A pan-Arctic synthesis of CH4 and CO2 production from anoxic soil incubations
Abstract
Permafrost thaw can alter the soil environment through changes in soil moisture, frequently resulting in soil saturation, a shift to anaerobic decomposition, and changes in the plant community. These changes, along with thawing of previously frozen organic material, can alter the form and magnitude of greenhouse gas production from permafrost ecosystems. We synthesized existing methane (CH4) and carbon dioxide (CO2) production measurements from anaerobic incubations of boreal and tundra soils from the geographic permafrost region to evaluate large-scale controls of anaerobic CO2 and CH4 production and compare the relative importance of landscape-level factors (e.g., vegetation type and landscape position), soil properties (e.g., pH, depth, and soil type), and soil environmental conditions (e.g., temperature and relative water table position). We found fivefold higher maximum CH4 production per gram soil carbon from organic soils than mineral soils. Maximum CH4 production from soils in the active layer (ground that thaws and refreezes annually) was nearly four times that of permafrost per gram soil carbon, and CH4 production per gram soil carbon was two times greater from sites without permafrost than sites with permafrost. Maximum CH4 and median anaerobic CO2 production decreased with depth, while CO2:CH4 production increased with depth. Maximum CH4 production was highest in soils with herbaceous vegetation and soils that were either consistently or periodically inundated. This synthesis identifies the need to consider biome, landscape position, and vascular/moss vegetation types when modeling CH4 production in permafrost ecosystems and suggests the need for longer-term anaerobic incubations to fully capture CH4 dynamics. Our results demonstrate that as climate warms in arctic and boreal regions, rates of anaerobic CO2 and CH4 production will increase, not only as a result of increased temperature, but also from shifts in vegetation and increased ground saturation that will accompany permafrost thaw.
Introduction
The northern permafrost region contains ~1300 Pg soil carbon (C) (Tarnocai et al., 2009; Hugelius et al., 2014), some of which has been stored in permafrost (ground that remains at or below 0° C for ≥2 years) for millennia. Climate warming in northern high latitudes will reduce the areal extent of permafrost (Zhang et al., 2008; Koven et al., 2012; Lawrence et al., 2012), making the large pool of permafrost C vulnerable to mineralization following thaw (Dutta et al., 2006; Schuur et al., 2009, 2013). Permafrost thawing in arctic and boreal regions has brought about changes in ecosystem C balance, often resulting in a net loss of C to the atmosphere (Johansson et al., 2006; O'Donnell et al., 2012; Natali et al., 2014). The magnitude, timing, and form of C release as a result of permafrost thaw will depend not only on changes in air and soil temperature, but also on associated landscape-level changes that can affect soil redox state, organic matter quality, environmental conditions, and plant and microbial community dynamics. While carbon dioxide (CO2) is the primary decomposition product in oxic soils, when soils are saturated, anaerobic decomposition results in the production of both methane (CH4) and CO2; the proportion of these anaerobic products is dependent upon methanogenic pathways that generate both CH4 and CO2 and other anaerobic pathways that generate CO2 (e.g., denitrification, sulfate reduction). Quantifying the relative amount of C released as CO2 or CH4 is essential for determining biological feedbacks from permafrost ecosystems to climate change because CH4 has a 28–34 times larger global warming potential (GWP) on the 100-year time horizon (Myhre et al., 2013).
Once permafrost thaws, the fate of soil C is determined in large part by soil moisture (e.g., Elberling et al., 2013), which can change abruptly following thaw due to collapse of surface soils, resulting in saturated conditions (Halsey et al., 1995; Jorgenson et al., 2001; Payette et al., 2004; Jorgenson & Osterkamp, 2005). Areas of permafrost thaw frequently become hotspots of CH4 flux to the atmosphere due to anaerobic decomposition (Christensen et al., 2004; Johansson et al., 2006; Walter et al., 2006; Wickland et al., 2006; Myers-Smith et al., 2007; Turetsky et al., 2007; Olefeldt et al., 2013). However, in some areas, permafrost thaw may result in soil drying, where CH4 emissions are reduced (Olefeldt et al., 2013), or in soils that can fluctuate between saturated and dry conditions. Fluctuations in soil saturation can inhibit CH4 production as a result of the oxidation and regeneration of alternate electron acceptors, or the depletion of substrate for methanogenesis due to consumption in more kinetically favorably reactions (Schlesinger, 1997; Knorr & Blodau, 2009).
In addition to changes in soil moisture, permafrost thaw can also impact the amount and composition of organic matter inputs to the anoxic zone, thereby altering substrate availability for fermentation and methanogenesis. Organic matter composition is driven by plant community composition and the form of organic matter inputs (e.g., roots vs. leaf litter) (Bergman et al., 1998; Kuder & Kruge, 2001; Hines et al., 2008; Nilsson & Oquist, 2009; Hodgkins et al., 2014). In permafrost regions, in addition to newly assimilated C from vegetation, there also can be inputs of organic material from thawing permafrost (Dutta et al., 2006; Waldrop et al., 2010; Lee et al., 2012; Schädel et al., 2014), which may be partly responsible for high rates of CH4 emissions observed following permafrost thaw (Olefeldt et al., 2013). Ultimately, microbial communities control both the production of substrates for methanogenesis from fermentation (e.g., acetate, hydrogen, formate, and possibly ethanol) and rates of methanogenesis itself, both of which have low energy yields and slow rates (c.f. Conrad, 1999) that can in turn limit CH4 production (Chanton et al., 1995; Bergman et al., 1998; Duddleston et al., 2002; Hines et al., 2008; McCalley et al., 2014).
Field and laboratory studies have also highlighted the importance of environmental conditions such as temperature, pH, anoxia, and redox potential on anaerobic decomposition. Methane production and anaerobic CO2 production positively relate to temperature in incubations of organic soils from the permafrost zone (Svensson, 1984; Yavitt et al., 2006; Lupascu et al., 2012; Treat et al., 2014). Methane production is also dependent on pH (Svensson, 1984; Valentine et al., 1994; Bergman et al., 1998), and while the exact mechanism is unclear, it is likely that fermentation products such as organic acids lower pH and inhibit methanogen growth. The pH optima of methanogens vary from acidic to neutral (Williams & Crawford, 1985; Dunfield et al., 1993).
A synthesis of CH4 emissions from the permafrost region identified plant community composition, water table depth, soil moisture, and soil temperature as key drivers of CH4 emissions (Olefeldt et al., 2013). These net CH4 emissions reflect CH4 production, transport, and oxidation processes. In this study, we examined how environmental and ecological drivers affect CH4 and anaerobic CO2 production through a synthesis of anaerobic incubation studies of soils from the permafrost region (Table 1, Fig. 1). We hypothesized that CH4 and CO2 production from permafrost region soils could be predicted by environmental controls (e.g., incubation temperature, relative water table position), soil characteristics (e.g., soil type, depth, active layer/permafrost layer, pH, and C:N ratio), and site characteristics (e.g., mean annual temperature, mean annual precipitation, biome, landscape position, vegetation type, and permafrost presence/absence). While CH4 and anaerobic CO2 production rates from incubations will not necessarily reflect field fluxes due to the concurrent processes of CH4 oxidation, alternative transport pathways, and plant–soil interactions, laboratory incubations allow the examination of soil properties and environmental conditions that control CH4 and CO2 production rates. We synthesized CH4 and anaerobic CO2 production rates across 20 independent studies from northern latitude sites in Alaska, Canada, Sweden, and Siberia to quantify effects of known controls on anaerobic processes that operate throughout the permafrost zone. Finally, we identify and discuss promising areas for future research.
| Author | Latitude | Longitude | Country | Permafrost zone | MAAT (°C)a | Biome | Landscape position | Soil type | pH | Depths (cm)b | Incubation temp. (°C) | Days measured | CO2c |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bellisario et al. (1999) | 55.67°N | 97.87°W | Canada | Discontinuous | −3.1 | Boreal | Wetland | Organic | 4.0–6.8 | 0–40 (10) | 21 | 5 | |
| Blazewicz et al. (2012) | 64.80°N | 147.90°W | USA | Discontinuous | −2.8 | Boreal | Wetland | Organic | 5.1 | 0–15 | 25 | 14–71 | |
| CapekU1 | 72.49°N | 101.65°E | Russia | Continuous | −13.4 | Tundra | Upland | Organic | 6.2 | 5–20 | 4, 12, 20 | 16–139 | x |
| ErnakovichU2 | 69.40°N | 148.70°W | USA | Continuous | −13.5 | Tundra | Upland | Mineral | 5.4–6.3 | 0–25 | 1, 15 | 1–91 | x |
| Ganzert et al. (2007) | 72.37°N | 126.47°E | Russia | Continuous | −14.7 | Tundra | Flood plain Wetland | Organic, mineral | No data | 0–52 (2–9) | 5, 18 | 7 | |
| 73.60°N | 117.33°E | Russia | Continuous | −14.7 | Tundra | Wetland | Organic, mineral | No data | 0–45 (5) | 5, 18 | 7 | ||
| 0–44 (5–8) | |||||||||||||
| Holland (1992) | 58.64°N | 93.82°W | Canada | Discontinuous | −7.6 | Boreal | Wetland | Organic | 7.0–7.8 | 0–25 | 15 | 5 | |
| 58.68°N | 93.84°W | Discontinuous | −7.6 | Boreal | Wetland | Organic | 7.0–7.8 | 0–25 | 15 | 5 | |||
| 58.66°N | 93.83°W | Discontinuous | −7.6 | Boreal | Wetland | Organic | 7.0–7.8 | 0–25 | 15 | 5 | |||
| 58.77°N | 93.86°W | Discontinuous | −7.6 | Boreal | Wetland | Organic | 7.0–7.8 | 0–25 | 15 | 5 | |||
| IversenU3 | 71.28°N | 156.61°W | USA | Continuous | −12.3 | Tundra | Wetland | Organic mineral | 4.3–5.8 | 0–27 (2–5) | 2, 12 | 1–28 | x |
| Kane et al. (2013) | 64.82°N | 147.87°W | USA | Discontinuous | −2.8 | Boreal | Wetland | Organic | 5.3 | 5–25 | 22 | 2–38 | x |
| Knoblauch et al. (2013) | 72.33°N | 126.28°E | Russia | Continuous | −14.7 | Tundra | Upland | Mineral | 4.0–8.1 | 58–2000d | 4 | 16–1248 | x |
| 72.37°N | 126.48°E | Continuous | −14.8 | ||||||||||
| Lee et al. (2012) | 63.88°N | 149.25°W | USA | Discontinuous | −4.1 | Boreal | Upland | Organic Mineral | 3.6–4.8 | 5–15 | 15 | 7–493 | x |
| 64.85°N | 149.72°W | USA | Discontinuous | −4.0 | Boreal | Upland | Mineral | 5.1 | 65–80 | 15 | 7–493 | x | |
| 68.63°N | 149.72°W | USA | Continuous | −11.4 | Tundra | Upland | Mineral | 7.2 | 1000 | 15 | 7–493 | x | |
| 68.80°N | 161.38°E | Russia | Continuous | −12.6 | Tundra | Upland | Mineral | 6.6 | 42–57, 100 | 15 | 7–493 | x | |
| No data | 1000 | ||||||||||||
| Lipson et al. (2012) | 71.32°N | 156.62°W | USA | Continuous | −12.3 | Tundra | Drained lake | Mineral | 4.6–5.2 | 0–15 | 4 | 1 | x |
| Lupascu et al. (2012) | 68.35°N | 18.82°E | Sweden | Discontinuous | −0.1 | Tundra | Wetland | Organic | 3.8–6.0 | 2–47d | 4, 14, 24 | 1 | x |
| Moore et al. (1994) | 51.18°N | 80.38°W | Canada | Discontinuous | −1 | Boreal | Wetland | Organic | No data | 0–10, 15–20 | 15 | 5 | |
| 51.28°N | 80.37°W | Discontinuous | −1 | Boreal | Wetland | Organic | 5.5 | 0–10, 15–20 | 15 | 5 | |||
| 51.29°N | 80.28°W | Discontinuous | −1 | Boreal | Wetland | Organic | 5.7 | 0–10, 15–20 | 15 | 5 | |||
| 51.31°N | 80.27°W | Discontinuous | −1 | Boreal | Wetland | Organic | 5.2 | 0–10, 15–20 | 15 | 5 | |||
| 51.50°N | 81.02°W | Discontinuous | −1 | Boreal | Wetland | Organic | 3.2 | 0–10, 15–20 | 15 | 5 | |||
| Svensson (1984) | 68.37°N | 19.05°E | Sweden | Discontinuous | −0.7 | Tundra | Wetland | Organic | 4.2–4.9 | 10–15 | 2, 5,10, 12, 15, 20, 24, 28, 37 | 30, 75 | |
| Treat et al. (2014) | 63.57°N | 157.73°W | USA | Discontinuous | −3.6 | Boreal | Lowland | Organic | 3.8–4.9 | 35–45, 90–100 | −0.5, 20 | 3–27 | x |
| 64.88°N | 147.78°W | USA | Discontinuous | −2.9 | Boreal | Lowland | Organic | 5.5 | 29–39, 83–92 | −0.5, 20 | 3–27 | x | |
| 68.61°N | 149.20°W | USA | Continuous | −11.5 | Tundra | Flood plain | Organic | 5.1–5.3 | 30–40, 90–100 | −0.5, 20 | 3–27 | x | |
| Waldrop et al. (2010) | 64.87°N | 147.86°W | USA | Discontinuous | −2.8 | Boreal | Upland | Organic | No data | 35–50, 85–100 | 5 | 30 | |
| 65.67°N | 149.08°W | USA | Discontinuous | −4.9 | Boreal | Lowland | Organic | No data | 35–50, 65–80 | 5 | 30 | ||
| 67.20°N | 150.27°W | USA | Continuous | −7.8 | Boreal | Upland | Mineral | No data | 35–50, 65–80 | 5 | 30 | ||
| Waldrop U4 | 64.69°N | 148.32°W | USA | Discontinuous | −2.8 | Boreal | Lowland | Mineral | No data | 26–41, 81–96 | 5, 23 | 0–378 | x |
| 64.70°N | 148.32°W | USA | Discontinuous | −2.8 | Boreal | Wetland | Organic | No data | 5–25, 20–35, 33–48, 40–60 | 5, 23 | 0–378 | x | |
| 65.35°N | 146.92°W | USA | Discontinuous | No data | Boreal | Lowland | Mineral | 4.7, 5.7 | 10–20 | 5, 23 | 0–378 | x | |
| 50–60, 90–100 | 5, 23 | 0–378 | x | ||||||||||
| Whalen & Reeburgh (2000) | 64.88°N | 147.50°W | USA | Discontinuous | −3.3 | Boreal | Wetland | Organic | 4.0 | 0–40 (5) | 1–12 | 2.5 | |
| Yavitt et al. (2006) | 55.85°N | 107.68°W | Canada | Discontinuous | −0.6 | Boreal | Wetland | Organic | 3.6–4.0 | 24–95d | 10, 22, 25 | 7, 54 | x |
| Zona et al. (2012) | 71.29 | 156.60°W | USA | Continuous | −12.3 | Tundra | Drained lake | Organic mineral | No data | 0–15 | 4 | 1–56 | x |
| No data | 15–30 |
- a Mean annual air temperature (MAAT) from WorldClim database (Hijmans et al., 2005).
- b Depth ranges used in incubation; parentheses indicate depth increments used if consecutive depths were incubated. CO2 and CH4 measurements were made for samples bulked over the sampling increment or depth.
- c Studies that also measured anaerobic CO2 production noted with ‘x’.
- d Multiple or irregular sampling depths were incubated; please consult reference for further information.
- Unpublished anaerobic incubation studies:U1Capek, C., H. Santruckova, K. Diakova, J.-E. Dickopp, J. Barta, B. Wild, J. Schnecker, G. Guggenberg, N. Gentsch, G. Hugelius, P. Kuhry, N. Lashchinsky, A. Gittel, C. Schleper, R. Mikutta, J. Palmtag, O. Shibistova, T. Urich, S. Zimov, A. Richter: Soil organic matter transformation in cryoturbated horizons of permafrost affected soils (in prep).U2Ernakovich, J. Sagwon Hills, Alaska; Ernakovich et al. (2014)U3Iversen, C.M., V. Sloan & R.J. Norby. Barrow Experimental Observatory, AK.U4Waldrop, M.W. et al. Interior Alaska.
Materials and methods
Data collection and site classification
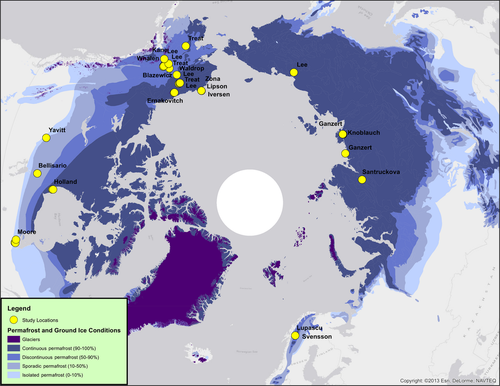
We compiled a database of anaerobic incubations that were conducted on soil samples within the geographic permafrost area (Fig. 1, Table 1; Brown et al. 1998, revised 2001). We identified potential published studies for inclusion in the database using Web of Science with the keywords ‘anaerobic or anoxic or methane or incubation’ and ‘arctic or boreal or permafrost’, and we actively solicited contributions of published and unpublished studies through the NSF-funded Vulnerability of Permafrost Carbon Research Coordination Network as a parallel study to Schädel et al. (2014). Twenty studies (16 published, 4 unpublished) fell within the northern high-latitude permafrost area and met the following criteria: (i) created anaerobic headspace using oxygen-free gas (most commonly N2 or He); (ii) measured CH4 on a per gram soil dry weight or per gram C basis; (iii) reported sample depth and incubation temperature. We included soils with either intact or homogenized soil structure but excluded soil slurries and incubations of lake sediments due to difficulties of comparing methods. These efforts resulted in a dataset of 2270 CH4 production measurements across 54 sites in Alaska (USA), Canada, Sweden, and Siberia (Russia) between 1984 and 2013 (Fig. 1). We extracted CH4 and CO2 production data from published studies using a data extraction program (Plot Digitizer), calculated the molar CO2:CH4 production ratio, and collected ancillary data that were commonly reported across studies.
We extracted information about site type and local conditions from the published studies and unpublished datasets. We classified sites by biome (tundra or boreal) and landscape position (drained lake basin, active floodplain, wetland, lowland, upland) based on site descriptions. The wetland classification included bogs, fens, mires, marshes, swamps, and polygonal tundra, while the lowland classification included low-lying black spruce forests without standing water. We classified dominant vascular vegetation within each landscape position at each site into graminoid, shrub, and tree categories using vegetation descriptions. Moss cover for each site was classified into three categories: no moss, dominance of Sphagnum mosses, or dominance by other mosses (e.g., Polytrichum spp., Drepanocladus spp.), based on site-level vegetation description. We also categorized water table position of each soil sample based on sample depth relative to reported water table depth. The three water table categories were dry (>2.5 cm above the mean water table depth), fluctuating (mean water table fell within 2.5 cm of the sample depth), and inundated (mean water table depth was within the sample depth). We classified soil type according to % soil C; soils with >20% C were classified as organic soil, while soils with <20% C were classified as mineral. Two samples with cryoturbation features were classified as a mix of organic and mineral soil (O/M). We also classified sites using two categories for permafrost: permafrost present/absent and active layer/permafrost layer. Permafrost present/absent included measurements only from active layer soils when permafrost was present, and the active layer/permafrost layer comparison excluded measurements from sites without permafrost. Finally, we extracted mean annual air temperature and mean annual precipitation (1950–2000) from WorldClim database (Hijmans et al., 2005). Vascular vegetation, moss cover, and water table position were not classified for samples from >1 m depth, and these samples were omitted from the vegetation and water table position analyses (N = 18).
Data assimilation and data processing
The anaerobic laboratory incubation studies ranged in incubation length from 1 to 1238 days, incubation temperatures ranged from −0.5 °C to 35 °C, and sample depth ranged from the soil surface to 10 m. There were often multiple C production measurements collected during an incubation for each sample (i.e., a unique site × depth × incubation temperature combination). Instead of using multiple production measurements taken over multiple days in the analysis, maximum CH4, mean CO2 production rates, and mean daily CO2:CH4 production ratios prior to 150 days were used as a single measurement for each sample (see Supplemental Materials), hereafter referred to as the aggregated dataset. We used maximum CH4 production rate from each sample instead of mean production (as we did for CO2) because different environmental conditions and potential differences in microbial communities resulted in varying lag times for CH4 production that, in part, drove mean CH4 differences. Similarly, lag time effects precluded us from obtaining relevant cumulative CH4 production rates. We excluded daily production measurements that showed CH4 uptake (consumption) from our calculations of the mean daily CO2:CH4 production. After finding mean and maximum CH4 and CO2 production rates for each sample, we identified 44 samples in the aggregated dataset that showed no CH4 production over the course of the incubation; all of these samples were removed from our study either because the incubation time (<7 days) was too short (33 samples) to recover from disturbance to the microbial communities due to sampling, transport, and handling (Nilsson & Oquist, 2009) or due to insufficient replication to validate the zero measurement (11 samples). This resulted in 303 measurements of CH4 production and 219 anaerobic CO2 production measurements in the aggregated dataset.
The dataset was highly unbalanced with regard to temperature, a known control on CH4 and CO2 production rates. We did not standardize measurements for incubation temperature using a Q10 derived from the full dataset because of the high variability associated with the calculation; Q10 derived from individual studies with multiple incubation temperatures (Table 1) ranged from 0.96 to 3.10 for CH4 production (median: 1.16) and from 0.67 to 4.10 for anaerobic CO2 production (median: 1.39). Instead, we included temperature as a covariate in our statistical analysis.
To directly compare the potential radiative forcing of CH4 and CO2 production, we calculated the CO2 equivalent (CO2e) of CH4 production using a GWP of 28 kg CO2 equivalents kg−1 CH4 over a 100-year time horizon (Myhre et al., 2013). While some of the CH4 produced under field conditions is oxidized before reaching the atmosphere (and thus has a GWP of 0), the calculation of CO2e allows us to compare the warming potential of these two greenhouse gases.
We estimated % C and bulk density values using an empirical relationship and calculated CO2 and CH4 production per gram soil C. Percent C was not reported for ~ 30% of measurements, and bulk density was not reported for ~ 70% of measurements. We estimated missing % C and bulk density values using an empirical relationship between depth, soil type, and % C or bulk density (Table S3, Fig. S5). We calculated missing CH4 or CO2 production per gram C from the reported C production per gram dry weight to investigate the relative differences in C quality among samples. We use the term, C quality, to refer to decomposability as determined from laboratory incubations, as has been performed in a recent synthesis of aerobic soil incubations (Schädel et al., 2014). While we mainly present results on a per gram C basis, results per gram dry weight followed similar trends. Carbon production rates on an areal basis were calculated to a 1 m depth using maximum CH4 and mean CO2 production per gram dry weight soil from the aggregated dataset and measured or estimated bulk density (see Supplemental Methods).
Lag analysis of CH4 production
To understand dynamics of CH4 production over time, we analyzed the lag time from the start of the incubation to maximum rates of CH4 production, hereafter referred to as lag time. Lag time integrates both redox potential and microbial community dynamics within the soils, as maximum rates of CH4 production are achieved under conditions when alternate electron acceptors are depleted and methanogen communities are established (Knorr & Blodau, 2009; Nilsson & Oquist, 2009). Microbial communities, and thus lag times, can be affected by disturbance when sampling, handling, and processing during the experimental setup. While the level of disturbance may vary across studies as a result of methodological differences, we do not expect systematic differences across sample or site types, which is the focus of our analyses. Lag time was calculated using a subset of the data with three or more CH4 production measurements during the incubation experiment (n = 120; Fig. S3).
K-value decay rate calculation
In addition to quantifying lag times, we calculated the exponential decay rate (k) of CH4 production following maximum rates of CH4 production using the same dataset. The exponential decay rate (k) represents the rate of relative decrease in CH4 production rates from maximum production rates over time to better understand potential changes in CH4 production due to substrate depletion of the labile C pool. We calculated relative decrease in CH4 production following maximum rates using the percentage of daily CH4 production relative to the maximum CH4 production among samples, which had several orders of magnitude of variation in maximum production rates (Fig. S1, Fig. S3). We then fit a log-linear regression to obtain k, the exponential decay rate of CH4 production over time. We tested for interactions between time and incubation temperature, vegetation, sample depth, and relative water table position that would indicate differences in the rate of labile C consumption among groups within those factors. In field conditions, substrate for methanogenesis is generally highly labile C compounds and recent photosynthates (King et al., 2002; Prater et al., 2007). k represents the depletion of labile C pool due to anaerobic metabolism. This approach assumes that CH4 is only derived from the labile C rather than multiple C pools of varying quality, as has previously been performed for CO2 production during incubations (Schädel et al., 2014). However, k provides little information on the absolute size of the labile C pool or about methanogenic substrate shifts from recently fixed plant photosynthates and highly labile C compounds to more recalcitrant compounds.
Temperature sensitivity analysis
We performed a temperature sensitivity analysis to better understand the response of CH4 and CO2 production to temperature for each soil sample. Apparent temperature sensitivity (Q10) was calculated using the equation: Q10 = (R2/R1) 10/(T2 − T1), where T1 and T2 represent two temperatures and R2 and R1 represent C production rates at temperatures T1 and T2. We calculated Q10 using the subset of data with incubations conducted at multiple temperatures as well as the entire dataset using the regression between incubation temperature and log-transformed mean CO2 and maximum CH4 production. We compared differences in apparent temperature sensitivity among groups using one-way anovas. There were insufficient data to evaluate differences in Q10 due to incubation temperature range (Hamdi et al., 2013).
Statistical analyses
We investigated differences in maximum CH4 production, mean CO2 production, CO2:CH4 production, CO2e, and lag times among environmental controls (incubation temperatures, relative water table position, pH), substrate controls (vegetation type, depth, permafrost, soil type), and landscape patterns (biomes and landscape position). We used mixed effects modeling (lmer, R package: lme4, Bates et al., 2014) with maximum likelihood estimation to account for the random effects of site and fixed effects of incubation temperature, pH, and depth. While we could have included these fixed effects as blocking factors in a linear modeling approach, the dataset was highly unbalanced and the mixed modeling approach was preferable. We tested for differences among groups using a chi-squared test against a null model including only fixed and random effects (incubation temperature, pH, and site). The chi-squared test is known to be conservative and agreed with model comparisons using AIC.
We hypothesized that CH4 and CO2 production could be predicted by a combination of environmental controls, soil characteristics, and site characteristics. We used a forward-selection stepwise multiple regression to identify which predictors explained the most variance among rates of maximum CH4 production, mean CO2 production, anaerobic CO2:CH4 production, and lag times based on AIC scores (stepAIC, R package: MASS, Venables & Ripley, 2002). Variables were selected for the model in the order of largest improvement in AIC scores from the null model with only an intercept and calculated the relative importance of the accepted predictors (calc.relimp, R package: Relaimpo, Grömping, 2006). Variables that resulted in higher AIC scores (i.e., poorer model fit) than the null model were rejected. Prior to running the stepwise regression, we tested for correlation among predictor variables and found that all correlations were <0.4. An additional ordination analysis was used for data visualization (Figs S8 and S9).
Data were log-transformed (+1 μg C gC−1 day−1) to meet assumption of normality for all statistical analyses. All statistical analyses were conducted using R (R Development Core Team, 2008). We were unable to use meta-analysis techniques on this dataset because there was no common incubation temperature or length across all incubations (i.e., no ‘control’ treatment; Table 1).
Results
General trends
The median value of the maximum CH4 production rates from incubations of northern permafrost zone soils was 0.6 μg CH4-C g soil−1 day −1 and normalized per gram soil C was 3.2 μg CH4-C gC−1 day −1. Maximum CH4 production per gram soil was positively related to % C in the soil (F1,205 = 36.7, P < 0.001, R2 = 0.15; Fig. S6). On an areal basis, median of the maximum CH4 production rates from laboratory incubations was 0.05 g CH4-C m−2 day −1.
The median rate of anaerobic CO2 production from incubations of northern permafrost zone soils was 18.0 μg CO2-C g soil−1 day−1 and normalized per gram C was 58.3 μg CO2-C gC−1 day−1. As with CH4 production, anaerobic CO2 production per gram soil was positively related to % C in the soil (F1,172 = 36.7, P < 0.001, R2 = 0.18; Fig. S6). On an areal basis, median anaerobic CO2 production from laboratory incubations was 1.5 g CO2-C m−2 day−1. Hereafter, we report all results for anaerobic CO2 and CH4 production as per gram soil C to account for differences in the amount of soil C among samples, unless otherwise noted.
CO2 and CH4 production rates varied among hierarchical landscape units. The CO2:CH4 production ratio was >50 times higher in boreal sites (median = 387) than tundra sites (median = 7; χ2 = 18.5, df = 1, P < 0.001), and while CO2 production was higher in the boreal biome than the tundra biome (153 ± 17 and 109 ± 16 μg CO2-C gC−1 day−1), these differences were not statistically significant (χ2 = 0.45, df = 1, P = 0.50). Anaerobic CO2 production and the CO2:CH4 production ratio differed among landscape positions (χ2 > 14, df = 4, P < 0.01; Table 2). Anaerobic CO2 production was largest from the wetlands while the CO2:CH4 production ratio was largest from the lowland forests and smallest from the wetlands (Table 2). Maximum CH4 production did not differ significantly between boreal and tundra sites (χ2 = 2.36, df = 1, P = 0.12) or among landscape positions (χ2 = 6.6, df = 4, P = 0.16; Table 2). However, the decay rates (k) of CH4 production differed significantly among landscape positions (Table 2).
| Category | Max. CH4 production (μg CH4-C gC−1 day−1) | nCH4 | Mean CO2 production (μg CO2-C gC−1 day−1) | nCO2 | Median CO2:CH4 production | Lag time (days) | Mean incubation temp. (°C) | k (% day−1) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | nlag | ||||||||
| Incubation temperature | ||||||||||
| <10 °C | 3.8 (0.5)a | 114 | 67 (16)a | 83 | 98a | 341 (65) | 28 | 56 | 3.6 | 0.14 (0.02) |
| 10–20 °C | 14.1 (2.6)a | 102 | 146 (27)a | 72 | 59a | 37 (8) | 20 | 54 | 15.1 | 0.10 (0.02) |
| >20 °C | 30.5 (4.5)a | 87 | 172 (17)a | 64 | 100a | 21 (14) | 0 | 10 | 24.4 | 0.09 (0.02) |
| Relative water table position | ||||||||||
| Dry | 2.4 (0.4)a | 48 | 68 (8)a | 45 | 153a | 49 (13)a | 20 | 28 | 12.6 | 0.07 (0.02)a |
| Fluctuating | 13.6 (3.3)a | 56 | 364 (60)a | 31 | 57a | 20 (2)a | 27 | 23 | 12.0 | 0.23 (0.05)a |
| Inundated | 20.3 (2.7)a | 165 | 105 (11)a | 115 | 32a | 11 (2)a | 10 | 31 | 15.6 | 0.11 (0.04)a |
| Depth | ||||||||||
| 0–20 cm | 23.7 (3.5)a | 124 | 181.0 (29.6) | 76 | 18a | 41 (9)a | 28 | 38 | 12.8 | 0.18 (0.04)a |
| 20–100 cm | 9.3 (1.4)a | 161 | 100.6 (10.3) | 125 | 153a | 95 (36)a | 17 | 54 | 14.6 | 0.09 (0.01)a |
| >100 cm | 5.0 (1.2)a | 18 | 42.6 (13.0) | 18 | 1635a | 765 (110)a | 880 | 18 | 5.8 | 0.03 (0.03)a |
| Landscape position | ||||||||||
| Drained Lake | 0.6 (0.3) | 7 | 125 (47)a | 7 | 34a | 19 (9) | 7 | 3 | 4.0 | 1.16 (0.37)a |
| Flood plain | 1.5 (0.5) | 18 | 41.7 (10.6)a | 8 | 353a | 17 (3) | 28 | 8 | 10.7 | 2.33 (1.19)a |
| Lowland | 0.7 (0.3) | 24 | 47.8 (9.5)a | 21 | 2336a | 37 (12) | NA | 21 | 10.8 | 0.06 (0.74)a |
| Upland | 8.2 (3.0) | 42 | 52.1 (9.2)a | 38 | 710a | 513 (83) | 10 | 38 | 8.2 | 0.03 (0.74)a |
| Wetland | 19.5 (2.2) | 212 | 159 (17)a | 81 | 19a | 15 (2) | 43 | 40 | 15.3 | 0.13 (0.74)a |
- a Indicates significant differences (P < 0.05) among groups.
Effects of substrate
Maximum CH4 and mean CO2 production rates per gram soil C were significantly different among mineral and organic soils and across soil depths. Maximum CH4 production was more than five times greater in organic soils than mineral soils (χ2 = 3.6, df = 1, P = 0.06; Table 3), and anaerobic CO2 production was three times greater in organic soils than mineral soils (χ2 = 20.5, df = 1, P < 0.001; Table 3). There was a trend toward lower anaerobic CO2:CH4 in mineral soils (median = 11.6) than in organic soils (median = 19.0; χ2 = 2.3, df = 1, P = 0.13). The decay rate (k) of CH4 production was significantly higher in organic soils than mineral soils (14.6 and 2.9 × 10−4 day−1, respectively; F3,748 = 51.8, P < 0.001). Lag times were significantly longer in mineral soils than organic soils (403 ± 72 days and 27 ± 5 days, respectively; χ2 = 4.5, df = 1, P = 0.03).
| % C | % N | Maximum CH4 production | Mean anaerobic CO2 production | |||||
|---|---|---|---|---|---|---|---|---|
| μg CH4-C g C−1 day−1 | μg CH4-C g DW−1 day−1 | mg CH4-C m−2 day−1 | μg CO2-C g C−1 day−1 | μg CO2-C g DW−1 day−1 | mg CO2-C m−2 day−1 | |||
| Soil Type | ||||||||
| Mineral | 6.8 (0.8)a | 0.3 (0.0)a | 3.3 (0.5)a | 0.2 (0.0)a | 71 (13) | 47.2 (7.4)a | 2.78 (0.47)a | 1070 (310) |
| Organic | 39.6 (0.8)a | 1.4 (0.0)a | 18.7 (12.1)a | 4.5 (0.7)a | 657 (102) | 145.8 (15.2)a | 57.1 (6.3)a | 5890 (690) |
| OM | 4.9 (1.7)a | 2.1 (1.9)a | 1.3 (1.0)a | 0.1 (0.0)a | NA | |||
| Depth (cm) | ||||||||
| 0–20 | 39.0 (1.6)a | 1.3 (0.1)a | 15.4 (2.7)a | 5.9 (1.1)a | 139 (24)a | 181 (30) | 74.6 (12.8) | 970 (110) |
| 20–100 | 30.1 (1.6)a | 1.1 (0.1)a | 3.2 (0.5)a | 0.9 (0.2)a | 833 (137)a | 101 (10) | 33.2 (3.7) | 7840 (890) |
| >100 | 4.2 (0.5)a | 0.3 (0.0)a | 5.0 (1.2)a | 0.2 (0.1)a | NA | 42.6 (13.0) | 1.2 (0.1) | NA |
- a Indicates significant differences among groups (P < 0.05).
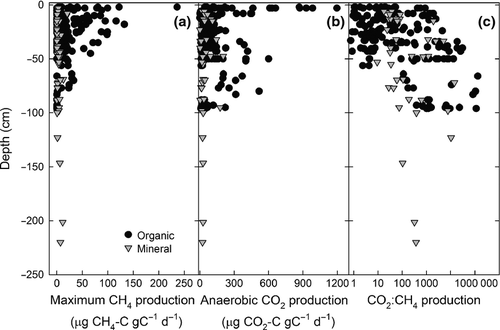
Both CH4 and anaerobic CO2 production decreased as a function of soil depth (Fig. 2). Maximum CH4 production was significantly higher in soil from the top 20 cm than deeper soils (>20 cm; χ2 = 17.0, df = 2, P < 0.001; Table 2). Anaerobic CO2 production also decreased at depth (χ2 = 6.5, df = 2, P = 0.04; Table 2), while CO2:CH4 production ratio increased with depth (Fig. 2c; Table 2). Soil depth also affected lag time, which was less than 45 days for surface soils and over 2 years for soils from >1 m depth (χ2 = 13.0, df = 2, P = 0.001; Table 2). The CO2e of anaerobic decomposition was largest from 20 to 100 cm depth soils, followed by surface soils (<20 cm), and then by deep soils (>100 cm) (1530, 680, and 340 μg CO2e g C−1 day−1, respectively; χ2 = 26.6, df = 2, P < 0.0001). The decay rate (k) of CH4 production significantly decreased with depth (Table 2; F5,746 = 35.3, P < 0.0001).
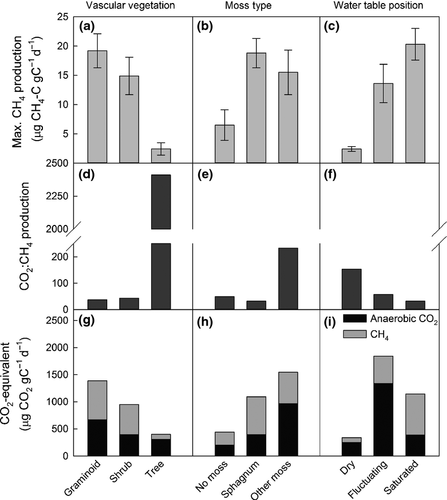
Rates of CH4 production and anaerobic CO2 production differed significantly among vegetation types. Maximum CH4 production was more than five times greater in soils from graminoid- and shrub-dominated sites compared to soils from treed sites (Fig. 3a; χ2 = 32.2, df = 2, P < 0.001). Maximum rates of CH4 production from sites with Sphagnum and other moss species were more than double sites without moss (Fig. 3b; χ2 = 21.1, df = 2, P < 0.001). The CO2:CH4 production ratio differed significantly among vascular plant types (Fig. 3d; χ2 = 188, df = 2, P < 0.001) and moss cover (Fig. 3e; χ2 = 78.4, df = 2, P < 0.001), as did CO2e (Fig. 3g–h; χ2 > 325, df = 2, P < 0.001). Lag times between the beginning of the incubation and day of maximum CH4 production were <30 days for graminoid- and tree-dominated sites, but were ~7 months for shrub-dominated sites (χ2 = 64.8, df = 2, P < 0.001). Decay rates (k) of CH4 production were larger in graminoid and shrub sites than treed sites (0.12 ± 0.02 and 0.04 ± 0.05 day−1, respectively).
Effects of environmental conditions
Environmental conditions – both within the incubation experiments themselves (i.e., temperature) and site-level conditions (pH and water table status) – significantly affected rates of CO2 and CH4 production. Incubation temperature significantly and positively related to CH4 and anaerobic CO2 production but explained relatively little variability compared to all variables considered (Table 4). The temperature sensitivities of anaerobic CH4 and CO2 production differed among studies with multiple incubation temperatures and across the whole synthesized dataset. The median apparent Q10 for CH4 production across all sites, depths, and temperatures was 1.18, and the median apparent Q10 for anaerobic CO2 production was 1.41. Lag times and decay rates (k) of CH4 production did not differ among incubation temperatures (Table 2).
| Predictor | Total % variance explained | |||
|---|---|---|---|---|
| CH4 | CO2 | CO2:CH4 | Lag time | |
| Landscape-level | ||||
| Biome | 11 | 5 | 32 | 6 |
| Landscape position | 7 | 9 | 8 | 20 |
| Permafrost: presence/absence | 3 | 0 | 0 | 0 |
| Mean annual precipitation (mm) | 0 | 0 | 3 | 9 |
| Mean annual air temp. (°C) | 0 | 5 | 0 | 12 |
| Substrate and soil characteristics | ||||
| Vascular vegetation type | 5 | 3 | 0 | 0 |
| Moss type | 4 | 12 | 0 | 0 |
| Soil type (organic/mineral) | 2 | 4 | 0 | 0 |
| Soil C:N ratio | 0 | 4 | 0 | 0 |
| pH | 0 | 5 | 19 | 0 |
| Depth | 0 | 0 | 8 | 10 |
| Soil environment | ||||
| Incubation temperature | 7 | 13 | 0 | 0 |
| Water table position | 0 | 16 | 0 | 0 |
| Regression statistics | ||||
| Total variance explained (r2) | 0.40 | 0.75 | 0.70 | 0.58 |
| F-value | 6.96 | 23.29 | 42.3 | 10.89 |
| Degrees of freedom | 12, 128 | 16, 122 | 7, 129 | 7, 56 |
| P | <0.001 | <0.001 | <0.001 | <0.001 |
| Number of measurements | 141 | 139 | 137 | 64 |
The relative water table position of a soil sample in the field also affected CO2 and CH4 production during the incubation experiments. Maximum CH4 production was more than five times higher from inundated samples than from dry samples (Fig. 3c, Table 2; χ2 = 43.8, df = 2, P < 0.001). Mean anaerobic CO2 production was more than three times higher in fluctuating water table samples than from inundated samples and more than five times higher in fluctuating samples than from dry soils (Table 2; χ2 = 77.3, df = 2, P < 0.001). The CO2:CH4 production ratio differed significantly among water table positions (Fig. 3f; χ2 = 123, df = 2, P < 0.001). Lag time was <20 days for inundated saturated samples or periodically inundated soils but >45 days for dry unsaturated soils (Table 2; χ2 = 113, df = 2, P < 0.001). Decay rates (k) of CH4 production rates were greatest in the zone of fluctuating water table followed by inundated samples (Table 2; Fig. S3).
Soil pH was significantly and positively correlated with CH4 production. Furthermore, the relationship between CH4 production and pH was dependent on incubation temperature (χ2 = 35, df = 4, P < 0.001; Fig. S7). The CO2:CH4 production ratio was negatively correlated with pH (slope = −0.64 ± 0.18, χ2 = 84, df = 1, P < 0.001).
Effects of permafrost
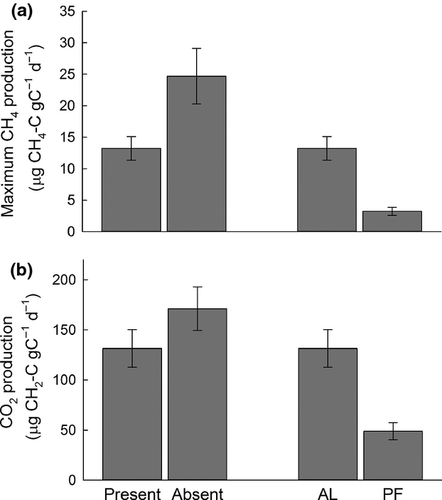
Presence of permafrost, both within and across sites, affected anaerobic CO2 and CH4 production. Maximum CH4 production rates from sites without permafrost were nearly double those from sites with permafrost (Fig. 4a; χ2 = 4.3, df = 1, P = 0.04). In sites with permafrost, maximum CH4 production rates from the active layer were nearly four times greater than from the permafrost layer (Fig. 4a; χ2 = 1.7, df = 1, P = 0.20). Anaerobic CO2 production was also higher from sites without permafrost than with permafrost (Fig. 4b; χ2 = 8.5, df = 1, P = 0.004) and was more than twice as large from the active layer than from the permafrost layer at sites that contained permafrost (Fig. 4b; χ2 = 3.1, df = 1, P = 0.08). In sites with permafrost, anaerobic CO2:CH4 production was significantly larger in the permafrost layer than the active layer (median = 1163 and 37, respectively; χ2 = 6.6, df = 1, P = 0.01). Lag times did not differ among any permafrost classification (P = 0.8, P = 0.28).
Relative importance of substrate, environmental, and landscape controls
The relative importance of substrate, environmental, and landscape controls differed for anaerobic CH4 and CO2 production. All variables considered explained 39% of CH4 production rates, with the highest amount of variance explained by landscape-level categorization (21%), followed by substrate controls (i.e., moss type, vascular vegetation type, and soil type) (11%) and incubation temperature (7%; Table 4). All variables considered explained 75% of anaerobic CO2 production. Environmental controls including incubation temperature (13%) and relative water table position (16%) explained 29% of variance, followed by substrate controls (moss type, vascular vegetation, pH, C:N ratio, and soil type) (28%) and landscape factors such as biome (5%), landscape position (9%), and mean annual air temperature (MAAT, 5%; Table 4). Site-level and landscape factors, such as biome, landscape position, and precipitation, explained most of the relative variance in CO2:CH4 production ratio (43%), followed by substrate controls (27%; Table 4). Lag time was best predicted by landscape factors (47% of total variance), followed by substrate controls, specifically depth (10% of total variance; Table 4). Permafrost, both within and across sites, was not a significant predictor of anaerobic CO2 production, CO2:CH4 production ratio, or lag time.
Discussion
Methane production in permafrost soils: insights from the synthesis
This synthesis of anaerobic incubations studies showed the complexities and also the generalizable patterns in CH4 and anaerobic CO2 production rates in soils across the permafrost region. Production of methane and anaerobic CO2, as observed from laboratory incubation studies, is largely controlled by factors that can be grouped into a few general categories: (i) substrate, (ii) environmental conditions, (iii) landscape-level factors, and (iv) microbial community dynamics (not examined in this synthesis; Table 4). The relative importance of environmental, substrate, and landscape controls differed for CH4 production and anaerobic CO2 production. We observed numerous legacy effects on CH4 production and anaerobic CO2 production from site factors such as landscape position, biome, relative water table position, and vegetation type (Fig. 3). These observed differences in CH4 and CO2 production among sites, under common incubation conditions, highlight the importance of soil and substrate composition for greenhouse gas production.
Substrate controls on CH4 production
Substrate composition controls the overall decomposability of soil organic matter, and therefore, substrate is a primary determinant of potential CH4 and CO2 production. Substrate quality often differs between depths due to fresh litter inputs from leaves and stems at the surface, and roots belowground. Because all soils were incubated under a strict anoxic atmosphere, we were able to examine the effect of organic matter quality at different depths on CH4 and CO2 production. Differences in organic matter quality on gas production rates were evident among depths and vegetation types. We observed higher CH4 and anaerobic CO2 production from surface soils as well as higher decay rates (k) of CH4 production, indicating higher organic carbon quality in this zone (Fig. 2, Table 2). Generally, higher quality organic C is expected closer to the ground surface due to root and litter inputs (Agren & Bosatta, 1996; Nilsson & Oquist, 2009); however, high-quality organic matter can be preserved under some permafrost formation conditions (e.g., Schuur et al., 2008).
Substrate controls on CH4 production are especially important in the permafrost zone because labile C can be added to soils not only from recently fixed plant inputs, but also as a result of permafrost thaw. Results from our synthesis show that vegetation type was a better predictor of CH4 production than sample depth (Table 4), that CH4 production generally decreased with depth (Fig. 3), and that production was higher from active layer soils than from permafrost. Previous studies have found relatively high rates of C production at depth (Waldrop et al., 2010; Treat et al., 2014) due to relatively high soil C lability, which depends on permafrost formation history (Waldrop et al., 2010; Harden et al., 2012; Lee et al., 2012; Treat et al., 2014) and processes such as cryoturbation that can mix undecomposed, relatively labile organic matter in mineral soils (Schuur et al., 2008) or in patterned tundra (Repo et al., 2009). Finally, landscape factors such as biome and landscape position affect substrate through vegetation composition, C and N inputs, and losses through leaching, as well as through permafrost formation history. For example, soil organic matter in permafrost was much more labile in tundra soils than boreal soils due, in part, to a legacy of more decomposition prior to permafrost formation in boreal soils (Treat et al., 2014), which may explain why landscape factors were strong predictors of anaerobic C production in this synthesis rather than depth and environmental conditions (Table 4).
Vegetation type also influenced CH4 production rates from incubations, similar to trends from field emissions (Olefeldt et al., 2013). Our synthesis results show that maximum CH4 production rates occurred in graminoid-dominated sites followed by shrub-dominated sites, while little to no CH4 production occurred in tree-dominated sites (Fig. 3). Similar patterns have been observed along a thaw gradient from thawed, wet, graminoid-dominated areas to dry palsa with intact permafrost due to changes in organic matter chemistry (Hodgkins et al., 2014). A synthesis of field measurements of CH4 fluxes showed similar trends with highest CH4 fluxes observed in graminoid-dominated sites and substantially lower CH4 fluxes from shrub- and tree-dominated sites (Olefeldt et al., 2013), which may be a function both of differences in organic matter composition and vascular transport of CH4 in sedge-dominated ecosystems. Tundra decomposition studies have shown that graminoid species decompose most quickly, followed by shrubs and finally mosses, a pattern tied to the high lignin:N ratios of woody tissues (Hobbie, 1996); our synthesis results show that k-values for CH4 production were largest in graminoid- and shrub-dominated sites and smallest in treed sites. Thus, dominant site vegetation resulted in differing substrate favorability for CH4 production in incubations through differences in the labile C pool, and this mechanism may be partially responsible for differences in CH4 fluxes among vegetation types in field measurements (e.g., Olefeldt et al., 2013). However, field measurements also capture effects of vegetation on gas exchange that are not reflected in laboratory incubations. For example, the proliferation of sedges can enhance CH4 emissions to the atmosphere through transport of CH4 through aerenchymous tissue (King et al., 1998) and also enhance CH4 oxidation through transport of oxygen to roots (Strom et al., 2005).
Favorable environmental conditions and microbial processes
Favorable environmental conditions for CH4 production include temperature, pH, and deficiency of oxygen and alternate electron acceptors (not addressed in this analysis); these factors act as a secondary control on CH4 production. Environmental conditions explained the most variance among CO2 production rates, but were less important for CH4 production rates (Table 4). Higher temperatures result in increased rates of CH4 and anaerobic CO2 production (Table 2), as has been shown in previous studies (Dunfield et al., 1993; Bergman et al., 1998; Whalen & Reeburgh, 2000; Yavitt et al., 2006; Lupascu et al., 2012). Previously, CH4 production had been found to be positively and negatively correlated with pH (c.f. Bergman et al., 1998), perhaps due to interactions between pH, temperature, and substrate type (Valentine et al., 1994; Bergman et al., 1998) or due to a pH optima of methanogenesis (Dunfield et al., 1993). This synthesis found a positive relationship between CH4 production and pH, and that relationship differed among incubation temperatures; pH can affect multiple processes simultaneously, including methanogenic pathway (Kotsyurbenko et al., 2007), and so although the specific mechanisms may be complex and interactive, pH is seen as a key variable controlling anaerobic metabolism across multiple landscape units.
Redox conditions play an important role for C cycling in anoxic soils. Dry, aerobic conditions or drying and re-wetting can result in a substantial lag time prior to CH4 production due to regeneration of alternate electron acceptors (Dise & Verry, 2001; Knorr & Blodau, 2009). In this synthesis of incubation studies, the lag time before maximum CH4 production was shortest in inundated sites, intermediate in fluctuating water table sites, and longest in dry sites (Table 2). Additionally, in chronically dry soils, methanogen populations may be substantially smaller than soils that experience inundation (Tveit et al., 2013) and require longer periods for population size to grow prior to measurable CH4 production. Observations of lag times suggest that environmental conditions, such as temperature and redox status, as well as methanogen populations, may limit CH4 production in fluctuating water table zones that can change over the course of a growing season in permafrost areas due to perched water tables. Furthermore, landscape changes associated with permafrost thaw, such as inundation, may not result in CH4 production until environmental conditions are suitable and methanogen communities are present.
Recommendations, scaling up, and climate change effects
Climate change in northern regions is affecting ecosystems in multiple ways, including deepening active layer depths, shifting vegetation dynamics, growing season length, and hydrology (Serreze et al., 2000; Hinzman et al., 2005; Myers-Smith et al., 2011; Overland et al., 2013). All of these factors have the potential to affect CH4 and anaerobic CO2 production. Our synthesis results show both direct effects of temperature on CH4 and anaerobic CO2 production (Table 2), as well as indirect effects of ecosystem shifts that may be associated with climate change.
CH4 production rates were more than twice as high in active layer soils compared to permafrost as well as from sites without permafrost compared to those underlain by permafrost, both of which suggest that CH4 production will increase as permafrost thaws. However, there are also important indirect effects of permafrost that will have profound impacts on anaerobic CO2 and CH4 production and emissions. Thermokarst thaw and permafrost thaw frequently result in wetter soils, especially in lowlands. This synthesis suggests that flooding and periodic inundation of surface soils due to permafrost thaw may result in higher CH4 fluxes, as has been observed in field CH4 emission studies (e.g., Olefeldt et al., 2013 and references therein). Vegetation changes, such as a transition from dry, treed sites to wet, graminoid sites, or from graminoid tundra sites to shrub or tree tundra, may also alter anaerobic CO2 and CH4 production rates (e.g., Hodgkins et al., 2014), although the effects of vegetation on CH4 production via substrate quality will operate at timescales of a growing season to decades while the environmental controls on CH4 production operate on daily to seasonal scales.
There is substantial uncertainty in the spatial and temporal variability of estimates of CH4 fluxes and CO2 exchange in the permafrost region (McGuire et al., 2009, 2012). This synthesis has several implications for modeling anaerobic CH4 and CO2 fluxes from permafrost region. Our results indicate that models of greenhouse gas emissions from thawing permafrost should consider several sources of variability in methanogenesis that are generally not taken into account: variation (i) between organic and mineral horizons, (ii) with depth within soil horizons, (iii) between active layer and thawed permafrost layer of soils in sites with permafrost, (iv) between permafrost sites and sites without permafrost, and (v) among plant functional types. Models generally consider some of the effects of water table position, but this study indicates that it is important to distinguish between chronically dry surface soil horizons and seasonally or chronically inundated soil horizons (e.g., Zhuang et al., 2004; Wania et al., 2010; Riley et al., 2011; Bohn et al., 2013). Similarly, models generally have different parameterizations for different anaerobic ecosystems, but there is no generally accepted scheme as to how those parameterizations should be developed. This synthesis indicates that parameterizations should be hierarchically organized by biome, landscape position, and vascular/moss vegetation types (Table 4). While models generally consider the effects of pH and temperature on methanogenesis, this study provides some additional insight into the relative importance of these effects on anaerobic metabolism (Table 4). The information on lag times in methanogenesis provided by this study can inform models that simulate the effects of thermokarst disturbance (e.g., from forested permafrost plateau to collapse scar bog) on methanogenesis because of possible lags in microbial colonization and population dynamics, which are ultimately shorter than the persistence of thermokarst wetlands. Finally, most models do not consider anaerobic CO2 production in modeling methane dynamics, and this study provides substantial insight into the variability in the ratio of anaerobic CO2 to CH4 production.
To improve estimates of CH4 production from soils across the permafrost zone, future incubation study designs should be modified to include longer incubations, a wider variety of sites, additional measurement parameters, and to include methane oxidation. The substantial lag times before maximum rates of CH4 production (Table 2) suggest that longer duration anaerobic incubation experiments are required to assess maximum CH4 production from permafrost soils; we suggest 30-day minimum incubation length and longer time periods if low incubation temperatures are used. Second, a sampling bias toward hot spots of CH4 production across the landscape (McClain et al., 2003), that is, wetlands, could affect our estimates of lag time and maximum CH4 production rates among some classifications we used, especially for permafrost presence/absence. Increased sampling of additional mineral or upland soils might reduce bias in our estimates of maximum rates of CH4 production and lag times in the permafrost zone. New methodologies need to be used to identify pathways of anaerobic CO2 and CH4 production, including use of stable isotopes and chemical characterization of organic matter (Corbett et al., 2013; Tfaily et al., 2013; Hodgkins et al., 2014). Furthermore, measurements of methanogen communities, redox status and pH in mineral or upland soils and both dry and saturated wetland soils may help clarify whether long lag times prior to maximum CH4 production rates are due to redox conditions or methanogen community abundances. Furthermore, we did not consider CH4 oxidation in this synthesis; additional research on rates of oxic and anoxic CH4 oxidation (Whalen & Reeburgh, 2000; Blazewicz et al., 2012; Haroon et al., 2013) will better constrain CH4 budgets from the permafrost zone.
Results from this synthesis highlight the importance of understanding the complex interactions among geochemistry, methanogen communities, and organic matter quality for determining ecosystem scale patterns of CH4 and CO2 production as the thaw regime and vegetation dynamics continue to change in northern latitude systems. Our results suggest that the largest source of anaerobic CO2 and CH4 produced will be from seasonally saturated surface soils rather than from the decomposition of newly thawed substrate found in permafrost soils. Therefore, increased wetland area associated with permafrost thaw will result in larger CH4 emissions than deeper seasonally thawed soils and underscores a need for capturing hydrologic changes associated with ecosystem transitions.
Acknowledgements
We thank the Permafrost Carbon Vulnerability Research Coordination Network (NSF Grant to EAGS) for helping us to organize this study. CT acknowledges funding from the U.S. DOE-SCGF and the University of New Hampshire Graduate School Dissertation Year Fellowship. Additional funding was provided by NSF OPP (ARC-1203777) to SMN; the Next-Generation Ecosystem Experiments (NGEE Arctic) project, supported by the Office of Biological and Environmental Research in the U.S. DOE Office of Science, to CMI, RJN, TRC, VLS; European Research Network CryoCARB (FWF – I370-B17) to AR; and USGS Global Change R&D program and the USGS Climate Science Center to MW. Craig Connolly and Adam Marquis provided assistance with GIS and data extraction. We thank Steve Frolking, Evan Kane, and four anonymous reviewers for comments that improved the manuscript. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.




