Autumn bird migration phenology: A potpourri of wind, precipitation and temperature effects
Abstract
Climate change has caused a clear and univocal trend towards advancement in spring phenology. Changes in autumn phenology are much more diverse, with advancement, delays, and ‘no change' all occurring frequently. For migratory birds, patterns in autumn migration phenology trends have been identified based on ecological and life-history traits. Explaining interspecific variation has nevertheless been challenging, and the underlying mechanisms have remained elusive. Radar studies on non-species-specific autumn migration intensity have repeatedly suggested that there are strong links with weather. In long-term species-specific studies, the variance in autumn migration phenology explained by weather has, nevertheless, been rather low, or a relationship was even lacking entirely. We performed a spatially explicit time window analysis of weather effects on mean autumn passage of four trans-Saharan and six intra-European passerines to gain insights into this apparent contradiction. We analysed data from standardized daily captures at the Heligoland island constant-effort site (Germany), in combination with gridded daily temperature, precipitation and wind data over a 55-year period (1960–2014), across northern Europe. Weather variables at the breeding and stopover grounds explained up to 80% of the species-specific interannual variability in autumn passage. Overall, wind conditions were most important. For intra-European migrants, wind was even twice as important as either temperature or precipitation, and the pattern also held in terms of relative contributions of each climate variable to the temporal trends in autumn phenology. For the trans-Saharan migrants, however, the pattern of relative trend contributions was completely reversed. Temperature and precipitation had strong trend contributions, while wind conditions had only a minor impact because they did not show any strong temporal trends. As such, understanding species-specific effects of climate on autumn phenology not only provides unique insights into each species' ecology but also how these effects shape the observed interspecific heterogeneity in autumn phenological trends.
1 INTRODUCTION
Every year, billions of animals migrate various distances across the Earth to increase their chances of survival and reproductive success (Bauer & Hoye, 2014; Dokter et al., 2018). Over the past decades, climate change has strongly influenced the timing of different aspects in the annual cycles and life stages of migratory animals (Parmesan & Yohe, 2003). These phenological changes could have consequences for their fitness and survival (Bairlein, 2016; Miller-Rushing, Høye, Inouye, & Post, 2010; Visser & Gienapp, 2019), which has resulted in further impetus to disentangle the specific relationships between weather and migration phenology.
While the influence of climate on many aspects of spring phenology in animals and plants has received a lot of attention, the relationship between climate and autumn phenology has been relatively neglected (Gallinat, Primack, & Wagner, 2015). Consequently, the climatic factors controlling autumn phenology are still poorly understood (Gallinat et al., 2015; Renfrew et al., 2013; Rivrud et al., 2016; Walther et al., 2002; Xu & Si, 2019). In general, spring (and summer) activities and events have shown a rather clear advancement in response to recent climate change across taxa (Menzel et al., 2006; Thackeray et al., 2010). The change in autumn events and activities, however, is much less uniform, with advancements, no change and delays all being observed regularly (Chambers, Beaumont, & Hudson, 2014; Lehikoinen, Sparks, & Žalakevičius, 2004; Menzel et al., 2006; Smith & Paton, 2011).
For birds in specific, it is also generally accepted that spring migration has been advancing in response to recent climate change (Haest, Hüppop, & Bairlein, 2018a; Jonzén et al., 2006; Knudsen et al., 2011), and evidence has been accumulating that temperature is the most important climatic driver of spring migration phenology (Haest, Hüppop, & Bairlein, 2018b; Usui, Butchart, & Phillimore, 2017; Van Doren & Horton, 2018). Notwithstanding, effects of weather and climate change are species- and likely even population-specific (Carey, 2009; Haest et al., 2018b; Shaw, 2016), and weather factors other than temperature cannot be ignored (Haest et al., 2018b). The link between migration and climate has, amongst all animal groups, by far been studied most intensively in birds (Shaw, 2016). Yet, both the general patterns and the relationships with weather have again been investigated much less in autumn than in spring bird migration (Bitterlin & Van Buskirk, 2014; Gordo, 2007; Jenni & Kéry, 2003; Miles et al., 2017). Similar to spring migration, autumn migration nevertheless plays an essential, albeit different, role in the full annual cycle of birds. Mortality during autumn migration, for example, has been suggested to have strong demographic consequences (Hewson, Thorup, Pearce-Higgins, & Atkinson, 2016; Klaassen et al., 2014). An increased understanding of the relationship between autumn migration phenology and weather, as well as the potential impacts of climate change, is hence a vital piece of the puzzle towards understanding the complete picture of the observed demographic changes in bird populations.
Several hypotheses have been proposed (and sometimes also opposed) based on ecological and life-history traits, to explain the observed differences between (and within) species in temporal trends in avian autumn migration phenology, such as moulting strategy (Kovács, Csörgo, Harnos, Fehérvári, & Nagy, 2011) (carry-over effects of) the timing of breeding (Lehikoinen, Saurola, Byholm, Lindén, & Valkama, 2010; McKinnon & Love, 2018; Mitchell, Newman, Wikelski, & Ryan Norris, 2012; Stutchbury et al., 2011; van Wijk, Schaub, & Bauer, 2017), dietary guild (La Sorte et al., 2015), average body size (Bitterlin & Van Buskirk, 2014), migration distance (Gatter, 1992), and the ability for multiple broods (Jenni & Kéry, 2003; Redlisiak, Remisiewicz, & Nowakowski, 2018; Van Buskirk, Mulvihill, & Leberman, 2009). Some patterns are indeed present, for example (short-distance) migrant birds both breeding and wintering within Europe seem to be delaying their autumn migration in response to recent climate change, while (long-distance) trans-Saharan migrants sometimes show advancements (Jenni & Kéry, 2003; Van Buskirk et al., 2009; but see the meta-analysis by Bitterlin & Van Buskirk, 2014). The explained variation in trends has, however, remained rather low and the mechanisms underlying many of the patterns rather elusive (Bitterlin & Van Buskirk, 2014; Charmantier & Gienapp, 2014; Chmura et al., 2019; Gill et al., 2013; Knudsen et al., 2011). To understand the patterns and causes of interspecific differences in trends, it seems vital, however, to first understand the species-specific (differences in) phenological responses to weather, and how these might be shaping the observed temporal trends. On the one hand, climatic effects on several specific aspects of autumn migration, for example, flight speed (Vansteelant et al., 2015), stopover transience and departure probability (Calvert, Taylor, & Walde, 2009), and migration onset (Shamoun-Baranes et al., 2006), are likely to influence overall migration timing and progress. In turn, many of the previously proposed ecological or life-history traits to explain interspecific differences in autumn migration trends, however, also refer to activities that have been suggested to be dependent on climate, for example (timing of) moult (Cockburn, Osmond, & Double, 2008, (carry-over effects of) timing of breeding (Dunn & Winkler, 1999; Gow et al., 2019; Parmesan & Yohe, 2003), breeding season length (Halupka & Halupka, 2017), and the prevalence of multiple brooding (Husby, Kruuk, & Visser, 2009). As such, interspecific differences in autumn phenology likely result from a complex interplay of multiple mechanisms of which many can be linked to different exposure and reactions to climate (Chmura et al., 2019).
Studies on the relationship between weather and avian autumn migration phenology have been performed at three different levels of biological detail: migration intensity in general using mostly radar, individual-specific migration using different tracking technologies, and species-specific migration using mostly long-term count, observation or ringing datasets. In many cases, very strong links have been found between overall autumn migration intensity and weather using radar technology (Erni, Liechti, Underhill, & Bruderer, 2002; Nilsson et al., 2019; Van Belle, Shamoun-Baranes, Loon, & Bouten, 2007). Extracting species-specific information using radar nevertheless remains notoriously challenging (Hüppop et al., 2019; Schmaljohann, Liechti, Bächler, Steuri, & Bruderer, 2008). Recent technological advancements in data acquisition with tracking devices are now providing ever-increasing information and insights into the migratory behaviour of individuals, populations and species that have hitherto been difficult, if not impossible (Bridge et al., 2011; Kays, Crofoot, Jetz, & Wikelski, 2015; Wilmers et al., 2015). At present, the potential for combining data for long-term insights, for example, relationships with climate change, however, still remains limited due to the overall limited coverage in time, that is, number of years, but also in number of individuals for each species or population (Bauer et al., 2019). Already existing long-term species- or population-level migration datasets on the other hand do have the potential to provide species-specific insights into the relationship between avian autumn migration and climate (change).
With a systematic literature search (sensu Nakagawa, Noble, Senior, & Lagisz, 2017; see Appendix S1 for a description of the literature search and the resulting literature list, as well as a summary by investigated weather variable types), we found 34 publications on the relationship between long-term species-specific avian autumn migration phenology and climate (change). Temperature has been investigated the most (28 out of 34 publications, i.e. 82%), followed by the North Atlantic Oscillation (NAO) index (14/34, i.e. 41%), precipitation (10/34, i.e. 29%) and wind-related variables (7/34, i.e. 21%). Of these, 57%, 43%, 60%, and 29% of the publications reported finding relationships with temperature, the NAO index, precipitation, and wind-derived variables respectively. Next to possible biological reasons, however, we identified a number of methodological limitations and statistical misconceptions that may have contributed to the large diversity in these reported relationships. First, while it is weather at the point of origin, that is, breeding or stopover area, that is the critical determinant for migration timing (Haest et al., 2018b; Lack, 1960; Schmaljohann, Lisovski, & Bairlein, 2017), many studies have used weather conditions at the location where the migration is measured. Second, even though there are large uncertainties in the exact timing and duration of the influence of weather on migration phenology (e.g. due to lag or indirect pathways; Gordo, 2007; van de Pol et al., 2016), most studies have made strong a priori assumptions on the time window of influence, for example, using monthly averages. Third, in at least half of the studies that did report finding relationships with autumn migration phenology, spurious correlations might have occurred due to (a) not accounting for shared trends or (b) not accounting for multiple hypotheses testing (Appendix S1; Haest et al., 2018a, 2018b; Iler, Inouye, Schmidt, & Høye, 2017). Contrary to radar studies on general migration intensity, the amount of variance in species-specific migration phenology explained by weather variables has moreover been rather low (e.g. Gordo & Sanz, 2006: <16%; Redlisiak et al., 2018: ≈35%; but see Shamoun-Baranes et al., 2006; for a notable exception for four soaring bird migrant species: >54%).
Large uncertainties, if not complete knowledge gaps, hence remain on which weather variables influence autumn migration phenology of which species, at which locations and at what time. We performed a spatially and temporally explicit analysis of weather data to systematically examine the relationship between climate and avian autumn migration phenology of 10 passerine species at Heligoland (Germany) over a 55-year period (1960–2014). Similar to Haest et al. (2018a) for spring migration phenology, we used as little as possible a priori assumptions or hypotheses on the what, where and when of the weather variables influencing autumn migration phenology. Instead, we took an exploratory data analysis approach to see what the data can tell us about possible weather influences and climate change effects on autumn migration phenology of birds.
2 MATERIALS AND METHODS
2.1 Autumn migration passage data
For over a century now, migrant birds are being ringed on the island of Heligoland (54°11′N, 07°53′E; often also spelled Helgoland). Since 1960, comparable efforts and methods have been in place with daily catches in the trapping garden throughout the whole year, resulting in an unusual long-term dataset on migration phenology. Barely any landbirds breed on Heligoland, and with no other landmass being present in a radius of almost 50 km, few birds reach the island during post-breeding dispersal (Hüppop & Hüppop, 2003, 2011; Hüppop & Winkel, 2006).
We used yearly mean autumn passage dates (MAPD) over the period 1960–2014 as a measure of autumn migration phenology (Table 1). To minimize potential bias due to the use of the Gregorian calendar, we converted trapping dates to Winter Solstice-based dates (WSD) instead of day-of-the-year (Sagarin, 2001). Yearly MAPD was calculated as the mean WSD of all birds ringed between WSD 223 and 344, that is, 1 August and 30 November approximately. For convenience, we report dates throughout the paper as approximate Gregorian calendar dates. We analysed MAPD data from 10 passerines of which six mainly migrate to wintering grounds within Europe or in the North of Africa, and four are trans-Saharan migrants (Table 1). The species in Table 1 are ordered by MAPD. We use this species order, instead of according to phylogeny, in all tables throughout the manuscript to allow comparison between species that are potentially subject to similar weather conditions prior to or during their migration.
| Species name | Scientific name | Average 5th percentile | Average MAPD | Average 95th percentile | Standard deviation MAPD (days) | Difference 95th and 5th percentile (days) | Total birds ringed 1960−2014 | Average birds/year |
|---|---|---|---|---|---|---|---|---|
| Willow Warbler | Phylloscopus trochilus | 10 Aug | 25 Aug | 15 Sep | 3.89 | 36 | 12,937 | 235 |
| European Pied Flycatcher | Ficedula hypoleuca | 11 Aug | 27 Aug | 18 Sep | 5.35 | 38 | 9,949 | 181 |
| Garden Warbler | Sylvia borin | 13 Aug | 31 Aug | 25 Sep | 5.31 | 43 | 23,090 | 420 |
| Common Redstart | Phoenicurus phoenicurus | 23 Aug | 10 Sep | 27 Sep | 3.90 | 35 | 13,613 | 248 |
| European Robin | Erithacus rubecula | 11 Sep | 1 Oct | 24 Oct | 5.67 | 43 | 22,086 | 402 |
| Dunnock | Prunella modularis | 17 Sep | 3 Oct | 20 Oct | 5.98 | 33 | 9,181 | 167 |
| Song Thrush | Turdus philomelos | 22 Sep | 4 Oct | 21 Oct | 3.96 | 29 | 75,734 | 1,377 |
| Common Chaffinch | Fringilla coelebs | 22 Sep | 12 Oct | 11 Nov | 7.57 | 50 | 25,692 | 467 |
| Redwing | Turdus iliacus | 30 Sep | 15 Oct | 7 Nov | 4.83 | 38 | 20,914 | 380 |
| Eurasian Blackbird | Turdus merula | 8 Oct | 27 Oct | 20 Nov | 5.02 | 43 | 53,288 | 969 |
Note
- Species are ordered by average MAPD.
Birds of a certain species that are captured at Heligoland likely stem from different populations (Dierschke, Dierschke, Hüppop, Hüppop, & Jachmann, 2011). The measured MAPD for each species is, hence, influenced by changes in the autumn phenology of each of these populations. This could obscure the relationship between the measured MAPD and the influencing weather conditions for each specific population. Different populations could, however, also be influenced by the same weather conditions at a certain time and place, for example, stopover areas or prior to crossing an ecological barrier. Breeding or stopover areas of a species also might have changed over the total analysed time period in response to climate change (Hitch & Leberg, 2007; Zuckerberg, Woods, & Porter, 2009). Yet if these areas are maintained for a long enough subset of the total analysed time period, or the shifts are not over large distances compared to the spatial resolution of the weather grids, it should be possible to link the observed MAPD to all of the different breeding or stopover locations. As such, using migration passage data has both advantages and disadvantages compared to breeding departure or winter area arrival data. Depending on the species, both of these latter autumn migration phenology metrics are, however, often difficult to estimate, particularly in passerine species.
2.2 Climate data
We acquired spatiotemporal data of air temperature, precipitation and wind from the NCEP Reanalysis I database (Kalnay et al., 1996; Kanamitsu et al., 2002) using the R package RNCEP (Kemp, Loon, Shamoun-Baranes, & Bouten, 2012). The spatial grid covered an area from roughly 48° to 72°N and 29°W to 44°E, ranging from northern Scandinavia in the North to southern Germany in the South, and from Iceland in the West to western Russia in the East. The spatial resolution of a grid cell ranged from 1.875° to 3.75°, depending on the weather variable (Table 2). Ocean grid cells were masked from the analysis. For each day and (land) grid cell, we derived four variables from the NCEP database: mean daily air temperature, daily precipitation sum, mean wind direction over 24 hr and wind direction at midnight. We analysed both mean daily winds and midnight winds, as most species in our analysis are known to mainly migrate during the night, but others possibly migrate during both day and night (e.g. Dunnock). The wind direction data were used to calculate the number of days for both winds originating from and in the direction of Heligoland within any given time window, by counting every day with a wind direction that fell between −45° and +45° of the angle between Heligoland and the centre of the grid cell under analysis. Depending on the location of the grid cell relative to Heligoland, we then interpreted these to be head- or tailwinds. We chose to test both measures for wind effects on migration as both hypotheses, that is, headwinds delay migration and tailwinds advance migration, represent different processes.
| Weather variable | NCEP variable | Spatial resolution (in degrees) | Number of analysed grid cells | Data pre-processing and comments |
|---|---|---|---|---|
| Temperature | ‘air.2m' | 1.875° | 293 | We calculated daily mean temperatures from the four 6-hr temperature values. |
| Precipitation | ‘prate.sfc' | 3.75° | 84 | Precipitation rate data were converted to mm/day. Spatial resolution is half that of the temperature data, because we took the mean over four grid cells. |
| Wind direction | (East-West) ‘wnd' and (North-South) ‘vwnd' at 925 hPa | 2.5° | 177 | The 925 hPa pressure level roughly corresponds to 750 m altitude. The 6-hr interval values of the two wind components were used to calculate a mean daily wind direction, as well as the midnight wind direction using the values of midnight only. |
The usefulness of large-scale climate indices such as the NAO index, to explain biological processes has recently been strongly questioned (Haest et al., 2018a; Mesquita et al., 2015; van de Pol et al., 2013). As the NAO has nevertheless also been frequently suggested to be related to autumn migration phenology (e.g. Calvert et al., 2009 and Therrien et al., 2017; see Introduction and Appendix S1), we also performed a time window analysis of daily NAO indices. The daily NAO data were downloaded from the website of the Climate Prediction Center of the National Oceanic and Atmospheric Administration (http://www.cpc.ncep.noaa.gov/products/precip/CWlink/pna/nao.shtml).
2.3 Ring recovery data
Next to the autumn migration phenology data at Heligoland, we also extracted ringing recovery data, consisting of birds ringed at Heligoland and recovered elsewhere, as well as birds ringed elsewhere and recovered on Heligoland. We extracted the locations and timing of the ringing recoveries from during the breeding and (Heligoland) autumn migration period. Depending on the species, the ring recoveries from the breeding period covered two or more months between May and September. The ringing recoveries from the (Heligoland) autumn migration period were 2 months long for all species and occurred between August and November (Table S1 and Figure S1). Even though we only analysed MAPD data from between 1960 and 2014, we included all of the recoveries since the start of the ringing activities in Heligoland in 1909. We did so because the total number of ring recoveries for certain species is already rather limited (Table S1). We used the ringing recovery data as a qualitative means only to help interpret the likelihood of the location and timing of identified weather signals.
2.4 Avoiding spurious correlations due to shared trends
If two time series both contain a trend over time, it is likely that correlating the two series without taking into account these shared trends will yield high, yet spurious, correlations (Haest et al., 2018a; Noriega & Ventosa-Santaulària, 2007). To avoid such spurious correlations due to the presence of trends alone, we determined whether a linear, quadratic, or cubic trend over time was most appropriate for the MAPD time series of each species. To do so, we compared the second-order Akaike information criterion (AICc) values (Burnham & Anderson, 2002) for linear, quadratic and cubic trend models. If a higher order MAPD trend model had an AICc value that was more than two units lower than the one from the previous order model, we judged the higher order model to be a better approximation of the trend over time. We applied augmented Dickey–Fuller tests (using the urca R package; Pfaff, 2008) to verify that the chosen trend models had successfully reduced the MAPD time series to stationarity. We checked for remaining autocorrelation in the residuals of the trend models with a Durbin–Watson test up to lag two (using the car R package; Fox & Weisberg, 2011). The identified trend model for each species was used as the base model (for comparison of reduction in AICc values by adding a weather variable) in the subsequent time window analyses.
2.5 Finding the ‘weather variable–location–time window' combinations that influence autumn migration phenology
To identify the weather variables that are most likely influencing the MAPD of each species at Heligoland, we applied a methodology similar to the one used in Haest et al. (2018a) for spring migration phenology. In summary, the method breaks down into two main chunks of analyses (Figure 1). First, a per grid cell search is performed of all possible time windows of any length between two given dates, for each weather variable and over a spatial grid covering a certain area of interest to the studied process. The time window analysis for each pixel was done with the R package climwin (Bailey & van de Pol, 2016; van de Pol et al., 2016). The spatial grid covered all somewhat likely geographic breeding or autumn stopover locations prior to Heligoland passage (see Climate data section). This first step ultimately results in a per species ‘long-list' of all potential ‘weather variable–location–time window' combinations, henceforth called candidate weather signals, that might possibly be influencing MAPD. This ‘long-list' nevertheless still includes many spurious candidate weather signals due to spatiotemporal (auto-)correlations in the weather variables. In the second step (right panel of Figure 1), the ‘long-list' of candidate weather signals is further analysed using a combination of variable importance methods in order to narrow down the candidates to a final list of ‘weather variable–location–time window' combinations that most likely influence autumn migration phenology. For more in-depth information on the methodology, we refer to Haest et al. (2018a), in which each step is explained in detail, including explanations and discussions on: (a) how spurious correlations are avoided; (b) choices for the maximum and minimum time window length for each of the weather variable types; and (c) specific (dis)advantages of each of the applied variable importance methods. Two small adjustments were made to the second part of the overall analysis (right panel in Figure 1) to further increase robustness of the methodology and to be able to more appropriately deal with a long set of candidate weather signals (as occurred in this study). For ease of reference, we summarized all of the settings and decision rules we used for the ‘weather variable–location–time window' analysis, as well as the method adjustments compared to Haest et al. (2018a), in Appendix S2.
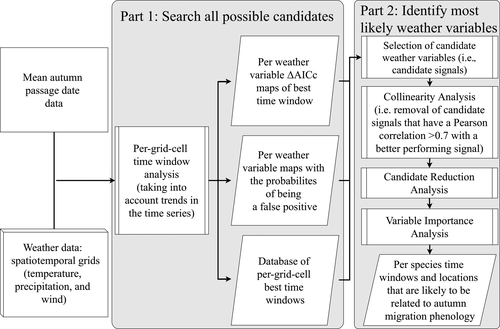
2.6 Assessing contributions of each weather variable to each species' trend over time
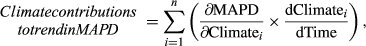
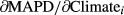 , and a simple linear regression between the respective weather variable and time, that is, years, to estimate
, and a simple linear regression between the respective weather variable and time, that is, years, to estimate 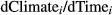 . Standard errors were calculated appropriately following error propagation rules (formula 3.18 in Taylor, 1997). Note that this approach by definition ignores any other (non-climatic) factors that might possibly affect changes in MAPD over time.
. Standard errors were calculated appropriately following error propagation rules (formula 3.18 in Taylor, 1997). Note that this approach by definition ignores any other (non-climatic) factors that might possibly affect changes in MAPD over time.2.7 Assessing relative importance of the weather variable types in terms of effect and temporal trend contributions
To get an idea about the relative overall importance of the three different weather variable types, that is, temperature, precipitation and wind, on the interannual fluctuations in MAPD at Heligoland across all species, we summed their respective mean relative variable importance values. To compare the importance of each climate variable in terms of temporal trends in MAPD over the past decades to the impact on interannual fluctuations in MAPD only, we similarly also calculated the relative contributions of each weather variable type to the temporal trends in MAPD as the sum of the absolute values of the trend contributions for each climate variable, divided by the total sum of the trend contributions. We did this across all species, but also across each migration strategy (TS: trans-Saharan vs. IE: intra-European migrants) to check for any possible patterns that might explain the frequently observed differences in trend directions of TS (advance) and IE (delay) migrants.
3 RESULTS
3.1 Trends in the MAPD time series
A linear trend was most appropriate to account for trends in the MAPD of eight of the 10 species (Table 3). Only for one trans-Saharan (Common Redstart) and one intra-European (Dunnock) migrant species, the trend was better described by including a quadratic term, that is, trends were nonlinear. The trends explained very little of the interannual variability in MAPD (R2 ≈ 0–0.17). Intra-European migrants seem to be delaying autumn passage at Heligoland over the last decades, while trans-Saharan migrants tend towards advancement. The AICc of an intercept-only model, that is, a null model without temporal trend, was more than two values lower for Willow Warbler and European Pied Flycatcher, indicating (together with the very low adjusted R2s) that the MAPD of these species currently do not necessarily seem to be changing strongly in a specific direction. Augmented Dickey–Fuller tests confirmed that all trend models successfully reduced the time series to stationarity (Table S2). No ‘significant' autocorrelations were found in the detrended residuals using Durbin–Watson tests (Table S3).
| Species name | Scientific name | Selected MAPD trend model | Adj. R2 | Int. | SE | Trend coefficients | |||
|---|---|---|---|---|---|---|---|---|---|
| Lin. | SE | Quad. | SE | ||||||
| Willow Warbler | Phylloscopus trochilus | Linear | 0 | 266.49 | 66.27 | −0.01 | 0.03 | — | — |
| European Pied Flycatcher | Ficedula hypoleuca | Linear | 0.01 | 352.16 | 90.01 | −0.05 | 0.05 | — | — |
| Garden Warbler | Sylvia borin | Linear | 0.08 | 459.99 | 85.85 | −0.10 | 0.04 | — | — |
| Common Redstart | Phoenicurus phoenicurus | Quadratic | 0.17 | 263.71 | 0.48 | −1.86 | 3.55 | −12.81 | 3.55 |
| European Robin | Erithacus rubecula | Linear | 0.08 | 35.97 | 110.62 | 0.13 | 0.06 | — | — |
| Dunnock | Prunella modularis | Quadratic | 0.14 | 286.11 | 0.75 | 5.08 | 5.55 | 17.34 | 5.55 |
| Song Thrush | Turdus philomelos | Linear | 0.10 | 101.06 | 76.49 | 0.09 | 0.04 | — | — |
| Common Chaffinch | Fringilla coelebs | Linear | 0.14 | −86.16 | 124.95 | 0.19 | 0.06 | — | — |
| Redwing | Turdus iliacus | Linear | 0.13 | 72.19 | 76.15 | 0.11 | 0.04 | — | — |
| Eurasian Blackbird | Turdus merula | Linear | 0.10 | 67.06 | 96.61 | 0.12 | 0.05 | — | — |
3.2 From candidate weather signals to the most likely weather influences
The spatiotemporal time window analysis initially resulted in a long-list of 306 candidate weather signals across all 10 species (12 to 46 candidates depending on the species; Table S4 and Figures S2–S7). From these signals, four were removed because the model without accounting for trends had an AICc compared to an intercept-only model that was less than two units lower. Subsequently, 119 candidate weather signals were removed from further analysis due to collinearity with another candidate that showed a bigger ΔAICc with an intercept-only model. For five species that still had more than 15 candidates left, we then removed another 38 candidate signals based on the variable importance outcome using the boruta method (Kursa & Rudnicki, 2010; Table S4). The final ‘long-list' of candidate weather signals just prior to the variable importance analyses as such consisted of 145 candidates across the 10 species (Table S5). Based on the output of the variable importance analysis (Table S5 and Appendix S2), we ultimately retrieved 32 signals across the 10 species that are very likely to be related to MAPD at Heligoland (Table S6).
3.3 Identified most influential ‘weather variable–location–time window' influences
The final identified weather influences for each species consisted of two to five signals, depending on the species (Figure 2, Figure S8, and Table S6). There was at least one temperature, precipitation, head-, and tailwind signal for six, eight, five, and eight of the ten species respectively. For three species (Garden Warbler, Dunnock and Eurasian Blackbird), we found both tail- and headwind influences. We did not find any obviously distinct pattern between trans-Saharan and intra-European migrants in terms of location and time windows of influence. The time window analysis of the daily NAO values resulted in a candidate signal for Garden Warbler only (Appendix S3). A variable importance analysis in combination with the final selected weather signals for Garden Warbler, however, showed that this NAO signal did not hold any additional information.
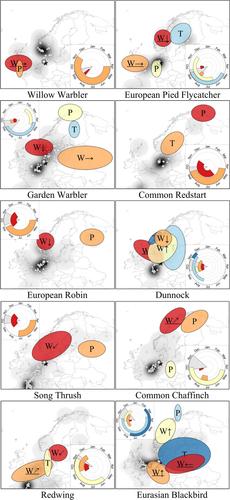
Wind conditions seem to influence MAPD both at likely breeding and autumn stopover areas. Temperature and precipitation influences were mainly located at likely breeding areas (6 out of 7 for temperature, and 8 out of 9 for precipitation; Figure 2; see also Table S7 for interpretations of the location and timing with respect to the species' lifecycles). One precipitation (European Pied Flycatcher) and one temperature signal (Redwing) were located at stopover areas close to and including Heligoland. Unambiguous identification of the location of a signal as a breeding or stopover area, however, was not always evident, that is, they could sometimes be either of both.
The influences of wind on MAPD at Heligoland invariably occurred during the autumn migration period. For four of the seven temperature signals, the best performing time window occurred during the start and earlier half of the autumn migration period at Heligoland (Figure 2, Figure S8, and Table S7). One occurred towards the end of the migration period (Dunnock in W Norway), one during spring migration (Eurasian Blackbird in S Finland–N Baltics), and one during the spring migration and the breeding period (Common Redstart in C Sweden). The timing of the identified best time windows for precipitation signals was highly variable. Only two (European Pied Flycatcher in the Netherlands–Belgium–N Germany, and Common Redstart in N Finland–NW Russia) occurred during the autumn migration period at Heligoland. Five occurred between the end of spring migration and the end of the breeding period (European Robin in E Finland, Song Thrush in W Russia, Common Chaffinch in N Finland and in E Germany, and Eurasian Blackbird in N Norway). Two precipitation time windows pointed towards even earlier periods in the year: during the start of migration at Heligoland for Willow Warbler in the UK, and during a 3-month period prior to spring migration at Heligoland for Garden Warbler in N Norway. Temporal autocorrelation caused large uncertainties in the exact periods of influence for most temperature (6 out of 7) and precipitation (7 of 9) signals, that is, there were many different time windows at those locations than performed similar, albeit slightly worse, in terms of AICc. Taking into account these uncertainties, the periods of influence for temperature and precipitation potentially covered much larger time windows. Timing of the wind influences was uncertain for only a few signals, and to a much lesser extent.
3.4 Weather variable types influencing autumn migration phenology
While temperature and precipitation each amounted to about a quarter of the total sum of the mean relative variable importance across all species, wind variables clearly seem to have a more important and frequent impact on MAPD at Heligoland, amounting to about half of the total sum of the mean relative variable importance (Table 4). For intra-European migrant species specifically, the same pattern seemed to hold, albeit perhaps with even more relative emphasis on wind-related variables. For trans-Saharan migrants, the importance in function of the weather variable types seemed more even, with each of the three weather variable types amounting to about a third of the mean relative importance sum. In terms of wind, it was mainly the occurrence of tailwinds that seemed to influence MAPD (Figure 2; 10 out of 16 wind signals). Frequency of headwinds seemed to influence the timing of MAPD much less (4 out of 16 wind signals). This suggests that in relation to wind, MAPD at Heligoland for these 10 species is mainly dependent on the relative occurrence of favourable winds to less favourable winds, that is, days with tailwinds, compared to days without tailwinds.
| Weather variable | Times selected | Sum of the mean RelImp | Percentage of the sum of all of the RelImp | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All species | TS | IE | All species | TS | IE | All species | TS | IE | |
| Temperature | 7 | 3 | 4 | 1.02 | 0.54 | 0.47 | 25 | 32 | 20 |
| Precipitation | 9 | 4 | 5 | 1.15 | 0.54 | 0.61 | 28 | 32 | 26 |
| Wind (summed) | 16 | 5 | 11 | 1.88 | 0.60 | 1.28 | 46 | 36 | 54 |
| Headwind | 6 | 1 | 5 | 0.68 | 0.12 | 0.55 | 17 | 7 | 23 |
| Tailwind | 10 | 4 | 6 | 1.20 | 0.47 | 0.73 | 30 | 28 | 31 |
- Abbreviations: IE, intra-European migrant species; RelImp, relative variable importance; TS, trans-Saharan migrant species.
3.5 Explained variance in MAPD
Depending on the species, between 43% and 80% of the variance in MAPD was explained by the model using all of the final identified weather signals (Table 5). On average across all species, 62% of the variance was explained, with eight of the 10 species having an adjusted R2 above 0.57. The similarity of the predictive R2 values (calculated using leave-one-out) to the adjusted R2 values furthermore confirms the robustness of the final identified weather signals.
| Species | Adjusted R2 | Predictive R2 |
|---|---|---|
| Willow Warbler | 0.48 | 0.44 |
| European Pied Flycatcher | 0.67 | 0.62 |
| Garden Warbler | 0.80 | 0.77 |
| Common Redstart | 0.43 | 0.39 |
| European Robin | 0.57 | 0.51 |
| Dunnock | 0.61 | 0.57 |
| Song Thrush | 0.66 | 0.62 |
| Common Chaffinch | 0.63 | 0.58 |
| Redwing | 0.61 | 0.58 |
| Eurasian Blackbird | 0.72 | 0.67 |
3.6 Direction of the effect of the weather variables on MAPD
The effect of summer and autumn temperatures on MAPD was distinctly different for trans-Saharan and intra-European migrants (Figure S8 and Figure 3, Tables S6 and S8): higher temperatures lead to earlier passage at Heligoland for trans-Saharan birds, while they result in delays for intra-European migrants. One spring (or early summer) temperature signal for Eurasian Blackbird was negatively related to MAPD. For precipitation, six out of the nine identified signals were positively related to MAPD, that is, increases in precipitation resulted in later autumn passage at Heligoland. Common Redstart and Eurasian Blackbird showed negative associations between precipitation and MAPD for areas in northern Scandinavia. Common Chaffinch had a negatively related precipitation signal for eastern Germany. Frequency of tailwinds was negatively associated with MAPD in six signals, and positively in four cases. Frequency of headwinds was associated positively with MAPD in five signals (all for intra-European species), and negatively in one (for a trans-Saharan species).
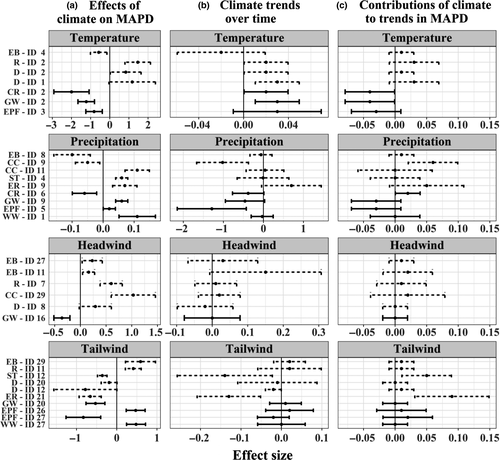
3.7 Contributions of climatic influences and trends to the temporal trends in MAPD
Contributions of weather variables' effects on MAPD to species-specific trends in MAPD at Heligoland are a complex combination of both the strength and direction of both the (a) effects of weather variables on MAPD and (b) trends over time in the weather variables (Figure 3 and Table 6). For intra-European migrants, all (20) weather influences have contributed towards a delay in MAPD, albeit to varying degrees. For trans-Saharan migrants, both positive (delay in MAPD) and negative (MAPD advancement) trend contributions occur. The contributions towards advancement in MAPD are, however, exclusively temperature and precipitation signals. All wind signals push towards MAPD delay.
| Species | Climate variable | ID |
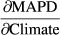
|
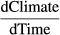
|
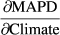 × × 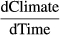 |
SE | ||
|---|---|---|---|---|---|---|---|---|
| Coef. | SE | Coef. | SE | |||||
| Willow Warbler | Precipitation | 1 | 0.11 | 0.03 | −0.03 | 0.14 | 0.00 | 0.02 |
| Tailwind in UK | 27 | 0.48 | 0.12 | 0.00 | 0.03 | 0.00 | 0.01 | |
| European Pied Flycatcher | Temperature | 3 | −0.82 | 0.22 | 0.03 | 0.02 | −0.03 | 0.02 |
| Tailwind in C Norway | 27 | −0.83 | 0.22 | −0.02 | 0.02 | 0.02 | 0.02 | |
| Tailwind in Ireland/UK | 26 | 0.47 | 0.12 | 0.02 | 0.03 | 0.01 | 0.02 | |
| Precipitation | 5 | 0.02 | 0.01 | −1.29 | 0.44 | −0.03 | 0.02 | |
| Garden Warbler | Precipitation | 9 | 0.06 | 0.01 | −0.46 | 0.25 | −0.03 | 0.02 |
| Temperature | 2 | −1.23 | 0.22 | 0.03 | 0.01 | −0.04 | 0.02 | |
| Tailwind in SW Norway | 20 | −0.53 | 0.12 | 0.01 | 0.02 | 0.00 | 0.01 | |
| Headwind in the Baltic states | 16 | −0.36 | 0.08 | 0.00 | 0.04 | 0.00 | 0.01 | |
| Common Redstart | Temperature | 2 | −2.00 | 0.47 | 0.02 | 0.01 | −0.04 | 0.02 |
| Precipitation | 6 | −0.06 | 0.02 | −0.38 | 0.20 | 0.02 | 0.01 | |
| European Robin | Tailwind in S and C Norway | 21 | −0.66 | 0.14 | −0.13 | 0.04 | 0.09 | 0.03 |
| Precipitation | 9 | 0.07 | 0.02 | 0.70 | 0.38 | 0.05 | 0.03 | |
| Dunnock | Temperature | 1 | 1.17 | 0.62 | 0.03 | 0.01 | 0.03 | 0.02 |
| Temperature | 2 | 0.83 | 0.41 | 0.02 | 0.01 | 0.01 | 0.01 | |
| Tailwind in C Norway | 12 | −0.78 | 0.40 | −0.02 | 0.01 | 0.01 | 0.01 | |
| Headwind in Norway/Sweden | 8 | 0.29 | 0.16 | −0.02 | 0.04 | 0.00 | 0.01 | |
| Tailwind in SW Norway | 20 | −0.19 | 0.10 | −0.01 | 0.05 | 0.00 | 0.01 | |
| Song Thrush | Precipitation | 4 | 0.06 | 0.01 | −0.01 | 0.33 | 0.00 | 0.02 |
| Tailwind in S Norway | 12 | −0.36 | 0.06 | −0.14 | 0.06 | 0.05 | 0.02 | |
| Common Chaffinch | Headwind in C Norway | 29 | 1.03 | 0.22 | 0.02 | 0.03 | 0.02 | 0.03 |
| Precipitation | 11 | 0.11 | 0.02 | 0.04 | 0.24 | 0.00 | 0.03 | |
| Precipitation | 9 | −0.05 | 0.02 | −1.02 | 0.33 | 0.06 | 0.02 | |
| Redwing | Tailwind in S Sweden | 11 | 0.41 | 0.10 | 0.02 | 0.04 | 0.01 | 0.01 |
| Headwind in N France | 7 | 0.60 | 0.11 | 0.01 | 0.03 | 0.01 | 0.02 | |
| Temperature | 2 | 1.46 | 0.34 | 0.02 | 0.01 | 0.03 | 0.02 | |
| Eurasian Blackbird | Precipitation | 8 | −0.10 | 0.03 | −0.06 | 0.14 | 0.01 | 0.01 |
| Tailwind in Poland/Baltics | 29 | 0.59 | 0.19 | 0.02 | 0.02 | 0.01 | 0.01 | |
| Temperature | 4 | −0.58 | 0.22 | −0.02 | 0.02 | 0.01 | 0.01 | |
| Headwind in C Norway | 11 | 0.16 | 0.06 | 0.15 | 0.08 | 0.02 | 0.02 | |
| Headwind in W Germany | 27 | 0.23 | 0.10 | 0.03 | 0.05 | 0.01 | 0.01 | |
Across all species, the relative contributions of each climate variable to the trends in MAPD (Table 7) remained largely similar to the relative weather variable importance for interannual variations in MAPD (Table 4), that is, wind has the strongest influence (41%), followed by precipitation (30%) and temperature (29%). The same pattern largely remains for intra-European migrants, albeit perhaps with even more emphasis on wind (55%) compared to precipitation (25%) and temperature (20%). For the trans-Saharan migrant species, the pattern of weather variable importance is completely flipped, with temperature having the strongest impact (48%) on the trends in MAPD, followed by precipitation (38%), and only then wind and to a much lesser extent (14%).
| Climate variable | All species (%) | TS (%) | IE (%) |
|---|---|---|---|
| Temperature | 29 | 48 | 20 |
| Precipitation | 30 | 38 | 25 |
| Wind (summed) | 41 | 14 | 55 |
| Headwind | 10 | 0 | 15 |
| Tailwind | 31 | 14 | 40 |
- Abbreviations: IE, intra-European migrants; TS, trans-Saharan migrants.
4 DISCUSSION
4.1 Which weather variable types influence interannual differences in avian autumn migration phenology?
Radar studies have repeatedly indicated strong relationships between non-species-specific migration intensity and weather conditions (Erni et al., 2002; Nilsson et al., 2019; Van Belle et al., 2007). Quantifying species-specific relationships between climate and autumn migration phenology has, nevertheless, proven challenging. While it has long been recognized that it is critical to use the right location and time of influence to assess the influence of climate on migration timing (Gordo, 2007; Lack, 1960; Shamoun-Baranes et al., 2006), large uncertainties in both space and time (due to a lack of sufficiently detailed data) have made it methodologically challenging to identify these areas and times of influence (Haest et al., 2018b; van de Pol et al., 2016). Our study shows that an exploratory data analysis approach is able to identify strong species-specific relationships between climate (change) and autumn migration timing of 10 passerine species at the island of Heligoland (Germany) by specifically addressing the spatial and temporal uncertainties in the weather influences.
Across all 10 species we studied, winds during the autumn migration period at both the likely stopover and breeding grounds were the most frequent and important climate influence on autumn migration timing (Table 4). Frequency of tailwinds had a bigger impact on migratory progress at Heligoland than frequency of headwinds. Notwithstanding, we did also find specific headwind frequency influences, including for one species (Dunnock) at roughly the same location and time as a tailwind frequency influence. We, hence, did not only provide further support for a ‘sit-and-wait-for-favourable-winds' strategy (Delingat, Bairlein, & Hedenström, 2008; Eikenaar & Schmaljohann, 2015; Gauthreaux Jr., Michi, & Belser, 2005; Kemp, Shamoun-Baranes, Gasteren, Bouten, & Loon, 2010; Kölzsch et al., 2016; Nilsson et al., 2019), but also for a ‘sit-and-wait-to-avoid-unfavourable-winds' strategy (see also Erni et al., 2002), with ‘favourable winds' here defined as tailwinds and ‘unfavourable winds' as headwinds, both independent of the wind speed (see Materials and Methods section).
Depending on the species, temperature and precipitation at presumed breeding areas also played important roles (Figure 2 and Table S6). The specific time windows of influence for temperature and precipitation were much less certain than for wind conditions. For temperature, the majority occurred just prior or during the autumn migration period at Heligoland. Timing of the precipitation influences was much more diverse, occurring during breeding periods, as well as autumn migration, but also during spring migration or even earlier. This variability in the timing of the precipitation effects once more points towards the complex amalgam of both direct and indirect (including time lagged, e.g. via food) effects through which precipitation likely influences the timing of biological events. Across taxa and biological events, similarly complex precipitation effects have been suggested for, for example, plant phenology (Gordo & Sanz, 2010; Peñuelas et al., 2004), insect migration and abundance (Evans, Salvatore, Pol, & Musters, 2019; Zipkin, Ries, Reeves, Regetz, & Oberhauser, 2012), avian spring migration and reproduction (Englert Duursma, Gallagher, & Griffith, 2019; Gordo, 2007; Gordo, Brotons, Ferrer, & Comas, 2005; Haest et al., 2018b) and mammal demography (Campos et al., 2017; Thibault, Ernest, White, Brown, & Goheen, 2010).
Our results show how species-specific combinations of precipitation, temperature, tail- and headwind influences at the breeding and stopover grounds (Figure 2) can explain between 50% and 80% of the interannual variation in autumn migration phenology (Table 5) in both intra-European and trans-Saharan migrant bird species. Similar to previous studies on the relationship between timing of (other) biological events and large-scale indices (Chambers et al., 2014; Haest et al., 2018b; van de Pol et al., 2013), we found no support for any influence of the NAO (index) on autumn migration phenology after more local weather influences were taken into account.
It is important to note that the relative importance of the different weather variables might only be representative for the studied species, geographical region and populations, as the influence of weather on bird migration phenology is species- and context-dependent (Calvert et al., 2009; Carey, 2009; Gordo, 2007; Haest et al., 2018b; Senner et al., 2018; Shaw, 2016). The larger relative importance of wind conditions at Heligoland might, for example, be influenced by its geographic location at an ecological barrier, that is, in the North Sea. For migration across large land masses, wind might be less important as birds can, at any time, land to rest or refuel (Bulte et al., 2014; Shamoun-Baranes, Liechti, & Vansteelant, 2017). Nonetheless, some of the wind influences we found were located far away from Heligoland and not always at any obvious ecological barrier (Figure 2). Furthermore, strong wind influences on autumn migration intensity (using radar) have recently also been shown across large parts of (continental) Europe (Nilsson et al., 2019).
4.2 Why are trends in autumn phenology so variable, including opposite directions?
Our analysis provides a clear illustration of how species- or population-specific responses to several climate (change) influences at the breeding and stopover grounds during and prior to autumn migration can bring about large interspecific variation in autumn migration phenology trends (Figure 3, Tables 6 and 7). The high variability in the time windows of the climatic influences between and within both species and weather variable types furthermore illustrates that the climatic influences, next to direct effects on migration departure and progress (Calvert et al., 2009; Shamoun-Baranes et al., 2006; Vansteelant et al., 2015), likely also work through impacts on (and carry-over effects of) several life-history and ecological events prior to autumn migration. Our findings, hence, confirm that interspecific differences in temporal trends in autumn migration phenology are very likely related to differences in ecological and life-history traits (Bitterlin & Van Buskirk, 2014; Jenni & Kéry, 2003), but also suggest that these differences are related to different exposure and reactions to climate during these prior life-history events (see also Chmura et al., 2019).
4.3 Long-distance versus short-distance migrants
One of the most frequent observed patterns in interspecific differences in autumn migration phenology trends is the advancement for long-distance (e.g. the trans-Saharan migrants in this study; TS) versus the delay for short-distance (e.g. the intra-European migrants in this study; IE) migrant bird species (Jenni & Kéry, 2003; Van Buskirk et al., 2009). Our results on the effects and trend contribution of each weather variable (Figure 3 and Table 6) provide some hints on the possible mechanisms through which climate (change) may be causing the apparent distinction in autumn migration trends of IE (delay) and TS (advance) migrants. For IE migrants, the climate contributions to MAPD trends (almost) unanimously pushed towards a delay in autumn migration. Climate contributions for TS migrant species were more heterogeneous, but particularly of interest is that contributions towards advancements were exclusively temperature and precipitation effects, not wind effects. The contrast in temperature trend contributions for IE and TS migrants seems to come about mainly due to the different direction of the impact of temperature on MAPD, as most (six of seven) of the influencing temperatures have increased over the past six decades. The contrast in precipitation trend contributions, on the other hand, seems to be mainly due to decreasing precipitation over time for TS migrants. As most of the precipitation influences had a delaying effect on MAPD, that is, a positive association, the decrease in precipitation over time translates into advancing trend contributions for the TS migrants. Interestingly also, the overall large importance of wind effects on interannual variation in MAPD (Table 4) is reflected in the relative contributions to trends in MAPD for IE migrants (Table 7). For TS migrants, however, the relative trend contributions of wind effects were strongly reduced because trends over time in the influencing wind variables were largely lacking.
These observations, in combination with the weather variable type-specific locations and timing (see Results section, Figures 2 and 3 and Table S7), fit with two previously suggested hypotheses to explain the contrasting patterns in autumn migration phenology trends between long- and short-distance migrants: (a) the optimal migration strategy (Gordo, 2007; Jenni & Kéry, 2003), that is, long-distance migrants depart earlier when they can to profit from resource peaks further along the migration route while short-distance migrants stay until deteriorating living conditions push the birds to move, seems to be reflected in the contrasting effects of temperatures; and (b) the ‘sit-and-wait-for-favourable-winds' strategy strongly affects interannual variability in migration timing of both long- and short-distance migrants (Gauthreaux Jr. et al., 2005), but for the species in our study, strong contributions to long-term trends in migration timing only occurred for short-distance migrants because the wind conditions that influence the long-distance migrants did not change in a specific direction. Given that responses are highly species- and context-dependent, however, further studies are needed on other species and locations to fully understand the mechanisms that drive interspecific differences in autumn migration trends, including if there are indeed explicit differences between long- and short-distance migrants.
4.4 All aspects of climate (change) are important
Climate change involves much more than merely an increase in temperatures. The potential role of altered precipitation, and especially of altered wind conditions, in the context of climate change impacts on migration phenology has, nevertheless, typically received much less attention (Gordo, 2007; Kemp et al., 2010), with only 29% and 12% of species-specific long-term studies investigating precipitation and wind respectively (Appendix S1). Our study shows how changes in both precipitation and wind conditions have already contributed to trends in autumn migration phenology at Heligoland over the past decades (Figure 3, Tables 6 and 7). A number of recent studies have also made projections on how future wind conditions are likely to impact migration progress and timing (e.g. La Sorte & Fink, 2017; La Sorte, Horton, Nilsson, & Dokter, 2019). Given the frequency and strength of precipitation and especially wind effects on autumn migration phenology that we (Table 4) and other studies have found (e.g. Calvert et al., 2009; Kemp et al., 2010; La Sorte et al., 2014; Laughlin, Sheldon, Winkler, & Taylor, 2016), it appears vital for future migration studies to investigate not only temperature but also other climate variables.
4.5 Implications for (autumn) migration in other animal taxa
There are migrant species in many animal groups, but the link between migration and climate (change) has been primarily studied in birds (Shaw, 2016). While the migration ecology of any animal group, species or population should be analysed within its own appropriate ecological and geographical context, our results do provide some suggestive patterns for migration in other animal taxa. Insect and bat migration timing, for example, are also highly dependent on a mixture of temperature, precipitation and wind conditions occurring prior or during migration, through both direct and indirect effects (Bauer et al., 2011; Pettit & O'Keefe, 2017; Shaw, 2016; Wikelski et al., 2006). Similarly complex contributions towards advancement and delays are hence to be expected and are likely also occurring already, potentially causing ecological mismatches in multiple trophic interactions (Thackeray et al., 2010; Visser & Gienapp, 2019).
Arguably, one could dismiss our approach as being too exploratory. Given the current uncertainties, if not complete knowledge gaps, about which weather variables influence autumn migration phenology of which species at which locations and at what time, however, an explorative approach does not only seem appropriate, but furthermore also generates hypotheses that can subsequently be tested using more confirmatory approaches. This applies to bird migration, but perhaps even more so to the migrant species in any of the other animal groups, as the link between climate (change) and migration has in most cases been studied even less. As such, we encourage other researchers with similar time series data to follow a similar approach in order to further unravel species- and population-specific responses of autumn migration timing to climate change. By doing so, new patterns may arise at a meta-analysis level that could ultimately contribute towards understanding the observed demographic changes in bird and other animal populations.
ACKNOWLEDGEMENTS
We are sincerely grateful to the numerous volunteers and the staff at the field station on Heligoland for the continuous efforts in collecting the standardized ringing data at the island of Heligoland. We are also most grateful to P. Gienapp, A. Phillimore and two anonymous reviewers for their helpful comments.




