Decadal warming causes a consistent and persistent shift from heterotrophic to autotrophic respiration in contrasting permafrost ecosystems
Abstract
Soil carbon in permafrost ecosystems has the potential to become a major positive feedback to climate change if permafrost thaw increases heterotrophic decomposition. However, warming can also stimulate autotrophic production leading to increased ecosystem carbon storage—a negative climate change feedback. Few studies partitioning ecosystem respiration examine decadal warming effects or compare responses among ecosystems. Here, we first examined how 11 years of warming during different seasons affected autotrophic and heterotrophic respiration in a bryophyte-dominated peatland in Abisko, Sweden. We used natural abundance radiocarbon to partition ecosystem respiration into autotrophic respiration, associated with production, and heterotrophic decomposition. Summertime warming decreased the age of carbon respired by the ecosystem due to increased proportional contributions from autotrophic and young soil respiration and decreased proportional contributions from old soil. Summertime warming's large effect was due to not only warmer air temperatures during the growing season, but also to warmer deep soils year-round. Second, we compared ecosystem respiration responses between two contrasting ecosystems, the Abisko peatland and a tussock-dominated tundra in Healy, Alaska. Each ecosystem had two different timescales of warming (<5 years and over a decade). Despite the Abisko peatland having greater ecosystem respiration and larger contributions from heterotrophic respiration than the Healy tundra, both systems responded consistently to short- and long-term warming with increased respiration, increased autotrophic contributions to ecosystem respiration, and increased ratios of autotrophic to heterotrophic respiration. We did not detect an increase in old soil carbon losses with warming at either site. If increased autotrophic respiration is balanced by increased primary production, as is the case in the Healy tundra, warming will not cause these ecosystems to become growing season carbon sources. Warming instead causes a persistent shift from heterotrophic to more autotrophic control of the growing season carbon cycle in these carbon-rich permafrost ecosystems.
Introduction
A major goal of terrestrial ecosystem warming experiments is to measure whether warming will cause an ecosystem to switch from being a carbon (C) sink to a C source, causing a positive feedback to climate change. This positive feedback is especially a concern in ecosystems underlain by permafrost (Schuur et al., 2013) because globally they store 1330–1580 Pg C in their soils—almost twice the amount of C currently stored in the atmosphere (Tarnocai et al., 2009; Hugelius et al., 2014). This C has been protected from decomposition for centuries to millennia (Dutta et al., 2006; Schirrmeister et al., 2011; Hicks Pries et al., 2012) due to freezing temperatures, but is becoming vulnerable to increased rates of mineralization as permafrost thaws. High northern latitudes, where most permafrost ecosystems are found, are especially vulnerable to warming with 5–9 °C temperature increases predicted over the next century (IPCC, 2013). This magnitude of warming is predicted to cause widespread permafrost thaw (Koven et al., 2013).
Whether an ecosystem becomes a C source with warming depends on the long-term balance between ecosystem respiration (Reco) and primary production, in the absence of disturbances such as fire. Globally, respiration increases with greater mean annual temperatures (Raich & Schlesinger, 1992). Respiration increases due to air or soil warming have been documented in many ecosystems (Wu et al., 2011), with the exceptions being ecosystems that are too dry (Liu et al., 2009), too wet (Oberbauer et al., 2007), or where labile soil C pools are limiting (Melillo et al., 2002). Numerous warming experiments in permafrost ecosystems have also found that Reco increases with warming (Hobbie & Chapin, 1998; Jones et al., 1998; Oberbauer et al., 1998, 2007; Dorrepaal et al., 2009; Huemmrich et al., 2010; Natali et al., 2014). Reco is composed of both heterotrophic respiration, associated with decomposition of organic matter, and autotrophic respiration, which scales with photosynthetic C assimilation (Waring et al., 1998). The relative responses of heterotrophic respiratory C loss and autotrophic C gain to warming determine whether or not a sustained positive feedback to climate change is possible in undisturbed ecosystems with negligible DOC losses. Increased autotrophic respiration generally does not cause a large positive feedback to climate change because, on the timescales over which anthropogenic climate change is occurring, plant respiration is generally balanced by production, whereas heterotrophic respiration is not (e.g., Giardina et al., 2014). Despite the plethora of studies on the response of Reco to warming in permafrost ecosystems, there are only a few that have investigated whether autotrophs or heterotrophs drive that response (Dorrepaal et al., 2009; Hicks Pries et al., 2013a).
To better predict climate change feedbacks from permafrost ecosystems, we need to understand their responses to warming across both time and space. Short-term responses of ecosystems to warming often differ from long-term responses (Bradford et al., 2008). Therefore, studies of long-term warming experiments in permafrost ecosystems are necessary to understand their response trajectories. Across space, permafrost ecosystems differ in their permafrost thermal dynamics and resulting landforms, climate, parent material, plant biomass, soil organic C quality, and nutrient availability, and they may therefore not respond similarly to climate change (Euskirchen et al., 2014). Once thawed, the large stores of organic C in most ecosystems underlain by permafrost will not all become equally available to microbes (e.g., Schädel et al., 2014). Rather, decomposition rates will be controlled by other limiting factors rather than by temperature alone. Across C-rich sites, differences in dominant vegetation, such as mosses versus vascular plants, affect litter quality (Lang et al., 2009; Hicks Pries et al., 2013b) leading to differences in soil organic C and nutrient availability. Such site differences likely affect both autotrophic and heterotrophic responses to warming. Comparing these responses across contrasting sites, and over longer periods, is thus necessary to understand how the climate change feedback varies spatially in order to make long-term global predictions.
Our objectives were to: (i) investigate how growing season autotrophic and heterotrophic respiration, including respiration of old soil C, respond to 11 years of experimental summer, winter, or annual warming in a subarctic peatland and (ii) compare growing season autotrophic and heterotrophic respiration responses to warming across space (Abisko, Sweden vs. Healy, Alaska) and time (short-term warming of <5 years versus decadal warming). We used natural abundance ∆14C to partition Reco into autotrophic and heterotrophic components at a long-term warming experiment in a subarctic bog in Abisko, Sweden (Aerts et al., 2004; Dorrepaal et al., 2004) and compared the results to similar partitioning studies from a short-term, subarctic tundra warming experiment (CiPEHR; Natali et al., 2011) and a long-term, natural permafrost thaw gradient (Schuur et al., 2009) in Healy, Alaska. We expected the Abisko site to have a stronger heterotrophic response to warming but a weaker autotrophic response than Healy due to its lower quality soil organic C, which can be more temperature-sensitive (Davidson et al., 2012), and its smaller amount of vascular plant biomass per area. This is the first study to partition Reco from a permafrost ecosystem after a decade of experimental warming. It is also one of the first studies to directly compare responses of respiration partitioning to arctic warming across continents in contrasting C-rich permafrost ecosystems that differ in climate, soils, and vegetation.
Materials and methods
We used two contrasting sites for this study and comparable Reco partitioning methods using natural abundance C isotopes. In Abisko, Sweden, we partitioned Reco into three sources—autotrophic; young surface soil (0–25 cm); and old, deeper soil (25–50 cm)—using natural abundance radiocarbon (14C). Radiocarbon separates respiration sources by age—on millennial timescales due to radioactive decay and on annual to decadal timescales using the bomb enrichment of atmospheric 14C (Levin & Hesshaimer, 2000; Trumbore, 2000). Generally, autotrophs have ∆14C values close to that of the current atmosphere due to photosynthesis, while young soils have more enriched values than the atmosphere characteristic of the bomb peak, and older soils have more depleted values than the atmosphere (Hicks Pries et al., 2013a). In Healy, Alaska, we previously partitioned Reco into four sources—aboveground autotrophic, belowground autotrophic, young surface soil (0–15 cm), and old, deeper soil (15+ cm) using natural abundance 14C and 13C as previously described (Hicks Pries et al., 2013a). For the comparison presented in this study, we combined the two autotrophic sources because we could not differentiate autotrophic sources at Abisko using only 14C. The young soil for both sites included enriched Δ14C from the past 50 years, and the old soil included soil sections that respired older, depleted Δ14C from the upswing of the bomb peak and earlier.
Study sites
The Abisko, Sweden site is a subarctic bog underlain by permafrost and dominated by Sphagnum fuscum peat moss with only 25% vascular plant cover (Table 1). Hexagonal open top chambers (OTCs; 50 cm high and 2.2 m in bottom diameter) placed on the moss surface have been used to warm this site since 2000 (Aerts et al., 2004; Dorrepaal et al., 2004). For our first objective, we investigated four main treatments, which differed based on the time of year the OTCs were on the plots. The control had no OTCs, the summer warming had OTCs from June through September, the winter warming had OTCs from October through April, and annual warming had OTCs year-round. The OTCs generally increased air and soil temperatures (to 20 cm deep) by about 1 °C during the height of the growing season (Dorrepaal et al., 2009). During the winter, OTCs doubled the snow depth to 3–12 cm (October–January) and 25–30 cm (February–April), creating an insulating layer that resulted in 0.5–2.2 °C warmer soil at 5 cm depth throughout the winter than in plots with ambient snow depth (Dorrepaal et al., 2004). Each treatment had five replicate plots distributed evenly among five blocks. For our second objective, the site by warming duration comparison, we used a fifth treatment in Abisko that consisted of five smaller (1.1 m in bottom diameter) OTCs that had warmed plots from May through September since 2006. These OTCs had similar warming effects as the larger OTC's. All Abisko sampling occurred during July 2011 because previous research found treatment effects on respiration were consistent throughout the growing season (Dorrepaal et al., 2009).
| Site | Abisko, Swedena | Healy, Alaska, USAb |
|---|---|---|
| Ecosystem | Ombrotrophic peat bog | Moist acidic tundra |
| MAT | −0.5 °Cc | −1 °C |
| Mean January T | −10.7 °C | −16 °C |
| Mean July T | 11.3 °C | 15 °C |
| MAP | 320 mm | 378 mm |
| Latitude | 68° 21′ N | 63° 53′ N |
| Elevation | 340 m | 700 m |
| Soil order | Histosol | Gelisol |
| Organic horizon | 60 cm | 45–65 cm |
| Active layer depth | 57 cm | 50 cm |
| Dominant vegetation | Sphagnum fuscum | Eriophorum vaginatum, Vaccinium uliginosum |
| Aboveground biomass | 650 g m−2 | 550–625 g m−2 |
| Vascular biomass | 220 g m−2 | 425–500 g m−2 |
| Other vegetation | Empetrum hermaphroditum, Betula nana, Rubus chamaemorus, Andromeda polifolia, Calamagrostis lapponica, and Vaccinium uliginosum | Pleurozium schreberi, Sphagnum spp., Carex bigelowii, Betula nana, Rubus chamaemorus, Empetrum nigrum, Rhododendron subarcticum, Vaccinium vitis-idaea, Andromeda polifolia, and Oxycoccus microcarpus |
The Eight Mile Lake sites in Healy, Alaska, are subarctic moist acidic tundra underlain by permafrost and dominated by the tussock-forming sedge Eriophorum vaginatum (Table 1). At the short-term warming site, we included the control and annual warming treatment of the CiPHER experiment (Natali et al., 2011), wherein snow fences (8 m long by 1.5 m tall) created a 1–1.5 m thicker snow cover to insulate soils during the winter since October 2008 and OTCs (60 × 60 cm) warmed air during the summer since 2009. OTC's were placed on bases cut 10 cm into the soil, and bases were also installed in control plots. During the growing season, soils were 1.5 °C warmer, thaw depths 10% deeper, and the air about 1 °C warmer in the annual treatment than in the control (Natali et al., 2014). Here, we present an average of data taken at the height of the growing season in 2010 and 2011. At the Healy long-term warming site, we included all sites from a natural thaw gradient where permafrost thaw has been observed for several decades (Osterkamp & Romanovsky, 1999) and much previous research has been carried out (e.g., Schuur et al., 2009; Trucco et al., 2012; Hicks Pries et al., 2013a). We present an average of data taken at the long-term warming site at the height of the growing season in 2008 and 2009.
Soil environment
In Abisko, in blocks one, three, and five, soil temperatures were continuously monitored at 5, 10, and 20 cm depths using NTC thermistor probes and data loggers (Axiom SmartReader Plus 8, Surrey, Canada). For the duration of the sampling (July 2011), additional temperature sensors (Tinytag Plus 2 with external NTC thermistors, Gemini Data Loggers, Chichester, UK) were installed at 5 cm depths in all plots in blocks two and four. In Healy, soil temperatures (5, 10, 20, and 40 cm depths) were measured in all plots using constantan-copper thermocouples and recorded in half-hour intervals using Campbell Scientific CR1000 data loggers. At all sites, thaw depth (the depth from the soil surface to the frozen soil) was sampled at three points in each plot using a metal probe pushed into the soil until it hit frozen ground. Gravimetric water content was measured in annual warming and control soil cores to 15 cm in Abisko, and volumetric water content (0–30 cm) was measured via Campbell CS616 water content reflectometer probes in the Healy short-term warming site (Natali et al., 2014); soil moisture was unavailable from the Abisko short-term and Healy long-term warming plots.
Ecosystem respiration
In Abisko, Reco flux measurements were made four times between July 14 and July 29, 2011. Respiration was measured using an EGM-4 (PP Systems, Massachusetts, United States) infrared gas analyzer over 2 min intervals. The analyzer was attached to 9.1 l opaque chambers placed onto permanently installed collars that went 10 cm into the soil (Dorrepaal et al., 2009). At Healy throughout the growing season, respiration was measured every 1.5 h with a Licor-820 infrared gas analyzer (LICOR Corp., Lincoln, Nebraska) connected to an autochamber (60 × 60 cm each) system at the experiment (Natali et al., 2014) or with a mix of weekly static chamber and continuous autochamber (70 × 70 cm each) measurements at the thaw gradient (Trucco et al., 2012). All analyzers were in line with a water trap to limit humidity effects on CO2 measurements.
At both sites, Reco ∆14C measurements were taken at each plot using the flux chambers (9.1 l Abisko, 10 l Healy) and permanently installed collars (Abisko: 23.1 cm in diameter inserted 10 cm in 2005; Healy: 25.4 cm in diameter inserted 6–7 cm in 2008). In brief, an opaque chamber was fit onto each collar encompassing aboveground plant biomass, and the respired CO2 was collected by pumping the chamber air through a molecular sieve (Alltech 13x, Alltech Associates, Deerfield, IL, USA) for 15 min. Prior to sample collection, the chamber headspace was scrubbed for 45 min with soda lime while maintaining ambient pCO2 to remove atmospheric contamination. The molecular sieves were baked at 625 °C to desorb CO2 (Bauer et al., 1992), which was purified using liquid N2 on a vacuum line and reduced to graphite by Fe reduction in H2 (Vogel et al., 1987). The graphite was sent to the UC Irvine W.M. Keck Carbon Cycle Accelerator Mass Spectrometry (AMS) Laboratory for radiocarbon analysis (precision ± 2.3‰, n = 102). ∆14C data were reported at the same δ13C value to correct for mass-dependent fractionation effects. Δ14C data were corrected for atmospheric contamination using each chamber's δ13C data in a 2-pool (atmospheric and Reco) mixing model (Schuur & Trumbore, 2006). Furthermore, respiration sampling only occurred under calm conditions to minimize intrusion of atmospheric air into the chambers.
Source respiration
Short-term incubations were used to measure ∆14C of autotrophic and heterotrophic source respiration using methods outlined in Hicks Pries et al. (2013a). Aboveground plant material was collected from an 18 × 18 cm quadrat, and belowground plant material was collected from an 18 × 9 cm rectangle to a depth of 20 cm. At Abisko, we collected this material from blocks 1, 3, and 5 outside the treatment plots because previous research had shown the ∆14C of autotrophic respiration did not differ among warming treatments (Hicks Pries et al., 2015). At Healy, we collected plant material from 5 × 10 cm quadrats from each treatment at six fences; samples from the same treatment were then combined by block, for a total of three replicates per treatment (Hicks Pries et al., 2015). At both sites, we clipped all live aboveground plant material, including the top of the moss layer down to where the moss turned brown, from each section and placed it in foil-covered mason jars (0.24L). Belowground roots and rhizomes (>1 mm diameter) were collected from the soil, rinsed, and shaken dry before being put into their own mason jars. We incubated plants as soon as possible after collection (within 5 min for aboveground and 30 min for belowground), to avoid changes to δ13C that can occur 40 min to an hour after excision (Midwood et al., 2006). The jar headspace was scrubbed through soda lime for 5 min at >1 l min−1 before starting the incubations. Δ14C incubations lasted 4 h after which headspace CO2 was collected into molecular sieves.
To measure the ∆14C of heterotrophic respiration at Abisko, we collected five ≈45 cm deep (the depth of the peat layer) soil cores—one from each block. We used a stainless steel 7 cm diameter corer to collect each core and then cut the core into sections: 0–5, 5–15, 15–25, 25–35, 35–45, and 45+ cm. At Healy, six surface cores per treatment (0–25 cm deep by 5 cm square) were cut from the tundra with a serrated knife in August 2010, and 12 deep cores (25–75 cm) were sampled in May 2009 while the ground was frozen using a SIPRE coring auger connected to a Tanaka TIA-340 (Hicks Pries et al., 2015). We removed all roots >1 mm in diameter and let the soils pre-incubate at room temperature in mason jars for 5 days before sampling. As in previous studies (Schuur & Trumbore, 2006; Hicks Pries et al., 2013a), we waited so the labile soil C pool would be more likely to be sampled than recent root-derived C. Soils were kept at field moisture and under aerobic conditions. We measured heterotrophic respiration flux during three short (~3 h) incubations prior to sampling CO2 for radiocarbon analysis in order to calculate a flux-weighted radiocarbon average from young and old soil (see below). For Δ14C sample collection, we scrubbed CO2 from the jar headspace, incubated the soils for 12 to 72 h (the time it took for 1.5 mg C to build up in the headspace), and pumped the headspace CO2 into molecular sieves. The Δ14C sample preparation and analysis were carried out as described for Reco.
Partitioning model
For partitioning Reco, heterotrophic respiration isotopes from the soil core depth sections were mathematically combined into two heterotrophic sources, young soil (YS) and old soil (OS), based on the depth of respired enriched ‘bomb peak’ CO2 and, for Abisko, the split from a previous 13C partitioning study (Dorrepaal et al., 2009). The depth of the split was 25 cm for Abisko and 15 cm for Healy. To calculate isotopic values of each core's YS and OS, we weighted the isotopic value of each incubated depth by its CO2 flux per g C (Table S1), corrected for each depth sections' average bulk density and average monthly field temperature using a Q10 of 2.5 (Schuur & Trumbore, 2006; Hicks Pries et al., 2013a). The Q10 was previously determined using a subset (12) of the collected soils (above) that were each split into two subsamples and simultaneously incubated at 2.5 and 12.5 °C over 10 days (see Hicks Pries et al., 2013a). Young soil and OS isotopic values were averaged separately across cores to obtain a range of heterotrophic source values. For the autotrophic source at Abisko, above- and belowground Δ14C were averaged together because they did not statistically differ (P = 0.34).

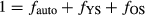
Data analysis
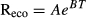
For the comparison of Abisko and Healy respiration, average daily Reco rates for July were used. To compare July old soil C loss, we first multiplied total July Reco flux by the old soil contribution at the plot level for each treatment and site. For the Healy control and short warming treatments, where more radiocarbon and Reco data were available than at Abisko, we present an additional estimate of the July old soil C loss based on a regression model that included the old soil contribution as a function of the Reco flux (Hicks Pries et al., 2015).
To investigate treatment differences after 11 years at Abisko, we performed analyses of variance (anova's) in R 3.1.0 (R Development Core Team, 2014) for soil temperature and thaw depth, radiocarbon values, and Reco source contributions. The main effects were the summer and winter warming treatments with a summer by winter warming interaction term. Block was a random effect. For repeated measures (Reco flux), plot was an additional random effect nested within block. To investigate responses across space and time, there were two main effects—duration of warming (control, short term, and long term) and site (Abisko and Healy)—and an interaction term. Data throughout the growing season at Healy were previously published (Hicks Pries et al., 2013a, 2015). To better compare with the Abisko data, only Healy data from the height of the growing season (mid July through early August) were used. Source contributions were logit transformed before analyses, and variances varied by location or treatment when necessary. All residuals were visually checked for normality and homogeneity of variances to ensure the assumptions of anova were met.
Results
Respiration responses after 11 years of warming at Abisko
Average annual soil temperatures were significantly warmer in the treatments with summertime OTCs (summer and annual warming) than in control or winter warming only treatments (Table 2, P < 0.001, n = 3). This difference in annual temperatures was greatest at 20 cm although there was not a significant interaction with depth. Neither summer nor winter warming affected soil temperature during the sampling period in July 2011 (Table 2, P > 0.1, n = 5), although thaw depth was marginally deeper in the treatments with summertime OTCs (Table 2, P = 0.08, n = 5). Annual warming caused significant 16% and 12% decreases in gravimetric water content compared to the control at 0–5 and 5–15 cm soil depths, respectively (Table S2, P = 0.01, n = 5).
| Depth | AmbientA | WinterA | SummerB | AnnualB | |
|---|---|---|---|---|---|
| Annual soil T (°C) | 5 cma | 0.9 ± 0.09 | 1.9 ± 0.10 | 1.5 ± 0.24 | 2.6 ± 0.31 |
| 10 cma | 1.4 ± 0.05 | 2.1 ± 0.24 | 2.0 ± 0.31 | 2.5 ± 0.17 | |
| 20 cmb | 0.7 ± 0.06 | 0.7 ± 0.20 | 1.2 ± 0.46 | 1.4 ± 0.18 | |
| July soil T (°C) | 5 cma | 12.1 ± 0.23 | 11.8 ± 0.17 | 12.2 ± 0.46 | 12.3 ± 0.30 |
| 10 cmb | 10.1 ± 0.55 | 10.1 ± 0.18 | 10.7 ± 0.80 | 10.3 ± 0.44 | |
| 20 cmc | 7.6 ± 0.78 | 6.2 ± 0.64 | 6.8 ± 1.6 | 8.1 ± 0.97 | |
| July thaw depth (cm) | 47 ± 5.8 | 44 ± 1.7 | 53 ± 5.2+ | 53 ± 5.1+ |
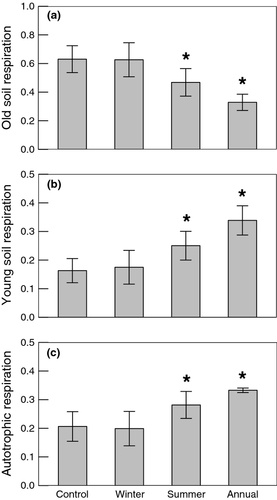
During the 2-week measurement period, summer warming enhanced Reco, but the effect was not significant (Fig. 1a, P = 0.3). Radiocarbon values were used to estimate the proportional contributions of each source to Reco. Ecosystem respiration radiocarbon values were significantly more enriched in treatments with summer warming, compared to control and winter warming only treatments, which had 14C values <20‰ (Fig. 1b, P = 0.035). These Reco values were less enriched than the average values of both young soil (62.4‰) and autotrophic (46.3‰) respiration, but more enriched than old soil (2.4‰) respiration (Table 3).

| Source | Δ14C |
|---|---|
| Autotrophs | 46.3 ± 6.3 |
| Young soil (0–25 cm) | 62.4 ± 10 |
| Old soil (25–50 cm) | 2.4 ± 15 |
The proportional contributions of respiration sources differed between treatments with summer warming and treatments without summer warming (Fig. 2). Summer warming significantly decreased the proportion of Reco from old soil respiration (P = 0.029) and significantly increased proportions from young soil and autotrophic respiration (P = 0.051) relative to treatments without summer warming. As a result, treatments with summer warming lost marginally less C from old soil than the control and winter warming only treatments (Fig. 1c, P = 0.09).
Comparing respiration responses across space and time
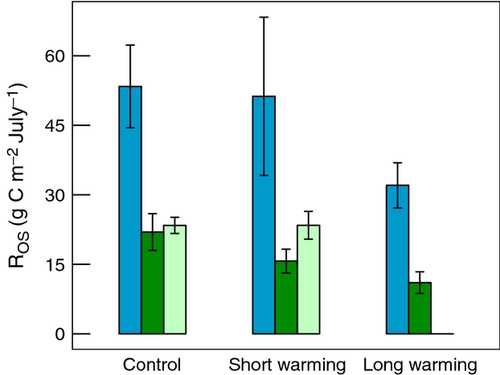
The permafrost ecosystems at Abisko, Sweden and Healy, Alaska, differed significantly in their Reco rates and partitioning of Reco (Fig. 3). During July, Abisko had an average respiration rate of 3.3 g C m−2 d−1, which was greater than Healy's 2.6 g C m−2 d−1 average (P = 0.051). A greater proportion of respiration at Abisko was coming from old soil than at Healy (0.44 as opposed to 0.22 on average, P = 0.0017), and a smaller proportion was coming from autotrophs (0.29 as opposed to 0.52 on average, P = 0.016). The largest difference was in the ratio of autotrophic to heterotrophic respiration, which was only 0.42 at Abisko compared to 1.22 on average at Healy (P = 0.011).
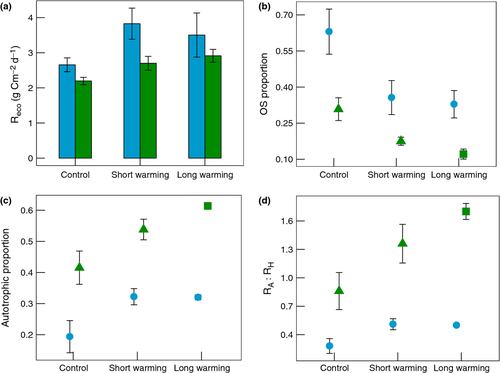
Despite these site-level differences, Abisko, Sweden and Healy, Alaska had broadly similar responses to warming (Fig. 3). Warming increased the daily Reco rate in July, but only significantly in the short term (P = 0.025), a trend driven by the lack of significant differences with long-term warming at Abisko (above). The proportion of respiration from old soil decreased and the proportion from autotrophs increased significantly with warming (P = 0.040 and P = 0.034, respectively). The ratio of autotrophic to heterotrophic respiration significantly increased with warming at both sites (P = 0.027). The responses appeared stronger with long-term thaw at Healy, compared to long-term warming at Abisko. However, short- and long-term warming did not differ significantly for any of the response variables except for the ratio at Healy (significant warming duration by site interaction, P = 0.012, all other interaction P-values >0.34).
The loss of old soil C during July was significantly greater in Abisko than in Healy (P < 0.0001). July old soil C loss decreased with duration of warming (P = 0.051) at both Abisko and Healy, based on the respiration multiplied by proportion estimate (Fig. 4). A Tukey post hoc test revealed that short-term warming had similar old soil C losses to the control (P = 0.69) but that long-term warming had smaller old C losses than the control (P = 0.048). Also, the regression-based estimate of July old soil C loss in the Healy control and short-term warming treatments indicated old soil C losses did not differ from the control in the short term (Fig. 4, P = 0.99).
Discussion
After 11 years of warming at the Abisko peatland, summer warming increased respiration rates due mainly to increased autotrophic contributions. However, C losses from old soil did not increase with long-term warming. Both the Abisko peatland and Healy tussock tundra responded similarly to warming with increased respiration rates, increased autotrophic contributions to Reco, and increased ratios of autotrophic to heterotrophic respiration. These responses were consistent with both short- and long-term warming.
Respiration responses after 11 years of warming at Abisko
Our first objective was to investigate respiration responses in a peatland subjected to 11 years of experimental warming during different seasons. In Abisko, during the height of the growing season, there was a nonsignificant increase in Reco in treatments with 11 years of summer warming. This result contrasts the doubling of respiration that occurred during the first 7 years of warming in this same peatland despite a similar 16% reduction in soil moisture (Dorrepaal et al., 2009) and contrasts results of other, but shorter term, warming experiments in arctic and subarctic systems (e.g., Hobbie & Chapin, 1998; Oberbauer et al., 2007; Sharp et al., 2013; Natali et al., 2014). The lack of large differences in Reco after 11 years may be due in part to similar soil temperatures among treatments during the 2-week measuring period in July 2011. Furthermore, the OTC's may not have been able to effectively raise air temperatures during this particularly warm period. However, while total Reco in July 2011 was not significantly affected by 11 years of warming, the underlying fluxes contributing to Reco were affected, indicating a shift in this peatland's C cycle.
Ecosystem respiration radiocarbon values in the treatments with summer warming increased after 11 years of warming at Abisko indicating that more C was being respired from isotopically enriched sources. Permafrost thaw has previously been shown to incre-ase Reco radiocarbon values due to increased contri-butions from a 14C enriched soil source in tundra (Nowinski et al., 2010). At other tundra sites, increased temperatures and thaw in permafrost ecosystems have caused decreases in Reco radiocarbon values due to increased contributions from old soil depleted in 14C (Schuur et al., 2009; Hicks Pries et al., 2013a, 2015). In contrast to these studies, summer warming at Abisko increased the proportion of respiration coming from the relatively enriched autotrophic and young soil sources while decreasing the proportion of respiration coming from old soil.
As a result of smaller old soil contributions in the treatments with summer warming and the lack of large Reco differences among treatments in July, the summer-warmed treatments lost marginally less old soil C at the height of the growing season than the control plots. In previous short-term studies, respiration from old soil increased with warming—up to 73% over control treatments (Hardie et al., 2009; Hicks Pries et al., 2013b, 2015; Lupascu et al., 2014). Previous studies at Abisko showed that deep (25–50 cm) soil contributed 69% to the aforementioned increase in Reco after 1 and 7 years of warming (Dorrepaal et al., 2009). The slight decrease in old soil respiration after 11 years of warming at this subarctic peat bog contradicts the prediction that permafrost thaw will cause large C losses (Schuur et al., 2013). However, these Abisko data represent only a snapshot at the height of the growing season and during a time at which there were no significant temperature differences among treatments. Old soil C contributions can increase as thaw deepens toward the end of the growing season (Hicks Pries et al., 2013a) and may increase during the winter because warming increases wintertime Reco, although wintertime source contributions are currently unknown (Natali et al., 2014). Thus, a more temporally resolved dataset would have allowed for a more robust estimate of old soil C losses at the Abisko site (Hicks Pries et al., 2013a, 2015).
Ecosystem respiration and its components did not respond to winter warming in this Abisko peatland. This result is opposite that of the CiPEHR warming experiment in Healy, Alaska, where the winter warming treatment increased Reco and autotrophic contributions to Reco, but summer warming with OTCs did not (Natali et al., 2014; Hicks Pries et al., 2015). The magnitude of winter warming differed among the two studies. In Abisko, the OTCs roughly doubled the winter snowpack to about 25 cm (see 2; Dorrepaal et al., 2004) while in Healy, the snow fences quadrupled the snowpack to over 1 m (Natali et al., 2014). The deeper snow pack allowed for greater insulation in Healy, thus keeping the soils warmer throughout the winter and leading to 10% deeper thaw depths during the growing season (Natali et al., 2014). In Abisko, treatments with summer warming had warmer soils annually and deeper July thaw much like the winter warming treatment in Healy. This similarity is likely due to an indirect effect of summer warming. The summertime OTCs only warmed the air (with little associated soil warming in July 2011) but had a large effect on Sphagnum growth, leading to a thicker moss layer (Dorrepaal et al., 2004; Keuper et al., 2011). This thicker moss layer can act as an insulator when dry (Soudzilovskaia et al., 2013) and if dry, may be a more effective insulator during winter than the doubled snowpack in the wintertime OTCs. In Abisko, Reco and its components are thus not only responding to warmer growing season air temperatures, but also to generally warmer soils year-round. Taking the two sites together, it thus seems that Reco has a stronger response to warmer soils than to warmer air temperatures.
Comparing respiration responses across space and time
Our second objective was to compare the respiration responses of the Abisko peatland after 11 and 5 years of warming to the short- and long-term responses of a different C-rich permafrost ecosystem in Healy, Alaska. One main difference between these sites is their vegetation biomass and composition. The moist acidic tundra in Healy has almost two times more aboveground vascular plant biomass per area than the bryophyte-dominated peatland in Abisko (Table 1). These differences in dominant vegetation and biomass were associated with clear differences in the ratios of autotrophic to heterotrophic respiration at the two sites (Fig. 3d). Abisko's Reco is mainly derived from heterotrophs, while at Healy, plants are responsible for more than half of the respiration. At Healy, belowground autotrophic respiration contributed to 25% of Reco during July (Hicks Pries et al., 2013a). At Abisko, smaller relative autotrophic contributions to Reco are likely because less vascular plant biomass means smaller contributions from root respiration. Heterotrophic dominance is a common characteristic of peatlands, which have the greatest contributions of heterotrophic respiration to soil efflux (over 80%) out of all ecosystem types globally (Subke et al., 2006).
The differences in dominant vegetation between the sites could influence their Reco responses to warming in other ways. The dominance of Sphagnum at Abisko leads to very high soil C:N ratios, as much as 80 at the surface and 90 at depth (RSPvL, unpublished data); whereas the C:N ratio in Healy is about 50 at the surface and 25 at depth (Hicks Pries et al., 2012). A recent meta-analysis showed permafrost soils with higher C:N ratios were more vulnerable to decomposition (Schädel et al., 2014). Furthermore, moss-derived organic matter is a low quality, inherently recalcitrant substrate as evidenced by its slow decomposition compared to vascular plant litter (Hobbie, 1996; Dorrepaal et al., 2005; Hicks Pries et al., 2013b). While moss-derived organic matter may be less easily decomposed by microbes, it is also potentially more responsive to warming based on kinetic theory (Davidson et al., 2012). However, despite these differences in vegetation and soil organic matter, which led to different respiration rates and different contributions of autotrophs to Reco, both Healy and Abisko had broadly similar responses to warming.
Warming in both locations strongly increased the proportion of autotrophic respiration in the short and long term (Fig. 3c). Increases in autotrophic respiration are likely due to both increased respiration associated with primary production and increased maintenance respiration as a result of more biomass. Warming and thaw have been shown to increase plant productivity around the Arctic (Elmendorf et al., 2012). The autotrophic respiration response was similar even though different plant functional types responded to warming at each site. At Healy, graminoid productivity increased due to warming in the short term (Natali et al., 2012), but shrub and moss productivity increased after decades of thaw (Schuur et al., 2007). At Abisko, Sphagnum fuscum growth increased in both the short and long term (Dorrepaal et al., 2004; Keuper et al., 2011). Positive plant responses to warming were also sustained in other long-term, high-latitude warming studies (Lamb et al., 2011; Sistla et al., 2013). Increased production, and thus autotrophic respiration, are likely due in part to increased nitrogen availability, the limiting nutrient of many subarctic ecosystems (Chapin et al., 1995; Shaver et al., 1998; Mack et al., 2004). At Healy, warming and thaw increased the amount of nitrogen in the plant canopy both in the short and long term (Schuur et al., 2007; Natali et al., 2012). At Abisko, summer warming increased fluxes of both organic nitrogen and ammonia in the surface soil (Weedon et al., 2012).
Healy and Abisko also shared the consistent trend that autotrophic respiration responds more strongly to warming and permafrost thaw than heterotrophic respiration, resulting in increased autotrophic to heterotrophic respiration ratios during the growing season. If the increase in autotrophic respiration in Abisko reflects greater net primary productivity, as is the case in Healy (Vogel et al., 2009; Trucco et al., 2012; Natali et al., 2014), and if DOC losses are minimal, the increase in the ratio at both sites suggests growing season heterotrophic losses are compensated for by increased plant growth, preventing these warming permafrost ecosystems from becoming net growing season C sources for the time being. The increase in this ratio indicates there has been a large shift in the C fluxes that drive each ecosystem's C balance as a result of warming.
The response trajectories of the source contributions, of the autotrophic to heterotrophic ratios, and of Reco rates were similar at the height of the growing season for both sites (Fig. 3a–d). Decadal warming sustained the same magnitude of response as short-term warming. Thus, the respiration components that were compared here seem to remain sensitive to warming in the long term and have not acclimated to warming in contrast to many laboratory studies (e.g., Crowther & Bradford, 2013).
At both sites, the old soil C as a proportion of Reco decreased, due to concurrent increases of both autotrophic and young soil respiration. Combining the Reco and old soil contribution data, our results show there is no detectable change in July old soil C loss due to both short- and long-term warming. This unresponsiveness occurs despite the large amounts of soil C that are potentially mineralizable based on short-term laboratory incubations (Weintraub & Schimel, 2005; Shaver et al., 2006; Elberling et al., 2013; Schädel et al., 2014) and despite increased nitrogen (Natali et al., 2012; Weedon et al., 2012), which can cause large soil C losses (Mack et al., 2004). Corroborating evidence showing soil C is not responsive to warming is scant (Lamb et al., 2011; Sistla et al., 2013). Furthermore, old soil C loss could be overwhelmed by the plant response during the growing season and may have increased during other seasons we did not measure. Although we caution against making definitive conclusions, this lack of a detectable old soil C response over time merits more investigations of long-term warming to predict the permafrost C climate change feedback.
Despite differences in plant community, vascular biomass, and climate, long- and short-term responses to warming were similar in the peatland and tussock tundra ecosystems. These similar responses occurred despite contrasting soil moisture responses to warming: Abisko became drier and Healy became wetter (Table S2). Warming causes a shift in the C cycle of these two permafrost ecosystems underlain by thick, organic soils by increasing autotrophic respiration more than heterotrophic respiration. These autotrophic responses to warming show no signs of abating after a decade, suggesting that arctic greening may be ongoing in these ecosystems. The evidence we have indicates the long-term response of old soil C to warming may not be as strong as previously predicted during the growing season. Thus, old soil C losses, which have the potential to cause large positive climate change feedbacks, should be investigated in situ in more long-term studies and across seasons.
Acknowledgements
This research was funded by a National Science Foundation Doctoral Dissertation Improvement Grant, Stiftelsen Oscar och Lili Lamms Minne, the Knut and Alice Wallenberg Foundation, and the University of Florida Graduate Research Abroad Program.




