Fertilized graminoids intensify negative drought effects on grassland productivity
Abstract
Droughts can strongly affect grassland productivity and biodiversity, but responses differ widely. Nutrient availability may be a critical factor explaining this variation, but is often ignored in analyses of drought responses. Here, we used a standardized nutrient addition experiment covering 10 European grasslands to test if full-factorial nitrogen, phosphorus, and potassium addition affected plant community responses to inter-annual variation in drought stress and to the extreme summer drought of 2018 in Europe. We found that nutrient addition amplified detrimental drought effects on community aboveground biomass production. Drought effects also differed between functional groups, with a negative effect on graminoid but not forb biomass production. Our results imply that eutrophication in grasslands, which promotes dominance of drought-sensitive graminoids over forbs, amplifies detrimental drought effects. In terms of climate change adaptation, agricultural management would benefit from taking into account differential drought impacts on fertilized versus unfertilized grasslands, which differ in ecosystem services they provide to society.
1 INTRODUCTION
Grasslands cover 30%–40% of the global land area (Blair et al., 2014), and play a pivotal role in the provision of multiple ecosystem services to human society, including biodiversity, fodder production, nutrient cycling, and carbon sequestration (Squires et al., 2018). Drought events can strongly affect grassland structure, function, and ecosystem service provisioning (IPBES, 2019), and these events are already observed (Dai & Zhao, 2017) and projected (Cook et al., 2020) to increase in frequency and magnitude around the world. The magnitude of drought impacts varies widely depending on local climate and ecosystem properties such as biodiversity (De Boeck et al., 2018; Isbell et al., 2015) and nutrient availability (Bharath et al., 2020; Friedrich et al., 2012). While the role of climate in determining sensitivity of communities and ecosystems to drought has been intensively studied for several years (e.gEstiarte et al., 2016; Knapp et al., 2015), the influence of nutrient availability, biodiversity, and especially their interdependencies remains unclear and has been studied in only a handful of experiments (e.gStampfli et al., 2018; Wang et al., 2018).
While it is well established that nutrient limitation can constrain the CO2 fertilization effect on plant growth (Terrer et al., 2019), and multiple studies have demonstrated the important role of nutrients in warming responses (Dieleman et al., 2012), the direction and magnitude of the influence of nutrient availability on ecosystem responses to drought remain uncertain and disputed. High nutrient availability may mitigate drought impacts (Saneoka et al., 2004) under moderate droughts (Shi et al., 2018), by stimulating production of N-based osmoprotectants (Gessler et al., 2017), increased water use efficiency through enhanced RubisCo production (Guo et al., 2016), or by promoting a deeper absolute rooting depth (Gessler et al., 2017). However, other studies point to increasing grassland sensitivity to drought with increasing nutrient availability (Bharath et al., 2020; Kübert et al., 2019). This could result from a nutrient-induced decrease in the root:shoot ratio (Blanke et al., 2012) or reduced investment in mycorrhizae (symbiotic fungi that improve plant nutrition and water uptake; Cleland et al., 2019). In addition, increased transpiration following higher early-season productivity in nutrient-rich conditions can aggravate soil moisture depletion and hence increase plant mortality during drought later in the season (Friedrich et al., 2012; Kübert et al., 2019). One recent multisite study found higher sensitivity of absolute aboveground biomass production in nutrient-enriched plots across North American grassland sites (Bharath et al., 2020), but the question remains open as to how such overall response depends on differential drought sensitivities among species or functional groups within the plant community.
The effect of drought on grassland productivity can depend strongly on plant community composition, as plant functional groups may differ substantially in their drought response. Graminoids generally possess higher phenotypic plasticity in drought impact-relevant traits (e.g., specific leaf area) than forbs, which enables them to better cope with drought (Wellstein et al., 2017). Moreover, in the recovery phase after drought, graminoids may have a competitive advantage compared to forbs because of more rapid growth following uptake of water and released nutrients (Van Sundert, Brune, et al., 2020), and subsequent shading of the forbs (Stampfli et al., 2018). Forbs could be especially drought-sensitive under high land-use intensity with regular fertilizer application, while graminoid responses to drought are expected to be less dependent on fertilization regimes (Stampfli et al., 2018). As a result, drought can increase the dominance of graminoids over forbs, both during and after drought (Breshears et al., 2016; Hoover et al., 2014; Stampfli et al., 2018), further amplifying the typical promotion of graminoids compared to forbs under nutrient addition (Cleland et al., 2019).
Within the functional group of forbs, drought resistance can also differ between legumes (Fabaceae) and nonlegumes and also this difference may depend on nutrient availability. In N-limited environments, the process of symbiotic N2-fixation can put legumes at an advantage compared to nonlegumes (Ledgard & Steele, 1992), provided that other environmental conditions to support N2-fixation and plant growth are met. Such environmental conditions include temperature (Houlton et al., 2008), water availability (Serraj et al., 1999), and nutrients other than N, such as P (Mitran et al., 2018; Spehn et al., 2002). Drought typically has a negative impact on N2-fixation (Serraj et al., 1999), retarding legume growth (Daryanto et al., 2015). In combination with an often higher specific leaf area (Craine et al., 2001) than nonleguminous forbs in grasslands, drought effects can be expected to be more pronounced for legumes than for nonlegumes. This may be especially apparent in conditions where N2-fixation would normally put legumes at an advantage, such as high P (or K) but low N availability environments, or where P (or K) but not N was added. Legumes may then lose their advantage when drought impairs the fixation process.
Compared to single sites, distributed experiments with standardized protocol such as those within NutNet offer the advantage of generality of tested ecological theories along gradients in geography, climate, and species composition. Results from distributed experiments are also interpretable with less ambiguity than these of meta-analysis, because the latter often summarize heterogeneous data from experiments designed with various research goals in mind (Borer et al., 2017). Here, we use 10 European sites of the multi-year (n = 3–9) full-factorial nitrogen, phosphorus, and potassium (NPK) addition experiment of the Nutrient Network (Figure 1; Borer et al., 2014; 2017) to test whether and how (i) nutrient addition influences grassland sensitivity to drought, and (ii) detect differential effects among plant functional groups. Through inter-annual comparison of fertilized and unfertilized plots, we tested if and how fertilization (n ≥ 3 plots per treatment), and especially pure N (overall + 18% ANPP vs. ambient) and the combined NPK addition (overall + 37% ANPP vs. ambient) mediated the response of grassland communities and functional groups to drought in terms of aboveground biomass production.
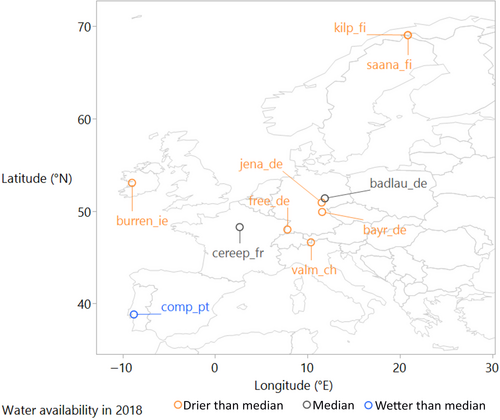
While fertilization and drought treatments were implemented at all sites (“main experiment”), a subset of sites implemented additional treatments (grazing exclusion, open roofs). These treatments were also included in our analyses and hypotheses were tested across both the “main” and other treatments, to verify the robustness of our results across the additional treatments. In addition to the inter-annual comparison of fertilized and unfertilized plots, we performed a separate analysis in which we focused on the year 2018 when large parts of the European continent experienced an exceptionally severe summer drought (Buras et al., 2020). We expected that (i) fertilization exacerbated the negative drought effect on annual community aboveground biomass production. We also expected (ii) negative drought effects to be more pronounced for forbs than for graminoids (i.e., grasses, rushes, and sedges), which would further promote dominance of graminoids under nutrient addition. Finally, we hypothesized (iii) more pronounced drought effects on legumes than on nonleguminous forbs, especially in treatments where P or K was added, but not N.
2 METHODS
2.1 Experiment description: The Nutrient Network (NutNet)
We used total and functional group aboveground net primary production (ANPP) data from 10 European grassland sites of the Nutrient Network, a standardized nutrient addition experiment (Borer et al., 2014; 2017), sampled from at earliest 2009, until 2018 (Figure 1—n = 3–5 plots per treatment and 3–9 years per site; Table 1). Grasslands were spread over Europe: four sites in Germany, two in Finland, and one each in Ireland, France, Portugal, and Switzerland. These sites were selected because all had actively running experiments during the European drought of 2018 and had more than 1 year of data.
| Site | Latitude (°) | Longitude (°) | Elevation (m) | Grassland type | n per treatment | Treatments other than nutrient addition | Approximatea sampling dates (DD-MM-YYYY) | N dep. (kg ha−1 year−1) | ANPPlive range (g m−2 year−1) | ANPPtotal range (g m−2 year−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| badlau_de | 51.39 | 11.88 | 120 | Old field | 3 | 03-07-2015d, 13-06-2016, 12-06-2017, 06-08-2018 | 14.0 | 200–650 | 256–808 | |
| bayr_de | 49.92 | 11.58 | 340 | Mesic grassland | 3 | Fencingb | 20-06-2016, 27-06-2017, 25-06-2018, 15-09-2016, 20-09-2017, 21-09-2018 | 15.7 | 48–372 | n/a |
| burren_ie | 53.07 | −8.99 | 112 | Calcareous grassland | 3 | Fencingb | 16-06-2015d, 02-08-2016, 20-07-2017, 01-08-2018 | 8.8 | 169–967 | 356–1380 |
| cereep_fr | 48.28 | 2.66 | 83 | Old field | 3 | Fencingb | 12-12-2012d, 05-09-2013, 09-10-2014, 10-09-2015, 06-09-2016, 22-09-2017, 21-09-2018 | 19.0 | n/a | 325–880 |
| comp_pt | 38.83 | −8.79 | 200 | Annual grassland | 3 | Fencingb | 11-06-2012d, 08-05-2013, 14-05-2014, 27-05-2015e, 20-05-2016, 10-05-2017, 10-05-2018 | 4.5 | 58–569 | 86–569 |
| free_de | 48.02 | 7.83 | 238 | Old field | 3 | Fencingb | 01-07-2016, 15-06-2017, 15-06-2018 | 4.0 | 175–869 | 304–1254 |
| jena_de | 50.93 | 11.53 | 320 | Mesic grassland | 3 | Roof controlc |
11-06-2014d, 08-06-2015, 06-06-2017, 04-06-2018 01-09-2014d, 31-08-2015, 30-08-2016f, 04-09-2017, 03-09-2018 |
15.5 | 127–789 | 153–863 |
| kilp_fi | 69.06 | 20.87 | 700 | Tundra grassland | 4 | Fencingb | 27-08-2013d, 13-08-2014, 04-08-2015, 30-07-2016, 28-07-2017, 29-07-2018 | 1.8 | 51–686 | 56–751 |
| saana_fi | 69.04 | 20.84 | 600 | Mountain grassland | 4 | Fencingb | 11-08-2014d, 05-08-2015, 31-07-2016, 31-07-2018 | 1.4 | 52–327 | 57–533 |
| valm_ch | 46.63 | 10.37 | 2320 | Mountain grassland | 3 | Fencingb | 06-08-2008d, 08-09-2009, 24-08-2010, 24-08-2011, 22-08-2012, 26-08-2013, 28-07-2014, 29-07-2015, 10-08-2016, 23-07-2018 | 18.9 | 51–712 | 0–978 |
| Site | Sand (%) | Silt (%) | Clay (%) | SOM (%) | Reference soil data | MAT (°C) | MAP (mm) | Median Pr (mm) | Median WB (mm) | Median Is | 2018 Pr (mm) | 2018 WB (mm) | 2018 Is |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| badlau_de | 38 | 56 | 7 | 4.3 | Altermann et al. (2005) | 9.3 | 523 | 97 | −172 | 60 | 97 | −209 | 56 |
| bayr_de | 40 | 44 | 16 | 6.3 | Personalg | 8.5 | 745 | 340 | −351 | 121 | 215 | −574 | 145 |
| burren_ie | 69 | 21 | 10 | 14.6 | UMNh | 9.8 | 1320 | 843 | +466 | 26 | 731 | +287 | 37 |
| cereep_fr | 70 | 20 | 10 | 2.5 | UMNh | 10.8 | 632 | 480 | −309 | 139 | 527 | −300 | 141 |
| comp_pt | 80 | 16 | 5 | 2.5 | Risch et al. (2019) | 16.6 | 564 | 606 | +132 | 99 | 606 | +258 | 60 |
| free_de | 30 | 51 | 20 | 5.0 | Personal1 + PIi | 11.4 | 934 | 345 | −54 | 46 | 345 | −54 | 46 |
| jena_de | 55 | 41 | 3 | 9.7 | UMNh | 8.6 | 654 | 283 | −397 | 127 | 282 | −509 | 135 |
| kilp_fi | 60 | 29 | 12 | 24.6 | Risch et al. (2019) | −3.3 | 569 | 101 | −16 | 12 | 38 | −57 | 12 |
| saana_fi | 61 | 34 | 5 | 27.1 | UMNh | −2.6 | 521 | 93 | −3 | 5 | 38 | −63 | 10 |
| valm_ch | 56 | 23 | 21 | 4.2 | UMNh | 0.1 | 681 | 283 | −73 | 49 | 226 | −116 | 57 |
- Abbreviations: ANPP, aboveground biomass production in control plots; Is, growing season soil drought index until sampling; MAP, mean annual precipitation; MAT, mean annual temperature; N dep., background atmospheric nitrogen deposition; Pr, growing season precipitation until sampling; SOM, soil organic matter concentration; WB, growing season water balance (Pr – PET) until sampling.
- a Exact dates varied across plots.
- b Fences excluding digging and medium-to-large-sized aboveground mammalian herbivores (Borer et al., 2014). In combination with Control and nitrogen, phosphorus, and potassium treatments.
- c Control rainout shelter treatment (no precipitation reduction), part of a combined drought experiment.
- d Pretreatment sampling. Data not included in the analyses.
- e Cattle disturbed the experiment. Data not included in the analyses.
- f Data from first sampling of the 2016 growing season were missing. Data from 2016 at this site were therefore not included in the analyses.
- g Samples collected and measurements performed by the lead author.
- h Samples processed at Waypoint Analytical (Memphis, TN, USA) at the behest of the University of Minnesota (USA), Department of Ecology, Evolution & Behavior.
- i Data provided by the principal investigator.
The sites were located between 38.83 and 69.06°N, with elevations ranging from 40 to 2320 m a.s.l., mean annual temperatures from −3.3°C to 16.6°C, and mean annual precipitation from 521 to 1320 mm. Grassland types varied, with temperate grasslands being the most common. Five of the sites were managed with varying mowing or grazing regimes. Site-specific information, such as grassland type, climate, sampling years and dates, soil organic matter content, and soil texture are given in Table 1.
The standardized treatment protocol involves a full-factorial annual N (10 g m−2 year−1), P (10 g m−2 year−1) and K (10 g m−2 year−1) addition experiment on 5 m × 5 m grassland plots. Along with K, a mixture of 100 g/m2 of micronutrients (1.5% Mg, 14% S, 0.2% B, 1% Cu, 15% Fe, 2.5% Mn, 0.05% Mo, 1% Zn) was added but only in the first year of treatment. At some of the sites, nutrient treatments were combined with a treatment of grazing exclusion (fencing), and/or “open roofs” that were used as controls of additional drought experiments. Data from such treatments were included in the present study to assess the robustness of our results across, for example, varying levels of grazing pressure.
Total biomass was sampled and separated into current year's graminoids (i.e., grasses, rushes, and sedges), leguminous and nonleguminous forbs, bryophytes, lichens, and woody vegetation once per year at most sites, usually at peak season, to estimate ANPP (g m−2 year−1). At sites bayr.de and jena.de, biomass was sampled both at the beginning of summer and at the end of the growing season; biomass values were then summed. Together with climate, background nutrient status, N deposition, species composition, etc., the sampling regime thus represents another source of uncertainty. Given that clear patterns emerged in spite of these differences among sites points toward a strong robustness of our findings. Two randomly placed 0.1 m2 quadrats were harvested on each plot, sorted to functional groups and dried at 60°C for at least 48 h.
2.2 General approach
To make responses and explanatory variables comparable across the sites, we normalized the annual biomass production and components per plot and year (nANPP) by taking the natural logarithm of each year's biomass divided by the mean value of all sampling years in that plot (n = 3–9 years per site; Extended Data Table 1). nANPP is similarly interpretable as the log-response ratio, a common metric used in meta-analyses, with values >0 indicating higher-than-average, and <0 lower-than-average biomass production. For the explanatory variable, we estimated a soil drought index (Is) that reflects the magnitude of drought experienced over the entire growing season at a site, based on daily weather (precipitation, potential evapotranspiration [PET]) and soil (texture, organic matter) data (see Section 2). For calculation of Is, we used the threshold proposed in Granier et al. (2007) and Vicca et al. (2012; relative extractable water [REW] = 0.4). In the absence of drought (i.e., REW > 0.4), Is equals zero. The longer and more intense a drought, the higher Is. For inter-site comparison, we expressed this as distance to the average by subtracting Is from the inter-annual mean (Diff Is = Is − mean Is for that site taken over all biomass sampling years; Diff Is > 0 means more drought stress than average, Diff Is < 0 means less drought stress than average). While actual soil moisture data were not available for most sites, such index based on “potential” soil moisture under unconstrained evapotranspiration offers the advantage that it takes into account among-site variation in the capacity of soils to store and supply water to biota. This soil influence is important for inter-site comparison but is ignored in analyses focused solely on water inputs (Vicca et al., 2014). We complemented our analyses using the soil drought index with results based on data of precipitation and the water balance (precipitation – PET) presented in Figure S2 and Table S1, which yielded qualitatively similar results.
2.3 Weather data and soil drought index
Daily minimum and maximum temperatures (Tmin and Tmax), and precipitation were measured on-site, or were collected from nearby weather stations which had data archived at the Global Historical Climatology Network (NOAA National Centers for Environmental Information). The growing season was then defined per calendar year as the period between the first span of at least 6 days with average temperature >5°C, and the first span of at least 6 days with average temperature <5°C (Zhang et al., 2018). Growing season degree days (GDD; °C days), precipitation (Pr; mm), water balance (WB; mm) and the soil drought index (Is) were then calculated from the start of the growing season until the day of sampling (the last sampling day was used where two biomass samplings per year occurred), to better represent the weather as experienced by the biota compared to annual full growing season meteorological data.
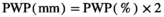
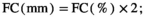
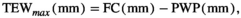
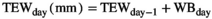
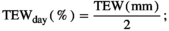
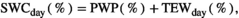
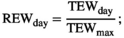
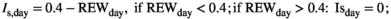
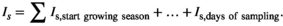
The “potential” SWC data (influenced by PET and Pr) were then compared to actual SWC measurements (influenced by actual evapotranspiration, Pr, horizontal and vertical water transport, etc.) from 2018 in Control plots at site free.de (Table 1), the only site and treatment with continuous SWC measurements over a full season. Our SWC estimates correlated strongly with actual SWC (Pearson's r = 0.81 for 5 cm soil depth; r = 0.73 for 20 cm soil depth; Figure S1).
In line with previous studies (Vicca et al., 2012), we suggest that drought stress indices from a soil moisture perspective (Is) better grasp the actual drought as experienced by the biota than solely meteorological data (Pr and WB). As advantages, we note for example (i) increases in the stress index only during periods when soil moisture is lower than a predefined threshold below which plants likely experience drought stress, (ii) higher suitability for inter-site comparison by taking into account differences in soil water holding capacity, (iii) avoiding a too strong influence of extreme precipitation events (e.g., a short heavy rainfall event cannot fully replenish soil water because some water is for example lost via runoff), and (iv) reduced sensitivity to slightly varying sampling times across years within one site (e.g., later than other years end-of-season sampling could be associated with higher precipitation compared to other years simply because of the longer growing season, while integrated soil moisture drought stress over the growing season and collected biomass may have been unaltered).
2.4 Data analyses
2.4.1 Nutrient treatment effects on plant community and functional group ANPP
In order to quantify overall effects of nutrient addition, and other treatment effects (fencing, roof control—included to verify the influence nutrient addition effects, irrespective of grazing exclusion) on the response variables of interest, linear mixed-effects models (packages lme4: Bates et al., 2015; lmerTest: Kuznetsova et al., 2017) were used. Fixed factors in the models were nutrient treatment, other treatments and their interaction. Random factors were site, year nested in site (since year, e.g., has different weather at different sites—year × site), and plot nested in site (plot × site), treatment (plot × treatment), and other treatment (plot × other treatment). For the ratio of graminoid to herbaceous (graminoid + forb) biomass production, constrained between zero and one, we used the function glmmTMB (Brooks et al., 2017) to perform mixed-effects beta-regression.
2.4.2 Interaction between drought and nutrients for community and functional group ANPP
We normalized the response (biomass) variables by taking the natural logarithm of the values per year and plot divided by the inter-annual average in that plot (normalized values abbreviated as “nANPP”). The explanatory variables (weather data) were normalized by subtracting the inter-annual average from the value (normalized values abbreviated as “Diff”). Normalized response and predictor variables were thus centered around “0,” hence allowing meaningful comparison of sites that differ in ANPP and climate. Based on these normalizations, we assessed how deviations from the average weather at a site influenced biomass production on a relative scale.
We derived statistics from the interaction between drought and nutrient additions from linear mixed-effects models. In the initial regression models, the influence of following fixed explanatory variables was tested on each of the normalized response variables: water availability (as Diff Is, Diff WB, or Diff Pr), nutrient treatment, other treatment (fencing, roof control), and the interactions water × nutrient treatment, water × other treatment, treatment × other treatment. The random structure included site, year (nested in site: year × site) and plot (nested in site [plot × site], treatment [plot × treatment], and other treatment [plot × other treatment]). Models were simplified based on backward elimination: clear nonsignificant interactions (p > 0.10) were removed from the fixed part, starting with the least significant interaction. Finally, in the models with Diff Pr, to test whether precipitation effects depended on temperature, also Diff GDD was included, with interactions with Diff Pr, treatment, other treatment, and Diff Pr × treatment. Diff GDD was not included in the Diff WB and Diff Is models because GDD, WB, and Is were all calculated partly based on the same Tmin and Tmax. Data of the year 2018 were analyzed analogously to the inter-annual analyses described here, but without including year and plot as random factors.
All statistical analyses were carried out in R version 3.6.0 (R Core Team, 2019). Model assumptions (linearity, normality of residuals, homoscedasticity, no outliers) were verified using standard functions in R. Marginal and conditional R2 from mixed models were calculated with function R.squaredGLMM from package MuMIn (Barton, 2019). Graphs were made with R package ggplot2 (Wickham, 2016), and the map with plotted locations of European NutNet sites was created using the JMP Pro 14 software (SAS Institute Inc., 2018).
3 RESULTS AND DISCUSSION
3.1 Graminoid response dominates nitrogen addition effect on community biomass production
Nutrient additions can alter functional group composition and thereby influence the drought sensitivity of the entire plant community. Nutrient-induced shifts in functional group dominance were indeed observed: fertilizer addition increased aboveground grassland biomass production and this response was mainly driven by the response of the graminoids (Figure 2; ANPPcommunity: F7,162 = 7.34; p < 0.001***); graminoids exhibited a clear, positive response to treatments that included N (ANPPgraminoid: F7,282 = 20.71; p < 0.001***), with the effect increasing when P and/or K were additionally included in the fertilizer mix. Forbs showed no nutrient addition effect on biomass production (ANPPforb: F7,123 = 1.00; p = 0.43), such that the dominance of graminoids in the herbaceous community increased under NK, NP, and NPK addition (ANPPgraminoid/herb: 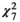 = 72.41; p < 0.001***). It has been suggested that strong positive effects of nutrient addition on ANPPgraminoid, but not ANPPforb can be ascribed to promoted growth of dominant grass species with resource-acquisitive traits (Blanke et al., 2012; La Pierre et al., 2016). Such grasses can then suppress a positive response of the forb functional group through increased competition for light (Hautier et al., 2009).
= 72.41; p < 0.001***). It has been suggested that strong positive effects of nutrient addition on ANPPgraminoid, but not ANPPforb can be ascribed to promoted growth of dominant grass species with resource-acquisitive traits (Blanke et al., 2012; La Pierre et al., 2016). Such grasses can then suppress a positive response of the forb functional group through increased competition for light (Hautier et al., 2009).
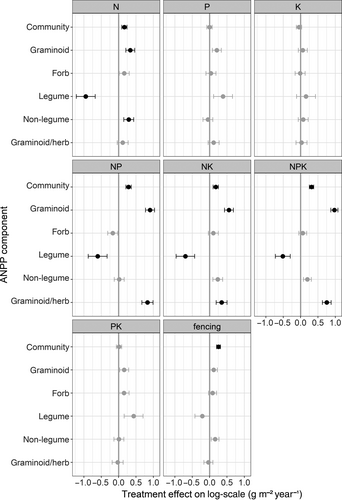
A shift occurred in the contribution of legumes versus nonlegumes to overall ANPPforb: ANPPlegume decreased under nutrient additions that included N (F7,252 = 7.14; p < 0.001***), while ANPPnonlegume showed no significant overall tendency (F7,249 = 1.62; p = 0.13). The advantage of legumes fixing atmospheric N2 thus appears less relevant when N is ample (Fu & Shen, 2016), increasing the impact of competition for light on the legumes (Hautier et al., 2009).
Fencing (grazing exclusion) treatments were combined with Control and NPK treatments at eight sites and were included in this study to verify the generality of our results on the interaction between drought stress and nutrient addition across European grasslands varying in grazing pressure, climate, plant species composition, etc. Grazing exclusion did not affect the ANPP of individual functional groups (p > 0.10) but had an overall positive effect on ANPPcommunity (F2,384 = 10.64; p < 0.001***), independent of NPK addition (F2,377 = 1.31; p = 0.27; Table 1). This result is in line with many earlier synthesis studies that found no interaction effect between nutrient addition and herbivory on community biomass (Borer et al., 2017; Gruner et al., 2008), despite that the interaction has been observed at the level of species composition and biodiversity (Borer et al., 2017; Brandt et al., 2019).
3.2 Drought effect on grassland ANPP depends on nutrient availability
Inter-annual analyses revealed that the soil drought index exhibited a significantly stronger negative effect on nANPPcommunity for NP and NPK addition than for other nutrient treatments (Figure 3a). In other words, NP and NPK addition aggravated drought effects on grassland ANPP. Also the water balance (precipitation minus PET) had a significant positive influence for NPK addition (+0.08 ± 0.04/100 mm), but not for other treatments (Table S1). Our results thus illustrate how NPK (and NP) addition can exacerbate the drought effect on aboveground biomass production.
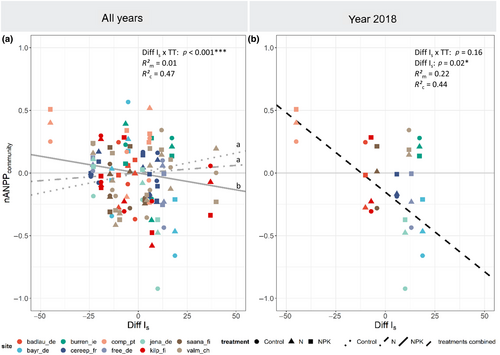
For the year 2018, when summer was extremely hot and dry at most sites, a strong negative relationship (−0.13 ± 0.05/10 drought index units) was found between the soil drought index and nANPPcommunity, irrespective of nutrient treatments (Figure 3b; Table S2). The overall drought effect was even stronger than in the NPK treatment of the inter-annual analysis. This observation may be ascribed to the particular extremity of the summer of 2018 (Buras et al., 2020), which seemed not completely grasped by the soil drought index (cf. the horizontal position of some points in Figure 3b). Substantial suppression of biomass production occurred at the dry sites, average production at a few sites with moderate drought and above-average biomass production at site comp.pt, which exhibited an anomalously wet growing season that year (Figure 1; Table 1). Drought effects did not depend on the nutrient treatment here, but this may be due to the limited sample size of this partial dataset.
Our continental-wide multisite comparison based on inter-annual data suggests a negative interaction effect between drought and nutrient availability. This interaction effect was clear not only on absolute, but also on relative-to-average values of aboveground grassland production (i.e., under drought, nANPP not only decreased more in NPK than in Control in absolute terms, but also in relative terms, suggesting a strong interactive effect). The negative interaction effect between drought and nutrients was most evident when comparing treatments including the most limiting nutrient (that is, N; Figure 2; Table S1) with ambient nutrient conditions. Our findings thus support previous, usually smaller scale or single-site studies suggesting that fertilization aggravates the impact of drought.
The negative interaction effect between drought and nutrients was reported in earlier studies focusing both on multi-species communities (Bharath et al., 2020; Kübert et al., 2019) as well as single species/monocultures (studies in grasslands but also other ecosystems; Friedrich et al., 2012; Meyer-Grünefeldt et al., 2015). A negative drought × nutrient interaction at the individual and population (i.e., within-species) level can, for example, occur through reduced root–shoot ratios under nutrient addition (Blanke et al., 2012) and increased pre-drought evapotranspiration associated with faster soil moisture depletion (Kübert et al., 2019). Such effects may counterbalance potentially positive nutrient influences on ANPP such as higher production of osmoprotectants (Saneoka et al., 2004), or deeper absolute rooting depth (Gessler et al., 2017). Our study used data from grassland communities where such individual species responses to drought, nutrient availability, and their interaction act concurrently with interspecific processes (e.g., release from competition).
Greater drought impacts on nutrient-rich than nutrient-poor grasslands can result in a shifting balance among ecosystem services at the landscape scale. Fertilized and non-fertilized grasslands differ in the services they provide; fertilization is typically more intensive in systems with provisioning services such as (fodder) production as primary use, whereas no input grasslands provide supporting or regulating services such as biodiversity and pollination (Zhao et al., 2020). In a future with more frequent and intense droughts, uncertainty of provisioning grassland services may therefore increase more than that of supporting and regulating ecosystem services. Depending on society's demands and preferences (e.g., maintaining high average but highly variable yield vs. more stable regulating services), shifts in the balance between high versus low intensity land use can be made as a climate adaptation strategy through adjustments of agricultural policies.
3.3 Nutrient addition intensifies negative drought effects on graminoids, but not forbs
Differential responses of plant functional groups played a significant role in shaping the drought impact on community productivity. Contrary to our expectations, drought had no effect on forb biomass but a negative effect on graminoids under some fertilization regimes (Figure 4a vs. 4b; Table S1). The negative impact of drought on graminoids (nANPPgraminoid) was found in NK (−0.09 ± 0.06/10 index units or +0.18 ± 0.09/100 mm), NP (−0.13 ± 0.06/10 index units or +0.18 ± 0.09/100 mm), and NPK (−0.13 ± 0.05/10 index units or +0.18 ± 0.09/100 mm) treatments but not in Control, N, P, and PK treatments (Figure 4a; Figure S2c; Table S1). Increasing drought resulted in a reduction of the proportion of graminoid to herbaceous (graminoid + forb) biomass production (nANPPgraminoid/herb) in fertilized N, NK, NP, and NPK treatments (Figure 4c; Table S1). Using the data subset from the extreme year 2018 only, increasing intensity of drought across sites was also associated with a reduction in the production of graminoids, albeit only marginally significantly (−0.15 ± 0.08/10 index units), and independently of nutrient addition (Figure 4d; Table S2). Forbs again showed no significant response to drought.
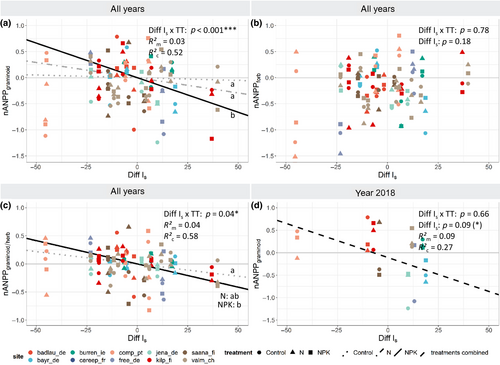
A higher impact of drought on graminoids than on forbs, and therefore reduced proportions of graminoids under drought (Figure 4c), contrasts with earlier studies that suggested stronger drought effects on forb biomass production than on graminoid biomass production (Breshears et al., 2016; Hoover et al., 2014; Reynaert et al., 2020; Stampfli et al., 2018; but see Kübert et al., 2019). We suggest that different mechanisms are at play that can either predispose or protect different functional groups against drought impacts. While higher phenotypic plasticity under drought in some traits such as specific leaf area may put graminoids at an advantage (Wellstein et al., 2017), their resource-acquisitive traits such as a shallow rooting system predispose common graminoids to reduced drought resistance, as opposed to forbs with deeper (tap)roots (Berendse, 1982; Zeiter et al., 2016). Moreover, also high initial evapotranspiration may promote soil moisture depletion in the shallow rooting zone of the graminoids, further amplifying drought stress (Kübert et al., 2019). Consequently, a graminoid-dominated plant community can become even more drought-sensitive under nutrient addition and this way drought can thus counteract graminoid dominance in nutrient-rich grasslands.
Besides variation in the role of mechanisms regulating drought sensitivity, differences in time scale could also partly explain apparent discrepancies in graminoid versus forb dominance between our study and others. The immediate drought response (resistance) may differ strongly from the response in the longer term that also includes recovery (Ingrisch & Bahn, 2018), and resistance and recovery often show opposite patterns (Bharath et al., 2020; Karlowsky et al., 2018; but see Wang et al., 2018). With our approach, we linked grassland responses to meteorological data of the same growing season. As a consequence, these responses reflect drought resistance, plus some within-year recovery depending on when the drought occurred relative to the biomass harvest. Recovery, however, is a phenomenon that occurs not only within a growing season, but continues over a multi-year time scale (e.g., Bharath et al., 2020). The yet limited time scale of the European NutNet data series does not allow disentangling resistance and recovery on a multi-year scale. Possibly, graminoid dominance recovers over longer periods (Stampfli et al., 2018), although a long-term drought-induced shift to a community with higher forb abundance is also possible, as was exemplified by a mesic meadow in Switzerland (Stampfli & Zeiter, 2004). In fact, such persistent changes may become more likely when droughts increase in frequency and intensity as a consequence of climate change (Frank et al., 2015).
Drought reduced aboveground biomass production of graminoids under NP and NPK addition, but not under ambient nutrient conditions (Figure 3a; Table S1). The differential drought sensitivity of graminoids versus forbs exclusively in fertilized plots therefore suggests that, in addition to the plant trait effects discussed above, a nutrient-induced increase in evapotranspiration associated with the graminoid biomass production (Figure 2) may have increased graminoid susceptibility to drought. Such fertilization effects cannot be captured by our drought indices and actual soil moisture data were not available for most sites. Further research is therefore needed to verify if soil moisture is indeed depleted more quickly in fertilized plots (e.g., Harpole et al., 2007), increasing drought severity and eventually, exacerbating reductions in biomass production.
The observation that no drought effect was found on forbs in fertilized plots, as opposed to negative drought effects on graminoids (Figure 3), may also be explained by release of forbs from competition (Connell, 1961). Under addition of N (plus P and/or K), graminoid biomass production is typically increased but not that of forbs because of shading by the graminoids (Hautier et al., 2009; Figure 2). However, when drought occurs, water limitation can suppress the growth of graminoids, and potentially also that of forbs. Reduced graminoid growth can then reduce competition for light which balances out the negative drought effect on forbs. Hence, whether experiments find apparent higher drought sensitivity of graminoids compared to forbs in a community may depend on the strength of competition between the functional groups, which in turn depends on nutrient availability. Such links among nutrient addition, drought, and shifts in interspecific interactions should be investigated further in experiments that manipulate all three factors.
3.4 Legumes are more responsive to drought than nonleguminous forbs
While the analysis on inter-annual variation showed no effect of the soil drought index on nANPPlegume or nANPPnonlegume (Figure 5a,b), the extreme year 2018 did reveal a significant positive effect of drought on legumes under NK and NPK addition, but not under other nutrient addition treatments (Figure 5c; Table S2). We suggest that the unexpected positive association between drought and legume production we observed may be an apparent effect, where drought suppressed biomass production of competitors (Figures 3 and 4), and in turn released legumes from competition (Connell, 1961). Interestingly, this effect seemed to be greatest where legume biomass was most suppressed (Figure 2).
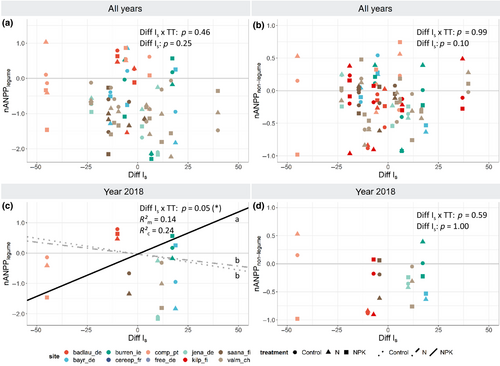
Although not the primary topic of this study, we note that a similar, positive interaction effect was found between fencing and drought on legumes, in both the 2018 dataset (+0.4 ± 0.1/10 index units; Table S2) and the inter-annual analysis (+0.13 ± 0.04/10 index units; Table S1). While fencing can have positive effects on legume abundance if herbivores strongly prefer grazing on N-rich legumes (Ritchie & Tilman, 1995), such positive fencing effect was not found in our study. Instead, we found a tendency for a negative influence of fencing (Figure 2). Possibly, fenced plots that prohibit grazing can strengthen competition for light from fast-growing graminoids and thereby reduce legume productivity. Drought may have suppressed such light competition effect on graminoids, causing an apparent positive effect on legumes. Interactions of drought with fencing on other functional groups and on the whole plant community were absent or more ambiguous than interactions with nutrient addition (Tables S1 and S2).
Legumes did not show an overall response to inter-annual variation in the soil drought index (Figure 5a,b), but the method using the water balance suggested a positive water availability effect on this functional group (+0.3 ± 0.2/100 mm), and the method using precipitation indicated positive precipitation effects on legumes only under P (+0.6 ± 0.2/100 mm) and PK addition (+0.4 ± 0.2 /100 mm). Nonlegumes were unresponsive (Table S1). This result would be in line with our hypothesis of increased drought impacts (or at least positive precipitation effects) on legumes compared to nonlegumes, especially under nutrient additions without N. These are treatments where legumes are expected to profit most from their N2 fixing capacity. Possibly, drier conditions suppressed symbiotic N2-fixation, either directly because of water limitation (Serraj et al., 1999), or indirectly due to hampered P uptake (Mariotte et al., 2020) by legumes with typically high P demand (Mitran et al., 2018). The legume advantage under P and PK addition over other forbs (Figure 2) may thus reduce with decreasing water availability. Further research is needed to verify these speculations.
3.5 Beyond functional groups: Biodiversity, nutrient addition, and drought impacts
We found that grassland responses to soil drought stress depend on both nutrient availability and functional group composition. The importance of plant community composition in shaping the drought response is in line with the diversity–stability hypothesis. Much of the literature on this hypothesis refers back to the work of Tilman and Downing (1994), who found greater drought resilience in more species-rich plots of a long-term N addition experiment with gradient design. Species richness declined under N addition in that experiment, but the positive richness–resilience link was found to be stronger than the negative N addition–resilience or grass biomass–resilience links, emphasizing the importance of plant diversity for drought resilience. Later studies pointed to components beyond richness underlying the diversity–stability link, such as species asynchrony and trait diversity that both have a strengthening effect on the relationship (Craven et al., 2018; Weisser et al., 2017). Nutrient addition has been shown to reduce species asynchrony and therewith community stability, even in the absence of substantial changes in overall richness (Hautier et al., 2014, 2020). For future biodiversity-oriented research, we suggest that in-depth evaluations of responses of grassland productivity, species traits and asynchrony to the global changes drought, nutrient addition, and their interaction can shed more light on how nutrients and biodiversity shape community drought responses.
4 CONCLUSION
Our findings demonstrate that nutrient availability and plant functional group composition can interdependently regulate grassland responses to climate change. High nutrient availability increased community and graminoid sensitivity to drought, likely driven by both increased soil moisture depletion and higher dominance of plants with resource-acquisitive traits. For future research, we recommend (i) taking into account functional groups when assessing ecosystem responses to global change, as well as (ii) nutrient availability (e.g., Folberth et al., 2016), for which nutrient status-related soil properties need to be measured (Van Sundert, Radujković, et al., 2020; Vicca et al., 2018). We also recommend (iii) measuring soil water content to allow a quantification of drought stress from a soil perspective. Taken together, our results highlight important interactive effects of nutrient enrichment and drought on plant biomass production. These have the potential to substantially alter future grassland functioning under global change and hence the ecosystem services that grasslands provide.
ACKNOWLEDGMENTS
This work was generated using data from the Nutrient Network (http://www.nutnet.org) experiment, funded at the site-scale by individual researchers. Coordination and data management have been supported by funding to E. Borer and E. Seabloom from the National Science Foundation Research Coordination Network (NSF-DEB-1042132) and Long Term Ecological Research (NSF-DEB-1234162 and NSF-DEB-1831944 to Cedar Creek LTER) programs, and the Institute on the Environment (DG-0001-13). We also thank the Minnesota Supercomputer Institute for hosting project data and the Institute on the Environment for hosting Network meetings. K.V.S., J.S., and S.V. acknowledge support from the Fund for Scientific Research (FWO), Flanders (Belgium). A.E. was funded by the Academy of Finland (projects 253385 and 297191). This work has benefited from technical and human resources provided by CEREEP-Ecotron IleDeFrance (CNRS/ENS UMS 3194) as well as financial support from the Regional Council of Ile-de-France under the DIM Program R2DS bearing the reference I-05-098/R. It has received a support under the program “Investissements d'Avenir” launched by the French government and implemented by ANR with the reference ANR-11-INBS-0001 AnaEE France and ANR-10-IDEX-0001-02 PSL. The German study site at the university of Bayreuth was supported by the German Ministry for Education and Research (BMBF) funding the SUSALPS project “Sustainable Use of Alpine and Pre-alpine Grassland Soils in a Changing Climate”; under Grant number: FKZ 031B0516C. We acknowledge Companhia das Lezírias (Portugal) for permission to undertake grassland research and FCT for funding CEF (UID/AGR/00239/2019).
AUTHOR CONTRIBUTIONS
Developed and framed research questions: Kevin Van Sundert, Ivan Nijs, and Sara Vicca. Analyzed data: Kevin Van Sundert. Contributed to data analyses: Kevin Van Sundert, Siddharth Bharath, Angelika Kübert, Ivan Nijs, Anita J. Porath-Krause, Dajana Radujkovic, and Sara Vicca. Wrote the paper: Kevin Van Sundert and Sara Vicca. Contributed to paper writing: Siddharth Bharath, Yvonne Buckley, Maria da Conceição Caldeira, Ian Donohue, Maren Dubbert, Anne Ebeling, Nico Eisenhauer, Anu Eskelinen, Alain Finn, Tobias Gebauer, Sylvia Haider, Amandine Hansart, Anke Jentsch, Angelika Kübert, Ivan Nijs, Charles A. Nock, Carla Nogueira, Anita J. Porath-Krause, Dajana Radujkovic, Xavier Raynaud, Anita C. Risch, Christiane Roscher, Michael Scherer-Lorenzen, Max A. Schuchardt, Martin Schütz, Julia Siebert, Judith Sitters, Marie Spohn, Risto Virtanen, Christiane Werner, and Peter Wilfahrt. Site coordinator (data collection): Mohammed A. S. Arfin Khan, Yvonne Buckley, Maria da Conceição Caldeira, Ian Donohue, Anne Ebeling, Nico Eisenhauer, Anu Eskelinen, Alain Finn, Tobias Gebauer, Sylvia Haider, Amandine Hansart, Charles A. Nock, Xavier Raynaud, Anita C. Risch, Christiane Roscher, Michael Scherer-Lorenzen, Martin Schütz, Julia Siebert, Marie Spohn, Risto Virtanen, and Christiane Werner. Nutrient Network coordinator: Peter Wilfahrt.
Open Research
DATA AVAILABILITY STATEMENT
R code and data header are available via https://github.com/KevinVanSundert/NutNet_2021_KVS. Data from the Nutrient Network are made publically available on a 3-year moving window (nutnet.org/data). The full dataset is available upon request.




