Short photoperiod reduces the temperature sensitivity of leaf-out in saplings of Fagus sylvatica but not in horse chestnut
Abstract
Leaf phenology is one of the most reliable bioindicators of ongoing global warming in temperate and boreal zones because it is highly sensitive to temperature variation. A large number of studies have reported advanced spring leaf-out due to global warming, yet the temperature sensitivity of leaf-out has significantly decreased in temperate deciduous tree species over the past three decades. One of the possible mechanisms is that photoperiod is limiting further advance to protect the leaves against potential damaging frosts. However, the “photoperiod limitation” hypothesis remains poorly investigated and experimentally tested. Here, we conducted a photoperiod- and temperature-manipulation experiment in climate chambers on two common deciduous species in Europe: Fagus sylvatica (European beech, a typically late flushing species) and Aesculus hippocastanum (horse chestnut, a typically early flushing species). In agreement with previous studies, we found that the warming significantly advanced the leaf-out dates by 4.3 and 3.7 days/°C for beech and horse chestnut saplings, respectively. However, shorter photoperiod significantly reduced the temperature sensitivity of beech only (3.0 days/°C) by substantially increasing the heat requirement to avoid leafing-out too early. Interestingly, the photoperiod limitation only occurs below a certain daylength (photoperiod threshold) when the warming increased above 4°C for beech trees. In contrast, for chestnut, no photoperiod threshold was found even when the ambient air temperature was warmed by 5°C. Given the species-specific photoperiod effect on leaf phenology, the sequence of the leaf-out timing among forest tree species may change under future climate warming conditions. Nonphotoperiodic species may benefit from warmer springs by starting the growing season earlier than photoperiodic sensitive species, modifying forest ecosystem structure and functions, but this photoperiod limitation needs to be further investigated experimentally in numerous species.
1 INTRODUCTION
The timing of leaf-out strongly affects the temperate deciduous trees’ fitness and distribution, as well as terrestrial ecosystem water and carbon balances (Chuine, 2010; Jeong, Medvigy, Shevliakova, & Malyshev, 2012; Peñuelas & Filella, 2009; Piao et al., 2017). Climate warming has substantially advanced the timing of spring leaf-out across temperate tree species (Cleland, Chuine, Menzel, Mooney, & Schwartz, 2007; Fu et al., 2014; Menzel et al., 2006; Peñuelas & Filella, 2001). A recent study found that the temperature sensitivity of leaf-out has significantly reduced over the past decades (Fu, Zhao et al., 2015), suggesting that the lengthening of the carbon uptake period due to earlier spring may slow down under future climate change conditions. The reduced temperature sensitivity of leaf-out may be related to chilling becoming insufficient in winter (Asse et al., 2018; Fu, Piao et al., 2015) and/or photoperiod limitation as leaf-out tends to occur now at a shorter photoperiod (Fu, Zhao et al., 2015). The winter precipitation may additionally have an indirect influnce on the temperature sensitivity of leaf-out (Yun et al, 2018). However, how the photoperiod cue coregulates the leaf-out process with temperature is still unclear and needs further investigations as it has critical implications in predicting ecosystem responses to ongoing climate change.
Previous studies have suggested that the interactions between winter chilling temperature required to break dormancy (hereafter called chilling), spring warming temperature (called hereafter heat requirement or growing degree days) and photoperiod, i.e. day length, regulate the timing of spring leaf-out of temperate and boreal tree species (Hänninen, 2016). Specifically, once a certain amount of chilling has accumulated, e.g. the chilling requirement, buds become sensitive to warmer temperatures and start developing after a certain accumulation of heat (called growing degree days, GDD). When chilling temperature is not sufficient, the forcing requirement increases (Hänninen, 2016). The seasonal increase of daylength co-occurring during the accumulation of GDD may compensate the chilling deficits by reducing the GDD requirement (Laube et al., 2014). In contrast a too short photoperiod in late winter may slow down the accumulation of heat to prevent a too premature leaf-out. However, how the photoperiod interacts with chilling and heat requirement is still largely unclear and currently debated (Chuine, Morin, & Bugmann, 2010, Korner & Basler, 2010, Laube et al., 2014, Zohner et al., 2016; Flynn & Wolkovich, 2018, Richardson et al, 2018). Recent studies have reported that winter warming reduces the chilling conditions at the southern range of the species area, and insufficient chilling during warm winter increases the requirement of GDD during spring (Fu, Piao et al., 2015; Vitasse et al., 2018), potentially delaying the date of leaf-out relative to similar spring temperatures following a warmer winter (Asse et al., 2018; Laube et al., 2014). However, during a cold spring, leaf-out occurs later at longer photoperiod. The increasing photoperiod could compensate partly insufficient chilling effect by reducing the heat requirement for leaf-out, and ensure the trees leaf-out not too late (Basler & Körner, 2014; Vitasse & Basler, 2013; Way & Montgomery, 2015). Therefore, despite higher GDD requirement due to insufficient chilling, trees could still leaf-out earlier under exceptionally warm spring conditions because of the rapid heat accumulation and the stimulating effect of increasing photoperiod on bud development (Basler & Körner, 2014; Vitasse & Basler, 2013).
In addition to the promoting effect of photoperiod beyond a certain threshold (i.e. by reducing the heat requirement or increasing the bud temperature sensitivity to temperature), a short photoperiod (relative to early leaf-out) may be a signal used by the tree to retard cell growth under favorable spring conditions. By advancing the leaf-out timing due to warmer temperatures, trees may become even less sensitive to warming due to a too short photoperiod that helps to prevent from potentially damaging frosts. However, the “short photoperiod limitation” hypothesis has not yet been well investigated (but see two recent studies in Malyshev, Henry, Bolte, MaA, and Kreyling (2018) and Flynn and Wolkovich (2018)), especially experimental efforts are needed.
Experimental studies investigating the interactive effects of chilling, heat requirements and photoperiod have some inevitable drawbacks (Wolkovich et al., 2012). For example, chilling, photoperiod and rate of heat accumulation are all altered simultaneously in temperature-controlled experiments (Laube et al., 2014; Malyshev et al., 2018; Zohner et al., 2016), and chilling is not controlled in cutting experiments because cuttings were sampled at different time, i.e. increasing photoperiod. Besides, the photoperiod treatments are generally set as constant values, rather than using a gradual increase that would mimic spring conditions (but see Basler & Körner, 2014). For instance, in Zohner and Renner (2015) and Malyshev et al. (2018), the photoperiod was fixed at 16 or 8 hr during the whole forcing period, which might be extreme, rather than standard photoperiod conditions for the studied tree species.
We, therefore, conducted an experiment during which the photoperiod and temperature were independently manipulated within transparent climate chambers for two European tree species: European beech, a typical late flushing and photoperiod-sensitive species (Basler & Körner, 2014; Laube et al., 2014; Zohner et al., 2016), and horse chestnut, an early flushing and photoperiod-insensitive species (Basler & Körner, 2014; Laube et al., 2014). We hypothesized that the leaf-out timing of horse chestnut would advance in response to warming but not to the shorter photoperiod treatment, whereas, for beech, the advance of leaf-out timing would be limited under shorter photoperiod, with the reduced photoperiod treatment increasing the heat requirement and thereby decreasing the apparent temperature sensitivity (ST, advancement of leaf-out in days per degree warming, days/°C). In addition, we expect the photoperiod effect to be visible only below a certain daylength which we aimed to quantify in this study.
2 MATERIALS AND METHODS
2.1 Experimental design and phenological measurements
The experiment was conducted at the Drie Eiken campus of the University of Antwerp, Belgium (51°19ʹN, 4°21ʹE). We transplanted beech (Fagus sylvatica) and horse chestnut (Aesculus hippocastanum) saplings ~1.5 m height into plastic pots (diameter 25 cm, depth 40 cm) in December 2015 and moved them into 12 outdoor transparent climate-controlled chambers on 1 January 2016 (Figure S1). These saplings came from a nursery near the experimental site and were grown from seeds collected from a local forest (Sonian Forest, Brussels). The saplings had been cultivated in the same field for one year prior to being transplanted into pots and used in our experiment. The pots were filled with an organic substrate with a pH of 6.0% and 20% organic matter (Universal potting soil, Viano, Aalst, Belgium). Chambers were artificially warmed by a climate-control unit in which a cooling and heating system was installed (Fu et al., 2016). Outside air was cooled or heated through the system, ensuring different levels of continuous (day and night) warming above the fluctuating ambient air temperature. The chambers were sunlit, facing south, with a transparent polycarbonate, 4 mm thick plate at the top (light absorption = 15%). The interior surface area was 200 × 150 cm, and the height was 180 cm on the south side and 200 cm on the north side (Figure S1). Temperature sensors (type QFA66; Siemens, Berlin, Germany) were used to continuously monitor the air temperature inside each chamber, logging every 30 min and stored as hourly data. The saplings were irrigated once or twice a week throughout the experimental period (from 1 January 2016 until leaf-out in spring 2016) as soon as the topsoil appeared dry.
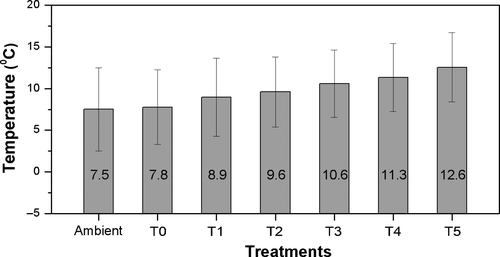
The experiment had five warming treatments (+1°C [T1], +2°C [T2], +3°C [T3], +4°C [T4] and +5°C [T5], two chambers per treatment) and a control treatment (+0°C [T0]). The chambers provided stable warming during the study period (1 January till leaf-out) within 5% of the prescribed value (Figure 1). Each chamber contained 11 saplings of both beech and horse chestnut, three of which were randomly selected and treated as controls for the photoperiod component of the experiment, four of which were covered by transparent bags, representing “shams” or “disturbance controls”, and four of which were covered by black opaque bags, representing a short-photoperiod treatment (Figure S2). In total, there were 132 saplings for each species, and 48 saplings treated with short-photoperiod treatment, and 48 saplings treated as disturbance controls and 36 saplings as control. During the experiment, no mortality was observed in the saplings. The bags were installed and removed in accordance with the daily time of sunrise and sunset: the bags were installed 1 hr before sunset and removed one hour after sunrise (Figure S3), i.e. reducing daylength by about 2 hr. The differences in air temperature in the bags were recorded using two Hobo data loggers (Onset Computer Corp., Bourne, MA) inside each bag (transparent and opaque) for each warming treatment. Air temperature did not differ between the transparent and opaque bags (Figure S4), because they remain only 1 hr at a time when the solar angle is very low. Leaf-out was observed on the terminal bud of each sapling and was defined as the date when the entire leaf blade and stalk were visible, following the guidelines provided by Fu et al. (2016). Phenology monitoring began on 1 February 2016, and data were collected every 2 days. Leaf-out dates were always identical in the control treatment and the treatment with transparent bags, so these two treatments were merged into the photoperiod control treatment.
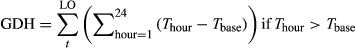 (1)
(1)The chilling hours were summed when the hourly temperature was between 0 and 5°C from 1 September 2015 until the date of leaf-out following previous study (Chuine, 2000), because temperatures below freezing point are not valid for chilling accumulation, and temperatures slightly above freezing are most effective (Hänninen, 2016).
2.2 Data analysis
Climate chamber was a nested factor, so we specified the nested effects of the chambers in the runs of the linear mixed models and found that the chambers did not significantly affect the results. The mean date of leaf-out, GDH and chilling-hour requirement were analyzed using all saplings per treatment, i.e. we averaged the climatic and phenological variables across all saplings of each treatment (two chambers per treatment). The temperature sensitivity of leaf-out (ST) was calculated using linear regression analyses (ordinary least squares regressions) of the dates of leaf-out against mean air temperature calculated from 1 January to the day of leaf-out for each species and each chambers. One-way ANOVAs were then applied to test pairwise comparisons of the leaf-out dates using all saplings between the long- and short-photoperiod treatments of each warming treatment. Differences in the temperature sensitivity of leaf-out between the short- and long-photoperiod treatments were tested with ANCOVAs (i.e. testing both the slope and intercept of the linear relationship). All statistical analyses were conducted using spss 16.0 (SPSS Inc., Chicago, IL).
3 RESULTS
We found that spring warming significantly advanced the dates of leaf-out in both species (Figure 2a,b), which is consistent with previous studies (Menzel et al., 2006; Peñuelas & Filella, 2001). No significant difference in temperature sensitivity between beech and horse chestnut was found, i.e. 4.3 ± 0.6 and 3.7 ± 0.5 days/°C for beech and horse chestnut, respectively (tested by ANCOVA, p = 0.501, df = 1,12, f = 0.47). The short-photoperiod treatment significantly decreased ST for beech (3.0 ± 0.4 days/°C, p = 0.020, df = 1.12, f = 6.445), but not for horse chestnut (3.7 ± 0.4 days/°C, p = 0.778, df = 1.12, f = 0.08, Figure 2c), in agreement with our hypothesis. The short-photoperiod treatment, however, delayed beech leaf-out only in the treatments that were warmed >4°C above ambient (Figures 2a & 3). Photoperiod thus significantly affected leaf-out only beyond a warming threshold, corresponding to a particular day of the year (DOY ~ 120, daylength ~ 14.7 hr), but not later.
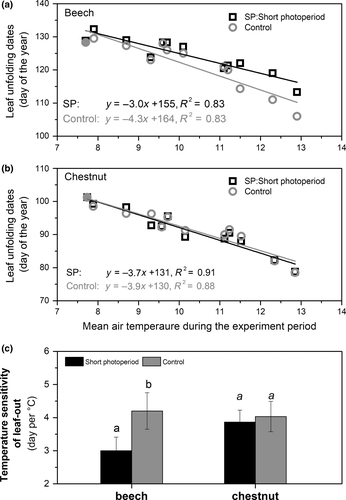
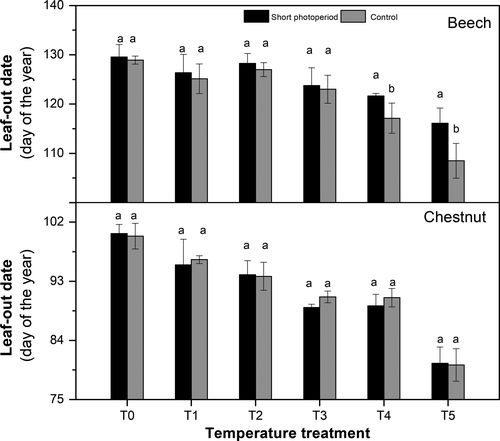
We further examined the relationship between chilling hours and heat requirement GDHs, (see Section 2) under the two photoperiod conditions (Figure 4) to explore the interaction between photoperiod, chilling and heat requirement. The heat requirement for leaf-out was substantially higher for beech than horse chestnut (Figure 4), with mean GDHs across all treatments 8.6 (±1.9) and 14.0 (±2.7) for horse chestnut and beech, respectively. The reduction in cumulative chilling induced by warming increased the heat requirement for leaf-out in both species and photoperiod treatments in an exponential manner, consistent with previous studies (Cannell & Smith, 1983; Hänninen, 2016). The heat requirement was higher in the short-photoperiod than in the control-photoperiod treatment, but only for beech and only in the highest warming treatments, in line with the postulated short photoperiod effect.
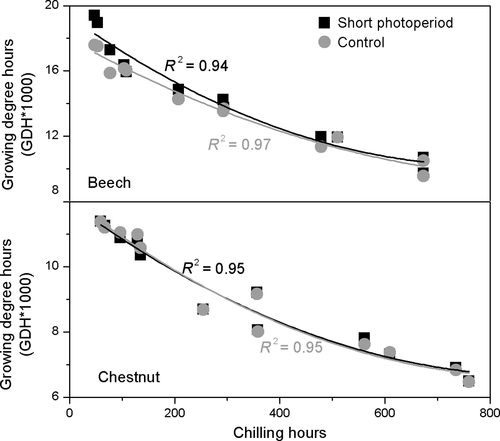
4 DISCUSSION
Changes in plant phenology induced by global climate change have direct effects on regional and global carbon and water cycle (Peñuelas & Filella, 2009; Piao et al., 2017). Previous studies have reported that a lengthening of the growing season in spring may potentially increase the carbon uptake by 0.5%–1% per day (Kimball, McDonald, Running, & Frolking, 2004; Piao, Friedlingstein, Ciais, Viovy, & Demarty, 2007; White et al., 1999). Changes in the spring leaf-out can also exert biophysical feedback on climate system by modifying the surface albedo and energy flux (Penuelas, Rutishauser, & Filella, 2009; Richardson et al., 2013). However, the underlying process of spring leaf-out phenology is still unclear. Previous studies have demonstrated that the gradual increase of photoperiod in spring, i.e. the day length after the winter solstice date, could compensate the chilling deficit (long photoperiod effect) by decreasing the heat requirement for leaf-out, and eventually prevent trees from a too late leaf-out which would lose favorable spring photosynthetic conditions (Caffarra, Donnelly, & Chuine, 2011; Vitasse & Basler, 2013). In line with these studies, we demonstrate experimentally that the photoperiod is an important environmental cue for regulating leaf-out timing of European beech. However, in contrast to previous studies, we reported a physiologically different photoperiod effect. Compared to the long photoperiod effect that promoted budburst (Basler & Körner, 2014; Laube et al., 2014; Vitasse & Basler, 2013; Zohner et al., 2016), here we found, for European beech, that shorter photoperiod below a critical threshold increased the heat requirement for leaf-out, a mechanism that has likely evolved to minimize the risk of damaging frosts. We therefore propose that two substantially different photoperiod effects exist in leaf-out process, at least for beech sapling, i.e. (a) a long photoperiod effect that promotes bud development in late spring preventing trees for a delayed late leaf-out so that they can benefit longer favorable photosynthetic periods and (b) a short photoperiod effect slowing down bud development in early spring allowing trees to avoid leaf-out too early and risking damaging frosts.
In contrast to previous studies, our study was able to manipulate photoperiod and temperature simultaneously by keeping a natural increase during the progression of spring, therefore were able to allow for the first time to better quantify the daylength threshold under which photoperiod is limiting further advance of spring phenology in response to warmer climate. In our present study, we found that the photoperiod threshold for beech saplings occur around the DOY 120 under +4°C warming treatments, which corresponds to a day length of 14.7 hr for the study location. Noteworthy, the mean ambient spring air temperature (from the 1st day of the year to the mean date of leaf-out, 7.5°C) on the study year 2016 was dramatically lower than the long term mean temperature (9.6°C), suggesting that a slight warming during spring, i.e. +2°C than long term average, will reach the photoperiod threshold for leaf-out of beech saplings at our study site, i.e. Antwerp, Belgium.
Our study confirms that the shortening photoperiod partially contributes to the declining apparent temperature sensitivity of leaf-out in European temperature deciduous trees observed over the last decades (Fu, Zhao et al., 2015). However, no photoperiod limitation has been observed for horse chestnut, even under the warmest treatment. Insufficient chilling to break dormancy (resulting in higher forcing requirement) may additionally explain the decline in temperature sensitivity, as recently suggested during warmest years at lowlands in the Alps (Asse et al., 2018; Vitasse et al., 2018). This suggests that a comprehensive understanding of the species-specific and ontogenetic differences of the short photoperiod effects on leaf-out is essential for improving our understanding of the responses of ecosystems to the ongoing climatic warming. To explain the species-specific photoperiod effect, we address the specific potential mechanisms for these two study species.
Horse chestnut (A. hippocastanum) is native to a small area in the Pindus Mountains mixed forests and Balkan mixed forests of South East Europe (Allen & Khela, 2017), but has been distributed over many parts of Europe. Evolutionary selection may reflect the original regions’ climate, and a shorter photoperiod threshold (relatively very early leaf-out dates) may exist, but the photoperiod threshold could have not been reached in Belgium at our experimental site (Antwerp) even when the air temperature was warmed by 5°C. Indeed, the differences in monthly temperature between Antwerp and Athens are 10.5, 11.6, and 13.0°C for March, April and May, respectively, in spring. Therefore, the range of the temperatures in our warming experiment was still in the coldest range of the temperature from the climatic origin of this species, so that for this species the photoperiod threshold limiting further phenological response to warming might be shorter than for European beech and not been reached in the present study. Consequently, we cannot exclude the possibility that there is no photoperiod effect for leaf-out process in horse chestnut saplings if the study would have been conducted in its area of origin. Besides young trees, as used in this study, may be more opportunistic to use warmer temperatures in early spring to outcompete other species or individuals and could thus be less photoperiodic sensitive and/or required less chilling for breaking dormancy compared to adult trees (Vitasse, 2013).
For beech, we found that the photoperiod effect only occurs after a photoperiod threshold when the warming increased above 4°C, which is consistent with a previous study that found that the advancement of leaf-out was significantly reduced when the warming treatment was above a certain threshold (Fu, Campioli, Deckmyn, & Janssens, 2013). We therefore propose that the interaction of temperature and short photoperiod may largely depend on a photoperiod threshold, i.e. a physiological parameter that could be a short range of days in spring. When the ecodormancy starts (second phase of dormancy and the cell starts to grow [Lang, 1987]) later than the photoperiod threshold (lack of chilling), trees will be more sensitive to warmer temperature (promoting photoperiod effect), while when the ecodormancy starts earlier than the photoperiod threshold (sufficient chilling), the temperature sensitivity for leaf-out will be significantly reduced (limiting photoperiod effect). The starting date of ecodormancy, however, cannot easily be determined as neither the range of the temperature that is effective to break dormancy (chilling) nor the amount of it is accurately know at the species level. It is therefore typically ignored in empirical studies, explaining the interaction between photoperiod effect and the start of ecodormancy on leaf-out process has not yet been well investigated (Chuine et al., 2016). To our knowledge, the photoperiod threshold has not yet been accurately quantified, likely because this date could hardly be estimated experimentally, as it required outdoor transparent climatic chambers. Considering the considerable differences in responses to environmental factors among species, and even among clones and provenances (Cook, Wolkovich, & Parmesan, 2012), we therefore propose that developing a practical method for measuring the dates of the start of ecodormancy and potential photoperiod threshold in temperate trees, and combining modeling with physiology at both the whole-tree and the molecular and ecosystemic levels is urgently needed.
Our manipulation experiment identified photoperiod as an additional environmental cue on the leaf-out process, and a short photoperiod significantly reduced the temperature sensitivity of leaf-out, and substantially increased the heat requirement to avoid leafing-out too early. Although this study was conducted on only two species, these two species use contrasting phenological strategies (e.g. one having typically a late and the other an early leaf-out timing) and could therefore reflect the two extreme ends of strategies of European tree species. However, this photoperiod effect should be further explored for other species to improve our understanding of the response of ecosystems to the ongoing global climate change. The species-specific photoperiod effect may result in substantial changes in the phenology dates among species in the future. We clearly showed that the temperature sensitivity of the photoperiod-sensitive species (beech) was higher under long-photoperiod conditions but lower under short-photoperiod conditions, suggesting that the rank of temperature sensitivity of leaf-out between photoperiod-sensitive and -insensitive species could be variable due to photoperiod limitation under warmer climate. This variability will affect the phenological sequence in temperate trees and thus substantially change the structure and function of temperate-forest ecosystems and their feedbacks to climatic systems.
ACKNOWLEDGMENTS
This study support by the National Key Research and Development Program of China (2017YFA06036001), the General program of National Nature science foundation of China (No.31770516) and the Thousand Talents Program for Young Professionals and the Fundamental Research Funds for the Central Universities. Ivan A. Janssen acknowledges support from the European Research Council through Synergy grant ERC-2013-SyG-610028 “P-IMBALANCE” and support from the University Of Antwerp Centre Of Excellence “GCE”.




