Species-specific responses to climate change and community composition determine future calcification rates of Florida Keys reefs
Abstract
Anthropogenic climate change compromises reef growth as a result of increasing temperatures and ocean acidification. Scleractinian corals vary in their sensitivity to these variables, suggesting species composition will influence how reef communities respond to future climate change. Because data are lacking for many species, most studies that model future reef growth rely on uniform scleractinian calcification sensitivities to temperature and ocean acidification. To address this knowledge gap, calcification of twelve common and understudied Caribbean coral species was measured for two months under crossed temperatures (27, 30.3 °C) and CO2 partial pressures (pCO2) (400, 900, 1300 μatm). Mixed-effects models of calcification for each species were then used to project community-level scleractinian calcification using Florida Keys reef composition data and IPCC AR5 ensemble climate model data. Three of the four most abundant species, Orbicella faveolata, Montastraea cavernosa, and Porites astreoides, had negative calcification responses to both elevated temperature and pCO2. In the business-as-usual CO2 emissions scenario, reefs with high abundances of these species had projected end-of-century declines in scleractinian calcification of >50% relative to present-day rates. Siderastrea siderea, the other most common species, was insensitive to both temperature and pCO2 within the levels tested here. Reefs dominated by this species had the most stable end-of-century growth. Under more optimistic scenarios of reduced CO2 emissions, calcification rates throughout the Florida Keys declined <20% by 2100. Under the most extreme emissions scenario, projected declines were highly variable among reefs, ranging 10–100%. Without considering bleaching, reef growth will likely decline on most reefs, especially where resistant species like S. siderea are not already dominant. This study demonstrates how species composition influences reef community responses to climate change and how reduced CO2 emissions can limit future declines in reef calcification.
Introduction
Coral reefs are diverse ecosystems that vary in their coral species composition. This in turn shapes the underlying habitat and the species they support (Hixon & Beets, 1993; Cheal et al., 2008). As a result of the 2 Tt of anthropogenic CO2 emissions since the start of the Industrial Revolution (Le Quere et al., 2015), oceans are experiencing unprecedented rates of pH decline (‘ocean acidification’) (Hönisch et al., 2012) and increased temperatures (Domingues et al., 2008). These changes deleteriously influence coral growth and cover through unfavorable chemistry and a disruption of the algal–coral symbiosis (‘bleaching’) (Hoegh-Guldberg et al., 2007). Declining coral cover and calcification (Perry et al., 2013) reduce the architectural complexity of reefs (Alvarez-Filip et al., 2009) and cause cascading negative effects on habitat, diversity, and biomass (Wilson et al., 2006; Paddack et al., 2009; Rogers et al., 2014).
Most studies conclude reef growth will slow significantly by the end of the century (Kleypas et al., 1999; Langdon et al., 2000; Kleypas & Yates, 2009; Hoeke et al., 2011; Chan & Connolly, 2013) or even cease by mid-century (Silverman et al., 2009). Assumed uniform coral responses to increased temperature and partial pressure of CO2 (pCO2) are often used in these modeling exercises (Buddemeier et al., 2008; Silverman et al., 2009; Evenhuis et al., 2015) because data on species-level responses are limited. In general, calcification has been measured as a Gaussian function of temperature (Jokiel & Coles, 1977; Marshall & Clode, 2004) at the organismal level and a linear function of temperature across latitude (Lough & Barnes, 2000). Bleaching often sets the upper thermal limit to calcification by depriving corals of photosynthate, a key energy source. Bleaching thresholds are usually 1–2 °C above summertime means (Coles et al., 1976; Jokiel & Coles, 1977). With respect to pCO2, calcification is often fit as a linear or power law function k(Ωarag - 1)n of aragonite saturation state (Ωarag), a carbonate chemistry parameter inversely proportional to pCO2. However, compilations and meta-analyses have shown the slope of the calcification–Ωarag relationship varies across published literature (Langdon & Atkinson, 2005; Kleypas & Langdon, 2006; Chan & Connolly, 2013). These differences can be attributed to both species and the experimental methods although species-specific responses have been measured within the same study where methods and conditions were consistent (Comeau et al., 2013). Variation in Ωarag sensitivity among species and the ability of corals to regulate the chemistry within the calcifying space (Mcconnaughey, 2003) indicate species composition will determine how reefs respond to climate change. Predictions based on uniform constants therefore may not reflect future CaCO3 precipitation of individual reefs.
Underscoring how reefs may vary in their responses to climate change, recent studies of reefs under elevated pCO2 have documented stable states ranging from reduced growth (Manzello, 2010; Fabricius et al., 2011) to tolerance (Shamberger et al., 2011, 2014) to phase shifts to macroalgae-dominated systems (Enochs et al., 2015). Furthermore, temperature often exerts a larger measurable influence on growth than Ωarag (Helmle et al., 2011; Carricart-Ganivet et al., 2012; Venti et al., 2014). This pattern is not surprising when the relative sensitivities of coral growth to temperature and Ωarag are compared to the annual variation of each parameter. In the Florida Keys, mean monthly temperature and Ωarag range from 23 to 30 °C and 3.1 to 4.6 units, respectively (Fig. S1) (Sutton et al., 2014; Kuffner et al., 2015). Beyond this 7 °C temperature range coral growth is limited (Vaughan, 1916; Vaughan & Wells, 1943; Jaap, 1984). In contrast, the 1.5 unit Ωarag range would only affect calcification by 23% (assuming a uniform 15% Ωarag−1 sensitivity from Chan & Connolly (2013)). Future increases in temperature could increase net annual calcification over certain timescales depending on corals’ thermal optima (Jokiel & Coles, 1977). However, sustained elevated temperatures can acutely stress corals and eventually induce bleaching and mortality. Conversely, decreased Ωarag will chronically depress calcification year-round. These different timescales and effect sizes highlight the need for studies testing coral responses to both increased temperature and pCO2.
The first goal of this experiment was to assay calcification of twelve common Caribbean species under elevated temperature crossed with elevated pCO2 in the same experimental conditions (seasonality, duration, feeding, light, etc.). The control temperature of 27 °C was chosen as representing the thermal optimum for these species in this region (Carricart-Ganivet et al., 2012; Kennedy et al., 2013) and is near the present-day mean annual temperature in the Florida Keys, where these species were collected. The elevated treatment temperature was set at 30.3 °C, near the 30.5 °C bleaching threshold for the Florida Keys based on NOAA's climatological bleaching model (Manzello et al., 2007). The study species were Acropora cervicornis, Agaricia agaricites, Dichocoenia stokesii, Montastraea cavernosa, Orbicella faveolata, Porites astreoides, Porites divaricata, Pseudodiploria clivosa, Pseudodiploria strigosa, Siderastrea siderea, Siderastrea radians, and Solenastrea hyades. These species were chosen because of their current or historic abundance and/or because data are sparse on their growth responses to increased temperature and pCO2. The second goal was to test whether reef-building species composition is important in determining potential changes in overall reef calcification. Empirical growth responses were coupled with benthic cover and climate model data to project end-of-century scleractinian community calcification at 43 reefs in the Florida Keys. Only two temperatures were tested to allow for a wider range in pCO2 levels, and therefore intra-annual sinusoidal temperature variation was not examined. Rather, the study focuses on calcification responses to shifts in mean annual temperatures, which are expected to increase steadily this century (IPCC, 2014).
Materials and methods
Experimental corals
Coral colonies were collected from sites in the Florida Keys (Table S1) in the summer of 2011 and brought to the University of Miami's Climate Change facility. Branching colonies were trimmed to approximately 5-cm-long fragments. Nonbranching colonies were cored into flat, circular 2.5-cm-diameter fragments. Coral fragments (hereafter referred to as corals) were glued to labeled ceramic plugs (Boston Aqua Farms, Boston, MA, USA) and allowed to recover at 27 °C and ambient pCO2 for at least one month before the experiment. Corals showed polyp extension as well as tissue growth over cut scars. Total number of corals per species ranged from 20 to 152 (Table S1). Coral surface areas were measured with imagej software (Abràmoff et al., 2004) using planar surface for mounding corals and cylindrical approximations for P. divaricata. The surface area of A. cervicornis was measured using 3D imaging at NOAA AOML's Ocean Chemistry and Ecosystems Division (Enochs et al., 2014).
Experimental system
Experiments were conducted at the University of Miami Corals and Climate Change Laboratory from October to December 2011. Three pCO2 treatments (400, 900, and 1300 μatm) were crossed with two temperatures of 27 and 30.3 °C. Mass flow controllers (Sierra Instruments Model 810C, Monterey, CA, USA) mixed pure CO2 with ambient air to achieve the treatment pCO2 levels. A pCO2 equilibrator coupled to a LICOR LI-820 gas analyzer (Lincoln, NE, USA) was rotated across tanks to monitor tank pCO2 levels. Temperatures were maintained by Omega Engineering temperature controllers (Model CN7833 Stamford, CT, USA) coupled with 1.5-kW heating elements and cooling coils.
Each tank consisted of a 60-L holding tank coupled to its own 200-L sump tank for a total volume of 260 L. Venturi injectors bubbled the sump tanks with the gas mixtures. In each tank, seawater was pumped from the sump to the holding tank where it gravity-fed back into the sump. Tanks were supplied with a steady 30 mL min−1 input of 10-μm filtered seawater from Bear Cut, Virginia Key. Bulk seawater exchanges associated with coral feeding and tank cleaning were >120 L week−1. Nutrients were not monitored in this study, but source water-dissolved inorganic nitrogen is <1 μm and phosphate is 0.1 μm (Devlin, 2015). HOBO U30 data loggers (Onset Computer, Cape Cod, MA, USA) recorded temperatures (T) in each tank every five minutes along with light from a centrally located photosynthetically active radiation (PAR) sensor. Light levels were adjusted with window screen such that average peak sunlight was 327 ± 14 μmol quanta m−2 s−1 (mean ± standard error, sample size n = 66) and total light exposure was 3.7 ± 0.2 mol quanta m−2 day−1 (n = 66).
Water samples were collected weekly from each tank to document chemical conditions. The total alkalinity (TA) was measured in duplicate on an automated Gran titrator and dissolved inorganic carbon (DIC) in duplicate using a coulometer (UIC, Inc., Joliet, IL, USA). Tris synthetic seawater buffer (Nemzer & Dickson, 2005) was used to calibrate the titrator pH sensor (Orion Thermo Fisher Scientific, Bothell, WA, USA) on the total scale (pHt). Salinity (S) was measured on a Guildline 8410A Salinometer. Total alkalinity and DIC were used to characterize carbonate parameters using the seacarb package v3.0.11 (Gattuso et al., 2015) in r v3.2.4 revised (R Core Team, 2016), using dissociation constants from Lueker et al. (2000), Perez & Fraga (1987), and Dickson (1990).
Procedure
The six treatments were each randomly distributed across twelve experimental tanks (two replicate tanks per treatment). Corals were then distributed among treatments using a stratified randomization based on parent colony. Treatments were ramped up from 27 °C and ambient 400 μatm pCO2 (control treatment) to target levels at rate of ~0.3 °C day−1 and 100–200 μatm pCO2 day−1. The buoyant weights of corals were recorded every two weeks with Sargent Welch SWT-403 and Mettler PB303-S balances (readability 1 mg). Growth rates, relative to surface area, were calculated as dry weight gain (Davies, 1989) between the last recorded weighing and the first weighing after the treatment ramping period, nominally six weeks. Corals were fed twice weekly a diet of live rotifers (~10 rotifers L−1) and larval feed (AP brand) composed of ~6 mg L−1 each of <100 μm particles and 250–450 μm particles. Aquarium pumps with diffusers kept food suspended in the feeding bins. Corals were fed starting late afternoon to early night (~4 h), after which the feeding water was discarded and bulk seawater replenishment was approximately 60 L.
Every two weeks, the treatments were randomly reassigned to tanks to account for any potential tank effects. The tank shuffling occurred while corals were isolated during feeding, with water changes of ~100 L and tank sumps rapidly equilibrating to new treatments during the four-hour feeding period. After feeding, corals were returned to the newly assigned tanks containing their treatment. Twice during the experiment, corals were randomly reassigned within-treatments/between-tanks to account for potential cohort effects. In other words, treatments did not covary with tanks or cohorts.
Growth analyses and projections
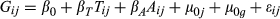 (1)
(1)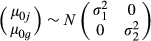 and residual εij ~ N(0, λ2). The cohort grouping is the unique combination of tank assignments for each coral, combining cohort, time, and tank to account for the periodic random shuffling of corals and tanks within treatments. Temperature and Ωarag were treated as additive linear effects because (i) temperature was limited to two treatments, (ii) previous studies indicate linear relationships between calcification and Ωarag (Langdon et al., 2000; Langdon & Atkinson, 2005; Chan & Connolly, 2013), and (iii) observed interactions between temperature and Ωarag were limited or insignificant (Langdon & Atkinson, 2005). If calcification did not appear to vary as a linear function of Ωarag, then calcification rates were fit to a generalized additive mixed model with Gaussian error distribution and identity link function, replacing the βAAij term with a smoothing function of Ωarag based on cubic regression splines. Model fits were evaluated from visual inspection of residuals (Fig. S3). The significance of linear mixed-model fixed effects was evaluated from profile likelihood 95% confidence intervals. Significance of general additive mixed model terms was evaluated from P-values based on Wald tests. Equation 1 was compared against models with a temperature-Ωarag fixed-effect interaction term or colony-location random intercept (Supporting Information). To test for tank effects, longitudinal versions of Eqn 1 with and without tanks as random intercepts (Supporting Information) were evaluated with likelihood ratio tests. Models were fit using the lme4 v1.1-11 (Bates et al., 2015) and gamm4 v0.2-3 (Wood & Scheipl, 2014) packages in the software program r v3.2.4 revised (R Core Team, 2016).
and residual εij ~ N(0, λ2). The cohort grouping is the unique combination of tank assignments for each coral, combining cohort, time, and tank to account for the periodic random shuffling of corals and tanks within treatments. Temperature and Ωarag were treated as additive linear effects because (i) temperature was limited to two treatments, (ii) previous studies indicate linear relationships between calcification and Ωarag (Langdon et al., 2000; Langdon & Atkinson, 2005; Chan & Connolly, 2013), and (iii) observed interactions between temperature and Ωarag were limited or insignificant (Langdon & Atkinson, 2005). If calcification did not appear to vary as a linear function of Ωarag, then calcification rates were fit to a generalized additive mixed model with Gaussian error distribution and identity link function, replacing the βAAij term with a smoothing function of Ωarag based on cubic regression splines. Model fits were evaluated from visual inspection of residuals (Fig. S3). The significance of linear mixed-model fixed effects was evaluated from profile likelihood 95% confidence intervals. Significance of general additive mixed model terms was evaluated from P-values based on Wald tests. Equation 1 was compared against models with a temperature-Ωarag fixed-effect interaction term or colony-location random intercept (Supporting Information). To test for tank effects, longitudinal versions of Eqn 1 with and without tanks as random intercepts (Supporting Information) were evaluated with likelihood ratio tests. Models were fit using the lme4 v1.1-11 (Bates et al., 2015) and gamm4 v0.2-3 (Wood & Scheipl, 2014) packages in the software program r v3.2.4 revised (R Core Team, 2016).Growth rates for Florida Keys reefs were projected to 2100 based on (i) the mixed-effects models from this experiment, (ii) Florida Keys reef composition data from the Coral Reef Evaluation and Monitoring Project (CREMP) (CREMP 2013, unpublished; Ruzicka et al., 2013), and (iii) temperature and Ωarag inputs from regridded Intergovernmental Panel on Climate Change (IPCC) AR5 climate model data (Van Hooidonk et al., 2014). Projections for each representative concentration pathway (RCP) scenario (Van Vuuren et al., 2011) were based on the temperature and Ωarag data from the 1° × 1° cell located at the center of the Florida Keys. The most recent CREMP estimates of coral cover from 2013 were averaged across stations within each reef. Six of 49 reefs were omitted because none of the study species were present, leaving 43 reefs for projections. Reefs were categorized as patch, offshore shallow (3–6 m), and offshore deep (10–20 m) reefs. The CREMP data combined benthic cover for certain groupings of corals: Agaricia agaricites/Undaria agaricites complex, Orbicella annularis complex, and Porites porites complex. Growth for these complexes was estimated by this experiment's mixed effect models for A. agaricites, O. faveolata, and P. divaricata, respectively. The coral calcification projections only apply to the portion of reefs covered by the study species, which was generally over three-quarters of the total scleractinian cover (Table S4). To facilitate intercomparisons across sites, calcification rates were scaled to the first year in the climate dataset (2006). Standard uncertainty of prediction was calculated by summing in quadrature the standard uncertainty in model coefficients and standard uncertainty due to colonies.
Results
Experimental conditions
Experimental tank conditions (Table 1) exhibited slight diurnal patterns in temperature and pCO2. The temperatures increased approximately 0.2 °C from morning to mid-afternoon as ambient heat energy outpaced the tank cooling mechanism while pCO2 concentrations decreased by approximately 50–200 μatm. Diurnal variations in temperature and pCO2 in reef environments, based on Cheeca Rocks MAPCO2 data, are approximately 0.4–1 °C and 20–80 μatm for comparison (Fig. S2). One tank from the high-temperature, high pCO2 treatment experienced a system failure toward the end of the experiment, and therefore the penultimate weighing was used to calculate calcification for its corals. Tank chemistry was stable despite biological activity from coral fragments and physical processes like evaporation because of the seawater replenishment from steady feeds and periodic bulk exchanges.
| Treatment | T (°C) | pCO2 (μatm) | pHt | TA (μmol kg−1 SW) | DIC (μmol kg−1 SW) | Ωarag | S |
|---|---|---|---|---|---|---|---|
| 1 | 27.0 ± 0.1 | 400 ± 60 | 8.06 ± 0.05 | 2377 ± 41 | 2057 ± 43 | 3.8 ± 0.3 | 33 ± 1 |
| 2 | 27.1 ± 0.1 | 899 ± 211 | 7.78 ± 0.10 | 2422 ± 47 | 2246 ± 41 | 2.3 ± 0.5 | 33 ± 1 |
| 3 | 27.0 ± 0.1 | 1343 ± 207 | 7.62 ± 0.06 | 2442 ± 33 | 2336 ± 33 | 1.7 ± 0.2 | 33 ± 1 |
| 4 | 30.4 ± 0.5 | 399 ± 49 | 8.06 ± 0.04 | 2406 ± 47 | 2052 ± 38 | 4.3 ± 0.4 | 33 ± 1 |
| 5 | 30.4 ± 0.5 | 946 ± 196 | 7.77 ± 0.09 | 2460 ± 52 | 2268 ± 54 | 2.5 ± 0.5 | 33 ± 1 |
| 6 | 30.3 ± 0.5 | 1292 ± 247 | 7.64 ± 0.08 | 2447 ± 77 | 2311 ± 77 | 2.0 ± 0.4 | 33 ± 1 |
Calcification
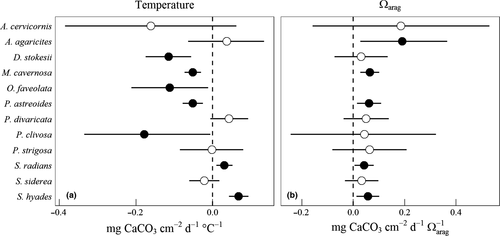
Calcification rates ranged from 0 to 2 mg CaCO3 cm−2 day−1 (Fig. 1). The highest rates in control conditions were 1.0–1.5 mg CaCO3 cm−2 day−1 for A. agaricites, O. faveolata, and S. siderea. Calcification responses to elevated temperature and pCO2 varied by species. Orbicella faveolata was evaluated with a general additive mixed-effects model (Table S3, Fig. S4) because mean calcification was near zero in the elevated temperature, mid-pCO2 treatment. Acropora cervicornis experienced early mortality, with no growth measurements in the high-temperature, highest pCO2 treatment. Significant negative responses to temperature were observed for D. stokesii, M. cavernosa, O. faveolata, P. astreoides, and P. clivosa, ranging from −0.18 ± 0.09 to −0.05 ± 0.01 mg CaCO3 cm−2 day−1 °C−1 (βT ± standard error, SE) (Fig. 2, Table S2). When two influential data points were excluded from P. clivosa, βT changed to a statistically insignificant −0.08 ± 0.07 mg CaCO3 cm−2 day−1 °C−1. Two species, S. radians and S. hyades, exhibited positive βT responses to elevated temperature of 0.03 ± 0.01 and 0.07 ± 0.01 mg CaCO3 cm−2 day−1 °C−1, respectively. Calcification was proportional to Ωarag for A. agaricites, M. cavernosa, P. astreoides, S. radians, and S. hyades, with βA terms ranging 0.04 ± 0.02 to 0.19 ± 0.08 mg CaCO3 cm−2 day−1 Ωarag−1 (βA ± SE). Coral colonies accounted for 20–70% of random-effects variance, while cohort grouping accounted for 0–30%. Acropora cervicornis was the exception to this pattern, with cohort grouping accounting for almost all of the random-effects variance. This is expected given corals came from mostly unique colonies. The temperature-Ωarag interaction term only improved the model fit for S. hyades (Χ2df=1 = 6.2, P = 0.01), but the original Eqn 1 model was retained for the projections (Supplemental Information). The colony-location random intercept term did not improve model fits (Χ2df=1 < 0.4, P > 0.05). No tank effect was observed based on likelihood ratio tests of the nested longitudinal models (Χ2df=1 < 0.1, P ~1 for all species).

Growth projections
The IPCC AR5 ensemble climate models predict end-of-century temperature increases in the Florida Keys from present-day 26.7 to 27.1 °C under the optimistic RCP 2.6 and 29.6 °C under business-as-usual RCP 8.5 (Fig. S5). The annual range in temperature is 6 °C. Mean annual Ωarag decreases from present-day 4.0 to 3.9 units (RCP 2.6) and 2.7 units (RCP 8.5) over the same time period. The annual range in Ωarag is 0.1–0.2 units. Standard deviations in temperature and Ωarag among climate models are 0.3–0.7 °C and 0.1–0.4 units, respectively, depending on year and RCP.
Scleractinian coral cover in the Florida Keys in 2013 ranged from <1% to 41% across 43 sites (Table S4). The twelve species from this study comprised 82 ± 16% (43), mean ± standard deviation (n reefs), of the total scleractinian coral cover. The most abundant of these species were Siderastrea siderea [11–42% interquartile range (IQR)], Orbicella annularis complex (0–23% IQR), Porites astreoides (3–17% IQR), and Montastraea cavernosa (1–11% IQR). Most of the other species contributed ≤10% relative coral cover at any given site. Applying laboratory measurements to species composition results in a baseline Florida Keys coral calcification rate of 0.25 ± 0.09 g CaCO3 cm−2 yr−1. This value is likely a lower estimate of coral calcification because it is constrained to the study species.
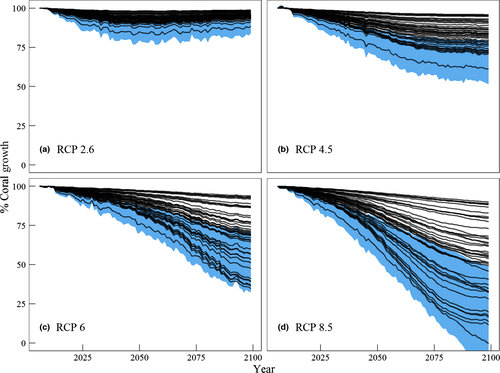
Ensemble mean scleractinian calcification rates in year 2100 were 96 ± 2% and 84 ± 8% (ensemble mean ± SD, n = 43) of present day for RCP 2.6 and 4.0, respectively (Fig. 3a, b, Table S4). End-of-century ensemble scleractinian calcification rates were 69 ± 18% and 55 ± 24% of present-day rates for RCP 6.0 and RCP 8.5, respectively. The median standard uncertainty in model coefficients was ±16%, and the median standard uncertainty of prediction was ±46%. Reefs with the largest projected declines in relative growth of 80 to 90% in RCP 8.5 (Fig. 3d) predominately consisted of O. annularis complex, P. astreoides, and M. cavernosa. These reefs included Eastern Sambo (shallow), Looe Key (shallow), Jaap, Black Coral Rock, Grecian Rocks, and Wonderland, where benthic coral cover is 5–40%. Reefs with higher relative proportions of S. siderea had projected calcification rates that were relatively unchanged across all emissions scenarios. These relatively unaffected sites include the patch reefs Dustan Rocks, Rawa, Thor, and West Turtle Shoal, as well as the deeper Molasses and Sombrero reefs (Fig. S6).
Discussion
Growth responses to elevated temperature and Ωarag varied among species (Fig. 1) with reef-builders such as O. faveolata, M. cavernosa, P. clivosa, A. agaricites, D. stokesii, and P. astreoides calcifying at decreased rates under treatment conditions. Two species, S. radians and S. hyades, had calcification rates that increased with increasing temperature but decreased with decreasing Ωarag. In contrast, S. siderea, A. cervicornis, P. strigosa, and P. divaricata did not show detectable responses to either temperature or Ωarag. The varied responses of these twelve species under the same experimental conditions suggest calcification is not solely an abiotic function of ambient temperature and chemical conditions.
Of the twelve study species, only the calcification rates of A. cervicornis and S. siderea have been tested in similar laboratory settings (i.e., including feeding) under climate change scenarios (increased temperature and pCO2) (Table 2). Fed A. cervicornis corals maintained calcification under elevated pCO2 (Towle et al., 2015). Although this study corroborates those prior results, A. cervicornis experienced increased mortality at elevated temperatures. Due to the protected status of this species, colonies were obtained from a sheltered coral nursery (Schopmeyer et al., 2012) and therefore may be less thermally robust than conspecifics from other sites. Unlike this study, Siderastrea siderea corals exhibited mixed, though not incompatible, responses to elevated temperature and pCO2 in two experiments (Castillo et al., 2014; Horvath et al., 2016). The corals from those studies were collected from the same location, and the experiments were conducted under similar conditions but different treatment levels. Other laboratory experiments on coral calcification under elevated pCO2 but constant temperature have documented null (Bedwell-Ivers et al., 2016) to negative (Renegar & Riegl, 2005; Enochs et al., 2014) pCO2 responses for A. cervicornis, negative responses for P. divaricata (Bedwell-Ivers et al., 2016), and negative responses for P. astreoides juveniles (Albright et al., 2008; Albright & Langdon, 2011; De Putron et al., 2011). The present study measured no growth for O. faveolata in the intermediate 2.5 Ωarag, 30 °C treatment, which may indicate an enzymatic ‘deadzone’ for this species (Wooldridge, 2008).
| Species | CO2 treatment | Calcification metric | Setting | n corals | n colonies | Ωarag | T (°C) | S | Light (mol m−2 day−1) | Nutrition | Recovery time | Ramping time | Duration | Linear ΔG % Ω−1 (baseline Ω = 4.6) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. cervicornis | pCO2 | BW | Laboratory | 20 | ND | 2.9–4.0 | 30 | ND | ND | not feda | 5 day | 0 day | 4 weeks | 13% | Bedwell-Ivers et al. (2016) |
| A. cervicornis | pCO2 | BW | Laboratory | 64 | 4 | 2.5–3.9 | 28 | 32 | 4.0–15.6 | ND | 7 day | 9 day | 42 day | 21–41% | Enochs et al. (2014) |
| A. cervicornis | pCO2 | BW | Laboratory | 192 | ND | 1.2–3.5 | 25 | 36 | NS | ND | ND | 0 day | 4 weeks | 22–29% | Renegar & Riegl (2005) |
| A. cervicornis | pCO2 | BW | Laboratory | 80 | 8 | 2.2–4.1 | 26–30 | 33 | 5.8 | Fed & not fed | 4 weeks | 0 day | 8 weeks | 0–17% | Towle et al. (2015) |
| O. faveolata | None | X-ray | Field | 7 | 7 | 4–4.5 | ND | ND | NS | In situ | NA | NA | 60 years | 0% | Helmle et al. (2011) |
| P. astreoides | pCO2 | Image | Laboratory | 102 | 102 | 2.3–3.9 | 28 | 36 | ND | Not fed | NA | 0 day | 49 day | 19% | Albright & Langdon (2011) |
| P. astreoides | HCl | Image | Laboratory | 35 | 35 | 2.2–3.2 | 25 | 35 | <0.4 | Not fed | NA | 0 day | 28 day | 36–39% | Albright et al. (2008) |
| P. astreoides | HCl/NaOH + Na2CO3 | BW; AA | Laboratory | 80 | 80 | 2.1–3.9 | 28–30 | 35 | 17–22 | Not feda | 24 day | 0 day | 35 day | 19–29% | Camp et al. (2016) |
| P. astreoides | None | CT | Field | 14 | 14 | 0.8–4.2 | 28 | 34 | ND | In situ | NA | NA | ND | 15% | Crook et al. (2013) |
| P. astreoides | HCl; pCO2 | DW | Laboratory | ND | ND | 0.1–4.2 | 29 | 37–38 | 2.6 | Not fed | NA | NA | 2 weeks | 14–20% | De Putron et al. (2011) |
| P. astreoides | None | AA; X-ray | Field | 9 | 9 | 2.8–3.5 | 19–28 | 36–37 | 7.4–55.5 | In situ | 6 mo | 0 day | 1.5 h; 18 months | 63% | Venti et al. (2014) |
| P. divaricata | pCO2 | BW | Laboratory | 20 | ND | 2.9–4 | 30 | ND | ND | Not feda | 5 day | 0 day | 4 weeks | 21% | Bedwell-Ivers et al. (2016) |
| P. strigosa | None | AA; X-ray | Field | 9 | 9 | 2.8–3.5 | 19–28 | 36–37 | 7.4–55.5 | In situ | 6 mo | 0 day | 1.5 h; 18 months | 63% | Venti et al. (2014) |
| S. radians | HCl + NaHCO3 | AA | Field | 10 | 10 | 1.1–5.7 | 20–31 | 32–47 | 3.5–50.0 | In situ | NA | NA | 1.5 h | 45% | Okazaki et al. (2013) |
| S. siderea | pCO2 | BW | Laboratory | 216 | 18 | 1.1–4.0 | 28 | 35 | 10.8 | Fed | 30 day | 15 day | 95 day | NAb | Castillo et al. (2014) |
| S. siderea | pCO2 | BW | Laboratory | 144 | 18 | 2.7–6.8 | 28–32 | 35 | 10.8 | Fed | 30 day | 14 day | 60 day | 10–43% | Horvath et al. (2016) |
| S. hyades | HCl + NaHCO3 | AA | Field | 7 | 7 | 1.1–5.7 | 20–31 | 32–47 | 3.5–50.0 | In situ | NA | NA | 1.5 h | 44% | Okazaki et al. (2013) |
| A. cervicornis | pCO2 | BW | Laboratory | 20 | 16 | 1.7–4.3 | 27–30 | 33 | 3.7 | Fed | 4 weeks | 10 day | 6 weeks | 29% | This study |
| A. agaricites | pCO2 | BW | Laboratory | 54 | 22 | 1.7–4.3 | 27–30 | 33 | 3.7 | Fed | 4 weeks | 10 day | 6 weeks | 13–14% | This study |
| D. stokesii | pCO2 | BW | Laboratory | 59 | 5 | 1.7–4.3 | 27–30 | 33 | 3.7 | Fed | 4 weeks | 10 day | 6 weeks | 5–11% | This study |
| M. cavernosa | pCO2 | BW | Laboratory | 145 | 9 | 1.7–4.3 | 27–30 | 33 | 3.7 | Fed | 4 weeks | 10 day | 6 weeks | 15–25% | This study |
| O. faveolata | pCO2 | BW | Laboratory | 130 | 17 | 1.7–4.3 | 27–30 | 33 | 3.7 | Fed | 4 weeks | 10 day | 6 weeks | NAc | This study |
| P. astreoides | pCO2 | BW | Laboratory | 152 | 10 | 1.7–4.3 | 27–30 | 33 | 3.7 | Fed | 4 weeks | 10 day | 6 weeks | 28% | This study |
| P. divaricata | pCO2 | BW | Laboratory | 115 | 71 | 1.7–4.3 | 27–30 | 33 | 3.7 | Fed | 4 weeks | 10 day | 6 weeks | 12–19% | This study |
| P. clivosa | pCO2 | BW | Laboratory | 43 | 6 | 1.7–4.3 | 27–30 | 33 | 3.7 | Fed | 4 weeks | 10 day | 6 weeks | −12–11% | This study |
| P. strigosa | pCO2 | BW | Laboratory | 42 | 9 | 1.7–4.3 | 27–30 | 33 | 3.7 | Fed | 4 weeks | 10 day | 6 weeks | 19% | This study |
| S. radians | pCO2 | BW | Laboratory | 146 | 44 | 1.7–4.3 | 27–30 | 33 | 3.7 | Fed | 4 weeks | 10 day | 6 weeks | 9–12% | This study |
| S. siderea | pCO2 | BW | Laboratory | 138 | 30 | 1.7–4.3 | 27–30 | 33 | 3.7 | Fed | 4 weeks | 10 day | 6 weeks | 3% | This study |
| S. hyades | pCO2 | BW | Laboratory | 138 | 22 | 1.7–4.3 | 27–30 | 33 | 3.7 | Fed | 4 weeks | 10 day | 6 weeks | 6–9% | This study |
- a Coral nutrition assumed from water changes.
- b Data fit to parabolic response.
- c Data fit to general additive model.
This study attempted to replicate general Florida Keys reef conditions at 10 m depth, but this approach carries inherent trade-offs. Coral species do not occupy the same niche, and therefore, the control treatment could be less optimal for some species than others. This appears to be the case for S. radians and S. hyades, which were mostly collected from Florida Bay where annual temperature maximums are regularly ≥2 °C higher than the Florida Reef Tract (Okazaki et al., 2013). These species exhibited positive responses to temperature from 27 to 30 °C. Beyond this range, calcification would eventually decrease as temperatures near these corals’ upper thermal limits. These species also appeared less sensitive to increased pCO2 in the laboratory setting than in the field (Table 2).
Simultaneously testing multiple species in a mixed assemblage has numerous advantages in comparison with meta-analyses, which are challenged to combine studies that differ in seasonality, duration, conditions, and methods. The mixed assemblage can be considered representative of actual reef environments. A multispecies approach is valuable for reducing potential publication bias (Møller & Jennions, 2001) from not reporting species with null responses (the ‘file drawer effect’) because it facilitates comparisons among species. Despite the robust evidence showing decreasing calcification under elevated pCO2, prior meta-analyses have found evidence for publication bias in ocean acidification research (Kroeker et al., 2010; Chan & Connolly, 2013).
The growth responses from these common but understudied Caribbean coral species can be used to refine estimates of reef CaCO3 precipitation based on species abundance (Perry et al., 2012) and to model changes in coral calcification of reefs over time. With respect to the latter, emissions scenarios and community composition determine the trajectories of scleractinian calcification. These trajectories were relatively stable under the optimistic RCP 2.6 and RCP 4.5 scenarios with <20% declines relative to present day (Fig. 3a, b). Under more realistic and extreme emissions scenarios of RCP 6 and RCP 8.5 where temperature increases by 1.5–3 °C and Ωarag decreases by 1–1.3 units, more pronounced differences in reef trajectories are a result of species composition (Fig. 3c, d). The most abundant corals in terms of benthic cover were M. cavernosa, O. faveolata, P. astreoides, and S. siderea. Consequently, their calcification rates and responses to treatments were the most influential on the projections (Table S4). Of these four species, calcification of S. siderea was unaffected by the temperature and pCO2 levels tested here. Reefs with relative scleractinian cover consisting of >40% S. siderea generally were the most stable. These reefs tended to be patch reefs or deeper (10–20 m) offshore reefs (Fig. S6). Siderastrea siderea is already noted for its tolerance to extreme temperatures (Lirman et al., 2011) and high relative abundance on the Florida Reef Tract (Lirman & Fong, 2007).
In comparison, uniform scleractinian sensitivity coefficients for temperature and Ωarag yield end-of-century calcification of 16% to −3% (net dissolution) relative to present-day under RCP 8.5 (blue shaded region in Fig. 3). These projections are based on additive, linear scleractinian calcification changes of 10 to 25% Ωarag−1 (Chan & Connolly, 2013) and a mean −24% °C−1 for temperatures above the thermal optimum (Clausen & Roth, 1975; Houck et al., 1977; Coles & Jokiel, 1978; Reynaud-Vaganay et al., 1999; Marshall & Clode, 2004; Al-Horani et al., 2005; Carricart-Ganivet et al., 2012). These declines based on uniform constants are steeper and more homogenous than the declines of 0% to 90% based on species-specific responses (Fig. 3d; Table S1). These results clearly show the importance of community composition in determining reef-wide calcification responses to climate change. However, in the absence of data on species sensitivities and reef composition, uniform assumptions/constants are still valuable for predicting aggregate, regional calcification trajectories, especially if they are based on the dominant coral species.
Several caveats must be considered with the projections. While calcification responses to elevated temperature and pCO2 were mostly negative, variability among colonies, communities, and climate models contributes uncertainty to predictions at a given reef. Therefore, efforts to measure baseline calcification rates and long-term monitoring of colonies at multiple sites are important (Kuffner et al., 2013). This experiment may also underestimate compensatory mechanisms that might allow corals to calcify at rates faster than measured here due to long-term acclimation or adaptation to these conditions over several decades (Wall et al., 2016). Only twelve of the ~70 coral species in the Caribbean were tested in this experiment, and calcification responses of the remaining species could influence the projected outcomes. Projections are also based only on scleractinian corals, which represent <8% benthic cover in the Florida Keys (Ruzicka et al., 2013). They do not consider nonscleractinian CaCO3 precipitation and dissolution processes which may contribute significantly to reef CaCO3 budgets (Perry et al., 2014).
The projections are likely conservative because they do not account for processes that are expected to reduce coral cover. Increased bleaching and mortality with increasing temperatures (Hoegh-Guldberg, 1999) will severely cripple or halt calcification (Van Hooidonk et al., 2014). To illustrate the importance of high-temperature stress, simulated coral cover in Hawaii over the next century was stable or increased when bleaching and mortality effects were ignored (Hoeke et al., 2011). The adverse effects of increased temperature and pCO2 on reproduction and recruitment (Hendriks et al., 2010; Kroeker et al., 2010; Albright, 2011; Anlauf et al., 2011; Chua et al., 2013) are expected to further reduce coral cover but they have not been well quantified at the community scale. Finally, actual CO2 emissions have consistently increased faster than the IPCC's most extreme scenarios (Peters et al., 2013), and therefore, future changes in temperature and Ωarag may be greater than predicted. Including all of these processes would likely result in more depressed calcification, but the focus of this study was calcification at sublethal temperatures.
Despite these caveats and conservative assumptions, the underlying pattern is lower, less stable growth from critical reef framework builders. In addition to bleaching, Caribbean reefs have already experienced large declines in scleractinian coral cover from reductions in herbivory, nutrient pollution, and disease (Aronson & Precht, 2001; Gardner et al., 2003; Kuffner & Toth, 2016). Current levels of coral cover are already associated with static reef growth or dissolution (Perry et al., 2013). Coupled with the prospect of more frequent bleaching and reduced aragonite saturation state driven by increasing anthropogenic atmospheric CO2, reefs are likely to experience continued declines. The species-specific responses measured here indicate that forecasts must take into account community composition. In addition to limiting pollution and overfishing (Wooldridge & Done, 2009; Kennedy et al., 2013), another local strategy for mitigating climate change is focusing management efforts on reefs with corals capable of resisting high temperatures and low Ωarag. However, this approach may amount to no more than a triage strategy for minimizing further losses. Alternatively, the growth projections under the different RCPs illustrate how reducing global CO2 emissions has the potential to benefit all reefs throughout the Florida Reef Tract.
Acknowledgements
We thank volunteers from University of Miami (UM) (J. Alpert, C. Beggs, R. Carlton, J. Cossavella, J. Diaz, J. Schacher), MAST Academy High School (G. Chacon, K. DeLisser, A. Fuste, C. Gladieux, B. Guerrero, E. Espinales, S. Khemlani, F. Rodriguez), and the public (C. Gallo, J. Grimm) for their assistance. We thank the Florida Keys National Marine Sanctuary, Keys Marine Lab, Naval Air Station Key West, UM Coral Futures Laboratory, UM Benthic Ecology Lab, Smithsonian Marine Station, Jay Fisch, Tom Capo, UM Experimental Hatchery, and NOAA AOML OCED for support. Research was conducted under permits from the National Park Service (EVER-2009-SCI-004), Florida Keys National Marine Sanctuary (FKNMS-2011-064), and Florida Fish and Wildlife Conservation Commission (SAL-11-1182A-SRP). The research was supported by the UM Stable Isotope Lab, Herbert W. Hoover Foundation travel award, and a RSMAS Alumni Award grant. RRO was supported by a UM Fellowship and National Science Foundation grant OCE0550588 to PKS and CL. PMEL contribution #4416.




