Pathways of mineral-associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry
Abstract
To predict the behavior of the terrestrial carbon cycle, it is critical to understand the source, formation pathway, and chemical composition of soil organic matter (SOM). There is emerging consensus that slow-cycling SOM generally consists of relatively low molecular weight organic carbon substrates that enter the mineral soil as dissolved organic matter and associate with mineral surfaces (referred to as “mineral-associated OM,” or MAOM). However, much debate and contradictory evidence persist around: (a) whether the organic C substrates within the MAOM pool primarily originate from aboveground vs. belowground plant sources and (b) whether C substrates directly sorb to mineral surfaces or undergo microbial transformation prior to their incorporation into MAOM. Here, we attempt to reconcile disparate views on the formation of MAOM by proposing a spatially explicit set of processes that link plant C source with MAOM formation pathway. Specifically, because belowground vs. aboveground sources of plant C enter spatially distinct regions of the mineral soil, we propose that fine-scale differences in microbial abundance should determine the probability of substrate–microbe vs. substrate–mineral interaction. Thus, formation of MAOM in areas of high microbial density (e.g., the rhizosphere and other microbial hotspots) should primarily occur through an in vivo microbial turnover pathway and favor C substrates that are first biosynthesized with high microbial carbon-use efficiency prior to incorporation in the MAOM pool. In contrast, in areas of low microbial density (e.g., certain regions of the bulk soil), MAOM formation should primarily occur through the direct sorption of intact or partially oxidized plant compounds to uncolonized mineral surfaces, minimizing the importance of carbon-use efficiency, and favoring C substrates with strong “sorptive affinity.” Through this framework, we thus describe how the primacy of biotic vs. abiotic controls on MAOM dynamics is not mutually exclusive, but rather spatially dictated. Such an understanding may be integral to more accurately modeling soil organic matter dynamics across different spatial scales.
1 INTRODUCTION
Soil organic matter (SOM), the largest terrestrial carbon (C) pool, accumulates through the continual deposition of plant C inputs into soil. While the majority of that C is mineralized by soil microorganisms and respired into the atmosphere over short timescales, a portion cycles through the soil slowly, persisting for centuries to millennia before turnover (Dungait, Hopkins, Gregory, & Whitmore, 2012). Much attention has been directed toward understanding how plant C inputs form this slow-cycling SOM, and how it will respond to environmental changes, including soil warming, the spread of invasive species, and land use changes (Crowther et al., 2016; Sanderman, Hengl, & Fiske, 2017; Tamura, Suseela, Simpson, Powell, & Tharayil, 2015). Indeed, the behavior of this slow-cycling SOM pool over the course of this century will help determine whether the soil acts as a C sink, or instead acts as a C source to the atmosphere, further accelerating climate change (Bradford et al., 2016).
Over the last two decades, substantial progress has been made in understanding how plant C inputs are transformed to slow-cycling SOM. These advancements, which have been collectively described as the “emerging view” (sensu Lehmann & Kleber, 2015), have helped displace long-held hypotheses around SOM formation and loss. Most notably, the current understanding is that the slowest cycling fraction of the SOM pool primarily consists of relatively small and recognizable biomolecules which interact with the surfaces of mineral particles, forming “mineral-associated organic matter” (MAOM) (Kelleher & Simpson, 2006; Piccolo, 2002; Sutton & Sposito, 2005). While not all MAOM persists in the soil over long-term timescales (e.g., Keiluweit et al., 2015), a subset of organic C compounds within the MAOM pool can persist for hundreds or thousands of years before turning over. The organic compounds within this slow-cycling MAOM pool generally exhibit both strong physiochemical sorption to the solid phase (e.g., through strong ligand exchange interactions) and spatial separation from soil microorganisms (e.g., via occlusion within microaggregates) (Dungait et al., 2012; Lützow et al., 2006; Mikutta & Kaiser, 2011).
Despite significant progress toward a synthetic understanding of the emerging view of slow-cycling MAOM formation (Cotrufo, Wallenstein, Boot, Denef, & Paul, 2013), there are still critical conceptual gaps. Together, these gaps impede a unified conceptual model of how different plant C inputs form MAOM. Here, we address two main unreconciled ideas, as they specifically relate to the formation of MAOM from relatively low molecular weight (LMW) C substrates—defined here as approximately <600 Da—as these are posited to be the dominant constituent of the slow-cycling MAOM pool (Lehmann & Kleber, 2015).
First, there are contrasting accounts around the importance of aboveground vs. belowground plant inputs as the primary source of C to the mineral soil. Several recent studies have shown the primacy of the root pathway in forming MAOM (Austin, Wickings, McDaniel, Robertson, & Grandy, 2017; Sokol, Kuebbing, Karlsen-Ayala, & Bradford, 2018), whereas others have demonstrated that aboveground inputs—especially dissolved organic matter from leaf litter leachate in high-leaching systems—can also significantly contribute to mineral soil C stocks (Michalzik, Tipping, & Mulder, 2003; Kalbitz & Kaiser, 2008). Second, once plant C reaches the mineral soil and is of sufficiently small size, there is conflicting evidence around the primary pathway by which these LMW C substrates are incorporated into the slow-cycling MAOM pool. Some authors assert that the majority of LMW C in MAOM is microbial-derived, because C inputs have undergone microbial assimilation, biosynthesis, and turnover prior to incorporation into MAOM—referred to as the “in vivo microbial turnover pathway” (sensu Liang, Schimel, & Jastrow, 2017) (Bradford, Keiser, Davies, Mersmann, & Strickland, 2013; Cotrufo et al., 2013;Gleixner, 2013; Kallenbach, Frey, & Grandy, 2016). Others, however, have shown that MAOM can be primarily composed of directly sorbed plant compounds—referred to as “the direct sorption pathway” (Kramer, Sanderman, Chadwick, Chorover, & Vitousek, 2012; Sanderman, Maddern, & Baldock, 2014). Even though these directly sorbed plant compounds are often partially oxidized and mobilized by the action of microbial extracellular enzymes prior to mineral-sorption, they do not pass through a microbial body and thus show a clear plant-derived signature within the MAOM pool (Sanderman et al., 2014).
It is critical to understand the relative importance of in vivo microbial turnover vs. direct sorption, because these two pathways are primarily governed by biotic vs. abiotic controls on MAOM dynamics, respectively. By extension, they should favor different biochemical and physicochemical properties of LMW C substrates for efficient incorporation into the MAOM pool. Specifically, through the in vivo microbial turnover pathway, microbial ecophysiology, as well as the biochemical quality of a C substrate, should act as the first set of controls on the efficiencey of MAOM formation, as they influence the efficiency by which the microbial community converts that C to microbial biomass and other metabolites vs. the amount that it respires as CO2 (termed “carbon-use efficiency”) (Bradford et al., 2013; Kallenbach et al., 2016). The greater production of microbial residues should translate to more C compounds that are subsequently available for mineral-sorption (Cotrufo et al., 2013). In contrast, through the direct sorption pathway, carbon-use efficiency does not play a key role. Rather, the mineralogy of the soil, as well as the “sorptive affinity” of a particular LMW plant C substrate to the solid phase, should determine whether a C compound will be retained as MAOM or leached from the mineral soil (Torn, Trumbore, Chadwick, & Vitousek, 2015).
Here, we aim to reconcile the divergent ideas around MAOM formation that arise from the in vivo microbial turnover vs. direct sorption pathways. We integrate and build upon prior conceptual frameworks (Cotrufo et al., 2013; Kaiser & Kalbitz, 2012; Guggenberger & Kaiser, 2003) to propose that biotic and abiotic controls on MAOM are not mutually exclusive, but rather operate in spatially distinct microregions of the upper mineral soil (i.e., in “horizontal soil space”), which are supplied by different sources of plant C input. As the main focus of this framework is on how LMW C substrates move from plant C source to the MAOM pool—primarily as dissolved organic matter (Cotrufo et al., 2015)— we do not extensively discuss the decomposition process that produces these LMW C substrates, nor on other forms of SOM formation, such as aggregation. Furthermore, we specifically discuss MAOM formation in moderately to well-drained mesic soils and hence under primarily oxic conditions, and thus do not examine other contexts of persistent SOM formation, such as slow-cycling SOC within anaerobic microsites (Keiluweit et al., 2017) or in environments where decomposition is temperature-limited (e.g., permafrost soils).
2 OVERVIEW
(Hypothesis I) The probability that a LMW plant C substrate will be incorporated into MAOM via the in vivo microbial turnover pathways vs. the direct sorption pathway is determined by the extent of microbial colonization on mineral surfaces at the substrate's point of entry to the mineral soil.
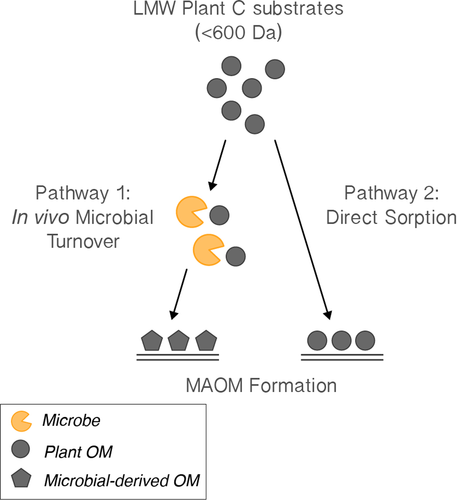
(Hypothesis II) The in vivo microbial turnover pathway and the direct sorption pathway select for different and opposing biochemical and physicochemical substrate properties.
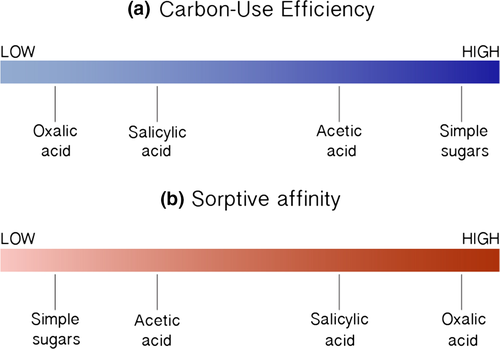
The optimal LMW C substrates to be transformed to MAOM via the in vivo microbial turnover pathway are those which must first be biosynthesized with high microbial carbon-use efficiency prior to mineral-sorption (Figure 3) (Cotrufo et al., 2013). The LMW C substrates which are efficiently biosynthesized by the microbial community lose a proportionally small amount of C as CO2 during biosynthesis (Geyer, Kyker-Snowman, Grandy, & Frey, 2016), and thus more C remains in the soil for incorporation into MAOM (Bradford et al., 2013; Haddix, Paul, & Cotrufo, 2016; Kallenbach et al., 2016). Of course, we stress that the carbon-use efficiency by which a LMW C substrate is biosynthesized is not a fixed value, but varies with several factors (e.g., microbial community composition, substrate concentration, pH, and temperature) (Manzoni, Taylor, Richter, Porporato, & Ågren, 2012). Despite this variation, there is evidence that certain LMW C substrates, such as simple sugars and certain amino acids, are generally associated with higher carbon-use efficiency values (i.e., ~40%–80%), while other LMW substrates, such as certain phenolic acids (e.g., salicylic acid and p-hydroxybenzoic acid) and certain polyvalent organic acids (e.g., oxalic acid), are generally associated with lower CUE values (i.e., 0%–30%) (Brant, Sulzman, & Myrold, 2006; Frey, Lee, Melillo, & Six, 2013; Sugai & Schimel, 2005).
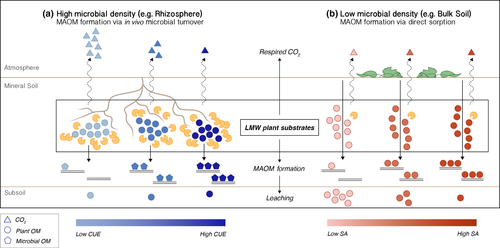
(Hypothesis III) Because the two MAOM formation pathways are posited to operate in distinct areas of horizontal soil space, the optimal biochemical vs. physicochemical properties for efficient MAOM formation should be explicitly linked to a substrate's point of entry to the mineral soil.
| Carbon-use efficiency | Sorptive affinity | ||
|---|---|---|---|
| Qmax (mg/kg) | k (L/mg) | ||
| Glucose | 40%–80%a,b | 35–190f | 0.02–0.03f |
| Acetic Acid | 40%–60%c | 0–2.7g,h,i | – |
| Alanine | 10%–60%d | 9–435f | 0.01–0.05f |
| Salicylic Acid | 21%–24%2 | 121–549f | 0.02–0.06f |
| Oxalic Acid | 2.4%–4.5%a,b | 825–2107f | 0.02–0.09f |
Note
- In order to control for all variables that can influence Qmax, all values shown here (except for acetic acid) are from a single study (Jagadamma et al., 2012), which standardized for substrate concentration and soil type. We only show k values from the sterile treatment in Jagadamma et al., 2012.
- aFrey et al. (2013); bBrant et al. (2006); cKeiluweit et al. (2015); Keiluweit et al. (2017); dApostel, Dipppold, Bore, and Kuzyakov (2017); eSugai and Schimel (2005); fJagadamma et al., 2012; gFischer and Kuzyakov (2010); hvan Hees et al., 2013; iThis data point is the only Qmax value not from the Jagadamma et al., 2012 study, as data were not available.
There are several common LMW substrates that show a negative correlation between their carbon-use efficiency vs. their polarity. Thus, we posit there is a common trade-off for many C compounds in their carbon-use efficiency vs. their sorptive affinity. For example, glucose is generally biosynthesized with high carbon-use efficiency but shows low sorptive affinity (Brant et al., 2006; Jagadamma, Mayes, & Phillips, 2012), whereas certain phenolic acids (e.g., salicylic acid and p-hydroxybenzoic acid) demonstrate high sorptive affinity, but are biosynthesized with low carbon-use efficiency (Cecchi, Koskinen, Cheng, & Haider, 2004; Sugai & Schimel, 2005). Importantly, this relationship is not a ubiquitous trade-off among all LMW substrates: There are several LMW substrates in soil that are common metabolic intermediates and enter directly into metabolic pathways (e.g., citric acid into the citric acid cycle), and which demonstrate both relatively high sorptive affinity and are biosynthesized with relatively high carbon-use efficiency (Jones & Edwards, 1998; Manzoni et al., 2012). Moreover, this relationship can be influenced by a range of factors and contexts; for example, some microbes may biosynthesize certain phenolic acids with high CUE.
Despite this complexity, we posit that for many LMW C substrates, incorporation into the MAOM pool can be efficient for a single compound at one point of entry to the mineral soil (e.g., glucose in a microbially dense region, like the rhizosphere), but inefficient for that same compound entering through a different point of entry (e.g., glucose into a region of low microbial density in the bulk soil). Therefore, there is not necessarily a single optimal substrate property that promotes efficient incorporation into the MAOM, rather it is contingent on a substrate's point of entry to the mineral soil (Figure 4).
We elaborate on the framework by exploring, in sequence, its four essential components: (a) aboveground vs. belowground C substrates; (b) their different point of entry to the mineral soil; (c) their incorporation into MAOM through either the direct sorption pathway or the in vivo microbial turnover pathway; and (d) the carbon-use efficiency vs. sorptive affinity of different LMW C substrates.
2.1 The relative importance of aboveground vs. belowground carbon sources
Much discussion has focused on whether aboveground vs. belowground inputs are the dominant supply of C to the mineral soil. A suite of studies over the past two decades have found that SOM formation from belowground C inputs can rival and even exceed those of aboveground inputs (reviewed in Jackson, Lajtha, Crow, Hugelius, & Kramer, 2017). This has been attributed to both greater overall C supply from the belowground vs. aboveground and more efficient retention of belowground C (Austin et al., 2017; Katterer, Bolinder, Andren, Kirchmann, & Menichetti, 2011; Kong & Six, 2010). Recently, these observations have prompted characterization of the belowground pathway as the dominant source of soil C (Pett-Ridge & Firestone, 2017; Poeplau, 2016; Rasse et al., 2005; Schmidt, Torn, & Abiven, 2011).
This assertion, however, may be premature. As Jackson et al. (2017) highlight, the majority of studies showing the primacy of belowground inputs have been conducted in annual row-crop systems. Certainly, in grassland ecosystems, root-derived inputs are likely the dominant C supply, as the majority of photosynthate is allocated belowground (i.e., >80% in temperate grasslands, Swift, Heal, & Anderson, 2017). Moreover, the majority of surface mineral soil in grasslands may be current or very recent rhizosphere, as roots generally turnover quite quickly, with complete turnover every 2–3 years (Fiala, 2010). However, the evidence has been more varied in forest ecosystems, including support for the primacy of aboveground inputs, for belowground inputs, and for roughly equal contributions from both (Bird & Torn, 2006; Crow et al., 2009; Lajtha, Bowden, & Nadelhoffer, 2014). Aboveground structural inputs are a less quantitatively significant source of C to the MAOM than previously thought (see Rasse et al., 2005), and evidence suggests that they primarily supply the fast-cycling particulate organic matter pool (Cotrufo et al., 2015). But dissolved organic matter from aboveground litter leachate can be a major source of C to mineral C stocks, especially in high-leaching forest ecosystems, where DOC can contribute up to 89% of C in mineral C stocks (Kaiser & Kalbitz, 2012; Michalzik et al., 2003; Sanderman & Amundson, 2008).
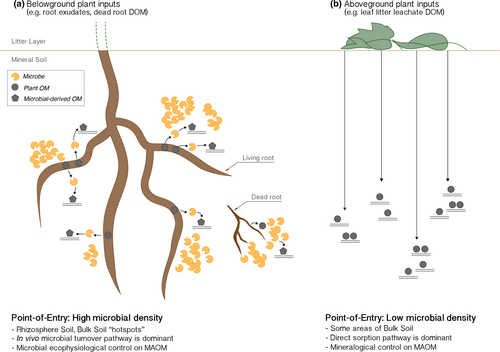
It then appears that both aboveground and belowground sources of C can be quantitatively significant contributors to the MAOM pool, with their importance varying across different ecosystem contexts. In our framework, we expect their importance to also vary at the fine spatial scales that characterize the soil matrix. The relative importance of aboveground vs. belowground inputs should be influenced not only by spatial location, but also by the unique patterns by which these different C inputs enter into the mineral soil (Rasse et al., 2005). For example, rhizodeposits (e.g., root exudates), as well as dissolved organic matter from dead roots, enter directly into the mineral soil. While the rate of rhizodeposit C input can demonstrate wide seasonal variation (Phillips, Erlitz, Bier, & Bernhardt, 2008), rhizodeposition generally enters more continuously throughout the growing season relative to the less frequent entry of aboveground DOC leachate (discussed below). Furthermore, rhizodeposition enters into a spatially constrained area of the soil (i.e., the ~2 mm around the root) (Nguyen, 2003). Because of this pattern of entry, a very dense and active microbial rhizosphere community can develop in the rhizosphere (Kuzyakov & Blagodatskaya, 2015). This high microbial abundance should increase the chance of microbial uptake and biosynthesis of incoming root C, prior to incorporation into the MAOM pool via the in vivo microbial turnover pathway (Sokol & Bradford, 2018; Figure 2).
In contrast to root inputs, dissolved organic matter of aboveground litter leachate (both fresh and decaying litter) moves down the soil profile during precipitation events (Kaiser, Guggenberger, Haumaier, & Zech, 2001) and thus enters the mineral soil as less frequent, more irregular, and more widely distributed pulses of C. In contrast to rhizodeposit C, dissolved organic matter from litter leachate can spread more diffusely throughout a greater soil volume (i.e., the bulk soil). This is because leachate can move down the soil profile through multiple flow regimes, that is, through both advective and diffusive flow. Specifically, Sanderman and Amundson (2008) described an initial flush of dissolved organic matter occurring after a precipitation event that moved through more defined pore networks (i.e., preferential flow pathways). But after initial draining, diffusive flow should carry dissolved organic matter into most pore spaces (Sanderman & Amundson, 2008).
It is important to note that, in forest ecosystems, preferential flow paths can persist for decades, becoming coated with biofilms and accumulating large microbial communities (Hagedorn & Bundt, 2002; Hagedorn, Mohn, Schleppi, & Fluhler, 1999). However, many flow pathways can also be much more dynamic and transient. Indeed, Bundt, Widmer, Pesaro, Zeyer, and Blaser (2001) found wide variability in the abundance of microbial biomass between flow paths and the adjacent soil matrix. Thus, while some flow paths may exhibit a high degree of microbial colonization (i.e., areas where the in vivo microbial turnover pathway may dominate), other areas of the bulk mineral soil should have much lower colonization of mineral surfaces, thus increasing the chance for incoming dissolved organic matter C substrates to directly encounter an uncolonized mineral surface.
2.2 Probability of substrate–microbe vs. substrate–mineral interactions
The predominant lifestyle of microbes in the soil is as sessile, surface-attached organisms to mineral particles, and not as planktonic or “free-floating” microbes (Or, Smets, Wraith, Dechesne, & Friedman, 2007). The physical and chemical features of mineral surfaces provide a suite of conditions for microbial growth (i.e., the establishment of microcolonies or biofilms), as well as a location to access resources in the surrounding soil matrix (Uroz, Kelly, Turpault, Lepleux, & Frey-Klett, 2005). Notably, while the abundance of microbes per gram of soil can be massive (e.g., up to 10^10 bacterial cells), the distribution of those microbes within the mineral soil is highly patchy and variable (Raynaud & Nunan, 2014; Vos et al., 1991). The majority of soil surfaces lack any microbial colonization—the total colonized surface area in mineral soil may be as low as 10^-6% (Young & Crawford, 2018). Thus, even in areas of high microbial abundance, the majority of mineral surface area is still uncolonized.
The greater abundance of soil microbes in the rhizosphere relative to the bulk soil—with estimates ranging from 10:1 to 100:1 (Prashar, Kapoor, & Sachdeva, 2013; Raynaud & Nunan, 2014)—suggests far greater colonization of mineral surfaces in the rhizosphere vs. the bulk soil (Figure 1). The difference between rhizosphere and bulk soil is, as discussed above, not binary: A continuum of microbial activity exists in the bulk soil (Richter, Oh, Fimmen, & Jackson, 2007). While there are regions of very low microbial biomass and activity, there are also microbial hotspots in the bulk mineral soil, including detritus (“the detritusphere”), biopores (such as earthworm burrows), and the surfaces of aggregates (Bundt et al., 2001; Kuzyakov & Blagodatskaya, 2015). The probability of substrate–microbe vs. substrate–mineral encounter will partially depend on the precise spatial distribution of these “hotspots.” The relative importance of direct sorption vs. the in vivo microbial turnover will reflect this continuum of microbial abundance throughout different microregions of the mineral soil. In Figure 2, we thus portray: (a) the rhizosphere soil, (b) a high-density region of the bulk soil (i.e., a “hotspot” around a decaying root), and (c) a low-density region of the bulk soil (e.g., an area of the bulk soil matrix adjacent to such a flow path). Overall, there is a higher probability for a LMW substrate exiting the root to enter the in vivo microbial turnover pathway while en route to mineral-sorption, relative to those substrates which enter into regions of the bulk soil with low colonization of mineral surfaces.
2.3 MAOM formation pathway: in vivo microbial turnover vs. direct sorption
If a LMW compound in the mineral soil is accessible and microbially assimilable, it will be assimilated by the soil microbial community, providing it is not metabolically toxic, and there are no severe constraints on microbial activity, such as temperature limitation or oxygen availability. Microbial uptake of LMW substrates can outcompete mineral-sorption (Fischer, Ingwersen, & Kuzyakov, 2010), but only when soil microbes are able to access a C substrate. Because soil microbes are mostly sessile, they therefore must wait for substrate to enter their habitat to be accessible (Hedges & Oades, 1997; Or et al., 2007). Following substrate assimilation, then microbial biosynthesis, growth, death, and turnover, soil microbes can directly contribute to the MAOM pool through the deposition of their senesced microbial biomass (necromass), exudates (e.g., extracellular polymeric substances), and other byproducts (e.g., stress compounds, such as osmolytes) (Schimel & Schaeffer, 2012). These microbial residues are prime targets for mineral-sorption due to the close spatial association that exists between minerals and microbes (Or et al., 2007; Pett-Ridge & Firestone, 2017). (For a discussion on the interactions between different microbial components and mineral surfaces, see Kögel-Knabner 2002, Kögel-Knabner, 2017.).
Overall, empirical support for the in vivo microbial turnover pathway has increased in recent years (Bradford et al., 2013; Kallenbach et al., 2016; Miltner, Bombach, Schmidt-Brücken, & Kästner, 2012), but microbial assimilation and turnover may not be a prerequisite for entry to the MAOM pool. Specifically, LMW compounds can be translocated as dissolved organic matter from a plant source to a mineral surface (Kalbitz & Kaiser, 2008; Michalzik et al., 2003; Neff & Asner, 2001; Sanderman & Amundson, 2008). The substrate can either enter the mineral soil intact, or—for more complex C compounds (e.g., lignin)—the substrate may first undergo partial oxidation by extracellular enzymes (e.g., laccases, peroxidases) before entry. This extracellular modification often occurs in the leaf litter layer, organic horizon, or upper soil horizon (e.g., in a grassland ecosystem or forest ecosystem with no organic horizon), before those LMW compounds are mobilized as dissolved organic matter (Kalbitz & Kaiser, 2008; Neff & Asner, 2001). Admittedly, only a proportion of the liberated LMW compounds will be available for transport, because the microbes performing litter degradation must assimilate at least some of them to maintain themselves and grow, hence reducing their transport to other areas of the soil (Schimel & Schaeffer, 2012).
The prevalence of direct sorption can vary widely, based on several factors including soil mineralogy (Sollins, Kramer, & Swanston, 2014), soil pH (Kothawala, Moore, & Hendershot, 2009; Mayes, Heal, Brandt, Phillips, & Jardine, 2012), vegetation type (forest vs. grassland) (Sanderman & Amundson, 2008), climate (Kramer et al., 2012), as well as the abundance and composition of the microbial community. Sanderman et al. (2014) suggested that while the in vivo microbial turnover pathway may be common in many soil types, one key context where direct sorption of plant compounds should dominate is in high-leaching forest soils with large proportions of reactive secondary minerals (e.g., oxyhydroxides or poorly crystalline short-range order minerals, like allophane), which can strongly bind a range of C substrates. In contrast, we posit that in grasslands that are generally drier, lower leaching environments, and have lower proportions of reactive secondary minerals, direct sorption of dissolved organic matter should be a less significant C source to mineral C stocks in these ecosystems (Sanderman & Amundson, 2008).
2.4 Physiochemical vs. biochemical properties of plant carbon substrates
Through the in vivo microbial turnover pathway, the soil microbial community acts as a “pump” (sensu Liang et al., 2017), regulating the flow of LMW C substrates en route to the MAOM pool. The efficiency by which a substrate is biosynthesized by the microbial community is determined by several factors (Manzoni et al., 2012), but we focus here on the biochemical properties of the substrate that are associated with high carbon-use efficiency at the microbial community scale (Geyer et al., 2016). In this framework, we explicitly deal with LMW substrates that are already microbially assimilable. As such, we do not discuss how carbon-use efficiency is affected by the number of enzymatic steps required to decompose a complex substrate until it is assimilable (often discussed in terms of activation energy, or Ea). Rather, we focus on the efficiency of microbial biomass synthesis from a particular LMW C substrate, based on the specific metabolic pathway it enters and the degree of reduction of C within the substrate (Manzoni et al., 2012; van Hees, Jones, Finlay, & Godbold, 2013). Higher respiration arises from the bioenergetic costs to metabolize compounds like certain phenolic acids vs. compounds like simple sugars and amino acids. The biochemical differences between these LMW substrates are reflected in a wide range of carbon-use efficiencies, several of which we show in Table 1.
Similar to carbon-use efficiency, sorptive affinity is not only a function of the substrate itself, but also a property of several features of the environment, including soil mineralogy, base saturation, and pH (Sollins, Homann, & Caldwell, 2009). However, we primarily focus on the properties of the LMW C substrate (i.e., “the sorbate”). Sorption to the mineral soil matrix can occur via a range of coulombic and non-coulombic associations, spanning weak to very strong interactions (Mikutta et al., 2007). Ligand exchange interactions form the strongest and most stable organo-mineral bonds, followed by cation bridging, hydrogen bonding, and van der Waals forces (Gu, Schmitt, Chen, Liang, & McCarthy, 1994; Mikutta, Kleber, Torn, & Jahn, 2006). Other forms of bonding may also be important: Nonpolar aromatic rings can also form very strong bonds with mineral surfaces via aromatic cation–pi and pi–pi electron donor–acceptor interactions (Keiluweit & Kleber, 2009).
Polyvalent carboxylic acids (e.g., citric acid and oxalic acid) and many phenolic acids and lignin monomers (e.g., vanillin, salicylic acid, and p-coumaric acid) show the strongest sorption across a range of mineral types (e.g., Jagadamma et al., 2012). Sustrates like simple sugars (e.g., glucose), certain amino acids (e.g., alanine), and monocarboxylic acids (e.g., acetic acid) consistently demonstrate low sorptive affinity, due to the relatively weak substrate–mineral associations they form (Jagadamma et al., 2012; Jagadamma, Mayes, Zinn, Gísladóttir, & Russell, 2014; Jones & Edwards, 1998; Kuzyakov & Jones, 2006). Sorption isotherm studies can be used to quantify sorptive affinity, through variables that include maximum sorption capacity (Qmax) and the binding coefficient (kd). As these values are difficult to compare across studies due to the wide range of conditions used (e.g., substrate concentration and mineral type), we show values in Table 1 from a single study with standardized conditions, to specifically compare the Qmax values of a suite of LMW C substrates.
From Table 1, it is apparent that several LMW C substrates that are biosynthesized with high carbon-use efficiency also show weak sorption with the mineral soil matrix (Jagadamma et al., 2012; Jones & Brassington, 1998; Jones, Dennis, Owen, & Hees, 2003). Sugars, amino acids, and acetic acid should then be optimal compounds for MAOM formation in areas of the soil with high microbial density, but these same compounds should also readily undergo physicochemical stripping from the soil matrix and hence leach down the soil profile in areas of low microbial density (Kaiser & Kalbitz, 2012). Similarly, several polar phenolic compounds exhibit strong mineral-sorption (Cecchi et al., 2004), but are generally inefficiently biosynthesized by microbial communities (Frey et al., 2013; Sugai & Schimel, 2005). As mentioned previously, this is not a universal trade-off. There are common metabolic intermediates in soil, such as citric acid, which are efficiently biosynthesized and have high polarity, causing strong sorption to the mineral phase (Jones & Hodge, 1999). Regardless, we stress the key point that there is generally not a single optimal substrate property that can be identified for efficient MAOM formation, but rather its point of entry to the mineral soil must be explicitly considered.
3 SYNTHESIS AND FUTURE DIRECTIONS
- Few studies have directly looked at how a suite of different LMW C substrates—which range in their sorptive affinity vs. their carbon-use efficiency (e.g., Table 1)—are incorporated into the MAOM pool across a range of relevant biotic conditions (e.g., different microbial densities) and abiotic conditions (e.g., mineralogy). We hypothesize that LMW C substrates with high carbon-use efficiency but low sorptive affinity should be efficiently incorporated into MAOM in areas of high microbial density (e.g., the rhizosphere), but poorly in areas of low microbial density, and vice versa. To address this hypothesis, one approach would be to use controlled laboratory-based experiments that manipulate microbial density in soil microcosms, and track the fate of isotopically labeled substrates which span a range in carbon-use efficiency vs. sorptive affinity.
- It is unclear how the interaction between C chemistry and point of entry in the mineral soil will influence the fate of a C compound in the MAOM pool, and how this interaction should be represented in SOM models (Mazzilli, Kemanian, Ernst, Jackson, & Piñeiro, 2015). Our framework puts forward hypotheses for how C chemistry can differentially impact MAOM formation based on whether it enters as dissolved organic matter from belowground vs. aboveground sources. Such hypotheses need to be tested to determine whether confidence in SOM model projections might be improved through the incorporation of input pathway (i.e., point of entry) as an important process-level control on MAOM formation.
- We posit that there should be different chemical signatures of newly formed MAOM in the rhizosphere vs. in low-density regions of the bulk soil. MAOM that is newly formed in the rhizosphere should have a more microbial-derived signature, dominated by microbial proteins, lipids, and amino sugars (Keiluweit et al., 2015). The MAOM in the bulk mineral soil, in contrast, should have a more plant-derived signature, dominated by phenolics and other aromatic lignin derivatives (e.g., Kaiser & Kalbitz, 2012).
- It is important to note that some authors have considered how the formation and chemical composition of MAOM can vary in vertical soil space. For example, Kaiser and Kalbitz (2012) put forward a conceptual framework on the vertical flow of dissolved organic matter in forest ecosystems and described direct sorption of lignin derivatives generally occurring in upper mineral horizons of forest soils. They posited that highly sorptive lignin derivatives preferentially accumulate in the surface horizon, and less sorptive microbial compounds (e.g., microbial sugars) accumulate in the subsurface, as they are repeatedly microbially processed and “cycled downwards” (Kaiser & Kalbitz, 2012). Here, we argue that—while differences between direct sorption are certainly present at these scales—they should also operate at much finer, millimeter or micrometer-size scales within the same horizon or depth increment (i.e., “horizontal soil space”). That is, there should be fine-scale variation in microbial hotpots and uncolonized mineral surfaces that favor different chemical properties of incoming LMW C substrates for MAOM formation. Only a few field studies thus far have looked at how the chemical composition of SOM also varies “horizontally” within soil space, based on proximity to a shoot or root (Angst, John, Mueller, Kögel-Knabner, & Rethemeyer, 2016; Angst, Kögel-Knabner, Kirfel, Hertel, & Mueller, 2016; Spielvogel et al., 1993). While some vertical differences in SOM content (i.e., topsoil vs. subsoil) have been observed based on shoot vs. root plant C source, no horizontal differences (e.g., rhizosphere vs. non-rhizosphere soil) in MAOM stock or composition have yet been observed in the few ecosystem contexts studied, and with the techniques employed (i.e., lipid biomarkers) (e.g., Angst, Kögel-Knabner, et al., 2016; Angst, John, et al., 2016). This is a key area of investigation, especially using finer scales of resolution (i.e., micrometer to millimeter scale) and different tracer techniques (e.g., 13C-labeled carbon compounds). Depending on the findings, there may be a need to integrate these fine-scale horizontal dynamics with vertical models of SOM formation and loss (e.g., Kaiser & Kalbitz, 2012).
- We posit that a different set of primary controls should operate on the rate of MAOM formation across horizontal soil space, that is, in areas of high microbial colonization (e.g., the rhizosphere) vs. low microbial colonization (e.g., certain areas of the bulk soil). Microbial vs. mineral control of soil C stabilization is known to vary across different ecosystems (Sollins et al., 2014), and we suggest that such controls operate at much finer scales in soil space. Indeed, microbial community structure and composition have been shown to be important for soil C dynamics in the high-activity rhizosphere, but are not significant factors in the bulk mineral soil with low microbial density (Nunan, Leloup, Ruamps, Pouteau, & Chenu, 2017). However, this binary categorization is likely an oversimplification, because biotic and abiotic controls are important for both MAOM formation pathways. For example, soil matrix interactions of microbial products are a key part of the in vivo microbial turnover pathway (see the “Microbial Efficiency Matrix Stabilization” framework by Cotrufo et al., 2013), and key questions thus surround which microbial residues are preferentially sorbed on mineral surfaces (Kögel-Knabner, 2017; Pett-Ridge & Firestone, 2017; Throckmorton et al., 2015). Similarly, the partial oxidation and mobilization of complex C compounds by microbial exoenzymes partially determine the flow of C in the direct sorption pathway, and microbes compete for these C products. Resolving the relative importance of biotic vs. abiotic controls in these two pathways is thus a key priority.
In conclusion, there is still a lack of empirical and conceptual understanding for how different plant C inputs are connected to patterns of MAOM formation and loss. Our framework advances spatially explicit hypotheses that connect plant C source and chemistry with pathways of MAOM formation. Building up a fine-scale understanding of MAOM dynamics is necessary to develop a robust conceptual model of how C flows through soil space and to understand how C substrates with different chemistries may be efficiently or inefficiently incorporated into MAOM based on their point of entry to the mineral soil. This understanding can then be scaled up to develop a more accurate understanding of soil C flows across ecosystem and landscape scales, and to understand and predict how environmental changes to plant and microbial communities may impact mineral SOM stocks through time.
ACKNOWLEDGEMENTS
The authors thank Elisa Iturbe for assistance with the illustrations, Marco Keiluweit for useful discussions surrounding carbon-use efficiency vs. polarity, and Sara Kuebbing, Emily Oldfield, Stephen Wood, Os Schmitz, and Rich Phillips for comments on the initial version of the manuscript. We also thank four anonymous reviewers, as well as Nishanth Tharayil, for their constructive feedback on the manuscript. The work was funded by grants to N.W.S. from the National Science & Engineering Research Council of Canada (Doctoral Scholarship “PGS D”) and the National Science Foundation (Doctoral Dissertation Improvement Grant). The authors declare they have no conflict of interest.




