Climate change alters elevational phenology patterns of the European spruce bark beetle (Ips typographus)
Abstract
The European spruce bark beetle Ips typographus is the most important insect pest in Central European forests. Under climate change, its phenology is presumed to be changing and mass infestations becoming more likely. While several studies have investigated climate effects across a latitudinal gradient, it remains an open question how phenology will change depending on elevation and topology. Knowing how an altered climate is likely to affect bark beetle populations, particularly across diverse topographies and elevations, is essential for adaptive management. We developed a time-varying distributed delay model to predict the phenology of I. typographus. This approach has the particular advantage of capturing the variability within populations and thus representing its stage structure at any time. The model is applied for three regional climate change scenarios, A1B, A2 and RCP3PD, to the diverse topography of Switzerland, covering a large range of elevations, aspects and slopes. We found a strong negative relationship between voltinism and elevation. Under climate change, the model predicts an increasing number of generations over the whole elevational gradient, which will be more pronounced at low elevations. In contrast, the pre-shift in spring swarming is expected to be greater at higher elevations. In comparison, the general trend of faster beetle development on steep southern slopes is only of minor importance. Overall, the maximum elevation allowing a complete yearly generation will move upwards. Generally, the predicted increase in number of generations, earlier spring swarming, more aggregated swarming, together with a projected increase in drought and storm events, will result in a higher risk of mass infestations. This will increase the pressure on spruce stands particularly in the lowlands and require intensified management efforts. It calls for adapted long-term silvicultural strategies to mitigate the loss of ecosystem services such as timber production protection against rockfall and avalanches and carbon storage.
1 INTRODUCTION
Forest ecosystem dynamics are being greatly impacted by climate change worldwide (Ayres & Lombardero, 2000; Dale, Joyce, McNulty, & Neilson, 2000; Dale et al., 2001; Fettig et al., 2013; Fuhrer et al., 2006). Disturbance events, such as storms, bark beetle infestations, droughts and wildfires, have become more frequent and severe (Schelhaas, Nabuurs, & Schuck, 2003; Seidl, Schelhaas, & Lexer, 2011; Seidl, Schelhaas, Rammer, & Verkerk, 2014). The phenology, distribution and interactions of many species have also changed (Battisti & Larsson, 2015; Parmesan, 2006; Walther et al., 2002). Particularly ectotherms like insects are strongly affected by climate change, because their development, reproduction and survival are directly related to temperature (Bale et al., 2002; Boggs, 2016; Logan, Régnière, & Powell, 2003; Volney & Fleming, 2000). Hence, in a warmer climate, outbreaks of forest insect pests are likely to become more frequent and intense (Logan et al., 2003). Therefore, it is crucial to assess such effects on economically important forest pest species. This is particularly challenging in areas where elevation, slope and aspect vary greatly resulting in considerable small-scale climate variability in, for example, the rugged topography of the Alps.
Increasing temperatures have been identified as the most significant climate change factor directly affecting insect herbivores (Bale et al., 2002; Volney & Fleming, 2000). They can lead to increased developmental rates and voltinism (Altermatt, 2009; Porter, Parry, & Carter, 1991; Pöyry et al., 2011), extended season lengths, increased winter survival and changes in interspecific interactions, for example, with their host plant and antagonists (Ayres & Lombardero, 2000; Bentz et al., 2010; Raffa et al., 2015; Wermelinger, Epper, Kenis, Ghosh, & Holdenrieder, 2012). Moreover, specific species may shift or extend their distribution range (Bale et al., 2002; Harrington, Fleming, & Woiwod, 2001) towards higher elevations and latitudes, and increase their voltinism at all ranges (Hlásny & Turčáni, 2009; Netherer & Schopf, 2010).
The European spruce bark beetle (Ips typographus L., Curculionidae, Scolytinae) is a major disturbance agent affecting the large-scale dynamics of Norway spruce forests (Picea abies [L.] Karst.), particularly in Central Europe and Scandinavia (Christiansen & Bakke, 1988). It is one of the few European insects considered to be a significant forest pest (Grégoire & Evans, 2004; Økland, Netherer, & Marini, 2015; Wermelinger, 2004). In coniferous forests, it is by far the most destructive species, capable of mass infestations killing thousands of mature Norway spruce trees. During the last few centuries, the spruce bark beetle has infested large areas of spruce forest all over Eurasia (Grégoire & Evans, 2004; Kausrud et al., 2012; Økland et al., 2015; Stadelmann, Bugmann, Wermelinger, Meier, & Bigler, 2013; Wermelinger & Jakoby, 2019), causing 8% of the total forest damage in Europe recorded between 1950 and 2000 (on average 2.9 million m3 wood per year; Schelhaas et al., 2003). Currently, such outbreaks occur almost exclusively after disturbance events such as storms or extensive drought periods.
While windstorms may provide large amounts of breeding substrate, temperature is the most important factor influencing the beetles’ life cycle (Annila, 1969). Thermal conditions directly drive development and reproduction rates (Netherer & Pennerstorfer, 2001; Wermelinger & Seifert, 1998, 1999), daily flight activity (Lobinger, 1994) and hence, the beetles’ phenology and voltinism. In Central Europe, I. typographus is usually bivoltine and starts spring swarming in April or May, but may even produce a third generation in extremely warm years (Wermelinger et al., 2012) given the required temperature sums at lower elevations. At higher elevations (and latitudes), beetle populations are to a large part univoltine and swarming starts later in the season. Global warming is likely to affect these patterns and thus the risk of outbreaks.
Modelling approaches are widely used to predict insect phenology and voltinism, especially in relation to climate change (e.g. Jönsson, Pulatov, Linderson, & Hall, 2013; Ziter, Robinson, & Newman, 2012). Particularly for bark beetles, modelling has been used in a number of studies (see Bentz & Jönsson, 2015; Seidl, Fernandes, et al., 2011 for overviews). Two phenology models have been developed for I. typographus in Central Europe and Scandinavia (Baier, Pennerstorfer, & Schopf, 2007; Jönsson, Harding, Bärring, & Ravn, 2007). Both are based on the accumulation of heat sums, which does not allow to represent the dynamics of the population structure, including the distribution of developmental stages, and, therefore, focus on single representative individuals. These two models have also been applied to investigate the effects of global warming on the beetles’ life cycle and disturbance regimes (Bentz et al., 2019; Berec, Dolezal, & Hais, 2013; Jönsson, Appelberg, Harding, & Bärring, 2009; Jönsson et al., 2007; Jönsson, Schroeder, Lagergren, Anderbrant, & Smith, 2012; Seidl, Schelhaas, Lindner, & Lexer, 2009; Temperli, Bugmann, & Elkin, 2013), but they focus on either hypothetical changes in climate of single locations or regional climate scenarios with a coarse spatial resolution. Also, most studies (apart from that of Jönsson & Bärring, 2011) did not account for uncertainty in climate model predictions, although there is great uncertainty, particularly about how local climate will change and how this uncertainty will propagate to the modelling results of forest insect pests (Neuvonen & Virtanen, 2015). For historically univoltine bark beetle populations, the latitudinal change in voltinism was analysed under climate change (Bentz et al., 2019; Jönsson et al. 2009, 2011). However, besides latitude, also topography, that is, elevation, slope and aspect, influences temperature which is the main driver of bark beetle development. For example, elevation was found a main factor driving the risk of bark beetle damage (Faccoli & Bernardinelli, 2014). Changes in bark beetle phenology in relation to slope, aspect and elevation have, however, never been studied across a wide topographical range.
We investigated the effects of climate change on the phenology of I. typographus in a highly diverse topography, taking Switzerland as a case study region representative for Central Europe. It has a rugged relief with a wide range of elevations, slopes and aspects and spruce covering a large elevational range from 250 m to more than 2,200 m a.s.l. Moreover, regional climate projections for Switzerland are available for different emission scenarios with a high resolution of 2 × 2 km (CH2011, 2011). We developed a new phenology model, which allows a detailed temporal representation of population structures and flight patterns at local, regional and national scales. In this study, we (a) analyse the effects of climate change on the phenology and voltinism of the European spruce bark beetle, (b) assess the influence of the varied topography on these effects and (c) draw conclusions about how a change in phenology and voltinism might affect the risk of bark beetle infestations in European forests.
2 METHODS
2.1 Study area
Switzerland has a diverse and rugged topography with a large range of elevations (Figure 1). It can be geographically structured into the Central Plateau (on average 400–600 m a.s.l.), Jura Mountains (up to 1,700 m a.s.l.), Northern Pre-Alps (up to 2,500 m a.s.l.), Central Alps (up to >4,000 m a.s.l.) and Southern Alps (up to 3,000 m a.s.l.), including deep valleys with steep slopes. This means that the temperature conditions greatly vary both within a given slope and between north and south facing slopes with different solar radiation.
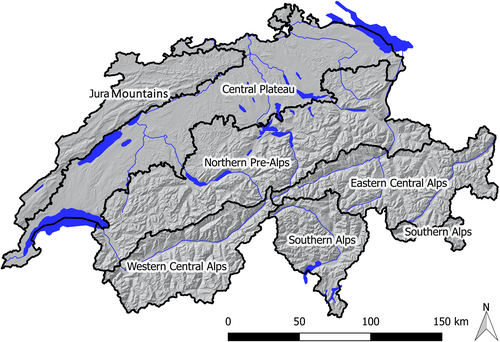
Norway spruce (Picea abies [L.] Karst) is by far the most abundant tree species in Switzerland and is present in all geographic regions. It is dominant in the higher elevations of the Alps and has the least proportion in the southern parts of Switzerland, being virtually absent in the southernmost areas of the country.
2.2 Description of phenology model
We used a time-varying distributed delay model (Manetsch, 1976) adapted to the biology of the European spruce bark beetle to analyse its phenology depending on climatic factors (for details on the beetles’ biology see, Christiansen & Bakke, 1988; Kausrud et al., 2012; Wermelinger, 2004). This approach is frequently used to describe the temporal development of insect and plant populations (e.g. Gutierrez, Pizzamiglio, Santos, Tennyson, & Villacorta, 1984; Gutierrez & Ponti, 2014; Stoeckli et al., 2012; Wermelinger, Candolfi, & Baumgärtner, 1992). The model simulates the seasonal distribution of developmental stages within a bark beetle population as well as the timing and distribution of adult flight according to local temperature. Simulations allow for an assessment of the potential number of generations (voltinism) and changes in the timing of swarming periods can be derived. The model allows both a short-term assessment of the population structure (i.e. distribution of developmental stages and generations) under the current climate and projections under long-term climate change scenarios. It should be noted that the model focusses on phenology, not on absolute population dynamics, and thus ignores population dynamical processes such as mortality and population increase.
2.3 Model structure
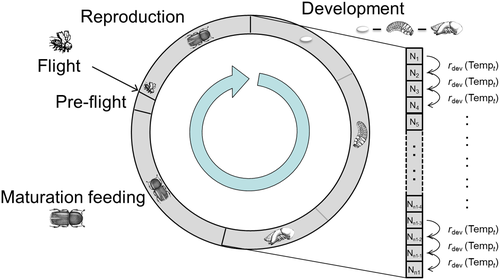
The entities in the model are individuals of I. typographus. They develop through different stages of the beetle's life cycle (Figure 2). Each stage is represented by a discrete number of substages in the developmental process (so-called slots). The individuals develop in hourly time steps from one substage to the next in a sequential order, with time-varying transition probabilities depending on temperature and, if applicable, photoperiod. The model covers four different life cycle stages (Figure 2): (a) the development stage from egg to pupa, (b) maturation feeding of teneral beetles, (c) fully developed beetles ready to fly (preflight) and (d) beetles during the flight and reproduction phase. After reproduction, a certain percentage psb of the beetle population produce a sister generation, that is, they conduct regeneration feeding (starting in slotsb of the maturation feeding stage), colonize another host tree and reproduce a second time. Depending on the length of the photoperiod and modulated by temperatures, adult beetles enter diapause in autumn, which prevents flight until the next year.
2.4 Detailed description of model processes
The time-varying transition probabilities r between substages (slots) are identical within one life cycle stage j, and depend on temperature and, if applicable, photoperiod. Accordingly, the number of individuals Ni in each slot i = 1, 2, …, n (n = total number of slots over all stages) at time t is determined by the number of individuals in this slot at the previous time step Ni(t − 1) plus those entering and minus those leaving the slot. The number of individuals that develop from one slot to the next is described by a stochastic, binomial process, where Ni(t − 1) is the number of trials and rj(t) is the success probability (i.e. the transition probability r of stage j at time t). The temperature-dependent transition probabilities in the different stages are described by nonlinear functions (Equations 1-4). All transition probabilities depend on hourly air or phloem temperatures. The hourly air temperatures T(t) are derived using a sine interpolation between the daily minimum (Tmin(t)) and maximum (Tmax(t)) air temperature. To convert air temperature to the corresponding phloem temperature Tp(t), the model applies a function using Newton's Law of Cooling (for details, see Trần, Ylioja, Billings, Regniere, & Ayres, 2007).
2.5 Temperature-dependent development, maturation feeding, and reproduction
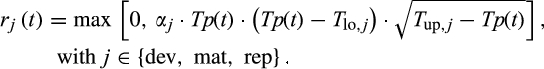 (1)
(1)The parameter α is a scaling factor. Tlo and Tup, respectively, define the temperatures below and above which no developmental progress occurs.
The number of substages (slots) required in each stage was calculated according to the method of Severini, Baumgärtner, and Limonta (2003) using data from laboratory experiments of Wermelinger and Seifert (1998, 1999). Accordingly, the stage of egg–larva–pupa development is subdivided into ndev = 71 slots, and the stage of maturation feeding into nmat = 8 slots. Using data from oviposition experiments (Wermelinger & Seifert, 1999) and assuming an additional average preoviposition period of 72 hr, we calculated a number of nrep = 11 slots between the start of swarming and oviposition.
2.6 Temperature-dependent flight
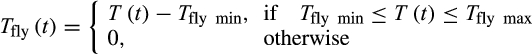 (2)
(2)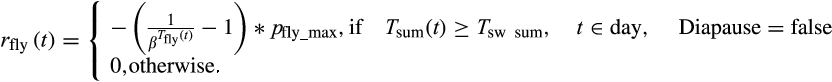 (3)
(3)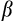 is a shape parameter. The diapause is terminated after overwintering when a minimal thermal sum
is a shape parameter. The diapause is terminated after overwintering when a minimal thermal sum 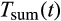 is reached, which is summed up beginning 1st January and calculated as:
is reached, which is summed up beginning 1st January and calculated as:
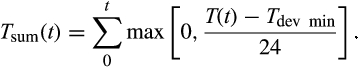 (4)
(4)In the preflight stage (i.e. the stage of developed beetles ready to fly), there is only one substage.
2.7 Diapause
Diapause is a special form of dormancy to cope with adverse environmental conditions or to synchronize population development. This phase of inactivity is initiated by recurring environmental triggers. The diapause of I. typographus starts if the length of the photoperiod decreases below a certain daylight threshold (Dolezal & Sehnal, 2007; Schroeder & Dalin, 2017) leading to a decrease in swarming activity. A modelling study of Jönsson et al. (2011) found a combination of day length and mean temperature thresholds more suitable than day length alone. Therefore, in the model, beetles initiate diapause (i.e. they do not fly anymore in the current year) if they have not finished their maturation feeding before a certain day of the year. This day lays in a time span determined by day length (DOYfirst to DOYlast), and is either the first day when the average daily air temperature drops below Tdia_min after DOYfirst, or at the latest DOYlast. Note that the model does not implement a potential obligate diapause (Schebeck et al., 2017).
In the following year, regeneration feeding after hibernation is necessary before spring dispersal (Dworschak, 2013). Accordingly, the diapause terminates when, starting from 1 January, a minimal annual thermal sum of Tsw_sum (Equation 4) has been reached and the beetles are placed in slotdia of the maturation feeding stage.
2.8 Model calibration
All model parameters (Table 1) were derived using Bayesian model calibration (O. Jakoby & C. Albert, unpublished data) based on data from bark beetle field monitoring nationwide (Wermelinger et al., 2014) and laboratory experiments (Wermelinger & Seifert, 1998, 1999).
| Parameter | Value | Unit | Description |
|---|---|---|---|
| T fly_min | 16.11 | °C | Minimum flight temperature |
| T fly_max | 31.29 | °C | Maximum flight temperature |
| T sw_sum | 100.00 | Degree days | Annual temperature sum required for swarming (Tsw_sum > 0) |
| T dev_min | 5.12 | °C | Minimum developmental temperature threshold for calculating the annual thermal sum Tsum(t) |
| slot dia | 6 | — | Maturation feeding slots, where individuals start regeneration feeding after diapause, that is, after Tsw_sum is reached |
| DOY first | 210 | Day of year | The first day of year after which diapause can be initiated (if average daily T < Tdia_min) |
| DOY last | 232 | Day of year | Day of year when diapause is initiated at latest |
| p fly_max | 0.01 | — | Maximum hourly flight probability of beetle (0 < pfly_max ≤ 1) |
| β | 1.36 | — | Curve parameter for swarming function (β > 1) |
| p sb | 0.30 | — | Percentage of sister breeding (0 ≤ psb ≤ 1) |
| slot sb | 4 | — | Maturation feeding slots where individuals start for regeneration feeding |
| k | 0.03 | — | Factor for the calculation of phloem temperature (k > 0); for details, see Trần et al. (2007) |
| T dia_min | 16.45 | °C | Average daily temperature under which diapause is initiated |
| α dev | 0.0000789 | — | Scaling parameter for Briere function—development |
| α mat | 0.0000101 | — | Scaling parameter for Briere function—maturation feeding |
| α rep | 0.0000255 | — | Scaling parameter for Briere function—reproduction |
| T lo,dev | 4.76 | °C | Maximal development temperature—development |
| T lo,mat | −4.43 | °C | Maximal development temperature—maturation feedinga |
| T lo,rep | −12.98 | °C | Maximal development temperature—reproductiona |
| T up,dev | 40.03 | °C | Maximal development temperature—development |
| T up,mat | 39.99 | °C | Maximal development temperature—maturation feeding |
| T up,rep | 36.00 | °C | Maximal development temperature—reproduction |
| n dev | 71 | — | Number of substages (slots)—development (egg to imago) |
| n dev | 8 | — | Number of substages (slots)—maturation feeding |
| n dev | 11 | — | Number of substages (slots)—reproduction (start of flight to oviposition) |
- a Calibrated values for minimal temperature below zero do not result in any beetle development at subzero temperatures but improve the shape of the fitted curve (see Figure S1 for details).
2.9 Climate projections
We used three climate projections for Switzerland (CH2011, 2011) based on the IPCC greenhouse gas scenarios A1B (rapid economic growth, emission reductions after 2050 due to declining population and technological innovations), A2 (larger emission than A1B due to a continuously growing population and no collaboration between countries) and RCP3PD (stabilizing CO2 concentration on a level preventing global warming of >2°C since the preindustrial period). They were developed on the basis of regional climate model simulations from the ENSEMBLES project (van der Linden & Mitchell, 2009). For each scenario, the data were calculated on a regular grid with a spatial resolution of 0.020833° (≈2 km) grid length and a daily temporal resolution (Zubler et al., 2014). Based on the 20-member ensemble of GCM-RCM combinations, the data set contains lower, median and upper estimates of the temperature change. According to these scenarios, the average annual temperature in Switzerland will increase by 0.9–2.0°C (RCP3PD), 2.3–4.5°C (A1B) or 2.7–5.2°C (A2) by the end of the century. In our analysis, we compared a reference period (1980–2009) with three 30 year periods centred around 2035 (2020–2049), 2060 (2045–2074) and 2085 (2070–2099).
2.10 Simulation settings
To estimate the relationship between topography and the phenology of I. typographus, we calculated the number of generations for those forested areas of Switzerland with a minimum spruce proportion of 30% on the 2 km rectangular grid. For the analysis, each 2 × 2 km grid cell was characterized by its elevation and a parameter (slasp) describing its slope and aspect (from steep northern over flat, east- or west-faced to steep southern slopes; cf. Bugmann, 1994). The model simulated the age structure of the population at each grid cell according to the local temperature regime. Each cell was initialized with a billion (109) adult individuals ready to fly at the beginning of each year. The number of generations was calculated for each year, and then averaged over the 30 year periods for the current climate and three scenario periods for each of the three climate scenarios (see 2.3).
3 RESULTS
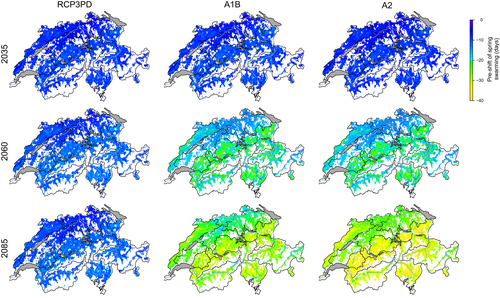
The number of generations of the spruce bark beetle per year and location varies according to the annual temperature regime. The start of spring swarming and the development time of the filial generations depend on spring and summer temperatures. As they vary in space, different numbers of generations can develop in a certain region (Figure 3a). This number relates also to topographical variables, including elevation, slope and aspect. On average, two generations develop at 500 m a.s.l., and 1.5 generations at about 1,000 m (i.e. in about 50% of all years two, otherwise one generation), but only one generation at 1,700 m (Figure 3b). Depending on the exact geographic location, slope and aspect, the potential number of generations varies by up to half a generation.
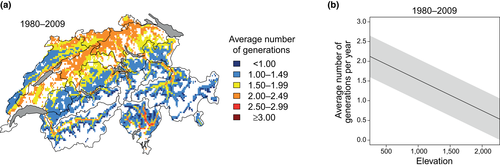
Under the current climate in Switzerland, usually two generations occur at low elevations on the Central Plateau and in the alpine valleys, and one generation is formed in the Alps, Pre-Alps and the higher elevations of the Jura Mountains (Figure 4, top row). This pattern varies between particularly cold years, that is, years with mostly below-average temperatures (when almost no second generation is started), and particularly warm years, that is, years with mostly above-average temperatures (when even a third generation can occur in the lowlands).

3.1 Effect of climate change on the potential number of generations
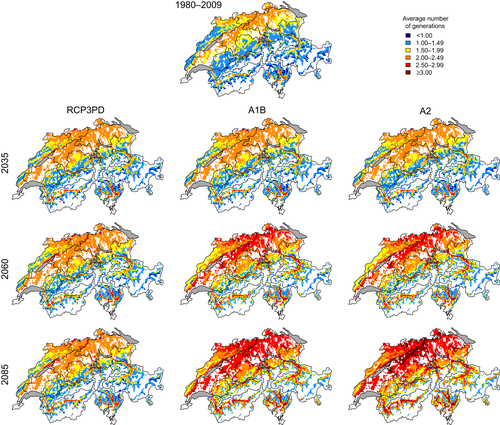
The projected increase in temperatures due to continuing climate change will lead to an increase in the number of beetle generations over almost all regions of Switzerland (Figure 4, bottom row). Under the A1B emission scenario, a year with average temperature conditions at the end of the century will correspond to a particularly warm year in the recent past, where a third generation occurs mainly in the central plateau. In a particularly warm year at the end of the century, three generations will occur over large parts of the country, except for high elevations in the Alps and the Jura Mountains.
The three emission scenarios differ with respect to the projected changes in the potential number of bark beetle generations (Figure 5). In case of immediate reduction of CO2 emission and moderate increase in temperature (RCP3PD), the average number of generations would only marginally increase in the first half of the 21st century, particularly in the pre-alpine regions. By the end of the century, virtually no further changes in generation numbers are projected. In contrast, under the assumption of a further CO2 emission increase until 2050 (A1B) or 2100 (A2), simulations show a continuous increase in the number of generations. This results in a third generation frequently occurring on the Central Plateau and in the alpine valleys, particularly in south-exposed locations.
This increase in the potential number of generations is usually more pronounced at lower elevations (Figure 6). Until around 2060, a corresponding increase in generation numbers is visible under all emission scenarios and at all elevations. At the end of the century, however, on average, one additional generation is expected at 500 m under the A2 scenario and slightly less than an extra generation under the A1B scenario. At 2,000 m, generation numbers increase only about half a generation under A1B and slightly more under A2. For the RCP3PD scenario, the increase in generation numbers at different elevations is limited to approximately 30% at 500 m a.s.l. and 20% at 2000 m a.s.l.
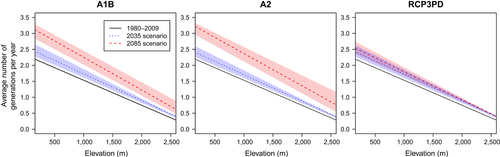
The uncertainty about the effect of minor temperature changes on the potential number of generations according to the climate models ensemble (all scenarios in the near future and RCP3PD) is much smaller at higher elevations, and amounting to less than half a generation (Figure 6; Figures S3–S5). However, if average temperatures increase more (A1B and A2 at the end of the century), the uncertainty is greater particularly at high elevations.
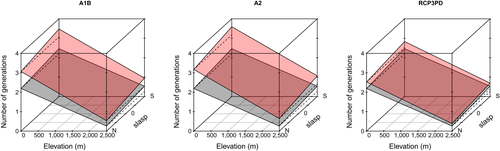
The slope and aspect (slasp) of a location have less effect on the number of generations than elevation (Figure 7). Global warming is likely to have most impact at the end of the century at low elevations and at southern slopes. The difference between south- and north-exposed slopes will be less than half a generation, but increases slightly compared to the current conditions for all scenarios.
3.2 Effects of climate change on the onset of spring swarming
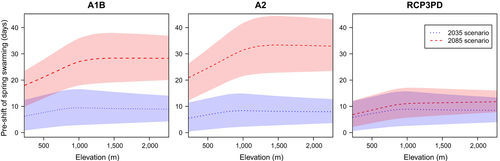
Climate change was found to induce a shift in the main flight periods of the spruce bark beetle. Spring swarming in particular is projected to start earlier in the year (Figure 8), beginning, on average, up to 10 days earlier in the first half of this century (2020–2049) for all emission scenarios. Until the end of the century (2070–2099), predictions for the average shift vary between 5–20 days (RCP3PD), 15–35 days (A1B) and 20–40 days (A2).
This shift towards earlier swarming will be lowest at low elevations, including the Central Plateau, the alpine valleys, and the Engadin (south-eastern part of Switzerland). At higher elevations, the shift in spring swarming will generally be larger (Figure 9). For the high emission scenario (A2), the average shift between locations at 300 m and 1,200 m differs by more than 10 days. Above approximately 1,200 m, the average shift remains constant. The uncertainty given from the three climate models results in a variation in the average shift of up to ±6 (PCP3PD), ±10 (A1B), and ±11 (A2) days around the average projection.
3.3 Effects of climate change on the distribution of development stages
The particular structure of the distributed delay model allows inspecting the annual dynamic of developmental stage distribution and flight pattern (Figure 10). The impact of climate change is exemplified for an intermediate temperature increase (A1B scenario at the end of the century) at representative locations on different elevations: In general, we found the annual flight pattern to be more scattered under cooler temperature conditions (usually observed for the flight of overwintering beetles). At higher temperatures (usually during summer), flight activity appears more continuous and more concentrated. This pattern, which is already implied under the average daily temperatures (Figure 10), is even more pronounced for temperature pattern of individual years (results not shown).
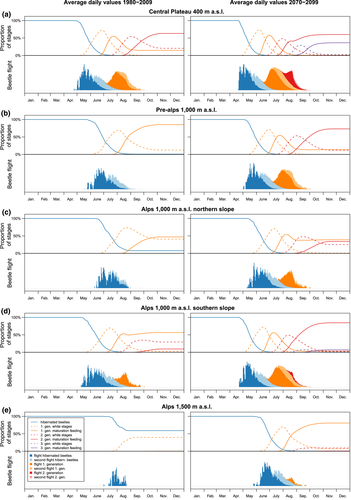
At the low elevations of the Central Plateau (Figure 10a), where currently a large proportion of the population overwinters as beetles of the second generation, an additional flight peak of the second generation has to be expected (on average about 40% of the population). Additionally, in contrast to the current situation, where a significant amount of larvae might not develop into the much more cold-tolerant teneral stage until winter, at the end of the century, almost all individuals of a second or third generation will be able to develop to teneral beetles due to longer warm periods after the initiation of diapause.
At mid elevations of the Pre-Alps, almost an entire additional generation has to be expected (Figure 10b). Under average temperature conditions at the end of the century, most individuals will overwinter as second-generation teneral beetles. Moreover, spring flight will occur almost one month earlier compared to today.
Particularly in the rugged topography of the Alps, differences in beetle development between northern and southern slopes (Figure 10c,d) are observed. At mid elevations of the Alps, the beetles develop faster and flight starts about 1 month earlier at the southern slopes than at the northern ones. At the end of the century, at northern slopes about two-thirds of the overwintering population will consist of individuals of the second generation with a considerable proportion of 'white' stages, whereas at southern slopes, it will consist exclusively of teneral beetles of the second (and, to a very small proportion, a third) generation.
At high elevations of the Alps (Figure 10e), where currently at most locations hardly any individuals can develop into the cold-resistant teneral stage, there will be regularly one fully developed generation of mostly teneral beetles by the end of the century (note that again there can be strong differences between north- and south-exposed locations).
In general, climate change will alter temporal population structure and flight pattern all over Switzerland. However, this will also strongly depend on local climate and will also differ between years.
4 DISCUSSION
4.1 Phenology and voltinism under climate change
Our projections show an upward shift in the occurrence of the European spruce bark beetle (I. typographus) and an increased potential for additional generations per year at all elevations in Switzerland. This corresponds to simulated future developments in other parts of Central Europe and Scandinavia (Bentz et al., 2019; Jönsson et al., 2011). In regions of high latitude in Scandinavia and at high elevations in Central Europe, where currently no full population can establish, one full annual generation is predicted to be found regularly by the end of this century. Outbreaks of such univoltine populations have been documented from several regions of Europe (Lausch, Fahse, & Heurich, 2011; Mezei et al., 2017). Additionally, at low elevations, a third generation, which today occurs only in years with particularly warm temperatures (Wermelinger et al., 2012), could become common by the end of this century (particularly under scenarios A1B and A2). However, according to our simulations, in line with the findings of Hlásny and Turčáni (2009) and Berec et al. (2013), more than three fully developed generations will remain very rare even under continuously increasing CO2 emissions until 2100 (A2 scenario).
Likewise, as presumed for Scandinavia (Jönsson et al., 2009, 2011), our model predicts that spring swarming will occur earlier and development will be faster under climate change. The shift in the annual flight pattern up to 40 days earlier is caused by diapause terminating earlier and the threshold air temperature for the beetles’ flight being reached earlier (Lobinger, 1994). Generally, this shift appears to be less pronounced at lower elevations, where swarming already occurs early in spring. Here, the time for terminating diapause (i.e. until reaching the required temperature sum) is the most important factor, whereas at higher elevations, reaching the flight temperatures is more crucial in spring.
The importance of topographical parameters at different scales for predicting bark beetle infestation risk has been stressed in several studies (Faccoli & Bernardinelli, 2014; Netherer & Nopp-Mayr, 2005; Seidl et al., 2016). Focussing solely on the number of generations, we found that slope and aspect were less important than elevation, probably because the large geographical variability between grid cells with similar slope and aspect overrides a distinct pattern. Nevertheless, comparing development and flight pattern at opposite slopes in the Alps reveals a distinct difference in the activity period, with an earlier onset of spring flight, a longer flight period and a faster development on southern slopes.
The potential changes in number of generations and phenology in a warming climate predicted in this study have been observed in many other insect (pest) species worldwide (e.g. Altermatt, 2009; Battisti et al., 2005; Parmesan, 2006; Ziter et al., 2012). The codling moth (Cydia pomonella L.), for example, a pest species feeding in apple orchards, is similarly expected having a third generation and a pre-shift in swarming that is larger at higher elevations in Switzerland (Stoeckli et al., 2012). At the turn of the last century, the mountain pine beetle (Dendroctonus ponderosae Hopkins), one of the most aggressive bark beetle species in the western United States, has altered its life cycle at higher elevations from semi- to univoltine generations due to warmer temperatures (Logan, Macfarlane, & Willcox, 2010; Logan & Powell, 2009), resulting in mass propagation and extensive forest dieback (Logan et al., 2003).
4.2 Implications for bark beetle population dynamics
The seasonal number of generations has a crucial impact on the propagation and thus outbreak risk of bark beetle populations. Given optimal conditions for brood development, for example, after large storm events or droughts that create extensive breeding resources, the population size can increase by a factor of 50 with each additional generation (Baier et al., 2007). The higher the abundance, the more likely infestations of vigorous spruce trees are (Mulock & Christiansen, 1986), and thus the higher the population increase. Hence, the increase in predicted generation numbers based on the climate scenarios considered in this study is likely to be associated with a higher risk of bark beetle outbreaks (Faccoli & Bernardinelli, 2014).
The predicted faster beetle development may also be counterproductive for population growth (Van Dyck, Bonte, Puls, Gotthard, & Maes, 2015) if an additional generation is started towards the end of the season and most individuals overwinter in the early, frost-sensitive life stages, that is, as larvae or pupae. However, winter temperatures are expected to rise with climate change, and immature bark beetle stages may continue their development even at low temperatures (Štefková, Okrouhlík, & Doležal, 2017). Particularly at low elevations, longer periods with higher temperatures will become more frequent and the whole population will reach the teneral stage. Accordingly, the risk of increased winter mortality due to the phenomenon described above is more relevant at higher elevations, when a second generation will be started in autumn, than for a third generation at low elevations.
However, the predicted occurrence of multivoltine populations at higher elevations might be delayed until the populations have genetically adapted to the warmer climate. The first generation individuals of I. typographus can belong to two different phenotypes (Schebeck et al., 2017): One reproducing within the same year depending on photoperiod and temperature, and the other entering an obligate diapause. This behaviour is likely to be genetically determined with obligate diapausing individuals occurring mainly at higher elevations and latitudes where environmental conditions usually do not allow the development of a second generation (Schroeder & Dalin, 2017). For Switzerland, there is no information on the occurrence of two different phenotypes. Therefore, the model does not include the option of an obligate diapause. All individuals that miss the facultative diapause threshold can potentially complete their development and emerge from the tree to start a new generation, depending on temperature. Thus, the percentage of individuals starting a new generation towards the end of the year might be overestimated at certain locations.
When bark beetle populations markedly increase and resources run short, intraspecific competition starts to limit their reproductive success and therefore could compromise population growth (Faccoli & Bernardinelli, 2011; Komonen, Schroeder, & Weslien, 2011). The effects of an additional I. typographus generation may be intensified if the phenology or overwintering behaviour of their natural enemies responds differently to a warmer climate (Wermelinger et al., 2012) which may lead to an increasing asynchrony of the two trophic levels (Schroeder & Dalin, 2017; Thomson, Macfadyen, & Hoffmann, 2010).
4.3 Impact of climate change on the infestation risk
Temperature was found to be the most important factor explaining bark beetle infestations and predicting outbreaks (Marini et al., 2017; Stadelmann, Bugmann, Wermelinger, et al., 2013). However, further factors influence not only the infestation risk, such as windthrow, precipitation and stand water supply, stand characteristics and management (Marini, Ayres, Battisti, & Faccoli, 2012; Marini et al., 2017; Overbeck & Schmidt, 2012; Stadelmann, Bugmann, Meier, Wermelinger, & Bigler, 2013) but also landscape-wide factors including the large-scale synchrony and connectivity of host and beetle populations (Seidl et al., 2016).
Under climate change, many forest ecosystems will be threatened more often by drought (Allen et al., 2010). Given an increase in population densities, the beetles can more easily overcome the threshold of a spruce tree's defence, which decreases as the tree is increasingly stressed by drought (Matthews et al., 2018; Netherer et al., 2015). Hence, with warm and dry summers, particularly at lower elevations, beetle populations can take advantage of their predicted faster developmental rate and increased number of generations because trees will be drought stressed, especially during the more condensed swarming period of the summer generation. Such warm and dry conditions have already been shown to be positively correlated with the severity of bark beetle outbreaks (Marini et al., 2012).
In recent decades, tree mortality due to storms and bark beetles has increased in Europe (Seidl, Schelhaas, et al., 2011). This trend is expected to continue, although the risk of storm damage in the Alps may decrease after 2020 (Seidl et al., 2014). The beetle damage at all elevations would intensify particularly after winter storms as the timeframe available for effective salvage logging will be reduced due to earlier spring swarming. Furthermore, the magnitude of bark beetle damage in Central Europe will depend greatly on the distribution and frequency of its host tree Norway spruce, which is likely to disappear at lower elevations, but will persist at medium and newly establish at higher elevations (Bugmann et al., 2014). Such spatial change, however, takes time and bark beetles are likely to promptly capitalize on the warmer and drier conditions. Thus, forest stands at low elevations are still likely to have the highest infestation risk in the coming decades.
4.4 Conclusions and outlook
An outstanding feature of our model is its ability to represent bark beetle population structure at any given time of the year. This implies two important advantages: First, it is capable of resolving the length of flight periods based on the continuous recruitment of adults from immature stages. This has important implications for the colonization of healthy trees since the predicted more concentrated flight of beetles, particularly in summer, increases the risks of successful attack. As a second advantage of representing population structure, our model describes the prevailing stages entering into winter diapause. Since different stages have differing cold tolerances (Dolezal & Sehnal, 2007; Faccoli, 2002), this provides a basis for analysing winter mortality, for example, in future multiyear studies.
Our simulations across a large elevational gradient and a diverse topography indicate that under global warming, insects like the European spruce bark beetle are likely to produce additional generations per year with earlier and more concentrated swarming across the whole of Central Europe. Additional beetle generations, more intense flight periods, potentially lower mortality in overwintering populations combined with other expected consequences of climate change such as increasing spruce drought stress will markedly raise the infestation risk of spruce-dominated forest stands and pose a challenge for forest management. Our results, therefore, should be integrated in comprehensive assessments of future outbreak risks. They can, for example, be included in a general stand predisposition assessment (Netherer & Nopp-Mayr, 2005) to derive overall risk predictions under global change based on biotic (e.g. stand characteristics, beetle phenology, management) and abiotic factors (e.g. drought, storms, site characteristics). Furthermore, multitrophic approaches and tools incorporating tree physiology, bark beetle population dynamics and interactions between trees, bark beetles and natural enemies are needed for more robust predictions of forest ecosystem responses to climate change (Anderegg et al., 2015). Finally, such models on the host–insect system will help to assess climate change impacts on spatio-temporal forest dynamics in general (Bugmann et al., 2014) on different spatial and temporal scales. By that, they will support short- and long-term decisions for better adapting multifunctional forest management to a changing climate.
ACKNOWLEDGEMENTS
This study was financed by the Swiss Federal Office for the Environment (FOEN) and the Swiss Federal Research Institute WSL in the framework of the research program “Forest and Climate Change.” The climate scenario data were obtained from the Center for Climate Systems Modeling (C2SM) at ETH Zurich. We thank Carlo Albert (Eawag) for his valuable support of model calibration, Beat Forster and Franz Meier from the Swiss Forest Protection group (WSL) for fruitful discussions, three anonymous reviewers for their valuable comments and all foresters involved in our bark beetle monitoring for providing valuable data for model calibration.




