Miami heat: Urban heat islands influence the thermal suitability of habitats for ectotherms
Abstract
The urban heat island effect, where urban areas exhibit higher temperatures than less-developed suburban and natural habitats, occurs in cities across the globe and is well understood from a physical perspective and at broad spatial scales. However, very little is known about how thermal variation caused by urbanization influences the ability of organisms to live in cities. Ectotherms are sensitive to environmental changes that affect thermal conditions, and therefore, increased urban temperatures may pose significant challenges to thermoregulation and alter temperature-dependent activity. To evaluate whether these changes to the thermal environment affect the persistence and dispersal of ectothermic species in urban areas, we studied two species of Anolis lizards (Anolis cristatellus and Anolis sagrei) introduced to Miami-Dade County, FL, USA, where they occur in both urban and natural habitats. We calculated canopy openness and measured operative temperature (Te), which estimates the distribution of body temperatures in a non-thermoregulating population, in four urban and four natural sites. We also captured lizards throughout the day and recorded their internal body temperature (Tb). We found that urban areas had more open canopies and higher Te compared to natural habitats. Laboratory trials showed that A. cristatellus preferred lower temperatures than A. sagrei. Urban sites currently occupied by each species appear to lower thermoregulatory costs for both species, but only A. sagreihad field Tb that were more often within their preferred temperature range in urban habitats compared to natural areas. Furthermore, based on available Te within each species' preferred temperature range, urban sites with only A. sagrei appear less suitable for A. cristatellus, whereas natural sites with only A. cristatellus are less suitable for A. sagrei. These results highlight how the thermal properties of urban areas contribute to patterns of persistence and dispersal, particularly relevant for studying species invasions worldwide.
1 INTRODUCTION
Habitat fragmentation and local-scale habitat modifications associated with urbanization have profound effects on air and surface temperatures in cities. This phenomenon is called the urban heat island effect, in which urban and developed areas are warmer and temporally more variable than less-developed and nearby natural habitats (Imhoff, Zhang, Wolfe, & Bounoua, 2010; Rizwan, Dennis, & Liu, 2008; Streutker, 2002). When humans construct and expand cities, vegetative cover is reduced and replaced with impervious, heat-absorbing artificial surfaces such as roads, parking lots, and buildings (Forman, 2014; Oke, 1982; Yuan & Bauer, 2007). At larger scales, once-continuous habitats become fragmented with increased surface area exposed to solar radiation (Delgado, Arroyo, Arévalo, & Fernández-Palacios, 2007; Mcdonald, Kareiva, & Forman, 2008). At smaller scales, the distribution of warm and cool microclimates is highly variable in cities, being influenced by diverse factors such as the placement of single trees and the socio-economic status of the neighborhood (Georgi & Zafiriadis, 2006; Jenerette et al., 2007; Kolbe et al., 2016). Thus, urbanization drastically alters thermal environments in cities compared to natural habitats, such as forests. Although the urban heat island effect is well studied from the perspective of its physical characteristics, very little is known about the consequences for organisms that inhabit cities (but see Angilletta et al., 2007). For ectothermic organisms in particular, whether the altered distribution of thermal microhabitats and increased temperatures of urban areas affect field body temperatures and related traits is an open question.
Environmental temperature increases in urban areas may have a direct impact on ectotherm fitness. Enzymatic activity that drives metabolism, movement, reproduction, and growth is usually positively correlated with body temperature up to an optimal functional temperature, after which performance sharply decreases as temperature increases (Angilletta, 2009; Huey & Kingsolver, 1989). Activity rates, including mating and foraging, change continuously with body temperature and reach their highest levels within the preferred temperature range (Gunderson & Leal, 2015, 2016). Because maximal performance, such as locomotion, can be critical for escaping predators, capturing prey, and defending territories (Irschick & Losos, 1998), the environmental temperature can have a strong impact on fitness. For example, when Anolis lizards were transplanted to a warmer habitat, those with maximal performance at higher temperatures and greater performance breadth had a survival advantage (Logan, Cox, & Calsbeek, 2014). Urban environments may be too extreme for some species, with temperatures regularly above their thermal tolerances or lethal temperatures (Kappes, Katzschner, & Nowak, 2012; Menke et al., 2011), and without enough cool refuges, some species will be excluded from these areas. However, some ectotherms have been shown to have increased thermal tolerance in cities; for example, urban ants in Sao Paulo, Brazil, could tolerate high temperatures (42°C) for longer than ants from rural areas (Angilletta et al., 2007). Alternatively, if urban areas are warmer but temperatures are within physiological tolerances, access to optimal and preferred body temperatures may increase, and constraints on activity time and performance may be reduced (Gunderson & Leal, 2015). For example, shade from landscaping vegetation increased activity time by nearly 400% for lizards in an arid ecosystem (Ackley, Angilletta, DeNardo, Sullivan, & Wu, 2015). Whether increased urban temperatures benefit or prove costly to organisms depends on how well ectotherms can use their habitat to regulate body temperatures.
Thermoregulatory costs may change for ectotherms in urban areas where the vegetation structure and thermal landscape (i.e., the spatial distribution of thermal microclimates) differ significantly from natural habitats. To regulate their body temperatures, ectotherms exchange heat with the surrounding environment (Angilletta, 2009), depending not only on ambient conditions (e.g., air and ground temperatures, and access to solar radiation), but also the spatial distribution of thermally variable microclimates (Sears & Angilletta, 2015; Sears, Raskin, & Angilletta, 2011). Some ectothermic organisms actively thermoregulate, moving between cool and warm microhabitats to achieve a preferred body temperature (Huey, Hertz, & Sinervo, 2003). Alternatively, the body temperature of an ectotherm in a thermally homogeneous habitat, such as a dense forest, conforms to the ambient temperature (Huey et al., 2009). The costs of each strategy, whether energetic (e.g., shuttling between basking sites) or opportunistic (e.g., metabolic or performance losses when outside their preferred temperature range), depend on both the amount of available sunny and shady microhabitats and their distribution in the habitat (Huey & Slatkin, 1976; Sears & Angilletta, 2015; Sears et al., 2016). For example, a population of Anolis cristatellus in warmer, more arid habitats in southwestern Puerto Rico actively thermoregulates, whereas lizards in cooler forested habitats with fewer basking sites thermoconform (Gunderson & Leal, 2012). Because the thermal landscape determines the relative ease of achieving optimal and preferred temperatures (i.e., cost of thermoregulation; Sears et al., 2016), maximal performance capacity and activity time, which are important proxies of fitness, could vary between habitats that differ in thermal quality. If warmer urban areas are more favorable thermal environments, this may reduce thermoregulatory costs, allowing organisms to expend less effort to reach preferred temperatures (Gunderson & Leal, 2012). Alternatively, thermoregulatory costs may increase if urban areas are too hot and ectotherms spend more time and energy seeking cooler microhabitats (Lagarde et al., 2012; Scheffers et al., 2013). Few studies have evaluated thermal ecology of ectotherms in cities, yet this factor should strongly influence the ability of these organisms to persist in this widespread and expanding type of environment.
Changes to the costs of temperature-dependent activity may influence the persistence of ectotherms in cities and determine the ability of non-native ectothermic species to expand their ranges. Due to habitat modification and extirpation of native species, among other urban phenomena, urbanized areas can function as points of entry and centers of population growth for introduced species (Blair & Johnson, 2008; Hufbauer et al., 2012). Furthermore, human activity and land development often contribute positively to invasion success (Roura-Pascual et al., 2011; Shochat et al., 2010). Because abiotic factors (e.g., temperature) play a major role in invasion success (Menke & Holway, 2006), when the thermal qualities of an urban area benefit an introduced species, such as those that prefer warmer conditions, urbanization may facilitate their spread (Menke et al., 2011; Piano et al., 2017). Alternatively, urban temperatures may exceed thermal tolerances, which may preclude introduced ectotherms from establishing in portions of urban habitats (Kolbe et al., 2016). Because invasive species can cause environmental damage and economic losses, understanding the mechanisms behind their spread and persistence is critical (Zenni & Nuñez, 2013).
Anolis lizards are an excellent system for evaluating the impact of urbanization on temperature-mediated traits in introduced species. Anoles have been used extensively in research on thermoregulation (e.g., Hertz, Huey, & Stevenson, 1993; Huey et al., 2003; Huey, 1974). Because of their relatively small size and small home ranges, their body temperatures can be compared to nearby models that represent their body temperatures for a non-thermoregulating population (i.e., the operative temperature: Te), allowing for an assessment of habitat choice and estimates of the costs of thermoregulation (Gunderson & Leal, 2012; Hertz, 1992; Huey et al., 2003). The temperature dependence of locomotion (i.e., thermal performance curve or TPC) is well studied in anoles (Gunderson & Leal, 2012; Huey, Niewiarowski, Kaufmann, & Herron, 1989), and warmer conditions have been shown to impose selection on thermal performance in anoles (Logan et al., 2014) as well as other lizard taxa (Gilbert & Miles, 2017). We studied the effects of urban environments on the thermal biology of two species, A. cristatellus and Anolis sagrei. Both species use similar portions of the structural habitat and are found in urban and natural habitats in both their native and non-native ranges. Where they co-occur in the Miami area, they have been shown to compete and affect each other's habitat use (Salzburg, 1984). Anolis cristatellus has lower reported thermal preferences and tolerances than A. sagrei and appears constrained to areas in Miami with high canopy cover (Corn, 1971; Kolbe et al., 2016). Anolis sagrei is widespread throughout urban areas of Miami as well as some natural forest locations (Battles, Moniz, & Kolbe, 2018).
We predict that the structural habitat changes because of urbanization will result in more open canopies in urban compared to natural areas. We also predict that urban areas will be warmer and more variable temporally than natural areas (Te), demonstrating an urban heat island effect at a scale relevant to lizards. Because thermal traits can acclimate or adapt to local conditions (e.g., Clusella-Trullas & Chown, 2014), we predict that urban lizards in both species will have higher thermal preferences (Tpref), higher optimal performance temperatures (Topt), and greater performance breadths than lizards from natural sites. Lizards may benefit in urban areas if they can maintain Tb within their Tpref range for more time during the day compared to natural areas. Alternatively, urban areas may present more extreme conditions (i.e., warmer overall and fewer cool spots) than natural areas, reducing or eliminating these potential benefits. Finally, we predict that the thermal characteristics, or thermal suitability, of a site largely dictate which of the two species is present, lending support to the hypothesis that abiotic factors influence the presence and spread of these invasive species.
2 MATERIALS AND METHODS
2.1 Study species and study sites
We studied two Anolis species, small insectivorous lizards found naturally in southern North America, Central and South America, and throughout the Caribbean (Losos, 2009). Several Anolis species have been introduced to the Miami metropolitan area (Kolbe et al., 2007), two of which are common in both natural forest and urban areas (See supporting information Appendix S1 for density estimates). Anolis sagrei is native to Cuba and the Bahamas, and non-native populations are now widely distributed in the southeastern United States with Miami area populations dating to the 1940–60s (Bell, 1953; Kolbe et al., 2004). Anolis cristatellus is native to Puerto Rico and was first documented in Miami in the mid-1970s (Bartlett & Bartlett, 1999; Kolbe et al., 2007; Powell, Henderson, Adler, & Dundee, 1996; Wilson & Porras, 1983). In contrast to the nearly ubiquitous A. sagrei, the distribution of A. cristatellus is more restricted, radiating out from two independent points of introductions (Kolbe et al., 2016).
We conducted our study in four urban and four natural sites throughout the Miami metropolitan area. Generally, natural sites were closed-canopy forests on upland hammocks, consisting of hardwood-oak overstory canopy with palmettos and saplings in the understory. All of the natural sites were forest patches within the urban matrix of metropolitan Miami. Urban sites are located within human-altered areas, generally along roadsides with bike paths, canals, and sidewalks. Sites vary in their intensity of urbanization with the Gables being the most urban. We are unaware of any urban sites in Miami that contain only A. cristatellus. See supporting information Appendix S2 for map and photographs of each site.
2.2 Operative temperature
We measured operative temperature (Te), the distribution of body temperatures in a non-thermoregulating population, which represents the available thermal environment for lizards. We made copper lizard models out of 28-gauge (0.32 mm) copper sheet, rolled into a cylinder and fitted with a cap from the same material on one end and flattened and folded to close the other end, and painted light brown to match lizard skin color and reflectance. Inside each model, we placed an iButton temperature logger (Thermochron model DS1921G-F5) that was wrapped in parafilm to increase waterproofing and then wrapped in cloth medical tape to buffer the iButton from directly touching the side of the model. The iButtons recorded temperatures every fifteen minutes for the duration of the study at each site. To place models at the natural sites, starting from near the center of the plot, we followed a random compass heading and distance and affixed a model on the nearest substrate at this location at a random height between 0 and 200 cm. This resulted in model placement on random orientations on tree trunks and branches, including underneath surfaces, where lizards regularly perch. In urban sites, we followed a transect parallel to the road, placing models evenly along the length of the transect at random heights between 0 and 200 cm, at a random distance from the road, and facing a random compass heading when on the substrate. At each site, we placed at least 30 operative temperature models. For all models, we recorded the substrate type and diameter of the vegetation. We calibrated the temperatures recorded by the models to more closely represent lizard Tb following Dzialowski (2005). We did this on sunny days from 28 August 2014 to 03 September 2014, which matched the weather conditions for days we collected data across all sites.
2.3 Body temperature
While the models were deployed at a site, we captured lizards and recorded their internal body temperature (Tb) with a small thermocouple (K-type, 36-gauge, 0.13 mm diameter) briefly inserted into their cloaca. We captured lizards on days when the sun was out, providing lizards with the opportunity to thermoregulate. We captured 12 lizards (mixed males and females) per hour between 07:00 and 18:00, for a total of at least 132 lizards per site. At sites with both species (Red and Crandon), we captured 132 individuals of each species. We only captured undisturbed lizards, and never those that we observed moving between sun and shade patches (i.e., only those lizards not changing basking status). We did not resample individuals, ensured by marking captured lizards with a small dot placed on the body with white correctional fluid (WhiteOut). When it took more than one day to reach the sample size at a site, we only captured lizards when overall weather conditions were similar across sampling days. We sampled sites between June and August in 2014 (see supporting information Appendix S3 for specific dates for each site).
2.4 Canopy openness
We measured canopy openness by taking hemispherical photographs facing upward from model locations and lizard capture locations with a handheld camera (10-megapixel Canon® Powershot SD1200 IS) and attached fish-eye lens. We analyzed these photographs with Gap Light Analyzer version 2.0 (Frazer, Canham, & Lertzman, 1999), calculating the percentage of pixels that were open sky.
2.5 Thermal preference
We measured the preferred temperature range, the central 50% of body temperatures, measured from lizards allowed to choose body temperatures in a thermal gradient free of other environmental constraints (Hertz et al., 1993), for male lizards caught in urban and natural sites (A. cristatellus: natural N = 24, urban N = 21; A. sagrei: natural N = 14, urban N = 15). Lizards were housed at the University of Rhode Island under a 12L:12D cycle for 5–42 days after capture, fed crickets every three days (except 24 hr before a trial) and misted three times per day. We measured thermal preferences by placing individuals in a thermal gradient comprised of eight visually and physically separated lanes, to run multiple lizards simultaneously. We placed an incandescent heat lamp at one end of the lane for basking at high temperatures, while underneath the opposite end of the gradient we placed a small plastic container filled with ice. The average temperature at the warm end of the gradient was 46.6°C (SE = 0.30; range = 44–52°C) and 15.4°C (SE = 0.28; range = 7–17°C) at the cold end. For both species, temperatures in all gradients always included the range of preferred temperatures and temperatures up to critical thermal maxima previously reported in the literature (Corn, 1971; Hertz et al., 1993). We measured internal body temperatures of lizards using a thermocouple (K-type, 36-gauge, 0.13 mm diameter) inserted in the cloaca and taped to the body, leaving lizards free to move throughout the thermal gradient. We recorded body temperatures every ten seconds, allowing continuous monitoring of body temperature without disturbance by observers. After acclimating to the lanes for 30 min, lizards were allowed to select body temperatures for between three and four hours. We excluded lizards from the experiment if thermocouples became detached or lizards showed abnormal behavior. In these cases, lizards were given one additional trial, but were completely excluded from analyses if they never performed.
2.6 Thermoregulatory effectiveness
To determine how accurately a lizard achieves a preferred body temperature, given the available thermal habitat, we calculated thermoregulatory effectiveness (E) for adult lizards observed in the wild with the following equations: E = 1 − db/de, where dband derefer to the mean deviation of Tb and Te from the preferred temperature range, respectively (Hertz et al., 1993). Values of E approaching one signify a highly effective thermoregulator, whereas values of E approaching zero represent a thermoconformer or an organism behaviorally passive in terms of temperature regulation. A negative E indicates avoidance of preferred thermal habitat. We set confidence intervals on E values through 1,000 replicates of bootstrap resampling of our field measurements of Te and Tbfor each site. We computed dband deby randomly drawing samples (with replacement) of n observations (total number of observations) of Te and Tb. We also calculated the percentages of models and lizards below, within, and above their Tpref range.
2.7 Thermal performance
We generated thermal performance curves by recording maximum sprint velocity on a standard racetrack, a 8.6-cm-wide board covered in window screen at a 37° angle to encourage quadrupedal movement, at five temperatures (15, 20, 25, 30, and 35°C) for A. cristatellus and six temperatures (15, 20, 25, 30, 35, and 40°C) for A. sagrei. We collected males of both A. cristatellus and A. sagreifrom urban sites, Red (N = 15) and Gables (N = 15), respectively, and natural sites, Matheson (N = 13) and Montgomery (N = 15), respectively. These were a different set of lizards than those used for the thermal preference experiment and were held under the same conditions. We kept lizards in an incubator for at least 30 min to reach the target temperature. Lizards were placed at the base of each track and allowed to run upwards. We encouraged movement with gentle taps near their tails when needed. We filmed all lizard runs at 240 frames-per-second with a digital camera (Casio Exilim Ex-zr1000) and used imagej (Schneider, Rasband, & Eliceiri, 2012) to determine maximum velocity. For A. sagreithermal performance curves, we anchored the high end of the TPCs with mean critical thermal maximum (CTmax) temperatures, calculated as the temperature at which righting response is lost, from a different data set for nearby urban (41.1°C) and natural (40.6°C) locations in Miami (Battles, unpublished data). For A cristatellus, we added CTmaxvalues for urban and natural A. cristatelluspopulations in Miami, which did not differ from each other (39.0°C; Leal & Gunderson, 2012). To estimate thermal performance curves, following Logan et al. (2014), we fitted data with 21 asymmetrical peak curves using tablecurve 2D (SysStat Software Inc, San Jose, CA). For each individual, we calculated AIC scores of the generated models and chose the best fit. When AIC values were too close to identify a single model, we chose the model with fewer parameters, and when models with the fewest parameters were indistinguishable, we chose the model with the highest R2value.
2.8 Statistics
We performed all statistical analyses in r (R Core Team, 2015) and analyzed species separately, except when specified otherwise. We performed an ANOVA of available canopy openness by site nested within site type (natural or urban), followed by Tukey-HSD post hoc analysis to determine differences among sites. To test whether the two species use different microhabitats from that available and from each other when they co-occur at the same urban sites (i.e., Crandon and Red), we compared the availability of canopy openness to locations used by lizards using ANOVA and Tukey-HSD post hoc tests. In urban sites with only A. sagrei(i.e., Gables and UM), we compared canopy openness availability and use with a t test. Natural sites lacked variation in canopy cover, so we did not test for differences between availability and use by lizards. To measure the effect of canopy openness on Te, we performed a mixed-model ANCOVA with site, model ID, and time of day as random effects. Then, for only the urban sites where canopy openness had an effect on temperature, we performed a mixed-model ANCOVA of Te by canopy openness with site and model ID as random effects, for each hour of the day.
To analyze thermal availability, we performed a mixed-model ANOVA testing for differences in Te by site type with time of day and model ID as random effects. To test for differences between Tb and Te, we performed a mixed-model ANOVA of temperature by type (Te [copper model] or Tb [A. cristatellus, and A. sagrei]) and site, and their interaction, with ID (lizards and model) and time of day as random effects. We used post hoc Tukey-HSD tests to test for differences between models and lizards in each site.
Following estimation of thermal performance curves (see above), we used t tests to compare moments on TPC: optimal performance temperature (Topt), maximal sprint speed (Pmax), and performance breadth (95% TBr and 80% TBr). The performance breadth is the range of temperatures at which sprint performance is at 95% and 80% of the maximal sprint speed, respectively. Next, we used chi-square tests to compare portions of the TPC (95% TBr, and 80% TBr) available and used in urban vs. natural sites.
3 RESULTS
3.1 Canopy openness
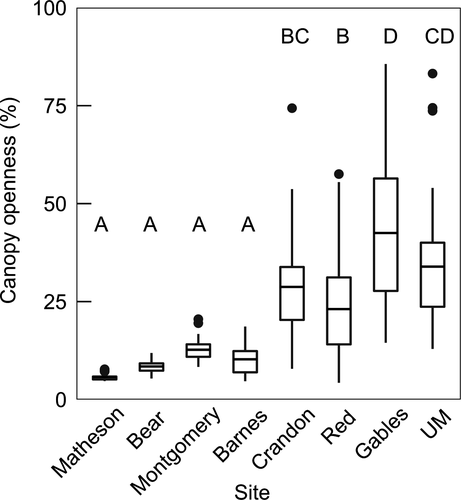
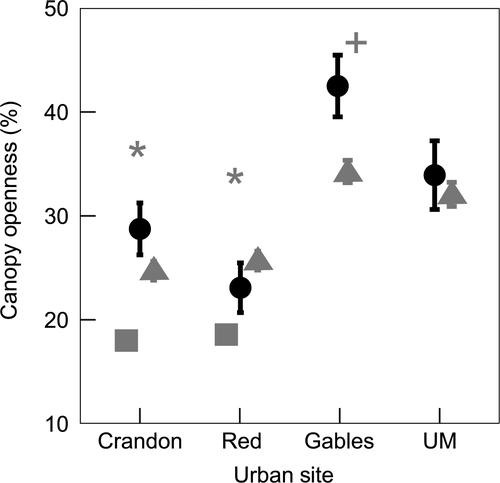
Urban areas had more open canopies than natural areas based on random model locations (F1,251 = 256.5, p < 0.001; Figure 1), with significant variation among urban sites, but not natural ones (Figure 1; F6,251 = 10.0, p < 0.001). Canopy openness strongly influenced Te variation within urban areas (F1,1,276 = 349.6, p < 0.001), overall explaining 13.6% of temperature variation not accounted for by variation between the sites, the time of day, and the model locations. The strength of the effect of canopy openness on temperature in urban areas changed throughout the day, with the strongest effects from late morning until mid-afternoon (Table 1). Because natural areas had more closed canopies and less variation in canopy cover, we did not find a canopy cover–temperature relationship in natural sites. In the urban sites with both species (i.e., Crandon and Red), A. cristatellusused locations with significantly more closed canopies than both A. sagrei and those available at random (Crandon: F2,276 = 24.4, p < 0.001; Red: F2,294 = 18.3, p < 0.001; Figure 2). In the urban site Gables, which had the most open canopy of all sites, A. sagrei used significantly more closed-canopy locations than randomly available (t = 2.6, df = 47.2, p < 0.05; Figure 2).
| Hour | F | Denominator df | Coefficient estimate | Variance explained (marginal R2) |
|---|---|---|---|---|
| 07:00 | 1.76 | 112.7 | 0.00 | 0.01 |
| 08:00 | 23.49** | 114.0 | 0.06 | 0.16 |
| 09:00 | 46.65** | 113.9 | 0.10 | 0.28 |
| 10:00 | 82.70** | 114.0 | 0.11 | 0.41 |
| 11:00 | 63.37** | 113.6 | 0.10 | 0.32 |
| 12:00 | 60.01** | 112.9 | 0.10 | 0.29 |
| 13:00 | 43.18** | 112.1 | 0.07 | 0.18 |
| 14:00 | 50.38** | 115.6 | 0.07 | 0.23 |
| 15:00 | 57.68** | 66.5 | 0.05 | 0.33 |
| 16:00 | 37.90** | 115.9 | 0.04 | 0.20 |
| 17:00 | 9.45* | 114.9 | 0.03 | 0.04 |
- *p < 0.01 **p < 0.001.
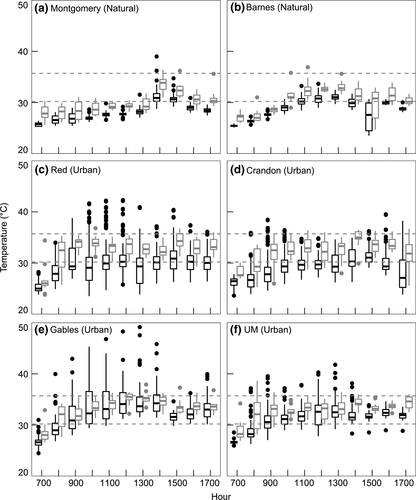
3.2 Field operative and body temperatures
Operative temperatures (Te) in urban areas were 1.5°C warmer on average than in natural areas (F1,264.7 = 120.3, p < 0.001; Figure 3). Anolis cristatellus Tb were on average 0.9°C higher in urban as compared to natural areas (F1,529 = 20.4, p < 0.001); however, lizard Tb at the Bear natural site did not differ from the urban sites. Anolis sagrei Tb were on average 2.5°C higher in urban compared to natural sites (F1,793 = 181.0, p < 0.001; Figure 3). The only pair of urban sites in which A. sagrei Tb differed significantly was Gables and Crandon (t = 3.2, df = 793, p < 0.05), with lizard Tbat Gables being 1.0°C higher.
3.3 Thermal preference
The preferred temperature range (middle 50% of temperatures from the laboratory gradient) for A. cristatellus was between 28.2 and 31.7°C, and the preferred temperature range for A. sagrei was between 30.2 and 35.7°C. Neither species differed in thermal preference between natural and urban populations. These preferences are consistent with previously reported preferred temperature ranges of A. cristatellus (Hertz et al., 1993; Huey & Webster, 1976) and A. sagrei (Corn, 1971).
3.4 Thermoregulatory effectiveness
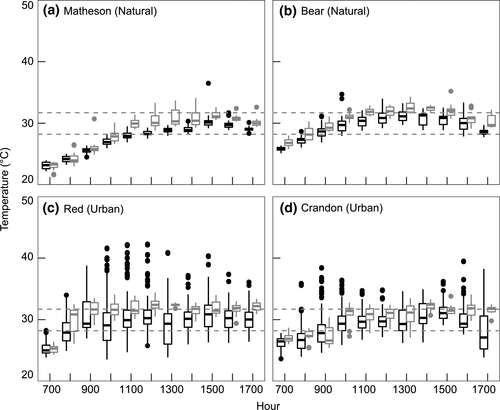
Overall, lizard body temperatures (Tb) exceed Te for both species (F2,1722 = 186.8, p < 0.001; Figures 3-5). For each species at each site, Tb were significantly higher than Te, except for A. sagrei at Gables (Figures 3-5; Tables 2 and 3).
| Site | t-value | d b | d e | de−db | E(95% CI) |
|---|---|---|---|---|---|
| Matheson (N) | 5.57 | 1.18 | 1.68 | 0.50 | 0.296 (0.290 to 0.302) |
| Bear (N) | 4.70 | 0.45 | 0.33 | −0.12 | −0.369 (−0.382 to −0.356) |
| Crandon (U) | 4.10 | 0.54 | 0.99 | 0.45 | 0.454 (0.450 to 0.458) |
| Red (U) | 6.10 | 0.77 | 1.11 | 0.34 | 0.304 (0.299 to 0.309) |
Note
- t-statistic = post hoc comparison from Tb vs. Te model (significant values, p < 0.05, in bold), db = mean absolute deviation of Tb from preferred temperature range, de = mean absolute deviation of Te from the preferred temperature range, E = effectiveness of behavioral thermoregulation (Hertz et al., 1993) and 95% confidence interval.
| Site | t-statistic | d b | d e | de−db | E (95% CI) |
|---|---|---|---|---|---|
| Barnes (N) | 6.64 | 0.84 | 1.37 | 0.53 | 0.391 (0.386–0.397) |
| Montgomery (N) | 6.40 | 1.00 | 2.45 | 1.45 | 0.587 (0.584–0.589) |
| Crandon (U) | 12.26 | 0.49 | 1.96 | 1.47 | 0.750 (0.747–0.753) |
| Red (U) | 11.68 | 0.48 | 1.65 | 1.17 | 0.711 (0.706–0.715) |
| Gables (U) | 1.36 | 0.31 | 0.94 | 0.63 | 0.667 (0.663–0.672) |
| UM (U) | 6.13 | 0.24 | 0.61 | 0.37 | 0.588 (0.582–0.594) |
Note
- t-statistic = post hoc comparison from Tb vs. Te model (significant values, p < 0.05, in bold), db = mean absolute deviation of Tb from preferred temperature range, de = mean absolute deviation of Te from the preferred temperature range, E = effectiveness of behavioral thermoregulation (Hertz et al., 1993) and 95% confidence interval.
For both species, in urban and natural areas, body temperatures were closer to the preferred temperature range than were operative temperatures (db < de), with exception of A. cristatellus at Bear, suggesting that lizards actively thermoregulate at most sites (Tables 2 and 3). Anolis cristatellus at urban and natural sites differ little in their thermoregulatory effectiveness (E), in contrast, A. sagrei appears to thermoregulate more effectively in urban areas than natural areas (Tables 2 and 3). The negative E value for A. cristatellus in the Bear natural site suggests that these lizards avoid available microhabitats within the preferred temperature range (Table 2). Furthermore, A. sagrei generally thermoregulates more effectively than A. cristatellus, which means that A. sagrei maintains Tb within Tpref despite Te being further outside the Tpref range of this species.
For A. cristatellus, the Matheson natural site had a higher percentage of models below the preferred temperature range than the other sites, and no models were above it (Figure 6a). As shown by the E values, A. cristatellus in natural and urban sites have similar percentages of lizards within their preferred temperature range (Figure 6, Table 2). Compared to A. sagrei, A. cristatellus at urban sites and the natural site Bear have higher percentages of lizard Tb above the preferred temperature range (Figures 7b–d and 7).
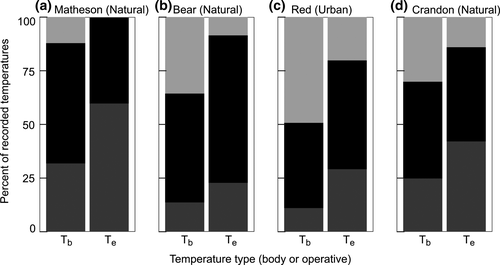
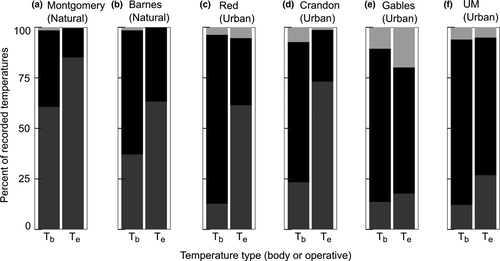
For A. sagrei, urban sites Crandon and Red were similar to natural sites in that they had a high percentage of Te below the preferred temperature range (Figure 7a–d). Despite having similar Te profiles as natural sites, lizards in the Crandon and Red urban sites are most often found within their preferred temperature range (Figure 7c,d). In contrast, the more urbanized sites of Gables and UM had low percentages of Te (and Tb) below the preferred range (Figure 7e,f). The percentage of lizard Tb within their preferred range was similar across all urban sites (Figure 7c–f).
3.5 Occupancy limitations
Both species co-occur at urban sites Red and Crandon. Where the species occur alone, a greater portion of Te values is within their preferred range compared to the preferred range of the other species, except for the natural sites where A. sagrei are found (Tables 4). At the time of this study, these natural sites (Barnes and Montgomery) were outside the distribution of A. cristatellus in Miami.
| Percentage of time Te is within Tpref | ||
|---|---|---|
| Site | Actual % for species present | Predicted % for species absent |
| Anolis cristatellus | Anolis sagrei | |
| Matheson | 40.1% | 3.1% |
| Bear | 68.8% | 34.1% |
| Anolis sagrei | Anolis cristatellus | |
| Barnes | 36.7% | 65.9% |
| Montgomery | 14.5% | 28.7% |
| Gables | 62.4% | 26.1% |
| UM | 68.1% | 38.7% |
3.6 Thermal performance
Optimal performance temperature, maximal sprint speed, and thermal performance breadth did not differ between urban and natural lizards for either species. However, compared to A. cristatellus, A. sagrei had a higher optimal performance temperature (Topt; 37.99°C vs. 33.96°C) and greater maximal sprint speed (1.74 m/s vs. 1.40 m/s). Furthermore, the temperatures at which A. sagrei could achieve 95% and 80% of Topt (performance breadth: Tbr) were higher than those for A. cristatellus (95% Tbr A. cristatellus: 30.85–34.95°C, A. sagrei: 34.38–38.37°C; 80% Tbr A. cristatellus: 25.66–35.58°C, A. sagrei: 29.70–39.22°C). See supporting information Appendix S5 for a TPC figure and table for TPC values.
For both species, compared to natural areas, operative temperatures in urban areas provide more access to the temperatures at which lizards can achieve 95% optimal performance (A. cristatellus: χ2 = 39.1, df = 1, p < 0.001; A. sagrei: χ2 = 105.3, df = 1, p < 0.001; Table 5). Operative temperatures in urban areas provide more access to the temperatures at which lizards can achieve 80% optimal performance for A. sagrei (χ2 = 100.3, df = 1, p < 0.001), but urban and natural sites were equal for A. cristatellus.
| Site type | Operative temperature | Body temperature | ||
|---|---|---|---|---|
| 95% TBr | 80% TBr | 95% TBr | 80% TBr | |
| Anolis cristatellus | ||||
| Natural | 13.5% | 76.2% | 39.0% | 90.2% |
| Urban | 22.3% | 75.6% | 63.9% | 95.5% |
| Anolis sagrei | ||||
| Natural | 0.3% | 31.0% | 4.2% | 60.2% |
| Urban | 8.9% | 48.0% | 26.4% | 86.9% |
Note
- 95% TBr: range of temperatures that confer 95% Pmax; 80% TBr: range of temperatures that confer 80% Pmax.
Lizards were found at body temperatures conferring 80% and 95% of optimal performance more often in urban areas than in natural areas (80% Tbr A. cristatellus: χ2 = 5.1, df = 1, p < 0.05; 80% Tbr A. sagrei: χ2 = 72.1, df = 1, p < 0.001; 95% Tbr A. cristatellus: χ2 = 32.2, df = 1, p < 0.001; 95% Tbr A. sagrei: χ2 = 55.1, df = 1, p < 0.001; Table 5).
4 DISCUSSION
Urbanization converts natural habitats into landscapes dominated by open space and human-made structures, altering the thermal environment for small ectotherms. As predicted, we found that canopies were over three times more open in urban areas (32%) than natural areas (9%; Figure 1), contributing to increased mean operative temperatures in urban habitats. These findings support patterns of higher temperatures in urban areas, which are strongly influenced by reduced tree cover (Georgi & Zafiriadis, 2006; Zhou, Huang, & Cadenasso, 2011). We further demonstrated this relationship at a scale relevant to small ectotherms, highlighting the variation in both canopy cover and thermal availability within urban areas. Not only were operative temperatures higher in urban areas, but lizard body temperatures were also higher. Our study is one of the first to demonstrate the consequences of urban heat islands for small ectotherms. Our mechanistic perspective revealed that urban and natural areas represent distinct thermal microhabitats in which ectotherms may experience shifts in thermoregulatory costs and changes in the constraints on temperature-dependent activity and performance, which should ultimately influence their ability to persist these habitats.
How increased temperatures in urban areas influence thermoregulatory costs for small ectotherms and thus the thermal suitability of urban habitats will vary both by a species' physiological traits and the availability of thermal microhabitat. Even though urban areas increase the availability of warm, sunny patches, for some species they could be distributed such that an urban area is too open, without enough nearby shade, increasing thermoregulatory costs for that species (Angilletta, 2009; Huey, 1974; Huey & Slatkin, 1976). However, in our sites, lower de values in urban sites (with exception in Bear natural site, discussed below) indicate reduced mean deviation of operative temperatures from the preferred temperature range for the species present. In this sense, energetic costs of moving to warm patches will be lower when the frequency of sunny patches is increased (Gunderson & Leal, 2012). Thermoregulation is also used to decrease body temperature, and therefore, ectotherms incur costs when operative temperatures exceed thermal preferences (common in our study) or tolerances (rare in our study). In these cases, such as for A. sagrei at the Gables urban site where lizards used locations with more canopy cover than randomly available, thermoregulatory costs may increase in urban areas, as lizards seek out scarce or widely separated cool, shaded spots to reduce Tb (Vickers, Manicom, & Schwarzkopf, 2011). In addition to the presence of sunny and shady microhabitats, their spatial distribution also determines thermoregulatory costs (Sears & Angilletta, 2015). Our study prioritized comparing operative and body temperatures for urban and natural sites, rather than their arrangement within sites, but future studies could evaluate how the spatial distribution of sunny and shady patches in urban areas influences thermoregulatory costs. We expect that buildings have a strong impact on thermoregulatory costs related to moving between patches, such that a single side of a building can be entirely shaded for several hours, while just a short distance away, perhaps around a corner, lizards could access full sun or a mixture of sunny and shady locations. Thermoregulatory costs are important to consider because they determine the ease with which ectotherms can achieve optimal temperatures for performance and maintain preferred temperatures, which should ultimately influence fitness (Gunderson & Leal, 2015; Huey & Berrigan, 2001).
For A. sagrei, urban areas increase access to preferred body temperatures, which should result in higher rates of activity. Temperature is one of the most important drivers of ectotherm activity (e.g., foraging, territory defense, and mating) and occurs at its highest levels when organisms are within their range of preferred body temperatures (Grant & Dunham, 1988; Gunderson & Leal, 2016). Despite increased mean temperatures in urban areas, urban populations of our study species did not have warm-shifted thermal performance curves or higher thermal preferences. Because A. sagrei can spend more time within Tpref in urban sites, this species may benefit from the thermal microhabitats of urban areas, likely reproducing at higher rates than in natural habitat (Huey & Berrigan, 2001). For example, in more open, warmer habitat compared to cooler, close-canopied forest, female A. cristatellus in Puerto Rico were more likely to be reproductive year-round, likely due to increased basking opportunities to achieve higher body temperatures (Otero, Huey, & Gorman, 2015). Furthermore, higher E values for A. sagrei in urban sites indicate that even when operative temperatures deviate far from preferred temperatures, lizards still precisely thermoregulate to maintain body temperatures within the preferred range (Hertz et al., 1993). Therefore, urban habitats are more favorable for A. sagrei, which may have trouble persisting in more close-canopied sites, such as forested habitats, that restrict their ability to achieve higher body temperatures. It is important to note that while costs of thermoregulation may be lower in urban sites, Basson, Levy, Angilletta, and Clusella-Trullas (2017) showed that even in a low-quality thermal habitat with high thermoregulatory costs in the laboratory, Cordylus lizards prioritized maintaining Tbwithin Tpref. It may be necessary to maintain warmer body temperatures that confer higher activity in urban habitats in the Miami area, even if costly, to successfully compete with multiple other introduced and native anoles (Kolbe et al., 2007), or manage urban predation pressure (Chejanovski, Avilés-Rodríguez, Lapiedra, Preisser, & Kolbe, 2017).
In contrast to A. sagrei, A. cristatellusmay not find urban sites more favorable than natural habitat. The thermoregulatory effectiveness (E) for A. cristatellus was not significantly different between urban and natural sites (Table 2). These values are similar to estimates for A. cristatellusin xeric habitats (warm and dry) in their native range, where lizards actively thermoregulate (Gunderson & Leal, 2012). However, thermoregulatory effectiveness was negative in the Bear natural site. Negative E values suggest lizards are avoiding or restricted from using sites with preferred temperatures, perhaps due to predation pressure or competitors (Hertz et al., 1993), but unfortunately we could not identify any obvious factors causing this at the Bear site. In urban areas, operative and body temperatures for A. cristatelluswere less often within preferred temperature range, which suggests that urban areas may constrain activity for this species. Similarly, Kaiser, Merckx, and Dyck (2016) found that a more-thermophilic butterfly species had increased survival and greater body size in urban areas than did a woodland species adapted for cooler conditions. Furthermore, if operative temperatures too frequently exceed thermal tolerances, or if the habitat lacks enough cool refuges, certain ectotherms could be excluded from urban areas altogether (Chown & Duffy, 2015). Interestingly, Hall and Warner (2017) found that female A. cristatellus from one of our urban sites, Red, had greater body condition and fecundity than lizards from one of our natural sites, Matheson. Our findings on operative and body temperatures suggest that factors other than temperature likely contribute to body condition and fecundity increases. Certainly, animals in urban areas have been found to express a longer reproductive period than in their natural habitat, but other determinants, such as food availability, may be important as well (Lowry, Lill, & Wong, 2013). However, Hall and Warner (2017) found that fecundity increased in urban areas because females began laying eggs earlier in their laboratory setting. We conducted our study in the summer, but urban areas may be more favorable to A. cristatellus during other seasons if urban areas reach preferred temperatures more often than natural areas, such as earlier in the spring when reproductive activity is beginning (Gorman & Licht, 1974; Hall & Warner, 2017; Lee, Clayton, Eisenstein, & Perez, 1989). The different responses to urban habitats of the lizard species in our study show that warmer urban habitats will not benefit all ectotherms equally.
Beyond the thermal suitability for a single species, competition on thermal niche axes can further limit persistence. For instance, in the Matheson natural site, which had the lowest mean operative temperatures, 40% of operative temperatures are within the preferred range of A. cristatellus, whereas only 3% would be within the preferred range of A. sagrei(Table 4). Similarly, in Puerto Rico, A. cristatellus and Anolis gundlachi both occupy forest habitats, but only A. gundlachi is found above ~300 m elevation (Gorman & Hillman, 1977). The mean available temperature in the forest above 300 m is at the low threshold for activity of A. cristatellus, but in the middle of activity range for A. gundlachi (Gorman & Hillman, 1977; Gunderson & Leal, 2016). Both species in our study co-occur along the edges of the Matheson forest patch, which is <1 km away from our urban site Red, but A. sagreiwas never found in the forest at Matheson. With conditions in almost the entire forest below its Tpref, A. sagrei cannot reach activity levels to forage, mate and defend territories effectively. This likely puts A. sagrei at a competitive disadvantage, and it may be excluded from large, forested areas with A. cristatellus in Miami. Similarly, at the Gables urban site, 62% of Te are within the preferred range of A. sagrei, but only 26% are within the preferred range of A. cristatellus (Table 4). With greater potential for higher activity rates, A. sagreihas a substantial competitive advantage. Yet, not all types of urban habitat exclude A. cristatellus. In the sites with both species (i.e., Crandon and Red), A. sagrei, which preferred warmer temperatures, selected microhabitats with more open canopies and achieved higher Tb than did A. cristatellus (Figure 2). These sites differed from the two urban sites with only A. sagrei in that the operative temperature distributions encompassed the Tpref ranges of both species. While competition between these two species could influence divergence in thermal traits, Tpref ranges did not differ between allopatry and sympatry in either species. Yet, divergence in Tpref allows them to partition the thermal niche, at least in some habitats (Magnuson, Crowder, & Medvick, 1979; Paterson & Blouin-Demers, 2017). Interestingly, the natural sites that A. sagrei occupies, Barnes and Montgomery, seem more favorable to A. cristatellus than for A. sagrei(Table 4). If A. cristatellus is ever transported to these natural sites or expands its distribution to include them, we predict that A. sagrei would be out-competed and displaced by A. cristatellus,which is better suited thermally for these forested areas (Kolbe et al., 2016).
The success and spread of introduced species will rely upon suitable thermal microhabitats for persistence. Abiotic factors, such as temperature, play an important role in where invasive species can or cannot persist (Bomford, Kraus, Barry, & Lawrence, 2009; Ulrichs & Hopper, 2008; Zenni & Nuñez, 2013). Because urbanization greatly increases species introductions (Shochat et al., 2010), the concurrent temperature increases associated with urban areas may enhance ectotherm invasion success for some species. In our case, numerous populations of A. sagrei have been documented outside their native range, often associated with human activity (Campbell, 1996; Godley, Lohrer, Layne, & Rossi, 1981; Kolbe, Ehrenberger, Moniz, & Angilletta, 2014; Norval, Mao, Chu, & Chen, 2002), and the favorability of urban thermal conditions for A. sagreiis likely a key factor in this species' invasion success (Angetter, Lötters, & Rödder, 2011). Outside of anoles, warmer conditions and human habitat modification improve the invasion success of the Argentine ant in many locations around the world (Roura-Pascual et al., 2011). However, even ectotherms that benefit from warmer temperatures are still limited by low temperatures due to seasonality or elevation (Angilletta, 2009; Sunday, Bates, & Dulvy, 2012). Urban areas may increase mean daily temperatures overall, but the magnitude and effect depend on the regional climate (Imhoff et al., 2010; Roth, Oke, & Emery, 1989). Yet, anole species have been shown to decrease their low-temperature tolerance by acclimation (Kolbe, VanMiddlesworth, Losin, Dappen, & Losos, 2012), and A. sagrei CTmin decreases with increasing latitude (Kolbe et al., 2014). Flexibility in low-temperature tolerance combined with increased activity time and reduced thermoregulatory costs may make cities more favorable for invasive species like A. sagrei.
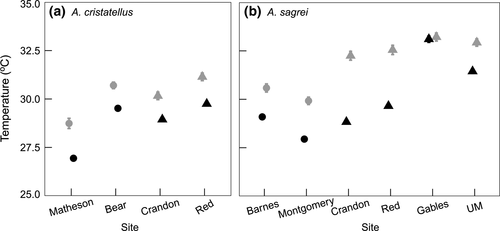
Alternatively, A. cristatellus represents an example of the opposite effect of urbanization on invasion. In a study exploring the spread of A. cristatellus in Miami, Kolbe et al. (2016) found that this species is positively associated with dense vegetation, high canopy cover, and forest patches, thus limiting its dispersal through the fragmented city. Our study corroborates these habitat requirements and explains a possible mechanism for why very warm urban sites, where A. sagrei are common, may exclude A. cristatellus. Hourly temperature (Te) for the urban site Gables, for example, reveal that A. cristatelluscould achieve preferred temperatures easily in the early mornings and evenings (Figure 4), but this would be difficult or impossible throughout the mid-day because of the lack of canopy cover (Figure 1). Therefore, high-temperature environments without enough thermal heterogeneity, such as this more open urban site, can limit activity time and reduce persistence for species like A. cristatellus (Lara-Reséndiz, Gadsden, Rosen, Sinervo, & Méndez-De la Cruz, 2015). Furthermore, if A. cristatellus were in Gables or similar urban locations throughout Miami, it would have the additional stress of more often needing to avoid its upper thermal tolerance (CTmax) compared to the urban sites where it is found (Crandon and Red). If high temperatures limit persistence, then dispersal of introduced species with similar thermal traits throughout urban areas will be restricted. Urbanization often exists as a gradient of intensity (Luck & Wu, 2002; McDonnell & Pickett, 1990), and some levels of urban development are suitable for introduced species while others are not (Crooks, Suarez, & Bolger, 2004; Grarock, Tidemann, Wood, & Lindenmayer, 2014). Research on the urban heat island effect shows a similar thermal gradient of intensity because of urban development (Rizwan et al., 2008), and variation in thermal quality may affect invasive ectotherms in a similar manner (Chown & Duffy, 2015). Indeed, our natural sites are part of an urban matrix and our urban sites are not entirely barren, suggesting our already significant results are conservative relative to more extreme comparisons on the urban-natural gradient.
Temperature, and other abiotic factors, likely play a major role in the persistence and spread of introduced ectotherms. Under the right thermal conditions, ectotherms can be more competitive and reproduce at greater rates than other species (Huey & Berrigan, 2001; Otero et al., 2015). In this study, we show that urbanization significantly alters thermal habitats for ectotherms, increasing both ambient temperature and the availability of warm microhabitats. Urban thermal habitat may confer decreased costs of thermoregulation for ectotherms, but urban areas in Miami impact the persistence and spread of two introduced species in opposing ways. With reduced thermoregulatory costs and increased time spent within Tpref, A. sagrei likely finds urban areas thermally superior to natural habitat in Miami. In contrast, because A. cristatellus Tb are within Tpref less often in urban habitats and A. cristatellusis at a disadvantage competing with A. sagrei from a thermal perspective, they are likely excluded from much of urbanized Miami, an effect that increases with urbanization. Many other factors, such as competition, predation, prey abundance, and disease, could influence persistence in urban areas. However, the thermal quality of urban habitats is certainly a fundamental aspect of urban environments for ectotherms. Our study provides a foundation for studying how the thermal characteristics of urban habitats influence ectothermic organisms. Future studies should consider these findings when evaluating and predicting the spread of introduced species.
ACKNOWLEDGEMENTS
We first thank M. Moniz for his help with data collection in the field. We thank the Miami-Dade Parks and Recreation department for permission to use Miami-Dade parks as study sites, J. Stroud for collecting and shipping lizards, and P. Griffith and the Montgomery Botanical Center access to research sites and support. This work was funded by grants from the National Geographic Society, the National Science Foundation (DEB-1354897), and the University of Rhode Island Enhancement of Graduate Research Award as well as funds from the University of Rhode Island. A. Battles was a National Science Foundation graduate research fellow while conducting this research. Protocols for use of lizards were approved by the URI Institutional Animal Care and Use Committee (AN11-09-005). We thank the Kolbe laboratory group for helpful comments on drafts of this manuscript. We have no conflicts of interest to declare.




