editing. Fabiano R. de Melo: Resources; writing – review and editing. Constanza Napolitano: Resources; writing – review and editing. Dede Aulia Rahman: Resources; writing – review and editing. Philip T. Robinson: Resources; writing – review and editing. Timothy Robinson: Resources; writing – review and editing. Carlo Rondinini: Conceptualization. Gono Semiadi: Resources; writing – review and editing. Karen Strier: Resources; writing – review and editing. Mauricio Talebi: Resources; writing – review and editing. William Andrew Taylor: Resources; writing – review and editing. Christine Thiel-Bender: Resources; writing – review and editing. Nelson Ting: Resources; writing – review and editing. Ingrid Wiesel: Resources; writing – review and editing.
ACKNOWLEDGEMENTS
M.P. thanks David Hewitt for providing valuable inputs on drawing species' past range maps. M.P. acknowledges financial support from Azione IV.6 ‘Dottorati e contratti di ricerca su tematiche green’. A.C. acknowledges financial support from the Research Development Fund Studentship Scheme provided by Northumbria University, project reference number RDF20/EE/GES/SUGGITT. M.L. has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 766417. C.N. wishes to thank funding from ANID PAI 77190064, ANID Fondecyt Regular 1220758, ANID/BASAL FB210018 and ANID/BASAL FB210006. D.C. thank the Government of Equatorial Guinea, the Universidad Nacional de Guinea Ecuatorial and the Bioko Biodiversity Protection Project, and has received funding from ExxonMobil Foundation, Mobil Equatorial Guinea, Inc. and the U.S. Fish and Wildlife Service.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest related to this research work.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.8362601, reference number 8362601.




