Plant stomatal closure improves aphid feeding under elevated CO2
Abstract
Stomata help plants regulate CO2 absorption and water vapor release in response to various environmental changes, and plants decrease their stomatal apertures and enhance their water status under elevated CO2. Although the bottom-up effect of elevated CO2 on insect performance has been extensively studied, few reports have considered how insect fitness is altered by elevated CO2-induced changes in host plant water status. We tested the hypothesis that aphids induce stomatal closure and increase host water potential, which facilitates their passive feeding, and that this induction can be enhanced by elevated CO2. Our results showed that aphid infestation triggered the abscisic acid (ABA) signaling pathway to decrease the stomatal apertures of Medicago truncatula, which consequently decreased leaf transpiration and helped maintain leaf water potential. These effects increased xylem-feeding time and decreased hemolymph osmolarity, which thereby enhanced phloem-feeding time and increased aphid abundance. Furthermore, elevated CO2 up-regulated an ABA-independent enzyme, carbonic anhydrase, which led to further decrease in stomatal aperture for aphid-infested plants. Thus, the effects of elevated CO2 and aphid infestation on stomatal closure synergistically improved the water status of the host plant. The results indicate that aphid infestation enhances aphid feeding under ambient CO2 and that this enhancement is increased under elevated CO2.
Introduction
Anthropogenic activities have caused a rapid increase in atmospheric CO2 concentration (IPCC, 2007), which increased from 280 ppm before industrialization to 401 ppm in June 2014 (Mauna Loa Observatory: NOAA-ESRL). Elevated CO2 has substantial effects on the biodiversity and function of ecosystems (Sala et al., 2000). Because CO2 is the substrate for plant photosynthesis, elevated CO2 typically increases the photosynthetic rate of C3 plants (Ainsworth & Long, 2005). Furthermore, plants exhibit reduced stomatal apertures and stomatal conductance in response to elevated CO2 (Ainsworth & Rogers, 2007). In FACE experiments, elevated CO2 has decreased stomatal conductance by an average of 22% for five functional plant groups that included 285 plant species (Ainsworth & Rogers, 2007). As noted, the decreased stomatal conductance reduces water loss from plants and increases plant water potential and water content (Wullschleger et al., 2002; Pritchard et al., 2007), and these effects are likely to alter the fitness of herbivorous insects (Roth & Lindroth, 1995).
Aphids damage plants by ingesting plant phloem sap for nutrition and by transmitting viral pathogens (Awmack et al., 1997). Some species of aphid exhibit enhanced feeding efficiency and performance under elevated CO2 (Robinson et al., 2012; Sun et al., 2013). The physiology and biochemistry underlying the reasons why some aphids are more effective under elevated CO2 than under ambient CO2, however, are not entirely understood. Furthermore, previous studies suggested that because the phloem in M. truncatula is more accessible (as a result of lower plant defenses) and contains more amino acids when grown under elevated CO2, aphids ingest more phloem saps from M. truncatula under elevated CO2 (Guo et al., 2013, 2014a; Zavala et al., 2013). The penetration phase (i.e., the phase before the aphid stylet reaches the phloem) and the phloem-feeding phase, however, cannot entirely explain the feeding efficiency of aphids. Sustainable feeding on phloem sap also relies on the duration of the xylem-feeding phase, which is closely associated with host water potential (Huberty & Denno, 2004; Pompon et al., 2010). Aphids feed almost on sugar-enriched phloem sap which has an osmotic pressure that is as much as 4–5 times greater than that of the aphid's hemolymph (Douglas, 2006). To avoid self-dehydration and osmotic stress in the hemolymph during the phloem-feeding phase, aphids must consume a certain amount of xylem sap, which has a lower osmolarity than phloem sap, to balance hemolymph osmolarity (Pompon et al., 2011). Given that host water status affects aphid feeding efficiency and hemolymph osmolarity (Jactel et al., 2012), it is reasonable to speculate that decreases in stomatal aperture and increases in plant water potential induced by elevated CO2 will facilitate xylem feeding and thereby decrease hemolymph osmolarity and enhance phloem feeding.
The opening and closing of stomata under various biotic and environmental conditions is regulated by several pathways involving abscisic acid (ABA) and carbonic anhydrase (Hu et al., 2009; Kim & Maik, 2010). While the ABA-dependent signaling pathway regulates stomatal aperture in plant responses to drought and pathogen invasion (Li et al., 2000; Zhang et al., 2006; Du et al., 2014), carbonic anhydrase, which acts independently of the ABA signaling pathway, serves as a key controller in CO2-induced stomatal closure (Hu et al., 2009). However, the effect of aphids on the stomatal apertures of their host plants has not been studied in details. Previous microarray data showed that aphid infestation could up-regulate ABA signaling pathway in sorghum (Zhu-Salzman et al., 2004). Thus, the interactive effects of aphid infestation and elevated CO2 on plant water status and in turn on phloem feeding by aphids should be evaluated.
The current study examined whether infestation by the pea aphid, Acyrthosiphon pisum, induces stomatal closure in the host plant Medicago truncatula, whether stomatal closure affects aphid performance, and whether these interactions between aphid and plant are altered by elevated CO2. To understand the mechanisms underlying these interactions, we used the sta-1 mutant of M. truncatula and its wild-type A17; the stomatal movement of sta-1 mutant is insensitive to ABA (Ding et al., 2008). The hypotheses tested were as follows: (1) aphid infestation decreases stomatal conductance and consequently enhances the water status of the wild-type plant by triggering ABA signaling pathway; (2) these effects reduce aphid hemolymph osmolarity, facilitate aphid feeding, and enhance aphid fitness; and (3) these aphid-induced changes are enhanced by elevated CO2.
Materials and methods
Control of CO2 concentrations
Experiments were performed in eight octagonal, open-top field chambers (OTCs) (4.2 m diameter and 2.4 m height) at the Observation Station of the Global Change Biology Group, Institute of Zoology, Chinese Academy of Science in Xiaotangshan County, Beijing, China (40°11′N, 116°24′E). The CO2 concentrations were set at the current atmospheric CO2 level (~400 μL L−1) and at an elevated CO2 level (700 μL L−1, which is the level predicted for the end of this century) (IPCC, 2007). Four blocks were used for each of the two CO2 treatments, and each block contained one pair of OTCs, one with ambient CO2and one with elevated CO2. CO2 concentration in each OTC was monitored and adjusted with an infrared CO2 analyzer (Ventostat 8102, Telaire Company, Goleta, CA, USA). The auto-control system for maintaining the CO2 concentrations, as well as specifications for the OTCs, is detailed in Guo et al. (2013).
Host plants
The sensitivity-to-ABA mutant (sta-1) of M. truncatula and its wild-type plant (cv. A17) were kindly provided by Professor Oldroyd, Department of Disease and Stress Biology, John Innes Centre, England. sta-1 is insensitive to ABA in terms of lateral root initiation and stomatal closure (Ding et al., 2008). The plants were pretreated in KCl-MES buffer (50 mm KCl and 10 mm MES, pH 6.12) under continuous white light (250 μmol m−2 s−1) for 90 min to ensure maximal stomatal opening and then treated with buffer alone or with buffer containing 25 μm ABA. With ABA treatment, all stomata close in the wild-type leaves but all stomata remain open in the sta-1 mutant leaves (Ding et al., 2008).
Medicago truncatula A17 and sta-1 plants were germinated and inoculated with Sinorhizobium meliloti 1021 as described previously (Guo et al., 2013). After grown in sterilized soil for 2 weeks, the M. truncatula seedlings were individually transplanted into plastic pots (35 cm diameter and 28 cm height) containing sterilized loamy field soil (organic carbon 75 g kg−1, N 500 mg kg−1, P 200 mg kg−1, K 300 mg kg−1) and were placed in OTCs on March 27, 2013. Each OTC contained 40 plants and 320 plants in total.
Aphid infestation
The pea aphid Acyrthosiphon pisum was originally collected from Pisum sativum L. in Yunnan Province and had been reared in the laboratory for 4 years. The nymphal instars from the same parthenogenetic pea aphid female were reared on Vicia faba with 14 h light (25 °C)/10 h dark (22 °C) in photoclimate chambers (Safe PRX-450C, Ningbo, China).
On May 10, 2013, after growing for 6 weeks, the plants were randomly divided into three groups for determining the plant response, aphid feeding behavior, and aphid physiological parameters. In the first group, six plants of each genotype per OTC (96 plants in total) were randomly selected, and each was infested with five apterous 4th instar pea aphid nymphs. The plants were placed in cages made with 80-mesh gauze, and the nymphs developed and reproduced offspring freely on each plant for 23 days (to 2 June 2013). Changes in aphid numbers on each plant were determined (as described in the next section). Aphids collected from these plants were also used for analysis of aphid water content and hemolymph osmolarity.
In the second group, six plants of each genotype per OTC (96 plants in total) were randomly selected, and each was infested with one apterous adult pea aphid for 12 h. These aphids were used to record feeding behavior (as described in the next section).
In the third group, four plants of each genotype per OTC (64 plants in total) were randomly selected, and each was infested with 50 apterous 4th instar nymphs. The nymphs were transferred to five mature trifoliate leaves (fourth to eighth terminal, mature, trifoliate leaves from the base of the shoot) with a soft brush and were caged on each plant with 80-mesh gauze. Another four plants of each genotype per OTC served as the uninfested control and were caged in the same way. After 24 h, the aphids were removed. The damaged leaves of two infested plants and two uninfested plants of each genotype per OTC were selected for measuring leaf gas exchange and water status. Damaged leaves of the other two infested plants and two uninfested plants of each genotype per OTC were harvested for scanning electron microscopy and for analysis of phytohormones and gene expression.
Aphid population
Aphid numbers on each plant in the first group were determined and recorded at the 7th, 11th, 15th, 19th, and 23th day. At the end of the experiment, the aphids were brushed from each plant and collected. Of the collected aphids, 50 adults per plant were used for hemolymph osmolarity analysis, and 35 adults per plant were collected for water content analysis (as described later).
Aphid hemolymph osmolarity and body water content
Aphid hemolymph was collected from aphids in the first group according to Shakesby et al. (2009). The osmolarity of 10-μL samples was determined with a pressure osmometer (model 5220, Wescor Inc., Logan, Utah, USA). To estimate water content, 35 adult aphids from two plants were collected and immediately weighed. Dry weights were obtained after the aphids were dried at 60 °C for 24 h. The water content of the aphids was determined by subtracting the dry weight from the fresh weight.
Aphid feeding behavior
The feeding behavior of pea aphids on the A17 and sta-1 plants of the second group was studied as described in Guo et al. (2014a) with some modifications. Twenty-four biological replicates were included for each A17 and sta-1 genotype under each CO2 level, and as noted earlier, one single trifoliate leaf on each plant was infested with one apterous adult pea aphid. An eight-channel amplifier was used to simultaneously monitor the feeding of eight aphids on separate plants for 12 h. Waveform patterns were scored according to the categories described by Tjallingii & Esch (1993) (Table S5).
Plant stomatal conductance, water transpiration rate, and water potential
As noted, plants in the third group were used to measure stomatal conductance, net photosynthetic rate, and water transpiration rate of uninfested plants and infested plants. These variables were measured on the fourth to eighth terminal mature trifoliate leaves from the base of the shoot with a Li-Cor 6400 gas exchange system (6400-40; Li-Cor Inc., Lincoln, NE, USA) between 7:30 hours and 10:30 hours. The incoming CO2 concentration was adjusted to 400 μmol mol−1 or 700 μmol mol−1. Relative humidity corresponded to ambient conditions (55–60%). Before gas exchange was measured, illumination was set to 90% red and 10% blue, the temperature was set to 25 °C, and photosynthetic active radiation (PAR) for the leaf in the measuring cuvette was 1200 μmol m−2 s−1. Measurements were taken when the CO2 assimilation rate was stable for at least 2 min. Another three leaves per plant were selected to measure leaf water potential with the PSYPRO water potential system (Wescor Inc., Logan, Utah, USA).
Scanning electron microscopy of stomatal apertures
To measure stomatal apertures, leaves from the third group of plants were fixed in 2.5% glutaraldehyde in 1 mm sodium phosphate buffer, pH 6.8, for 12 h, and then were washed in 50, 60, 70, 90, 100, and 100% ethanol (15 min for each wash). The samples were subjected to critical point drying in CO2 and were coated with gold using an Eiko 1B.5 sputter coater (Eiko, Tokyo, Japan). The samples were then examined with a Hitachi s570 scanning electron microscope (Tokyo, Japan) at an accelerating voltage of 5–15 kV, and stomatal apertures were measured (Zhang et al., 2005).
ABA content and gene expression
Approximately 200 mg of leaf tissue (fresh weight) per plant in the third group was pooled for ABA quantification as described previously (Fu et al., 2012). The RNA Easy Mini Kit (Qiagen, Hilden, Germany) was used to isolate total RNA from M. truncatula leaves, and 1 μg of RNA was used to synthesize cDNAs. mRNAs of the following three target genes involved in regulating stomatal movement were quantified by real-time quantitative PCR: 9-cis-epoxycarotenoid dioxygenase (NCED), ABA-responsive protein (ABR), and β-carbonic anhydrase1 (β-CA1). NCED and ABR are in the ABA signaling pathway (Iuchi et al., 2001; Colditz et al., 2004), while CA1 is in an ABA-independent pathway and regulates stomatal aperture in response to CO2 level (Hu et al., 2009). Specific primers for genes were designed from the M. truncatula EST sequences using PRIMER5 software (Table S1). The house-keeping gene β-actin was used as the internal qPCR standard to analyze plant gene expression. The fold changes of the target genes were calculated using the 2−ΔΔCt normalization method. Each combination of aphid infestation, plant genotype, and CO2 level was represented by four biological replicates, and each biological replicate contained four technical repeats.
Statistical analyses
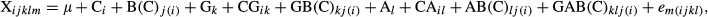
Results
Aphid infestation induces stomatal closure, and the effect is associated with the ABA signaling pathway
To determine whether M. truncatula stomata respond to aphid infestation, we infested wild-type A17 leaves with 50 adults. After 24 h, the average width of the stomatal aperture decreased relative to that of noninfested control (Fig. 1a; Table S2). The stomatal conductance (gs) and photosynthetic rate of A17 plants also decreased in response to aphid infestation (Figs 1c and 2a; Table S2).
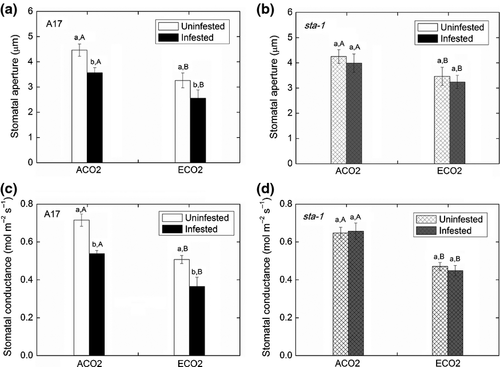
To determine whether the aphid-induced decrease in stomatal aperture requires components of the ABA signaling pathway, we examined the stomatal response to aphid infestation in sta-1 plants, which are insensitive to ABA in stomatal closure. Infestation of sta-1 plants for 24 h did not induce stomatal closure (Fig. S1c, d). The stomatal conductance (gs) and photosynthetic rate of sta-1 plants were unaffected by aphid infestation (Figs 1d and 2b). Furthermore, ABA content was increased and expression of NCED and ABR was up-regulated by aphid infestation of A17 plants (Fig. 3a–c). These results are consistent with the hypothesis that a decrease in stomatal aperture in response to aphid infestation depends on the ABA signaling pathway.
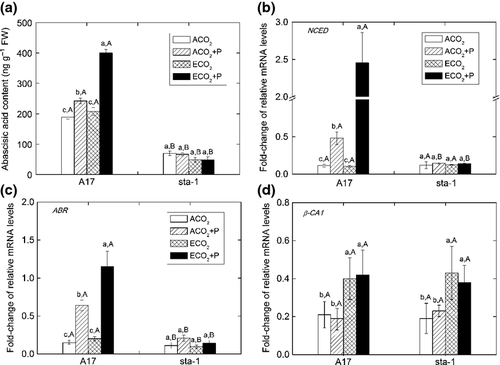
The decrease of stomatal aperture induced by elevated CO2 is independent of the ABA signaling pathway
In the absence of aphid infestation, elevated CO2 decreased the stomatal aperture and stomatal conductance but increased the photosynthetic rate for both genotypes (Figs 1, 2a, b and S1). Elevated CO2 did not affect the ABA content and expression of key genes involved in ABA biosynthesis (NCED) and in the ABA-responsive pathway (ABR), but elevated CO2 up-regulated the expression of CA1 in both genotypes (Fig. 3; Table S3). These results suggest that the decrease in stomatal aperture induced by elevated CO2 relies on carbonic anhydrase, which is ABA-independent. When the plants were infested with aphids, elevated CO2 also decreased the stomatal aperture and conductance and increased photosynthetic rate for both genotypes (Figs 1, 2a, b and S1). In addition, elevated CO2 increased the ABA content and expression of NCED and ABR in aphid-infested A17 plants, which suggested that aphid infestation could induce more stomatal closure in A17 plants grown under elevated CO2 than under ambient CO2 (Fig. 3a–c).
Aphid-induced and CO2-induced stomatal closure improve the water status of M. truncatula
The primary physiological role of stomatal closure is to minimize loss of water vapor during transpiration (Hetherington & Woodward, 2003). Aphid infestation decreased the transpiration rate of A17 leaves but did not affect the transpiration rate of sta-1 leaves (Fig. 2c, d). This result suggested that, by stimulating the ABA signaling pathway, aphid infestation decreased the transpiration of wild-type M. truncatula.
To test whether the decreased transpiration rate improved plant water status, we measured the plant water potential (Table S2). Under ambient CO2, aphid infestation decreased the water potential of sta-1 plants (Fig. 2e) but not of A17 plants (Fig. 2f). Under elevated CO2, aphid infestation increased the water potential of A17 plants but decreased the water potential of sta-1 plants (Fig. 2e, f). These results suggested that aphid-induced stomatal closure helps plants prevent water loss and maintain water potential and that elevated CO2 had a positive synergistic effect on aphid-induced increases in plant water potential.
Regardless of plant genotype, elevated CO2 decreased transpiration and increased leaf water potential (Fig. 2c, e). Moreover, aphid-infested A17 plants grown under elevated CO2 had the highest water potential among the treatments, suggesting that elevated CO2 and aphid infestation have an additive effect in promoting plant water status.
Stomatal closure facilitates the xylem feeding by aphids, which in turn increases individual feeding efficiency
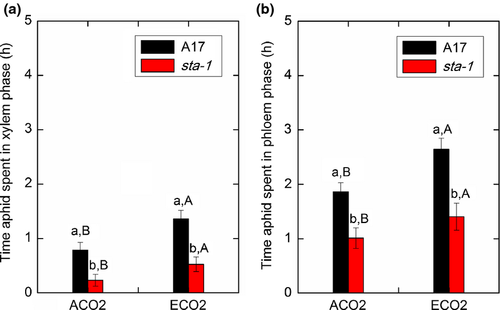
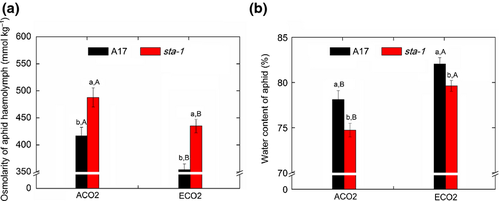
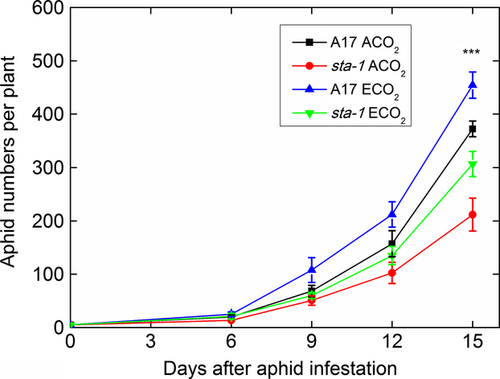
To balance the high osmotic pressure of sugar-enriched phloem sap, aphids must intermittently ingest xylem sap, the water content of which is determined by the water status of the whole plant. We therefore determined whether the improvement of plant water status resulting from stomatal closure affected aphid xylem feeding, hemolymph osmolarity, phloem feeding, and abundance. Regardless of CO2 level, the duration of xylem feeding was shorter on sta-1 plants than on A17 plants (Fig. 4a). The reduced time spent by aphids taking up xylem sap was paralleled by an increase in the duration of the nonpenetration phase of feeding on sta-1 plants (Table S5). The reduced time spent taking up the xylem sap of sta-1 plants led to a higher hemolymph osmolarity and a lower water content in aphids reared on sta-1 plants than on A17 plants (Fig. 5; Table S4). This suggested that stomatal closure triggered by aphids could facilitate aphid uptake of water from the xylem and decrease aphid osmotic pressure. Furthermore, elevated CO2 increased the xylem-feeding time and subsequently decreased the hemolymph osmolarity and increased the water content of aphids (Figs 4a and 5), which suggested that elevated CO2 helps aphids avoid dehydration. Our results also showed that aphids spent less time in the phloem phase when feeding on sta-1 plants than on A17 plants (Fig. 4), which led to lower population abundance on sta-1 plants than on A17 plants regardless of CO2 level (Fig. 6). Moreover, elevated CO2 increased the duration of phloem feeding and subsequently increased the numbers of aphids reared on both genotypes. This result suggested that aphids suffered less from dehydration and experienced better phloem feeding on M. truncatula grown under elevated CO2 than under ambient CO2.
Discussion
As hypothesized, aphid feeding under ambient CO2 did trigger the ABA signaling pathway and did induce stomatal closure in wild-type M. truncatula plants. Although stomatal aperture was decreased under elevated CO2 in the absence of aphid feeding, the aphid effect was not reduced under elevated CO2; indeed, aphid feeding significantly reduced stomatal aperture and increased transpiration rate under both ambient and elevated CO2. The effect of elevated CO2 and aphid infestation on stomatal closure synergistically improved the water status of the host plant, which in turn facilitated aphid xylem feeding and osmoregulation. The results indicate that aphid-induced stomatal closure enhances aphid feeding and that this enhancement is further increased under elevated CO2.
Stomata not only act as gateways for gas exchange between plants and the atmosphere, but also can be closed to help prevent pathogen invasion. Recent research has shown that bacterial pathogens can trigger stomatal closure, which prevents bacterial penetration through these pores, and that this closure involves the triggering of the ABA signaling pathway (Melotto et al., 2006; Montillet & Hirt, 2013). Likewise, we found that aphid infestation triggered stomatal closure, and enhanced the ABA signaling pathway of wild-type M. truncatula, which is in accordance with Zhu-Salzman et al. (2004), whose microarray data showed that infestation by the greenbug aphid triggered the ABA signaling pathway in sorghum. Furthermore, because aphid infestation did not trigger stomatal closure or ABA accumulation in the ABA-insensitive mutant sta-1, we infer that the ABA signaling pathway has a central role in aphid-induced stomatal closure in M. truncatula (Figs 1 and S2). In addition to failing to accumulate ABA or to close stomata in response to aphid infestation, the ABA-insensitive mutant sta-1 also supported a higher transpiration rate and lower water potential than the wild type (Fig. 2c–f). It seems that, like the salicylic acid signaling pathway (Moran & Thompson, 2001), the ABA signaling pathway is triggered by aphid infestation, resulting in stomatal closure and an increase in the water status of the host plant.
Due to the enhancement of carbon uptake, the current data showed that elevated CO2 increased the photosynthetic rate (Ainsworth & Long, 2005; Leakey et al., 2009). However, elevated CO2 decreased the stomatal conductance and transpiration rate of M. truncatula (Figs 1c, d and 2c, d). A reduction in stomatal apertures seems to be a common response of plants to elevated CO2 (Ainsworth & Rogers, 2007). Although aphids induce stomatal closure via the ABA signaling pathway, it is uncertain whether elevated CO2 induces stomatal closure via the ABA signaling pathway. Zou et al. (2007) found that elevated CO2 increased the ABA content and gene expression in the ABA signaling pathway in Larrea tridentata. In contrast, we found that although elevated CO2 decreased stomatal aperture and conductance in M. truncatula, it did not affect ABA content or the expression of the marker genes in the ABA signaling pathway in wild-type plants. These results suggest that an ABA-independent pathway is responsible for stomatal regulation when M. truncatula is exposed to elevated CO2. Furthermore, in agreement with Hu et al. (2009), carbonic anhydrase was up-regulated in both M. truncatula genotypes under elevated CO2. It seems that elevated CO2 stimulates carbonic anhydrase rather than the ABA signaling pathway to induce stomatal closure in M. truncatula.
At the outset of this study, we hypothesized that the decrease in stomatal aperture under elevated CO2 would suppress the decreases in stomatal aperture induced by aphids. Surprisingly, aphid infestation induced a decrease in stomatal aperture and stomatal conductance under both ambient and elevated CO2. The effect was approximately additive, and stomatal closure was greatest (and ABA content and associated gene expression were highest) when wild-type plants were grown under elevated CO2 and were infested with aphids. Aphid-induced and elevated CO2-induced decreases in stomatal aperture synergistically enhance the aphid feeding efficiency because they decrease transpiration and increase the water potential of the host plant. Although aphid infestation did not trigger stomatal closure of sta-1 plants, elevated CO2 decreased the stomatal aperture, decreased water loss, and increased the water potential of sta-1 plants. These results suggest that elevated CO2 improves the water status of aphid-infested M. truncatula plants by decreasing stomatal aperture and that this effect of elevated CO2 is independent of the ABA signaling pathway.
Herbivorous insects can perceive the changes in host plant water status and avoid feeding on plants suffering from a water deficit (Chown et al., 2011; McQuate & Connor, 1990a,b). Furthermore, the changes in the water status of the host can even mediate the interaction between below- and above-ground herbivorous insects. For example, corn plants infested with rootworms decreased their foliar water content, which in turn negatively affected the fitness of the leaf-chewing insect Spodoptera littoralis (Erb et al., 2009). Unlike chewing insects, aphids and other phloem-sucking insects are highly specialized for feeding on phloem, which makes them more vulnerable than leaf-chewing insects to fluctuation in host water status for two reasons. First, to feed on phloem, aphids require that the host's cell maintain a high turgor pressure (Huberty & Denno, 2004). Second, aphid must absorb xylem sap to balance the osmotic pressure of the sugar-rich phloem sap (Daniels et al., 2009; Nalam et al., 2012). Therefore, a host plant with sufficient water content benefits aphid by providing sufficient cell turgor pressure and sufficient xylem sap. In current study, we had speculated that stomatal closure induced by aphid infestation would benefit aphids by increasing the duration of xylem feeding. As expected, aphids spent less time on the xylem-feeding phase when reared on sta-1 plants (whose stomata did not close in response to aphid infestation) than on wild-type plants (Fig. 4), which apparently led to a higher osmolarity and lower water content in aphids reared on sta-1 plants than on wild-type plants (Fig. 5). In addition, sta-1 plants had lower nitrogen fixation ability than A17 plants (Ding et al., 2008), which led a lower N nutrition status in sta-1 plants when compared with A17 plants. Thus, considering the combination effect of water and nutrition status, aphids reared on sta-1 plants spent less time in the phloem-feeding phase and had lower numbers than aphids on wild-type plants. We also found that, regardless of plant genotype, elevated CO2 increased the xylem-feeding phase, decreased hemolymph osmolarity, increased aphid water content, increased the duration of phloem feeding, and increased pea aphid numbers on M. truncatula. We conclude that pea aphid-induced stomatal closure in M. truncatula facilitates xylem feeding and thereby promotes phloem feeding and increases in aphid numbers. These effects were enhanced by elevated CO2.
By decreasing host resistance and increasing host amino acid concentration, elevated CO2 enables stylet of the pea aphid to reach the M. truncatula phloem more easily and to consume more phloem sap (Guo et al., 2013, 2014a,b). The increased phloem-feeding phase, however, means that aphids must absorb more water from the xylem to balance the osmotic pressure of the hemolymph. The current study shows that, in addition to favoring aphid feeding on M. truncatula by reducing host resistance and by increasing host nutrient content, elevated CO2 also favors aphid feeding on M. truncatula by increasing host water potential and therefore increasing xylem-feeding time, which further improved the feeding efficiency of aphids (Fig. S2).
Our study has revealed a novel strategy by which aphids induced the host plant to benefit their own feeding behavior. We found that aphids can trigger stomatal closure, decrease leaf transpiration, and maintain the water potential of the host plant. This manipulation of host plant's stomata helps aphids absorb water from the xylem to neutralize phloem osmotic pressure. Moreover, stomatal apertures are further decreased by elevated CO2. As a result, passive feeding and osmotic pressure regulation of the aphids are further enhanced under elevated CO2, resulting in improved aphid performance. The results suggest that elevated CO2 will make the management of aphid pests in agriculture more difficult.
Acknowledgements
We thank Prof. Bruce Jaffee (University of California at Davis, CA, USA) for reviewing a draft of the manuscript. We also thank the anonymous reviewers for their valuable comments. This project was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (No. XDB11050400) and the National Nature Science Fund of China (No. 31170390 and 31221091).




