A meta-analysis of soil salinization effects on nitrogen pools, cycles and fluxes in coastal ecosystems
Abstract
Salinity intrusion caused by land subsidence resulting from increasing groundwater abstraction, decreasing river sediment loads and increasing sea level because of climate change has caused widespread soil salinization in coastal ecosystems. Soil salinization may greatly alter nitrogen (N) cycling in coastal ecosystems. However, a comprehensive understanding of the effects of soil salinization on ecosystem N pools, cycling processes and fluxes is not available for coastal ecosystems. Therefore, we compiled data from 551 observations from 21 peer-reviewed papers and conducted a meta-analysis of experimental soil salinization effects on 19 variables related to N pools, cycling processes and fluxes in coastal ecosystems. Our results showed that the effects of soil salinization varied across different ecosystem types and salinity levels. Soil salinization increased plant N content (18%), soil NH4+ (12%) and soil total N (210%), although it decreased soil NO3− (2%) and soil microbial biomass N (74%). Increasing soil salinity stimulated soil N2O fluxes as well as hydrological NH4+ and NO2− fluxes more than threefold, although it decreased the hydrological dissolved organic nitrogen (DON) flux (59%). Soil salinization also increased the net N mineralization by 70%, although salinization effects were not observed on the net nitrification, denitrification and dissimilatory nitrate reduction to ammonium in this meta-analysis. Overall, this meta-analysis improves our understanding of the responses of ecosystem N cycling to soil salinization, identifies knowledge gaps and highlights the urgent need for studies on the effects of soil salinization on coastal agro-ecosystem and microbial N immobilization. Additional increases in knowledge are critical for designing sustainable adaptation measures to the predicted intrusion of salinity intrusion so that the productivity of coastal agro-ecosystems can be maintained or improved and the N losses and pollution of the natural environment can be minimized.
Introduction
Sea-level rise (SLR), increased groundwater abstraction and decreased sediment loads by embanked rivers cause saltwater intrusions in coastal regions (Milly et al., 2005; Van Dijk et al., 2009; IPCC 2013; Rasmussen et al., 2013), which drive widespread soil salinization in coastal ecosystems (Werner et al., 2013; Chandrajith et al., 2014). Agro-ecosystems in coastal regions feed over 40% of the world's population (Burke et al., 2000). However, these systems are currently threatened by soil salinization, which is further aggravated by salinity intrusion because of climate change (Smajgl et al., 2015). Particular concerns include the salinization effects on nitrogen (N) cycling in coastal ecosystems (Herbert, 1999; Herbert et al., 2015) because N is an essential nutrient for crop production and natural ecosystem functioning as well as affecting environmental quality from regional to global scales because of gaseous and hydrological N losses from ecosystems. Soil salinization can mediate ecosystem N cycling through direct impacts on microorganisms and their communities and functions (Santoro, 2010; Bernhard et al., 2015) as well as through alterations of abiotic factors (e.g., soil cation and anion compositions) in coastal ecosystems (Marton et al., 2012; Ardon et al., 2013; Teixeira et al., 2013). Therefore, soil salinity is likely a key regulator of plant and soil N pools, cycling processes and fluxes in coastal ecosystems, although one has to assume that the underlying regulatory mechanisms are complex.
Increases in soil salinity may decrease net photosynthesis and induce physiological stress on plant growth (Hester et al., 2001), thereby resulting in decreases in plant biomass productivity in coastal ecosystems (e.g., Erickson et al., 2007; Sutter et al., 2014). In the Chesapeake Bay (USA), the plant biomass production was significantly decreased because of the inhibition of plant carbon assimilation at high salinity levels in a tidal freshwater wetland (Sutter et al., 2014), although in the same study, the salt marsh plant biomass remained unchanged with increasing salinity, which was likely because the dominant species (e.g., Spartina) is more salt tolerant (Hester et al., 2001). Soil salinization tended to enhance plant tissue N in a freshwater wetland (e.g., Erickson et al., 2007) because plants can conserve nitrogen more effectively under salt stress, thereby resulting in a relative increase in plant N (Neubauer et al., 2000). Comparable observations were also reported by Sutter et al. (2014) for a freshwater wetland where increasing soil salinity significantly increased plant tissue N, although such an effect was not found for a salt marsh. However, across different coastal ecosystems, the salinization effects on plant biomass and plant tissue N are inconsistent (Matamala & Drake, 1999; Erickson et al., 2007; Sutter et al., 2014).
Several experiments have shown that soil salinity is a key regulating factor of N mineralization in coastal ecosystems (Fang et al., 2005; Noe et al., 2013; Brouns et al., 2014). Noe et al. (2013) found that N mineralization in a tidal freshwater forested wetland was stimulated by increasing salinity from 0.1 to 3.5 parts per thousand (ppt), which was likely because of plant stress, accelerated senescence and related additional plant biomass input to the soil. However, soil N mineralization as a biologically mediated process was inhibited by increasing soil salinity because microbial activity tends to be reduced at higher salinities (e.g., Pathak & Rao, 1998; Rath & Rousk, 2015). A review of soil salinization effects on N mineralization in coastal wetlands also suggests that N mineralization processes in coastal wetlands may be greatly impacted by soil salinization, although the underlying mechanisms are complex and depend on the ecosystem type and the soil's physical, chemical and biological properties (Bai et al., 2012).
Nitrification that transforms NH4+ to nitrate (NO3−) via microbial processes can also be affected by salinization (Joye & Hollibaugh, 1995; Osborne et al., 2015). Increasing soil salinity not only constrains the physiological fitness of nitrifiers but also affects the availability of soil oxygen, thereby resulting in a decrease in nitrification rates in coastal freshwater ecosystems (Joye & Hollibaugh, 1995; Rysgaard et al., 1999; Giblin et al., 2010). However, Magalhaes et al. (2005) found that nitrification rates were stimulated when the soil salinity increased from 0 to 15 ppt in the Douro River Estuary of Portugal, which suggested that nitrifying bacteria in this estuary were salt tolerant. These conflicting findings indicate that the salinization effects on nitrification may vary across different coastal ecosystems.
Similar to nitrification, soil salinization has been shown to have contrasting effects on microbial denitrification through its differential effects on the physiology of soil microbes in various coastal ecosystems (Seitzinger et al., 1991; Fear et al., 2005; Giblin et al., 2010; Bruesewitz et al., 2013). For example, decreases in denitrification rates with increases in soil salinity were observed in the Parker River Estuary, Massachusetts, USA, which was explained by the inhibitory effect of soil salinization on the nitrification process and the reduced availability of nitrate as substrate for denitrification (Giblin et al., 2010). However, Fear et al. (2005) found that increases in salinity (2–24 ppt) did not significantly stimulate or inhibit denitrification rates in the Neuse River Estuary of North Carolina (USA), which was likely because of the simultaneous change in other regulating parameters of denitrification, such as soil available oxygen and organic carbon (Rath & Rousk, 2015).
The relative importance of the dissimilatory nitrate reduction to ammonium (DNRA) for NO3− reduction has become increasingly evident in numerous coastal ecosystems (Dong et al., 2011; Giblin et al., 2013; Yang et al., 2015), although it is not been well understood. Compared with denitrification, the DNRA transforms NO3− to NH4+; hence, N can be retained if the DNRA outcompetes denitrification for NO3− (Tobias et al., 2001). The balance of the two processes is regulated by various environmental factors, such as salinity level as well as NO3− and organic C availability (Brunet & Garciagil, 1996; Giblin et al., 2010; Dong et al., 2011; Algar & Vallino, 2014). Increasing salinity has been found to favor the DNRA (e.g., Osborne et al., 2015), and various reduced sulfur compounds (H2S, FeS, S2O32−) have been shown to serve as electron donors for the DNRA in coastal ecosystems (Brunet & Garciagil, 1996). Previous studies have also demonstrated that the DNRA is favored over denitrification with high organic C availability (Gardner & Mccarthy, 2009), although it may also be shifted to denitrification with increased in soil salinity (e.g., Giblin et al., 2013). Thus, the balance of the DNRA and denitrification responds differently to soil salinity depending on the ecosystem and is tightly related to other environmental factors (e.g., NO3− and organic C availability).
The microbial N cycling processes drive and regulate the exchange of N compounds (e.g., N2O, NO3− and NH4+) across the atmosphere–soil–hydrosphere interfaces in coastal ecosystems, which contribute to regional and global N pollution (Teixeira et al., 2013; Cornwell et al., 2014; Bernhard et al., 2015). Soil salinization, therefore, could directly affect gaseous and hydrological N losses from coastal ecosystems through alterations in soil microbial communities and their biochemical activities. Furthermore, soil salinity could also change other soil environmental parameters, such as soil available oxygen (Noe et al., 2013), or ionic strength (Weston et al., 2010). These changes might drive increased N losses from coastal ecosystems along hydrological and atmospheric pathways. Nevertheless, general patterns on how gaseous and hydrological N losses respond to soil salinization in coastal ecosystems have not yet been identified.
The inconsistent findings from different studies emphasize the need to synthesize available results to reveal a general trend of soil salinization effects on ecosystem N pools, cycling processes and fluxes in coastal ecosystems. Therefore, we conducted a meta-analysis to examine the responses of ecosystem N pools (soil total nitrogen, NH4+, NO3− and microbial N pools, plant productivity and canopy N concentration), microbial N cycles (N mineralization, nitrification, denitrification, DNRA and Anammox) and gaseous and hydrological N fluxes (N2O emission and hydrological NH4+, NO3−, NO2− and DON fluxes) to salinization in coastal ecosystems.
Materials and methods
Data compilation
We searched for peer-reviewed journal articles published before May 2015 and listed in the ISI Web of Science. The search terms were ‘nitrogen’ and ‘seawater intrusion’; ‘nitrogen’ and ‘sea level rise’; ‘nitrogen’ and ‘salinity’ and ‘coastal ecosystems’; and ‘nitrogen’ and ‘salinity’ and ‘delta’. In total, 938 publications were found. The following criteria were applied to select appropriate studies: (i) only studies including no salinization (control) and salinization (treatment) experimental plots were selected; (ii) at least one of the selected variables was measured; (iii) only control and salinization treatment data were used for multifactorial studies and the interacting effects were excluded; and (iv) means and sample sizes had to be reported. We screened the publications in accordance with the above criteria, and 21 papers that reported the responses of N cycling processes to soil salinization in coastal ecosystems were selected from the 938 peer-reviewed publications (Table 1). These publications summarized 551 observations, and the compiled database included 19 variables related to ecosystem N pools [soil total nitrogen, NH4+, NO3− and microbial biomass N, plant tissue N (a percentage of dry biomass) as well as plant below- and aboveground biomass], N cycling processes (N mineralization, nitrification, denitrification, DNRA and Anammox) and N fluxes (N2O emission and hydrological NH4+, NO3−, NO2− and DON fluxes) (Appendix S1).
| Study number | Reference | Country | Geographic location | Ecosystem types | Experimental method | Number of variables |
|---|---|---|---|---|---|---|
| 1 | Antheunisse et al., (2007) | Netherlands | 51°47°N, 4°13°E; 51°44′N, 4°21′E | Grassland | Mesocosm experiment | 6 |
| 2 | Ardon et al., (2013) | United States | 35°54′N, 76°09′W | Freshwater wetland | Field and microcosm experiments | 3 |
| 3 | Brouns et al., (2014) | Netherlands |
52°08′N, 4°50′E; 52°52′N, 5°48′E; 52°08′N, 4°48′E; 52°59′N, 6°24 E |
Peat meadow | Laboratory experiment | 2 |
| 4 | Dausse et al., (2012) | United Kingdom | – | Salt marsh | Field experiment | 6 |
| 5 | Dong et al., (2011) | Thailand; Indonesia; Fiji |
13°24′N, 99°59′E; 13°23′N, 100°00′E; 6°01′S, 106°37′E; 6°00′S, 106°38′E; 6°00′S, 106°38′E; 6°00′S, 106°38′E; 18°06′S, 178°32′E; 18°07′S, 178°31′E; 18°08′S, 178°30′E |
Estuary | Field and laboratory experiments | 3 |
| 6 | Fear et al., (2005) | United States | – | Estuary | Laboratory experiment | 2 |
| 7 | Gao et al., (2014) | China | 37°35′, −38°12′N, 118°33′, −119°20′E | Freshwater wetland | Laboratory experiment | 2 |
| 8 | Koop-Jakobsen & Giblin, (2009) | United States | – | Estuary | Laboratory experiment | 2 |
| 9 | Marton et al., (2012) | United States | 31°21′N, 81°32′W; 31°59′N, 81°17′W; 30°57′N, 81°53′W | Freshwater forested wetland | Laboratory experiment | 1 |
| 10 | Nielsen et al., (2009) | Germany | – | Estuary | Field and laboratory experiment | 3 |
| 11 | Noe et al., (2013) | United States | – | Freshwater forested wetland | Field and laboratory experiment | 8 |
| 12 | Osborne et al., (2015) | United States | – | Freshwater wetland | Field, microcosm and laboratory experiments | 1 |
| 13 | Person & Ruess, (2003) | United States | – | Salt marsh | Field and laboratory experiment | 1 |
| 14 | Qin et al., (2015) | China | – | Salt marsh | Laboratory experiment | 3 |
| 15 | Reddy & Crohn, (2014) | United States | 33°35′N, 116°06′W | Nonvegetation | Laboratory experiment | 5 |
| 16 | Singh et al., (2002) | India | – | Rice | Field experiment | 3 |
| 17 | Sutter et al., (2015) | United States | – | Freshwater wetland | Field experiment | 3 |
| 18 | Sutter et al., (2014) | United States | – | Freshwater wetland | Field experiment | 3 |
| 19 | Teixeira et al., (2013) | Portugal | 41°32′N, 8°47′W; 41°20′N, 8°44′W | Estuary | Laboratory experiment | 1 |
| 20 | Thomas & Christian, (2001) | United States | 41°21′N, 70°23′W; 37°54′N, 75°36′W; 31°19′N, 81°18′W | Salt marsh | Field experiment | 3 |
| 21 | Zhang et al., (2013) | China | 37°35′, −38°12′N, 118°33′, −119°20′E | Estuary | Field experiment | 4 |
Because most studies reported more than one salinity level, one salinity level was defined as one treatment in this meta-analysis and all comparisons were recorded as independent observations. For this meta-analysis, we specifically selected studies with high-quality data (e.g., replicated randomized experiments, direct comparison of different, well-defined salinity levels with no salinity), justifying the consideration of the different salinity levels as independent treatments. For each study, we extracted information on the experimental location, ecosystem type, experimental method as well as the response variables. Data were extracted by getdata graph digitizer software (version 2.26; http://www.getdata-graph-digitizer.com/download.php) if the data were reported as figures in the original publication. We calculated the standard deviation from the sample size and standard error if not reported. Soil salinity data were also collected, and the salinity value was transformed to the unit of parts per thousand (ppt) if not already reported accordingly. Each study was grouped into one of the four ecosystems: freshwater wetland [e.g., freshwater forested wetland, river estuary, rice paddy (only one study with two observations)], salt marsh, grassland and vegetation-free system (i.e., bare soil). We also grouped the collected data into three soil salinization categories in accordance with the recommendation of the FAO (FAO, 1985): <6 ppt (slightly saline), 6–12 ppt (medium saline) and >12 ppt (highly saline).
Statistical analyses
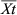 ) to that in the control group (
) to that in the control group (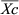 ) (Douxchamps et al., 2011) (Eqn 1). The logarithm of RR was calculated (Hedges et al., 1999) to indicate the effects of soil salinization as follows:
) (Douxchamps et al., 2011) (Eqn 1). The logarithm of RR was calculated (Hedges et al., 1999) to indicate the effects of soil salinization as follows:
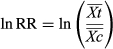 (1)
(1)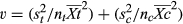 (2)
(2)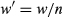 (3)
(3)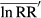 for all observations were calculated using Eqns 4 and 5:
for all observations were calculated using Eqns 4 and 5:
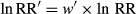 (4)
(4)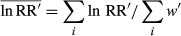 (5)
(5)where ln RRi′ and wi′ are the ln RR′ and w′ of the ith observation, respectively.
Bootstrapping procedures within the metawin 2.1 software (Arizona State University, Tempe, AZ, USA) were used to generate 95% confidence intervals for the weighted effect sizes using 4999 iterations (Rosenberg et al., 2000). The soil salinization effects were considered significant if the 95% confidence intervals did not overlap with zero. The potential effects of publication bias were excluded by conducting a Kendall's tau rank correlation and fail-safe number tests within metawin 2.1 (Rosenberg et al., 2000). We conducted a categorical randomized-effects model meta-analysis to compare the responses among ecosystem types and soil salinity gradients using a framework similar to an anova, and the between-group heterogeneity was assessed using randomization procedures based on 4999 replications (Adams et al., 1997). We also applied a continuous randomized-effects model meta-analysis to test the relationships between the variables’ effect size (ln RR′) and soil salinity levels across various coastal ecosystems. The statistical results for the total heterogeneity of the effect size among studies (QT), the differences among the group cumulative effect sizes (QM), the residual error (QE), and the slope and the probability value of the regression were reported.
Results
Increasing soil salinity significantly increased the plant N content by 17.6% across all coastal ecosystems, although it did not significantly affect the plant above- or belowground biomass (Fig. 1). However, if the salinization effects were analyzed separately for freshwater and salt marsh ecosystems, a significant increase in plant N could only be demonstrated for salt marsh ecosystems (95% CI of effect size: 0.027–0.202) (Fig. 2). Furthermore, plant N was significantly enhanced with increasing salinity under highly saline conditions but not under slightly saline conditions (Fig. 3).
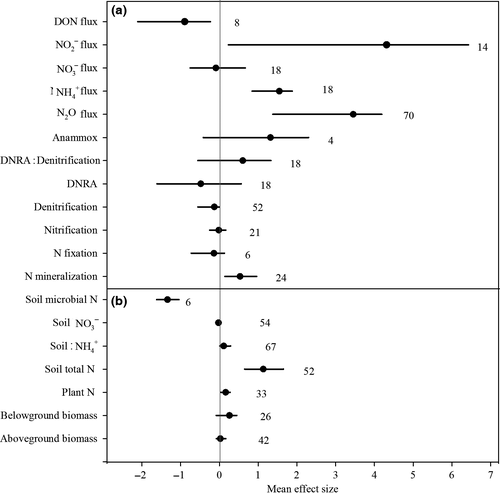
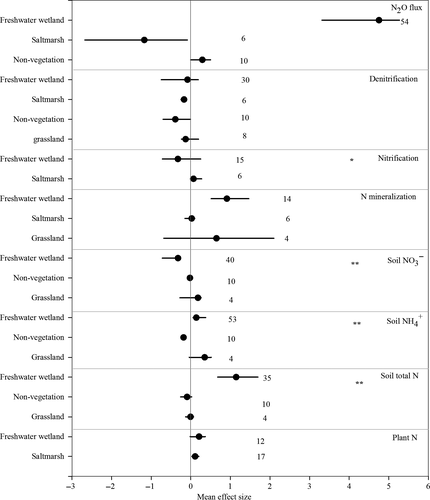
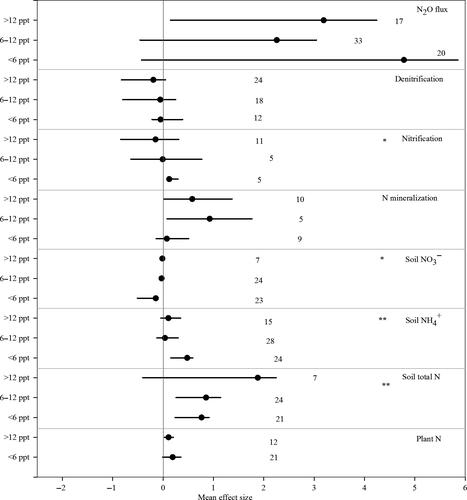
We also found that soil salinization significantly increased the total soil nitrogen (TN) (209.9%) and soil NH4+ (11.7%) and decreased the soil microbial N (−73.8%). However, the soil NO3− was not significantly affected by salinization (Fig. 1). With regard to the ecosystem categories, the soil salinization effects did not change the soil total N and NH4+ in the grassland and nonvegetated ecosystems, whereas it did have a significantly positive effect on both the soil total N and NH4+ in freshwater wetlands (Fig. 2). However, we found evidence that the soil total N and NH4+ responses to salinization were dependent on the salinity level (Fig. 3). Although there were no general patterns in the salinization effect on soil NO3− concentrations (Fig. 1), the responses were significantly negatively affected by salinity in the freshwater wetlands (Fig. 2) primarily under slightly saline conditions (Fig. 3).
On average, increasing the soil salinity significantly stimulated the net N mineralization (hereafter referred to as N mineralization) and anaerobic ammonium oxidation (Anammox) but did not change the biological N2 fixation, net nitrification (hereafter referred to as nitrification), denitrification and the DNRA (Fig. 1). It should be noted that soil salinization increased the ratio of the DNRA to net denitrification (hereafter referred to as denitrification) by 82.5% (Fig. 1). A comparison of the different ecosystem types indicated that N mineralization was only stimulated by increasing soil salinity for freshwater wetlands and not for salt marsh and grassland ecosystems (Fig. 2). Potential denitrification was inhibited by increases in the soil salinity in the vegetation-free and salt marsh ecosystems, although it was not significantly affected in the freshwater wetlands and grasslands. The soil N mineralization was significantly stimulated by medium and high salinity, whereas nitrification was only significantly positively affected by slightly saline conditions (Fig. 3).
Increasing soil salinity significantly stimulated soil N2O fluxes (Fig. 1), although the effect sizes varied across the various coastal ecosystems (Fig. 2). Soil N2O fluxes were significantly stimulated by increasing salinity in the freshwater wetlands, whereas they were significantly inhibited by increasing soil salinity in the salt marshes. Increasing soil salinity also significantly enhanced hydrological NH4+ and NO2−fluxes and significantly decreased DON fluxes, although they did not induce changes in NO3− fluxes (Fig. 1).
Discussion
Plant biomass and plant N
Our meta-analysis indicated that soil salinization significantly increased plant N content and did not change the plant above- and belowground biomass (Fig. 1). Although soil salinity has been shown to induce physiological stress on plant growth and decrease plant biomass in most ecosystems (Hester et al., 2001), the lack of change in plant biomass under soil salinization in the present meta-analysis might have been caused by shifts in the plant species community composition (Crain et al., 2004; Sutter et al., 2014). The plant biomass of salt marshes was reported to be unaffected (Erickson et al., 2007) or even significantly enhanced (Sutter et al., 2014) with increasing soil salinity in the Chesapeake Bay of USA. Therefore, salt-tolerant salt marsh plants, such as Spartina, may replace other less salt-tolerant species, such as P. hemitomon (e.g., Hester et al., 2001). However, for most freshwater wetlands, soil salinization has been found to suppress plant growth and decrease plant biomass through declines in net plant CO2 assimilation (Munns, 2002; Sutter et al., 2014).
Compared with its effects on plant biomass, soil salinization significantly increased the plant N concentrations (Fig. 1). Different mechanisms could be responsible for this phenomenon:
- the demonstrated increase in soil total N and soil NH4+ which allows plants to more easily acquire N from soils (Fig. 1).
- plant physiological responses. For example, some studies have found that freshwater wetland plants tend to conserve nitrogen more efficiently under soil salinization and show decreases in CO2 assimilation and increases in photorespiration (e.g., Sutter et al., 2014). Moreover, root exudation seems to be increased by salinization (Neubauer et al., 2013). These two factors together have been hypothesized to result in higher plant N content. Notably, this plant physiological response, that is, enhanced carbon respiration and decreased CO2 assimilation, to salinity intrusion is a positive feedback to global climate change because increasing temperatures will indirectly result in increased salinization of coastal ecosystems through sea-level rises.
- change in plant species composition due to salinity. In addition to a physiological explanation for increased plant N concentrations, Ryan & Boyer (2012) argue that the response of total ecosystem plant N concentrations to salinization could also be related to changes in the plant community composition toward more salt-tolerant plant species. For example, plant N was significantly enhanced with increasing salinity in a salt marsh in the San Francisco Bay (USA), which was likely because of the increased abundance of Spartina (salt-tolerant species) in the salt marsh system (Ryan & Boyer, 2012).
However, as indicated in our meta-analysis (Fig. 4), targeted studies that use salinity gradient approaches to test the effects of salinization on the plant N content of coastal ecosystems are rare, and this knowledge gap directly pertains to the variability in the responses of plant N content and biomass under salinity intrusions in coastal ecosystems.
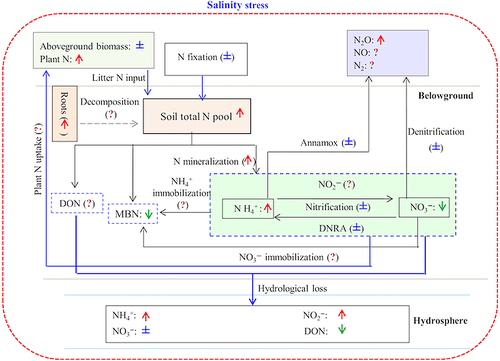
Soil N
Elevated salinity significantly increased the soil total N and NH4+, decreased the microbial biomass nitrogen (MBN) and did not change the soil NO3− across the various coastal ecosystems (Fig. 1). The increased soil total N could be related to the increased plant litter N input to the soil organic matter pool because soil salinization tends to enhance plant N content and results in increased plant litter input to soils because of the increased senescence of aboveground plant biomass following salt accumulation in leaves and other plant parts (Person & Ruess, 2003; Dausse et al., 2012). The increased soil NH4+ might be explained by the observed stimulation of the net N mineralization (Fig. 1). In addition, the increase in soil NH4+ concentrations might also be explained by decreased plant NH4+ uptake and/or microbial NH4+ immobilization with increasing salinity. However, reports on the effects of salinization effects on these parameters in coastal ecosystems are not available (Fig. 4). The indicated knowledge gaps also constrain our understanding of why soil salinization decreased soil microbial biomass nitrogen (MBN) across studies (Fig. 1). We hypothesize that the decrease in soil MBN is likely related to the effect of osmotic stress at high salinity on the abundance and community composition of soil microorganisms.
N mineralization
Our meta-analysis showed that soil salinization on average increased N mineralization by 70.6% (P < 0.05, Fig. 1), which may be explained by the stimulated plant litter N input from dead and stressed plants because of salinization, which was discussed in the above sections. The increased input of detritus with greater N content can stimulate soil N mineralization rates, which likely occurs through priming effects (Kuzyakov et al., 2000; Antheunisse et al., 2007; Noe et al., 2013). Studies have found that soil salinization enhances the relative abundance and activity of the specific microbial functional groups responsible for N mineralization (Muhammad et al., 2006; Wong et al., 2008), which could be another mechanism underlying the stimulation of net N mineralization found in this meta-analysis. The decrease in soil microbial biomass N observed in our meta-analysis (Fig. 1) suggests that microbial N immobilization is inhibited by increasing salinity in coastal ecosystems. Some studies found the increase in net N mineralization was along with the decrease in soil microbial biomass N likely due to the inhibited microbial N immobilization (e.g., Shaw & Harte, 2001; Kooijman et al., 2009). Thus, the inhibited microbial N immobilization could be one cause of increased net N mineralization by soil salinization in coastal ecosystems.
Although the response of plant N to salinization in coastal ecosystems was revealed by the present meta-analysis, plant N uptake was found to be generally decreased by salinization in most inland crop systems (e.g., Van Hoorn et al., 2001). Therefore, we assumed that the reduction in microbial N immobilization and plant N uptake may also be responsible for the increased net N mineralization by salinization in coastal ecosystems.
Overall, our meta-analysis showed a significant salinization effect on net N mineralization, and the magnitude of the salinization effect was ecosystem dependent. The soil salinization effects on net N mineralization were significant in the freshwater wetlands, although they were not significant in salt marshes (Fig. 2). As discussed above, this phenomenon may be caused by the decrease in plant N assimilation because of the lower salt tolerance of plant species in freshwater wetlands compared with those in salt marshes. This finding can also explain the relatively larger effect size of N mineralization observed at higher salinity across different coastal ecosystems (Fig. 3).
Nitrification and Anammox
Although the general effect of salinization on nitrification was not significant across the different coastal systems (Figs. 1 and 2), soil salinization still significantly increased nitrification rates under slightly saline conditions (Fig. 3). Culture experiments showed that estuarine isolates of nitrifiers exhibited optimum nitrification rates at salinity levels of 5–10 ppt (Jones & Hood, 1980). Therefore, as observed in our meta-analysis (Figs 1-3), nitrification can be expected to increase if soil salinity is within the optimum of 5–10 ppt and inhibited if soil salinity exceeds the optimum salinity range. It is noteworthy that nitrification rates are highly dependent on soil oxygen and NH4+ availability, which may also be altered by salinization in coastal ecosystems (Rysgaard et al., 1999; Noe et al., 2013). For example, increasing salinity can reduce the oxygen available to nitrifying bacteria to oxidize ammonium to nitrate, which was observed in a Danish estuarine ecosystem (Rysgaard et al., 1999). Our meta-analysis highlights the gap in knowledge on the response of nitrification to soil salinization, which impedes our ability to predict the effect of salinity intrusions on nitrification and nitrogen cycling and the losses in coastal ecosystems under future climatic conditions.
Our meta-analysis also revealed that soil salinization on average stimulated Anammox more than twofold (Fig. 1) because Anammox bacteria in general are highly tolerant to salt stress (Xiao & Roberts, 2010). A recent review also revealed that Anammox could be stimulated by increasing salinity below 15.0 ppt, although it may be inhibited at higher salinity (Jin et al., 2012), which may explain our observations that Anammox was enhanced when the salinity was between 3 and 15 ppt. Furthermore, it should be noted that the effect size of Anammox was governed by the salinity level as well as the salt type. Dapena-Mora et al. (2007) reported that Anammox was not inhibited at salinity levels below 8.8 ppt but was significantly inhibited at salinity levels of 7.0 ppt if the salt composition was shifted from NaCl to KCl and/or Na2SO4. Although previous studies typically focused on the response of Anammox to salinity during wastewater treatment, few studies have been conducted in coastal ecosystems (Schmid et al., 2007; Jin et al., 2012) despite the universal finding of Anammox in anoxic marine environments (Xiao & Roberts, 2010). This knowledge gap limits our ability to quantitatively predict the response of Anammox to salinity intrusions in coastal ecosystems.
Denitrification and dissimilatory nitrate reduction to ammonium (DNRA)
Overall, soil salinization consistently decreases denitrification across coastal ecosystems, although its effect is not statistically significant (Figs 1-3). Denitrification occurs preferentially under anoxic conditions and is the primary N cycling process for N removal in coastal ecosystems (Dong et al., 2011). The identified positive correlation between salinity levels and salinization effect size on denitrification rates in our meta-analysis (Table 2) indicates that soil salinity is a key regulating factor of microbial denitrification in coastal ecosystems, although denitrification is also controlled by temperature, oxygen, dissolved organic C and nitrate availability (e.g., Butterbach-Bahl et al., 2013). Soil salinity natural gradients and laboratory manipulation experiments have shown that elevated salinity primarily results in sulfide toxicity to denitrifiers and changes in microbial composition (Santoro et al., 2006; Santoro, 2010), which decreases denitrification rates (Dong et al., 2011; Marton et al., 2012). Moreover, soil salinization may also indirectly affect denitrification by shifting soil redox conditions and changing the availability of C and N substrates (Noe et al., 2013; Morrissey et al., 2014; Osborne et al., 2015). For instance, decreases in nitrification rates by increases in soil salinity resulted in a lower availability of soil NO3− as a substrate for denitrification (Fig. 1; Noe et al., 2013). Decreases in the release of dissolved organic carbon (DOC) under soil salinization could also constrain denitrification through a reduction of organic C availability (Rath & Rousk, 2015). Furthermore, a decrease in soil respiration by soil salinization (Wong et al., 2008) could reduce soil oxygen consumption and inhibit denitrification as a result of alterations in the aeration status of the soil.
| QT | QM | QE | Slope | P value | |
|---|---|---|---|---|---|
| N pools | |||||
| Plant N | 20.2846 | 3.3143 | 16.9703 | 0.0072 | 0.94614 |
| Soil total N | 87.0357 | 0.0044 | 87.0313 | 0.0008 | 0.00124 |
| Soil NH4+ | 298.8553 | 39.3227 | 259.5326 | 0.0229 | 0.0000 |
| Soil NO3− | 50.8337 | 0.8515 | 49.9822 | −0.0044 | 0.91547 |
| N cycles | |||||
| N mineralization | 18.2022 | 0.2224 | 17.9799 | 0.0098 | 0.37619 |
| Nitrification | 16.0101 | 1.0596 | 14.9506 | −0.0200 | 0.31276 |
| Denitrification | 43.4929 | 6.8344 | 36.6585 | −0.0405 | 0.01235 |
| N fluxes | |||||
| N2O flux | 40.1332 | 0.0987 | 40.0345 | 0.0194 | 0.97734 |
| NH4+ flux | 13.8164 | 5.2932 | 8.5232 | 0.0540 | 0.68005 |
| NO3− flux | 22.2664 | 0.0328 | 22.2336 | 0.0110 | 0.17469 |
| NO2− flux | 6.8399 | 1.2840 | 5.5560 | 0.1492 | 0.91021 |
Soil salinization on average decreased the DNRA by 38.2%, although this effect was not significant (Fig. 1). Under anoxic soil conditions, the DNRA can consume 5–40% of the total nitrate pool (Osborne et al., 2015), although most of the N can be retained in coastal ecosystems (e.g., as reviewed by Giblin et al., 2013). The soil aeration status, NO3− and organic C availability as well as the soil pH have been identified as the regulating factors of DNRA in most ecosystems (e.g., as reviewed by Rutting et al., 2011). Therefore, the decreased DNRA by soil salinization, as found in our meta-analysis, can be explained by either the direct inhibition of the related microbial DNRA activity or indirectly by the decreased availability of soil NO3− because of inhibited nitrification (Jin et al., 2012). However, the DNRA was positively correlated with soil salinity in the freshwater wetlands of Massachusetts (USA) because of the interaction of soil salinity and nitrate availability (Giblin et al., 2010). Thus, the mechanism underlying the DNRA response to soil salinity appears to be complex because the effect of soil salinity is often coupled with other factors that regulate the DNRA. However, the number of observations reported in the literature is limited.
Although increased soil salinity inhibited denitrification and the DNRA, soil salinization favored the DNRA relative to denitrification across coastal ecosystems (P < 0.05; Fig. 1). Similarly, several studies reported that increasing soil salinity tended to favor the DNRA over denitrification, which was likely because of an increase in sulfate reduction (e.g., Brunet & Garciagil, 1996; Giblin et al., 2010). In addition to an increase in sulfide concentrations, elevated soil salinity favors anoxic conditions, which likely induce denitrification in coastal ecosystems. Therefore, it is unclear whether increasing salinity triggers a switch of denitrification to DNRA. Nevertheless, previous studies have demonstrated that the balance between denitrification and DNRA, which are two processes that compete for nitrate, is also dependent on various other factors, such as the soil redox status, temperature, soil C and N availability (Dong et al., 2011; Giblin et al., 2013; Osborne et al., 2015). For instance, Dong et al. (2011) reported the relative importance of the DNRA and showed that denitrification was dependent on both the availability of organic C and nitrate in the soil and temperatures in a tropical estuary. At high soil temperatures and low organic C and nitrate concentrations, the DNRA was the dominant nitrate reduction process relative to denitrification. It is noteworthy that NO3− can be retained in the soil rather than lost to the atmosphere as N2O if the DNRA outcompetes denitrification for soil nitrate (Tobias et al., 2001). Overall, the response of the ratio of DNRA to denitrification to soil salinity in coastal ecosystems is not clarified in the current literature.
N loss
On average, increasing soil salinity significantly stimulated soil N2O emissions (Fig. 1), although the magnitude of the response varied across different ecosystems and salinity levels (Fig. 2; Fig. 3). Soil N2O can be produced by several biotic processes, such as microbial nitrification and denitrification (as reviewed by Butterbach-Bahl et al., 2013), and abiotic processes, such as the chemical reactions of nitrite and hydroxylamine (as reviewed by Heil et al., 2016), that are regulated by various environmental factors. There are several possible explanations for the increase in soil N2O emissions with increasing soil salinity. First, higher soil salinity can directly inhibit the last step of the denitrification chain of specific denitrifiers, including the reduction of N2O to N2, which is likely because osmotic stress induced by salinization can suppress the denitrifiers responsible for reducing N2O to N2 (Reddy & Crohn, 2014). Similarly, Marton et al. (2012) found that soil N2O emissions positively responded to elevated salinity in a tidal freshwater wetland of the Satilla River delta in southern Georgia (USA) because the enzyme N2O reductase was inhibited at higher salinity levels, which resulted in greater N2O emissions. Second, increasing soil salinity can stimulate sulfate reductions, thereby leading to elevated H2S concentrations that can effectively inhibit N2O reductase (Sorensen et al., 1980). However, previous studies often used the ratio of N2 to N2O as an indicator of the N2O reduction activity of denitrifying communities because a direct quantification in the field was not possible (e.g., Sorensen et al., 1980). A major unknown variable in this context is the response of N2O reductase activity to soil salinity levels as well as salt species. Third, increasing salinity levels can reduce microbial activity and limit the availability of labile organic carbon (e.g., reviewed by Rath & Rousk, 2015). Thus, soil salinization can indirectly inhibit N2O reduction, and several studies have demonstrated that the addition of labile C tends to stimulate the reduction of N2O to N2 via denitrification (Sanchez-Martin et al., 2008; Senbayram et al., 2012). However, Wang et al. (2009) found that in a freshwater wetland of the Yangtze River Estuary in China, high soil salinity inhibited nitrification and decreased nitrate production, which could lead to a reduction in denitrification and soil N2O emissions. A fourth possibility could be that because of the stronger inhibition of nitrite oxidizers relative to ammonia oxidizers by salinity, the associated nitrite accumulation (e.g., Zhao et al., 2014) could lead to a higher abiotic (chemical) formation of N2O in the soil, such as through the reaction of nitrite with hydroxylamine, another reactive nitrification intermediate (see Heil et al., 2016, for a more detailed introduction into the various chemical reactions in soil leading to N2O formation).
Increasing soil salinity significantly increased the hydrological NH4+ and NO2− fluxes and decreased the DON fluxes but did not significantly change the NO3− fluxes (Fig. 1). Consistent with our meta-analysis, increases in NH4+ fluxes at elevated salinity have also been observed in several studies (Dausse et al., 2012; Ardon et al., 2013; Noe et al., 2013). The soil salinization effects on hydrological NH4+ fluxes exhibited the same pattern as the net N mineralization (Fig. 1), suggesting that increased net N mineralization is a likely cause of the increased hydrological NH4+ fluxes with increasing salinity. The increased hydrological NH4+ fluxes caused by increasing soil salinity may also be explained through a promotion of the displacement of NH4+ adsorbed to cation exchange sites because of the intrusion of salt cations in coastal ecosystem (e.g., Weston et al., 2010). Nevertheless, positive salinization effects on net N mineralization may include an increased release of NH4+ from organic N compounds, which could also explain the decrease in DON fluxes found in the meta-analysis (Ardon et al., 2013).
As revealed by a recent review (Dendooven et al., 2010), the increase in NO2− fluxes with increasing soil salinity observed in our meta-analysis is likely caused by the inhibitory effects of salinization on the oxidation of NO2− to NO3− (i.e., second step of nitrification) because nitrite oxidizers react more sensitively to salt stress compared with ammonia oxidizers, which results in a mismatch of NO2− production and consumption in favor of production.
Overall, our analysis indicates that the improved N status (e.g., increased soil total N) is due that salinization tends to stimulate the N cycling processes which conserves reactive nitrogen in the system, for example, reduced nitrification, stimulation of dissimilatory nitrate reduction to ammonium or reduced denitrification, although these stimulation effects are not statistically significant (Fig. 1). However, there are still major knowledge gaps as the magnitude of N2 emissions was hardly assessed and effects of salinization on nitrate leaching and/or NH3 volatilization were hardly explored. That means, in contrast to other terrestrial ecosystems, a direct positive link between ecosystem N status and environmental N losses cannot be drawn in coastal ecosystems on the basis of available knowledge. Therefore, it is so far not clear whether the positive salinization effect on ecosystem N status is directly resulting in increase in N losses.
Limitations and perspective
As mentioned above, in addition to the soil salinity level, other factors have also been shown to control the responses of N pools, cycling process and fluxes under soil salinization in coastal ecosystems, and these factors include the salt ion species and the relative abundance and community of plant species (e.g., Osborne et al., 2015; Yang et al., 2015). Unfortunately, the interactions of these factors with salinity level could not be revealed in our meta-analysis because of insufficient data, which may constrain our conclusions to some extent. Nevertheless, using the available information, we formulated a conceptual framework that highlights the current knowledge gaps on the response of ecosystem N pools, cycles and fluxes to soil salinization in coastal ecosystems (Fig. 4). However, the changes in the soil gross N turnover processes and rates in coastal ecosystems under increasing salinity intrusions resulting from sea-level rise are not well understood because the salinization effects on microbial N transformation (e.g., ammonification, nitrification, denitrification and DNRA) and N immobilization have not been simultaneously quantified. Moreover, the role of plant–microbe interactions in mediating the responses of ecosystem N pools, cycling processes and fluxes to salinity intrusions in coastal ecosystem is still unclear despite their importance in most ecosystems (Robertson & Vitousek, 2009).
Although nitrogen is an indispensable nutrient for agricultural production, agriculture largely contributes to N pollution through the insufficient management of N loss processes. Salinization significantly affects crop productivity and N cycling in agricultural soil, which has been primarily demonstrated for inland arable land (Funakawa et al., 2000; Dendooven et al., 2010), which accounts for approximately 10% of the global arable land (Szabolcs, 1989). Among the available salinization effects on inland agro-ecosystems, most studies have focused on crop productivities, and only limited attention has been paid to N cycling and losses (e.g., Funakawa et al., 2000). Moreover, to the best of our knowledge, all of the available studies in coastal regions have focused on natural and/or seminatural ecosystems. Thus, it remains unclear whether our current understanding of ecosystem N cycling and flux responses to soil salinization for these ecosystems (i.e., inland agro-ecosystems and natural/seminatural coastal ecosystems) can be extrapolated to coastal agro-ecosystems, which are also major food production areas worldwide because of the formation of soils on fertile sediments and the high availability of water (Atapattu & Molden, 2006). Thus, further research is needed on the responses of ecosystem N cycling and fluxes in coastal agro-ecosystems to soil salinization because such knowledge is critical for implementing sustainable agricultural production while minimizing N pollution.
Acknowledgements
We thank the anonymous reviewers for their very constructive comments on previous versions of this manuscript. This study was supported by the German BMBF project ‘DeltAdapt’ (Sustainable adaptation of coastal agro-ecosystems to increased salinity intrusion, grant no. 031A287C), the National Natural Science Foundation of China (grant no. 41573079) and the Opening Fund of the State Key Laboratory of Environmental Geochemistry (SKLEG2016909).




