Refining predictions of population decline at species' rear edges
Abstract
According to broad-scale application of biogeographical theory, widespread retractions of species' rear edges should be seen in response to ongoing climate change. This prediction rests on the assumption that rear edge populations are “marginal” since they occur at the limit of the species' ecological tolerance and are expected to decline in performance as climate warming pushes them to extirpation. However, conflicts between observations and predictions are increasingly accumulating and little progress has been made in explaining this disparity. We argue that a revision of the concept of marginality is necessary, together with explicit testing of population decline, which is increasingly possible as data availability improves. Such action should be based on taking the population perspective across a species' rear edge, encompassing the ecological, geographical and genetic dimensions of marginality. Refining our understanding of rear edge populations is essential to advance our ability to monitor, predict and plan for the impacts of environmental change on species range dynamics.
1 INTRODUCTION
Climate change impacts species performance and distribution across the globe (Parmesan & Yohe, 2003). Biogeographical theory suggests that rising global temperatures should drive species to move polewards and upwards in elevation as they track the climates to which they are adapted. Therefore, it is reasonable to expect that population loss and range retractions should be seen in the most low-latitude, drought-prone areas of a species' distribution (the rear edge, Hampe & Petit, 2005), given that widespread climate-driven extinction has been predicted (Thomas et al., 2004; Urban, 2015). However, assumptions of declining rear edge population performance are a long-lasting legacy of uncritical application of the centre–periphery hypothesis (Brown, 1984; Safriel, Volis, & Kark, 1994). This prediction assumes that rear edge populations are fundamentally at higher risk of extinction than those populations at the core of the species' range. This elevated extinction risk is attributed to the expectation that they occur in less favourable climates (or habitats) and are more at risk from demographic stochasticity because of lower and highly variable population sizes. Consequently, widespread “marginality” is predicted at the species' rear edge, i.e., decreased population performance because populations occur at the limits of the species' physiological and ecological tolerance.
The assumption of rear edge population decline in response to climate change appears well supported in the literature (e.g. Allen et al., 2010; Carnicer et al., 2011; Feeley et al., 2011; Lesica & Crone, 2016; Marqués, Camarero, Gazol, & Zavala, 2016; Reich et al., 2015). However, such support is often derived from an amalgamation of case studies of decline, risking inaccurate predictions when attempting to extrapolate regionally across the rear edge of a species distribution. “Marginality” at the population level is determined by the interaction of a variety of constraints, including climate and local-scale environmental conditions, habitat fragmentation, species traits, physiology and biotic interactions, as well as population demography and genetics. At the same time, anthropogenic land-use changes shape how species are distributed, and their legacies strongly influence population dynamics. All these together result in ecological and evolutionary mechanisms that are dependent upon far more than the biogeographical location of a population (Hampe & Petit, 2005; Pironon et al., 2016; Sexton, Mcintyre, Angert, & Rice, 2009). Consequently, conflicts between predictions and observed population responses are increasingly accumulating (e.g. Bertrand et al., 2011; Cavin & Jump, 2017; Doak & Morris, 2010; Granda et al., 2018; Rabasa et al., 2013; Rapacciuolo et al., 2014). Here we examine the potential reasons for this disparity by decomposing the causes of marginality and discuss why simplifying assumptions on marginality have implications for predicting species' range shifts. We propose a generally applicable rationale for research design and analysis to better integrate population-level responses into a biogeographical context of species decline. Our focus is on plant – and especially tree – species because of the abundance of data available and the key roles forests play in global carbon and hydrological cycles and maintaining biodiversity. We argue that, as data availability increases, greater emphasis should be placed on recognizing the scale dependency of the factors determining population dynamics, which is fundamental in highly heterogeneous regions like the rear edges, where global change is strongly altering the structure and function of forest ecosystems.
2 EMPIRICAL EVIDENCE IN AGREEMENT WITH BIOGEOGRAPHICAL THEORY
A broad range of studies in the literature provides empirical evidence of declining rear edge populations relative to those of the range-core or across low-altitude relative to high-altitude areas in concordance with biogeographical predictions. For example, sudden population mortality associated with elevated drought stress at species rear edges has been observed in forest ecosystems across the globe (Allen et al., 2010). Equally, evidence of population decline that heralds range retractions is often provided by dendroecological approaches. For example, Scots pine (Pinus sylvestris) forests in the Gúdar range (southern Iberian Range, Iberian Peninsula) are representative populations of the species' rear edge. The species occurs in a mountainous orography, where low-altitude, dry-edge populations coexist with a more drought-tolerant pine species, the black pine (Pinus nigra subsp. salzmannii). In accordance with biogeographical predictions, Scots pine growth is enhanced by temperature at mid- and upper elevations, and constrained because of enhanced drought stress at low-elevations. In these low-altitude areas, where both species co-occur, black pine is more resilient than Scots pine to extreme drought events, suggesting that future changes in species composition are likely (Marqués et al., 2016). Experimental evidence of species' responses to climate manipulation also supports biogeographical predictions. For example, in situ experimental warming in northern Minnesota, North America, showed reductions in photosynthesis and growth near warm range limits and increases near cold range limits in juvenile trees of 11 boreal and temperate forest species (Reich et al., 2015). Species' range shifts predicted by biogeographical theory have been observed in biodiversity hotspots like the Tropical Andes. Elevational shifts during a 4-year period were assessed for 38 tree genera across an elevational gradient from 950 to 3,400 m in Manu National Park in south-eastern Peru. Mean migration rate was 2.5–3.5 vertical metres upslope per year and low-elevation genera also increased in abundance in most of the study plots. However, the rate of elevational migration was lower than predicted according to the temperature increase in the region, suggesting a lagged response to climate change of primary tropical montane forests (Feeley et al., 2011).
3 WHY DISPARITIES BETWEEN BIOGEOGRAPHICAL THEORY AND POPULATION ECOLOGY MATTER
Four complementary explanations drawn from empirical evidence clarify why rear edge population performance can deviate from biogeographical predictions:
3.1 Geographical and ecological edges do not always overlap at the population scale
Assuming a complete overlap of geographical and ecological range limits at the rear edge of a species' distribution may explain counterintuitive population responses. For example, decline in the abundance of plant species with an arctic-alpine and boreal distribution across western North America has been observed across rear edge populations occurring in the northern Rocky Mountains. Although the overall trend of species' abundance decline is in agreement with biogeographical predictions, 50% of monitored populations remained stable or even increased in abundance (Lesica & Crone, 2016). Therefore, decreased population performance at rear edges cannot be assumed because ecological and geographical range margins do not always overlap.
3.2 Interactions among ecological factors determine population dynamics
Species distributions and population dynamics are determined by complex interactions of ecological factors (Harper, 1977). For example, soil phosphorus strongly limits tropical tree distributions along a gradient of dry-season moisture along the Panama Canal (Condit, Engelbrecht, Pino, Pérez, & Turner, 2013) and, in Mediterranean communities, several plant species only survive at the drier edge of their ranges in communities beneath the facilitative effects of the shrub “retama amarilla” (Retama sphaerocarpa) (Armas, Rodríguez-Echeverría, & Pugnaire, 2011). However, such complexity is typically simplified in large-scale studies because of methodological limitations when trying to represent population-level processes over broader spatial scales. Consequently, disparities between population responses and biogeographical predictions are likely to be common. For example, elevational range shifts inferred from adult and juvenile abundance in Mediterranean, temperate and boreal tree species in Europe are idiosyncratic rather than being consistent with temperature-based predictions (Rabasa et al., 2013). Similarly, downslope shifts in elevation are as common as upslope shifts across a broad range of taxa in California (Rapacciuolo et al., 2014). Common explanations for these unexpected responses are factors such as human land-use, water balance or soil quality, species physiological and dispersal traits, demographic dynamics and biotic interactions (Rabasa et al., 2013; Rapacciuolo et al., 2014).
3.3 Decoupling between microclimates and macroclimates
Large-scale predictions from bioclimatic models are generally derived from coarse-gridded climatic data because fine resolution or microclimatic data are rarely available over large spatial scales. Organisms, however, respond to their local environment. For instance, microclimatic variation due to topographic factors is generally not captured by the resolution of interpolated climatic data while differences between regional free-air and local temperatures may amount to several degrees (Dobrowski, 2011). At finer scales, biophysical processes have impressive effects. For example, structural characteristics of old-growth forests may provide microclimates cooler by as much as 2.5°C across forest stands (Frey et al., 2016). Therefore, it is not surprising that climate at resolution of 100 or more metres poorly explains variation of leaf and wood traits across populations of temperate and Mediterranean trees (Vilà-Cabrera, Martínez-Vilalta, & Retana, 2015). In the context of marginality, a highly illustrative example of mismatch between micro- and macroclimates is the persistence of rear edge populations such as the stands of pedunculated oak (Quercus robur L.) in Jerte valley, western Iberian Peninsula (Moracho, Moreno, Jordano, & Hampe, 2016) which has a regional climate significantly hotter and dryer than that tolerated by this species. Consequently, a decoupling between micro- and macroclimates has strong implications for climate-based predictions on population decline (Hampe & Jump, 2011).
3.4 Evolutionary processes
Populations (or genotypes) are adapted to a specific range of ecological conditions and, consequently, each individual within a species may experience stress from climate change (Harte, Ostling, Green, & Kinzig, 2004). Therefore, the existence (or lack) of genetic adaptations to climatic stress may also explain some of the former unexpected responses. For example, greenhouse experiments show that dry-edge populations of the spurge olive (Cneorum tricoccon), a Mediterranean evergreen shrub with a narrow distribution, exhibits more drought-tolerant phenotypes, and growth of individuals inhabiting drier habitats is less affected by drought stress (Lázaro-Nogal et al., 2016). However, most empirical evidence on spatial variation of key species traits comes from observations across broad latitudinal gradients. For example, rear edge populations of the European beech tree show higher resistance to xylem embolism relative to midlatitude, range-core populations (Stojnić et al., 2018). Yet, a proper understanding on whether variation in this and other traits relevant for species persistence occurs across rear edge populations, is lacking.
The former explanations point to two subtly interrelated aspects that, if not acknowledged, strongly limit our understanding of marginality, and our ability to predict population loss. First, marginality is a multidimensional property of populations that encompasses ecological, geographical and genetic components. Second, methodological limitations and lack of data restrict our capacity to link population ecology with biogeography (but see SDMs accounting for phenotypic plasticity and local adaptation in Benito Garzón, Robson, & Hampe, 2019). Consequently, local predictions of rear edge decline only based on distribution patterns at the regional scale become unrealistic (Thuiller et al., 2008). Overcoming such limitations is essential to reconcile population ecology with biogeographical theory at species' rear edges to enable a predictive understanding of their dynamics, function and management (Mouquet et al., 2015).
4 REFINING OUR PREDICTIVE UNDERSTANDING OF REAR EDGE POPULATION DECLINE
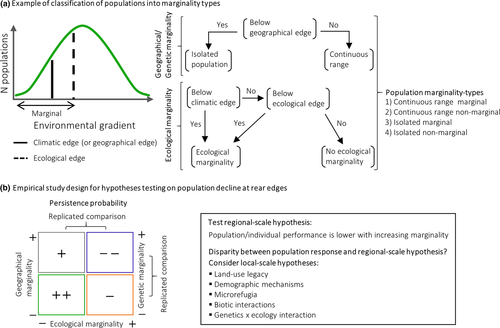
We propose a rationale that integrates the ecological, geographical and genetic dimensions of marginality to determine the regional- and local-scale mechanisms shaping the probability of persistence (or extinction) of rear edge populations (Figure 1). Importantly, the scale dependency of ecological mechanisms influencing the persistence probability of populations may result in contrasting predictions between the regional and local scales. Consequently, we argue that a hypothesis-driven approach is necessary, with population decline tested rather than assumed according to predicted marginality. At the core of the rationale lies a data-driven methodology that permits the incorporation of increasingly available data sources into experimental study design. Essentially, each marginality dimension can be inferred from multiple ecological components (e.g. climatic range, landscape connectivity, community composition, human-driven habitat degradation, etc.) across the species' rear edge. The distribution and edges of these components and their interactions can be identified and populations categorized across marginality types (Figure 2a) ensuring that, at the regional scale, the entire rear edge structure is represented (Figure 1). At the same time, population and individual parameters need to be measured with replication within and compared across marginality types to ensure a balanced sampling and accurate parameter assessment (Figure 2b). Observed population responses are then contrasted with regional-level predictions and, if disparities arise, local-scale mechanisms need to be considered (Figure 2b). We demonstrate how application of this rationale improves understanding of marginality and highlights the need to consider the scale dependency of ecological suitability.
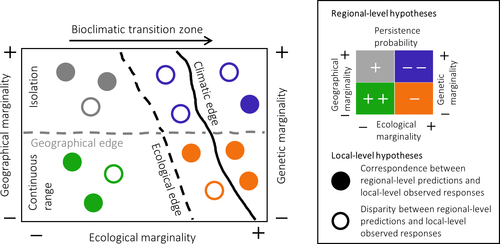
4.1 Conceptualizing the dimensions of marginality
Our understanding of marginality as a multidimensional concept, the rear edge structure, as well as the regional- and local-level hypotheses of population decline are illustrated in Figure 1. In analogy with the limits of the realized niche (Hutchinson, 1957), abiotic and biotic factors define ecological marginality at the regional and local scales. The regional climate (or macroclimate) of the population location relative to the edge of the species' climatic distribution (or the threshold of species' climatic tolerance) is used to infer ecological marginality at the regional scale, while the range of population-scale habitat characteristics (e.g. microclimate, soil quality, land-use history) is used to derive local ecological marginality. Population decline is thus predicted to occur at the extremes of these factors, for example, drier climates, poor soils or intense disturbance. Rear edge populations occur along bioclimatic transition zones (Jump, Mátyás, & Peñuelas, 2009), where species climatic suitability decreases and habitat heterogeneity is high over small spatial scales. Consequently, changes in the composition of communities can occur abruptly with shifts in habitat quality such that community composition can be used alongside abiotic conditions to infer ecological marginality. At the landscape scale, the composition of communities surrounding the focal rear edge population is used to infer regional scale ecological marginality, which increases approaching the transition between bioclimatic zones. At the local scale, the community composition is used to infer interactions among organisms – within or across trophic levels – potentially determining ecological marginality. If co-occurring species, relative to the focal one, are competitors under an ecological advantage (e.g. drought-tolerant) or antagonists (e.g. biotic agents), such biotic interactions result in increased local ecological marginality. On the contrary, biotic interactions result in decreased local ecological marginality if beneficial effects can emerge from species coexistence (e.g. facilitation, mutualism or complementarity).
The rear edge is typically made up of populations of variable size and connectivity, defining a fragmented landscape (Hampe & Petit, 2005; Jump et al., 2009). Therefore, the spatial distribution, size and connectivity of populations (i.e. habitat configuration) are used to infer regional scale geographical (and genetic) marginality. Increased fragmentation and isolation as a consequence of either natural processes or anthropogenic impacts, result in decreased population performance. This detrimental effect is associated with an altered habitat leading to edge effects (Murcia, 1995), increased metapopulation dynamics due to dispersal limitation (Hanski, 1991), disrupted biotic networks and novel interactions or invasion (Hagen et al., 2012) and the loss of genetic variation and individual fitness because of increased chance of genetic drift and inbreeding (Templeton, Shaw, Routman, & Davis, 1990). However, in parallel with deviation of local ecological conditions from the regional scale, population responses that are the product of local-scale mechanisms (e.g. local adaptation) or biotic interactions (e.g. mutualistic symbioses) may contradict predicted marginality based on habitat configuration alone.
4.2 Quantifying marginality and testing regional scale hypotheses of population decline
Marginality can be quantified along multiple axes at the regional scale using existing data sources, allowing hypothesis testing on the regional mechanisms determining population decline (Figure 1). Climatic and geographic range edges may not completely overlap (Cavin & Jump, 2017; Chardon, Cornwell, Flint, Flint, & Ackerly, 2015). Consequently, while geographical ranges frequently correlate with climate at the continental scale, it cannot be assumed that all rear edge populations are climatically limited. This idea can be understood, for example, from the variable relationship between the climatic characteristics and geographical location of populations of the European beech (Fagus sylvatica L.) tree from the Iberian Peninsula to Northern Scotland. Populations inhabiting dry and wet sites relative to the species' climatic distribution can be found at the rear edge with contrasting implications for population performance (Cavin & Jump, 2017). Large-scale forest inventories or remotely sensed data layers such as land-cover maps can be used to determine geographical marginality, with gridded climate data used to infer ecological marginality relative to the climatic distribution of the species (Figure 2a). The interaction between both types of marginality results in variable predicted extinction risk across the rear edge (Figure 1).
At rear edges, abrupt bioclimatic transitions may not be explained by climate alone. For example, the pine–cloud forest ecotone on the windward slopes of the Cordillera Central, Dominican Republic, is primarily a result of high-elevation fire regimes. Declining temperature and precipitation with elevation together with trade wind inversion, and small-scale variation in topography and vegetation determine fire occurrence and ecotone formation (Martin, Sherman, & Fahey, 2007). Existing data sources that incorporate species composition data (e.g. inventories and land-cover maps) can be used to infer bioclimatic transitions at the landscape scale, and thus refine predictions on ecological marginality based on climate alone (Figures 1 and 2a). This idea can be exemplified by the exceptional range retraction of ponderosa pine (Pinus ponderosa) after a severe drought in mid-1950s at the ecotone between this species and piñon–juniper woodland (Pinus edulis and Juniperus monosperma) in northern New Mexico (Allen & Breshears, 1998). Forest dieback predominantly concentrated in low-altitude, drought-prone populations, but more climatically favourable areas along the entire altitudinal gradient were also affected likely because of a competitive disadvantage relative to more drought-tolerant species. The interaction between climate and community composition at the regional scale reflects a mosaic of ecological conditions at rear edges not only dependent on climate (Figure 1), and should, therefore, be incorporated into empirical study design (Figure 2a).
Populations at similar levels of ecological marginality are at higher risk of extinction with increasing geographical (and genetic) marginality at the regional scale (Figure 1). Spatial pattern and landscape connectivity GIS analyses (e.g. Wegmann et al., 2018) on land-cover maps and other remote-sensing-derived sources can be used to accurately infer habitat configuration and test predictions of decreased population performance (Figure 2a). Population fragmentation is associated with ecological edge effects (Murcia, 1995). For example, in tropical montane forests in the Bolivian Andes, temperature gradients from the edge to the interior of forest patches are equivalent to a 100-m shift in elevation. Higher temperatures at forest edges cause warmer and drier habitats with corresponding elevation of drought stress, changes in species composition and increased fire risk (Lippok et al., 2014). Fragmentation may also strongly decrease individual fitness and alter population dynamics through rapid genetic changes. For example, loss of large-vertebrate dispersers because of human-driven habitat fragmentation across Brazilian Atlantic rainforests is associated with a rapid (<100 years) evolutionary seed size reduction in a keystone palm species (Euterpe edulis). Seed size reduction results in increased seed vulnerability to desiccation and decreased seedling growth. At the same time, genetic diversity among seedlings in fragmented (defaunated) sites is lower than in nonfragmented sites. Altogether, these impacts have strong implications for population dynamics under predicted drier conditions in the studied forests (Carvalho, Galetti, Colevatti, & Jordano, 2016; Galetti et al., 2013).
5 SHIFTING TO THE POPULATION PERSPECTIVE: REFOCUSING ON LOCAL-SCALE HYPOTHESES
Framing hypotheses of population decline based on marginality predicted at the regional scale can result in disparities between regional predictions and observed population responses. Such disparities demonstrate the need to refocus studies exploring rear edge performance on local-scale hypotheses (Figures 1 and 2b). Below we first address the strong influence that anthropogenic land-uses and their legacies have on our understanding of marginality and their likely prominent role to explain the mismatch between predictions and observations. Thereafter, we illustrate with selected examples from the literature how rapidly increasing data availability can be harnessed for the evaluation of local-scale mechanisms across marginality-types (Figure 2b), thereby refining our predictive understanding of rear edges.
5.1 Anthropogenic land-uses and their legacies
Anthropogenic land-use during the last few hundred years has altered the realized niche of species and consequently their contemporary distribution is often not in equilibrium with the range of ecological conditions they are able to exploit. For example, using “pre-settlement” vegetation estimations inferred from survey records (1830–1910), and historical climate and contemporary data, Goring and Williams (2017) demonstrated that human land conversion shifted the past distribution of some tree genera in Midwestern United States, from drier and warmer climates in the past to wetter and cooler conditions today. Land-use changes and associated habitat modifications, therefore, complicate the identification of “ecological edges” of a species' distribution (Figure 1). Anthropogenic land-use also interacts with climate change impacts on population dynamics. For example, human-driven forest loss prevails in warmer (low-latitude or altitude) regions and, rather than climate change, recent habitat loss – quantified from ~30-m resolution data generated from Landsat image analysis – explains the biotic attrition observed in these areas (Guo, Lenoir, & Bonebrake, 2018). On the other hand, tree species plantations for wood or food production and fire suppression can contribute to species expansion beyond their climatic limits, but increase the risk of dieback episodes and wildfires during extreme dry years (Maranz, 2009; Nowacki & Abrams, 2015; Sánchez-Salguero, Navarro-Cerrillo, Swetnam, & Zavala, 2012). At the same time, socioeconomic changes can lead to widespread forest expansion over abandoned land (Meyfroidt & Lambin, 2011). For example, the combination of forest inventory data with historical and modern land-cover maps generated form aerial images shows that the ~25% of current forests in the Iberian Peninsula, the rear edge of several temperate and boreal tree species, are growing on former agricultural and grazing land abandoned after the 1950s (Vilà-Cabrera, Espelta, Vayreda, & Pino, 2017). Consequently, anthropogenic habitat modification and its legacies represent a critical dimension of marginality as they may intensify, confound or delay climate-driven population decline at rear edges.
5.2 Population demography and structure
Forest inventory networks are very useful for assessing recent demographic dynamics over large geographical scales. However, the spatiotemporal resolution and the quantity of data are limited and need to be complemented with more detailed data and studies. Long-term population responses can be better understood taking advantage of the increasing availability of dendroecological data over large geographical areas (e.g. Sánchez-salguero et al., 2017), while field-based investigations can inform on particular persistence mechanisms such as compensatory changes in demographic rates (Doak & Morris, 2010) or stabilizing processes (e.g. competition release) after extreme drought events (Lloret, Escudero, Iriondo, Martínez-Vilalta, & Valladares, 2012). However, detailed information on population structural characteristics including human uses needs to be assessed using inventory data and, together with observed population demography, explicitly placed in the context of past management and its legacy. Such characterization of population structure is essential given that, for example, regular forest management (e.g. thinning) can assist a species to persist under chronic climatic stress (Linares, Camarero, & Carreira, 2009), delaying or even concealing the decline of the species if the less vigorous individuals are removed. However, when forest management is abandoned and the stand matures this beneficial effect can reverse due to greater physiological constraints associated with larger trees (D'Amato, Bradford, Fraver, & Palik, 2013). If coupled with long-term acclimation to favourable water availability, such structural shifts (i.e. bigger stems and higher leaf area) may lead to greater demand of water resources that are not available during extreme drought (Jump et al., 2017), resulting in increased population decline even across better-quality habitats (Figure 1).
5.3 Local-scale environmental conditions
Rear edges mostly occur within areas of high habitat heterogeneity at small spatial scales (Hampe & Petit, 2005). Microtopography is an important driver of small-scale variation in habitat quality, and it can be modelled from existing data such as high-resolution digital elevation models (DEM) derived from remote sensing. For example, Adams, Barnard, and Loomis (2014) used 1-m resolution DEM to show how microtopographic control on moisture conditions mediates tree growth and water-use responses to drought near the elevational range limits of lodgepole pine (Pinus contorta) and ponderosa pine (P. ponderosa) in the Gordon Gulch catchment, Colorado. Such topographic variability together with a range of other physical (e.g. lithology, edaphic characteristics) and biophysical factors (e.g. vegetation structure and traits) facilitates the existence of microrefugia (Figure 1; McLaughlin et al., 2017). For instance, rock outcrops and associated habitat can create microclimates 4.9°C cooler, 12% wetter and less variable than the climate of the surrounding habitat. This microclimate is associated with the persistence of a rear edge population of Podocarpus lambertii at the species' drier range edge located in a semiarid region in Brazil (Locosselli, Cardim, & Ceccantini, 2016). Microclimate data can be derived from local networks of climate data loggers and combined with remotely sensed topographical and vegetation structural data. Improvements in data resolution are essential in highly variable regions in terms of habitat conditions, where the potential for microclimatic buffering strongly relies on microrefugia occurrence and human impacts on habitat structure. For example, along a land-use intensity gradient in Borneo, from unlogged old-growth forests to mature oil-palm plantations, canopy structure and topography are strong drivers of small-scale variation in understory temperature and vapour pressure deficit. Assessing and modelling variation in microclimatic conditions is critical in regions like the lowland tropics, where many species reach their thermal tolerance limits (Jucker et al., 2018).
5.4 Biotic interactions
Alterations to species coexistence can reflect an altered habitat, for example, such that more drought- and shade-tolerant species gain a competitive advantage. For example, the local coexistence between the boreal pine species Scots pine (P. sylvestris) and Mediterranean oak species (e.g. Quercus ilex and Q. pubescens) can be observed along altitudinal gradients in many European mountain systems, such as the Pyrenees. Oak seedling abundance and performance are higher under drought-induced Scots pine decline but this association is not only restricted to the most drought-prone stands at low-altitudes. Habitat deterioration and past species-selective management explain observed community dynamics at the local scale (Galiano, Martínez-Vilalta, Eugenio, Granzow-de la Cerda, & Lloret, 2013). The local community composition can be directly obtained from inventory data or field-based sampling, directly informing on ecological marginality and supporting a better understanding of marginality type (Figures 1 and 2a).
Large-scale inventories are useful to assess how variation in biotic interactions scale-up over broad geographical areas, for example, those involving antagonistic interactions such as insect and fungal damage on trees (e.g. Carnicer et al., 2011). Although these large-scale analyses are often based on categorical data or species relative abundance, they provide a first identification of the spatial variation in species assemblages and should be used for setting more detailed experiments and studies on relevant biotic interactions. For example, uncommon or novel interactions can be established if climate change or anthropogenic land-uses, like fire suppression, shift the identity of coexisting species. Experimental evidence demonstrates that the performance of populations failing to migrate as temperature increases will be strongly reduced by novel competitors migrating upwards in elevation (Alexander, Diez, & Levine, 2015). Other more complex situations, for example, coevolution in mutualistic symbioses, need specific approaches but existing information can support hypothesis development and experimental design. For example, the structural characteristics of drought-tolerant, moth-susceptible pinyon pine (P. edulis) individuals differ from drought-intolerant, moth-resistant ones at the edge of the pine species' physiological tolerance in Northern Arizona. This information supported Gehring, Sthultz, Flores-Rentería, Whipple, and Whitham (2017) to demonstrate that under drought stress, interactions between plant genotype, resistance to herbivory and mutualistic fungi operate differentially among individuals, providing an interpretation for landscape-scale patterns of population decline. Drought-tolerant, moth-susceptible trees have higher growth and survival than drought-intolerant, moth-resistant ones, and this differential performance correlates with distinct, genetically based ectomycorrhizal communities.
5.5 Population genetics matters but within a context of ecological change
The putative long-term stability of relict populations during Quaternary climatic oscillations – the result of microrefugia occurrence and evolutionary processes (Hampe & Jump, 2011; Hampe & Petit, 2005; Woolbright, Whitham, Gehring, Allan, & Bailey, 2014) – is an excellent example of the mismatch between predictions and observed responses at rear edges (Figure 1). Relict populations reinforce the idea that species' extinction risk depends on the interaction between population genetics and ecology. However, it has long been recognized that negative ecological impacts (e.g. demographic decline, restriction to dispersal, disruption of community dynamics) can often outweigh genetic factors in a context of rapid environmental change (Lande, 1988). Studies addressing questions of genetic marginality primarily need to account for species-specific ecological requirements and demography. For example, along fragmented forests in southern Australia, decreased pollen diversity and increased selfing associate with fragmentation for two insect-pollinated eucalypt tree species, but not for a bird-pollinated one (Breed et al., 2015). Moreover, where fragmentation drives decreased genetic diversity and increased risk of inbreeding, population performance is not necessarily reduced if, for instance, functional genetic variation is not altered (Reed & Frankham, 2001), genotypes are adapted to the local habitat (Kawecki, 2008) or the mating system evolves to ensure population viability (Ouayjan & Hampe, 2018). Furthermore, the amount of genetic variation (functional or neutral) and the degree of evolutionary adaptation to a marginal habitat may not matter when rapid environmental change drives abrupt shifts in population demography and increases species' regional extinction risk (Lande, 1988) (Figure 1). Consequently, while population genetics can contribute towards refining predictions of rear edge population decline, it should be considered in the context of population ecology, with the focus on variation of functionally relevant phenotypic traits and demographic performance.
6 A POPULATION-FOCUSED STUDY AT THE SPECIES' REAR EDGE
The European beech (Fagus sylvativa L.) tree is drought-sensitive and it is expected to be particularly vulnerable to deteriorating water balance across rear edge populations occurring in the north-eastern Iberian Peninsula. To highlight this approach to experimental design we used different existing data sources: (a) three regional forest inventories (the Ecological and Forest Inventory of Catalonia, the Spanish National Forest Inventory, and the Catalan Inventory of Singular Forests); (b) an 8 m2 resolution land-cover map (Land-Cover Map of Catalonia); and (c) 1 km2 resolution gridded layer of the ratio of annual precipitation to potential evapotranspiration derived from the WorldClim database. Using these data, we selected 40 beech populations classified into four main population types according to ecological marginality, based on climate and community composition, and geographical (genetic) marginality, based on plot spatial distribution (Figures 1 and 2). At each location we assessed population decline parameters, that is, adult mortality and canopy defoliation based on measurements in one point in time (see Supporting information Appendix S1) and tested regional hypotheses on population decline (Figure 1). The direct comparison among marginality types provides evidence on two fundamental aspects. First, population decline seems to be occurring regionally but especially across ecologically marginal areas within the continuous range (Figure 3a), rather than at geographical edges where population extinction is first predicted to occur. Second, isolated populations inhabiting marginal habitats show lower levels of mortality and canopy decline than expected, which also are comparable to those observed in populations occurring across better-quality habitats. This mismatch between predictions and local observation is consistent with recent evidence showing high stability of rear edge beech populations (Cavin & Jump, 2017; Hacket-Pain & Friend, 2017; Stojnić et al., 2018).

We also show that differences across populations are mediated by the variability of decline along gradients resulting from interactions among marginality dimensions (Figure 3b). First, fragmentation and climate interact to explain patterns of population decline, evidencing regional population loss and local population retention. Second, climate and landscape-scale community composition interact to explain trends in population decline that might seem counterintuitive based on the effects of the dimensions separately. Broadly, mortality increases while approaching the transition area between bioclimates (i.e. from temperate to Mediterranean) across populations located in relatively wet habitats and, to the contrary, it decreases while approaching the transition area between bioclimates across populations located in dry habitats, with a trend from continuous range to isolated populations (Figure 3b). All together, these results provide evidence on three main aspects. First, the mosaic of ecological conditions at the species' rear edge where climate alone cannot explain population responses. Second, the putative persistence of some relict populations across the species' rear edge. Third, the uneven but predictable pattern of population decline across populations that can occur also in better-quality habitats.
This simple study-case application demonstrates that some disparities between predictions and observations can be reconciled accounting for simple interactions among marginality components, and that the potential scale dependency of the mechanisms involved in population decline is a critical issue for modelling species distributions and regional biodiversity patterns at rear edges (Figure 1). By incorporating existing data sources to better infer the ecological structure of species rear edges through marginality-type classification and taking a hypothesis-driven approach, the rationale provided is flexible enough to be applicable to field-based approaches, in situ or controlled-condition experimentation, population genetic studies and approaches accounting for land-use changes, and allows better integration of population ecology and biogeography.
7 CONCLUSIONS
Taking the population perspective on marginality is challenging for empirical studies, yet it is both possible and essential for our understanding of rear edge dynamics. It is of primary importance to determine interactions among ecological mechanisms driving population decline and the influence of anthropogenic land-use. Similarly, scaling-up the complexity of marginality to broader scales presents a critical challenge for biogeographical studies. The problem of data resolution driving a mismatch between regional predictions and local observations can be improved as data availability increases, which is critical to plan for climate change impacts. For example, if management and conservation decisions are to be based on predictions and the actions implemented “locally,” we must know the spatial resolution of data that is needed to accurately predict rear edge dynamics. At the same time, data availability is distributed unevenly across spatial scales, systems and world regions, with regional scales, plant species and the Northern Hemisphere over-represented. Local environmental monitoring is essential to avoid scale-dependent hazards, and large-scale and systematic sampling protocols in the Southern Hemisphere and across taxa other than plants are needed. Increasingly, application of remote sensing methodologies and modelling can help fill data gaps, although ground truth data are still required. Importantly, the rationale presented allows the incorporation of other marginality dimensions not considered here. For example, it is critical to account for biological invasions, including novel competitors and pathogens, or nitrogen deposition and nutrient limitation. Such progress is essential to better understand and predict the impacts of a warming climate and how it interacts with other environmental changes to drive population retention or loss at species' rear edges.
ACKNOWLEDGEMENTS
We thank people involved in the study-case presented: S. García, C. Mercer and S. Nieto contributed in fieldwork sampling, and P. Ruiz-Benito and A. Guardia kindly provided and helped in managing forest inventory and land-cover data. We also thank local stakeholders for their support in providing valuable information. The comments of four anonymous reviewers contributed to improve the manuscript. A.V.C. was funded by the European Union's Horizon 2020 Research and Innovation Programme under Marie Skłodowska-Curie grant agreement no. 656300, and the 50th Anniversary Fellowship programme of the University of Stirling. AJ and AP thank Santander Universities for travel funding.




