CTFS-ForestGEO: a worldwide network monitoring forests in an era of global change
Abstract
Global change is impacting forests worldwide, threatening biodiversity and ecosystem services including climate regulation. Understanding how forests respond is critical to forest conservation and climate protection. This review describes an international network of 59 long-term forest dynamics research sites (CTFS-ForestGEO) useful for characterizing forest responses to global change. Within very large plots (median size 25 ha), all stems ≥1 cm diameter are identified to species, mapped, and regularly recensused according to standardized protocols. CTFS-ForestGEO spans 25°S–61°N latitude, is generally representative of the range of bioclimatic, edaphic, and topographic conditions experienced by forests worldwide, and is the only forest monitoring network that applies a standardized protocol to each of the world's major forest biomes. Supplementary standardized measurements at subsets of the sites provide additional information on plants, animals, and ecosystem and environmental variables. CTFS-ForestGEO sites are experiencing multifaceted anthropogenic global change pressures including warming (average 0.61 °C), changes in precipitation (up to ±30% change), atmospheric deposition of nitrogen and sulfur compounds (up to 3.8 g N m−2 yr−1 and 3.1 g S m−2 yr−1), and forest fragmentation in the surrounding landscape (up to 88% reduced tree cover within 5 km). The broad suite of measurements made at CTFS-ForestGEO sites makes it possible to investigate the complex ways in which global change is impacting forest dynamics. Ongoing research across the CTFS-ForestGEO network is yielding insights into how and why the forests are changing, and continued monitoring will provide vital contributions to understanding worldwide forest diversity and dynamics in an era of global change.
Introduction
Forests play key roles in biodiversity maintenance and climate regulation. Globally, they support over half of all described species and provide a range of valuable ecosystem services (Groombridge, 2002; Pan et al., 2013). Forests play a particularly significant role in climate regulation; they contain ~45% of carbon (C) in the terrestrial biosphere and influence climate on local to global scales through their low albedo and high rates of evapotranspiration (Snyder et al., 2004; Bonan, 2008; Anderson-Teixeira et al., 2012; Pan et al., 2013). Global change pressures – including climate change, pollution, agricultural expansion, logging, nontimber forest product extraction, hunting, and the spread of invasive species – are affecting forests worldwide, threatening biodiversity, altering community composition, and driving feedbacks to climate change (Foley et al., 2005; Chapin et al., 2008; Wright, 2010). Understanding and predicting such changes will be critical to biodiversity conservation, management of ecosystem services, and climate protection.
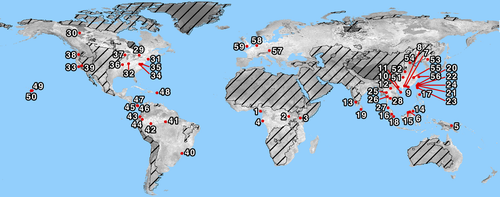
The Center for Tropical Forest Science (CTFS) – Forest Global Earth Observatory (ForestGEO) is a global network of forest research sites that is strategically poised for monitoring, understanding, and predicting forest responses to global change. This international partnership currently includes 59 long-term forest dynamics research sites in 24 countries (Fig. 1), which have been monitored continuously since as early as 1981 (Barro Colorado Island; Condit, 1995). The network applies a unique standardized tree census protocol across all of the world's major forest biomes, allowing comparison across sites (e.g., Condit, 2000; Muller-Landau et al., 2006a,b; Chave et al., 2008; Chisholm et al., 2013, 2014). Supplementary measurements, also following standardized procedures, provide additional information on plants, animals, and ecosystem processes, making it possible to identify ecological interactions that might otherwise be missed (e.g., Harrison et al., 2013). This review describes the defining features of a CTFS-ForestGEO plot, the distribution and representativeness of CTFS-ForestGEO sites, supplementary measurements and their applications, global change pressures across the CTFS-ForestGEO network, and the impacts of these drivers documented to date.
Attributes of a CTFS-ForestGEO plot
The unifying measurement at all CTFS-ForestGEO sites is an intensive census of all freestanding woody stems ≥1 cm diameter at breast height (DBH), typically repeated every 5 years, that characterizes forest structure, diversity and dynamics over a large spatial area (Table 1). Plot sizes are large, ranging from 2 to 120 ha, with a median size of 25 ha and 90% ≥10 ha (Table 2). Following standardized methodology, each individual (genet) is mapped, tagged, and identified to species when it first enters the census. In the case of multistemmed individuals, each stem ≥1 cm DBH (ramet) is censused. On each stem, diameter is measured at breast height (1.3 m) or above stem irregularities (Manokaran et al., 1990; Condit, 1998). The census includes both trees and shrubs; henceforth, the term “trees” will refer to all individuals in the census. An accompanying fine-scale topographic survey allows identification of topographically defined habitat types (e.g., ridges, valleys, slopes; Condit, 1998). This core CTFS-ForestGEO protocol has proved useful for a wide range of analyses (Table 1).
| Attribute | Utility |
|---|---|
| Very large plot size | Resolve community and population dynamics of highly diverse forests with many rare species with sufficient sample sizes (Losos & Leigh, 2004; Condit et al., 2006); quantify spatial patterns at multiple scales (Condit et al., 2000; Wiegand et al., 2007a,b; Detto & Muller-Landau, 2013; Lutz et al., 2013); characterize gap dynamics (Feeley et al., 2007b); calibrate and validate remote sensing and models, particularly those with large spatial grain (Mascaro et al., 2011; Réjou-Méchain et al., 2014) |
| Includes every freestanding woody stem ≥1 cm DBH | Characterize the abundance and diversity of understory as well as canopy trees; quantify the demography of juveniles (Condit, 2000; Muller-Landau et al., 2006a,b). |
| All individuals identified to species | Characterize patterns of diversity, species-area, and abundance distributions (Hubbell, 1979, 2001; He & Legendre, 2002; Condit et al., 2005; John et al., 2007; Shen et al., 2009; He & Hubbell, 2011; Wang et al., 2011; Cheng et al., 2012); test theories of competition and coexistence (Brown et al., 2013); describe poorly known plant species (Gereau & Kenfack, 2000; Davies, 2001; Davies et al., 2001; Sonké et al., 2002; Kenfack et al., 2004, 2006) |
| Diameter measured on all stems | Characterize size-abundance distributions (Muller-Landau et al., 2006b; Lai et al., 2013; Lutz et al., 2013); combine with allometries to estimate whole-ecosystem properties such as biomass (Chave et al., 2008; Valencia et al., 2009; Lin et al., 2012; Ngo et al., 2013; Muller-Landau et al., 2014) |
| Mapping of all stems and fine-scale topography | Characterize the spatial pattern of populations (Condit, 2000); conduct spatially explicit analyses of neighborhood influences (Condit et al., 1992; Hubbell et al., 2001; Uriarte et al., 2004, 2005; Rüger et al., 2011, 2012; Lutz et al., 2014); characterize microhabitat specificity and controls on demography, biomass, etc. (Harms et al., 2001; Valencia et al., 2004; Chuyong et al., 2011); align on the ground and remote sensing measurements (Asner et al., 2011; Mascaro et al., 2011). |
| Census typically repeated every 5 years | Characterize demographic rates and changes therein (Russo et al., 2005; Muller-Landau et al., 2006a,b; Feeley et al., 2007a; Lai et al., 2013; Stephenson et al., 2014); characterize changes in community composition (Losos & Leigh, 2004; Chave et al., 2008; Feeley et al., 2011; Swenson et al., 2012; Chisholm et al., 2014); characterize changes in biomass or productivity (Chave et al., 2008; Banin et al., 2014; Muller-Landau et al., 2014) |
| No. | Site | Country | Köppen Climate zoneb | MAT (°C)c | MAP (mm yr−1)d | Dominant Soil order(s)d | Dominant vegetation type(s)e | Natural Dist. Regimef | N species | Plot Size (ha) | Year establishedg |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Afrotropics | |||||||||||
| 1 | Korup | Cameroon | Am | 26.6 | 5272 | Ult, Ox | BE | W | 494 | 55 | 1996 |
| 2 | Ituri (Edoro and Lenda) | Democratic Republic of Congo | Af | 24.3 | 1682 | Ox | BE | W; A | 445 | 40 | 1994 |
| 3 | Rabi | Gabon | Aw | 26.0 | 2282 | Ox | BE | W | 342 | 25 | 2010 |
| 4 | Mpala | Kenya | Cfb | 17.9 | 657 | Alf; Ve | BdD | Fi, A | 22 | 120 | 2011 |
| Australasia | |||||||||||
| 5 | Wanang | Papua New Guinea | Af | 26.0 | 3500 | Alf; In | BE | L; E | 500a | 50 | 2009 |
| Indo-Malaya | |||||||||||
| 6 | Kuala Belalong | Brunei Darussalam | Af | 26.5 | 5203 | Ult | BE | L | 850–1050a | 25 | 2009 |
| 7 | Dinghushan | China | Cfa | 20.9 | 1985 | BE | 210 | 20 | 2005 | ||
| 8 | Heishiding | China | Cfa | 22.0 | 1744 | BE | 245 | 50 | 2013 | ||
| 9 | Hong Kong | China | Cwa | 23.3 | 2399 | Ox | BE | H | 67–147a | 21 | 2012 |
| 10 | Jianfengling | China | Aw | 19.8 | 1657 | Ult | BE | H | 291 | 60 | 2012 |
| 11 | Nonggang | China | Cwa | 22.0 | 1376 | Ox | BE; BdD | D | 223 | 15 | 2011 |
| 12 | Xishuangbanna | China | Cwa | 21.8 | 1493 | Ox | BE | W; D | 468 | 20 | 2007 |
| 13 | Mudumalai | India | Aw | 22.7 | 1255 | Alf | BdD | Fi; A; D | 72 | 50 | 1987 |
| 14 | Danum Valley | Malaysia | Af | 26.7 | 2822 | UIt | BE | D; A | a | 50 | 2010 |
| 15 | Lambir | Malaysia | Af | 26.6 | 2664 | Ult | BE | L; D | 1182 | 52 | 1991 |
| 16 | Pasoh | Malaysia | Af | 27.9 | 1788 | Ult | BE | W | 814 | 50 | 1986 |
| 17 | Palanan | Philippines | Af | 26.1 | 3380 | Ult; In | BE | H | 335 | 16 | 1994 |
| 18 | Bukit Timah | Singapore | Af | 26.9 | 2473 | Ult | BE | A | 347 | 4 | 1993 |
| 19 | Sinharaja | Sri Lanka | Af | 22.5 | 5016 | Ult | BE | W | 204 | 25 | 1993 |
| 20 | Fushan | Taiwan | Cfa | 18.2 | 4271 | Ult; In | BE | H | 110 | 25 | 2004 |
| 21 | Kenting | Taiwan | Am | 25.4 | 1964 | BE | H | 95 | 10 | 1996 | |
| 22 | Lienhuachih | Taiwan | Cwb | 20.8 | 2211 | Ult | BE | H; L | 144 | 25 | 2008 |
| 23 | Nanjenshan | Taiwan | Aw | 23.5 | 3582 | Ult | BE; BdD | W; H | 125 | 6 | 1989 |
| 24 | Zenlun | Taiwan | Am | 22.7 | 2620 | NE | H | 12 | 2005 | ||
| 25 | Doi Inthanon | Thailand | Aw | 20.9 | 1908 | UIt | BE | – | 162 | 15 | 1997 |
| 26 | Huai Kha Khaeng (HKK) | Thailand | Aw | 23.5 | 1476 | Alf | BE; BdD | Fi; D | 251 | 50 | 1992 |
| 27 | Khao Chong | Thailand | Am | 27.1 | 2611 | Ult; In | BE | W; L | 593 | 24 | 2000 |
| 28 | Mo Singto | Thailand | Aw | 23.5 | 2100 | BE; BdD | W | 262 | 30.5 | 2000 | |
| Nearctic | |||||||||||
| 29 | Haliburton | Canada | Dfb | 4.2 | 962 | BcD | – | 30 | 13.5 | 2007 | |
| 30 | Scotty Creek | Canada | Dfc | −3.2 | 369 | Ge | NE | PT; Fi | 12–15a | 21 | 2013 |
| 31 | Harvard Forest | USA | Dfb | 9.0 | 1050 | In | BdD | H | 60 | 35 | 2010 |
| 32 | Lilly Dickey Woods | USA | Cfa | 11.6 | 1203 | In; Ult; Alf | BcD | W; D; Ic | 35 | 25 | 2009 |
| 33 | Santa Cruz | USA | Csb | 14.8 | 778 | Mo | BE; NE | Fi,W | 33 | 16 | 2007 |
| 34 | Smithsonian Conservation Biology Institute (SCBI) | USA | Cfa | 12.9 | 1001 | Alf | BcD | W, Ic | 64 | 25.6 | 2008 |
| 35 | Smithsonian Environmental Research Center (SERC) | USA | Cfa | 13.2 | 1068 | Ult; In; En | BcD | H; W | 79 | 16 | 2007 |
| 36 | Tyson Research Center | USA | Cfa | 13.5 | 957 | Alf | BcD | D; Fi; Ic; W | 42 | 20 | 2013 |
| 37 | Wabikon | USA | Dfb | 4.2 | 805 | Alf | BcD | W | 42 | 25.6 | 2008 |
| 38 | Wind River | USA | Csb | 9.2 | 2495 | An | NE | Fi; W; In | 26 | 25.6 | 2010 |
| 39 | Yosemite National Park | USA | Csb | 10.2 | 1065 | Alf | NE | Fi; W; D; In | 23 | 25.6 | 2009 |
| Neotropics | |||||||||||
| 40 | Ilha do Cardoso | Brazil | Cfa | 22.4 | 2100 | S | BE | 106 | 10.2 | 2004 | |
| 41 | Manaus | Brazil | Af | 26.7 | 2600 | Ox | BE | W | 1440a | 25 | 2004 |
| 42 | Amacayacu | Colombia | Af | 25.8 | 3215 | Ult | BE | Fl | 1133 | 25 | 2006 |
| 43 | La Planada | Colombia | Cfb | 19.0 | 4087 | An | BE | W | 240 | 25 | 1997 |
| 44 | Yasuni | Ecuador | Af | 28.3 | 3081 | Ult | BE | – | 1114 | 50 | 1995 |
| 45 | Barro Colorado Island (BCI) | Panama | Am | 27.1 | 2551 | Ox | BdD; BE | D; W | 299 | 50 | 1981 |
| 46 | Cocoli | Panama | Am | 26.6 | 1950 | Ox; In | BdD; BE | D; W | 176 | 4 | 1994 |
| 47 | San Lorenzo/Sherman | Panama | Am | 26.2 | 3030 | BE | D; W | 238 | 6 | 1996 | |
| 48 | Luquillo | Puerto Rico, USA | Am | 22.8 | 3548 | Ox; Ult | BE | H; L | 138 | 16 | 1990 |
| Oceania | |||||||||||
| 49 | Laupahoehoe | USA | Cfb | 16.0 | 3440 | An | BE | W | 21 | 4 | 2008 |
| 50 | Palamanui | USA | Cfb | 20.0 | 835 | Hi | BE | W | 15 | 4 | 2008 |
| Palearctic | |||||||||||
| 51 | Badagongshan | China | Cfa | 15.9 | 1410 | In | BE; BdD | Fl | 238 | 25 | 2011 |
| 52 | Baotianman | China | Cwa | 15.1 | 886 | BdD | A; D | 126 | 25 | 2009 | |
| 53 | Changbaishan | China | Dwb | 2.9 | 700 | Alf | NE; BcD | 52 | 25 | 2004 | |
| 54 | Donglingshan | China | Dwb | 4.7 | 570 | Alf | BcD | Fi | 58 | 20 | 2010 |
| 55 | Gutianshan | China | Cfa | 15.3 | 1964 | Ult | BE; BdD | Ic | 159 | 24 | 2005 |
| 56 | Tiantongshan | China | Cfa | 16.2 | 1375 | Ox | BE | H; D | 153 | 20 | 2008 |
| 57 | Zofin | Czech Republic | Cfb | 6.2 | 866 | S; In; Hi | BcD; NE | W; In | 12 | 25 | 2012 |
| 58 | Speulderbos | Netherlands | Cfb | 10.1 | 833 | In | BcD | W; A | 13 | 27 | 2013 |
| 59 | Wytham Woods | UK | Cfb | 10.0 | 717 | E | BcD | 23 | 18 | 2008 | |
- a Measurement in progress.
- b Af: Tropical with significant precipitation year-round; Am: Tropical monsoon; Aw: Tropical wet and dry; Csb-Dry-summer subtropical/mid-latitude climate with dry summers (a.k.a.: Warm-summer Mediterranean); Cfa: Humid subtropical/mid-latitude climate with significant precipitation year-round; Cwa: Humid subtropical/midlatitude climate with dry winters; Cfb: Oceanic with significant precipitation year-round; Cwb: Oceanic with dry winters; Dfb: Humid Continental with significant precipitation year-round; Dwb: Humid continental with dry winters; Dfc: Subarctic.
- c Climate data are the best available for each site (based on judgment of site PIs; years vary). For sites where local data are not available or not reported, values (italicized) are mean 1950–2000 climate from WorldClim at 30 arcsecond resolution (Table S4; Hijmans et al., 2005).
- d Categorical following the USDA Soil Taxonomy System (Soil Survey Staff, 1999): Alf, Alfisols; An, Andisols; E, Entisoils; Ge, Gelisols; Hi, Histosols; In, Inceptisols; Ox, Oxisols; Ult, Ultisols; S, Spodosols; Ve, Vertisols.
- e BE, broadleaf evergreen; BdD, broadleaf drought deciduous; BcD, broadleaf cold deciduous; NE, needleleaf evergreen.
- f A, animal activity (destructive); D, Drought; E, Erosion; Fi, Fire; Fl, flood; H, hurricane/typhoon; Ic, Ice storms; Ininsect outbreaks; L, landslides; PT, permafrost thaw; W, wind storms (local); ‘–’, no major natural disturbances.
- g When census spanned multiple years, the first year is listed.
Site distribution and representativeness
This core tree census protocol has been applied to 59 sites distributed among all of the world's major forest biomes, making CTFS-ForestGEO the only international forest monitoring network with global distribution (Fig. 1; Table 2). In total, 1653 ha of forest (>5.68 million individuals) are currently monitored, with a cumulative sum of >17 000 ha-years of forest monitoring.
CTFS-ForestGEO sites cover a wide diversity of physical and biotic environments (Figs 1 and 2; Table 1, Table S1). The network spans latitudes 25°S–61°N, with sites in every biogeographic realm (sensu Olson et al., 2001; Table 1, Table S1). Climate varies widely (Fig. 2; Table 1, Table S2): mean annual temperature (MAT) ranges from −3.2 °C (Scotty Creek, Canada) to 28.3 °C (Yasuni, Ecuador), and mean annual precipitation (MAP) from 369 mm yr−1 (Scotty Creek, Canada) to 5272 mm yr−1 (Korup, Cameroon). Elevation ranges from 3 m.a.s.l. (Ilha do Cardoso, Brazil) to 1911 m.a.s.l. (Yosemite, USA), and relief from 4 m (SERC, USA) to 298 m (Tiantongshan, China; Table S1). According to the Soil Survey Staff (1999) soil classification, 11 of the world's 12 soil orders are represented (the exception is Aridisols; Table 1), with corresponding marked variation in fertility.
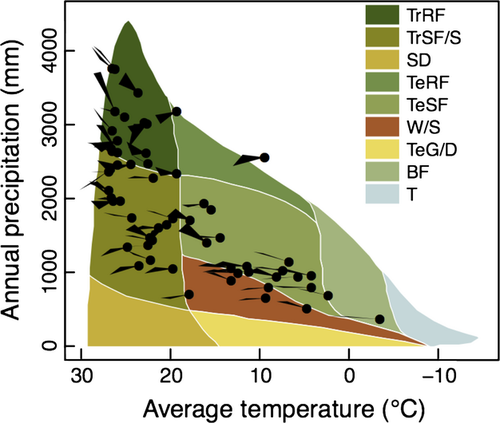
The CTFS-ForestGEO network is generally representative of the range of bioclimatic, edaphic, and topographic conditions experienced by forests globally (Fig. 1), as evidenced by a multivariate spatial clustering analysis with 4 km resolution (Hargrove et al., 2003; Hoffman et al., 2013; Maddalena et al., 2014; Appendix S1). Particularly well-represented regions include tropical rain forests on upland or ‘tierra firme’ habitats – especially in the Indo-Malay biogeographic zone – and temperate forests of Eastern China and Eastern North America. Underrepresented regions include temperate forests in the Southern Hemisphere; seasonal forests and woodland savannas south and east of the Amazon and in Africa; the Rocky Mountains of North America; and boreal forests – particularly in the Palearctic biogeographic zone. On a finer scale, many of the CTFS-ForestGEO sites in Asia, Europe, and North America are on more topographically complex terrain compared to the original forest distribution, as are most remaining intact forests in these regions. Forests with extreme edaphic conditions – for example, mangrove, swamp, and peat forests – remain almost completely unrepresented.
Dominant vegetation types of the CTFS-ForestGEO sites include broadleaf evergreen, broadleaf drought deciduous, broadleaf cold deciduous, and needle-leaf evergreen forests (Table 1). Floristically, the network has extensive coverage, with >10 000 tree and shrub species (and >14 000 unique site-species combinations). Unique tree floras that are not yet represented include the high-endemism forests of Madagascar; southern temperate forests in New Zealand, Australia, and southern South America; and dry forests in Africa and the southern and eastern Amazon.
The sites are generally in old-growth or mature secondary forests and are commonly among the most intact, biodiverse, and well-protected forests within their region. They are subjected to a range of natural disturbances (Table 1), and a number of sites have experienced significant natural disturbances in recent years (e.g., fire at Yosemite, typhoons at Palanan). In addition, most sites have experienced some level of anthropogenic disturbance (discussed below; Table S5).
Supplementary measurements and applications
At all sites, the core census is complemented by one or more supplementary measurements that provide further basis for standardized comparisons across the world's major forest biomes. Supplementary measurements provide additional information on plants, animals, and ecosystem and environmental variables (Table 3). In this section, we review CTFS-ForestGEO -specific protocols and other relatively standard measurements that are comparable across sites. The Supplementary Information section provides further information on methodologies (Appendix S2) and details which measurements have been made at each site (Tables S6 and S7).
| Measurement | N sitesa | Description | Utility |
|---|---|---|---|
| Plants | |||
| Lianas | 7 (15) | Lianas are included as part of the core census; they are mapped, identified to species, and measured at breast height (1.3 m) | Characterize liana abundance and diversity and changes therein (Schnitzer, 2005; DeWalt et al., 2015; Thomas et al., 2015); understand liana impacts on tree community (Ingwell et al., 2010). |
| Functional traits | 33 (39)b | Traits characterized include three dimensions (maximum height and crown diameter); leaf traits [size, specific leaf area, thickness, (N), (P), dry matter content]; wood traits (stem wood density, C content); and reproductive traits (dispersal mode; fruit, diaspore, and seed fresh and dry masses). | Characterize species’ differences in physiology and ecological roles (Condit et al., 1996; Santiago & Wright, 2007; Muller-Landau et al., 2008; Kraft et al., 2010; Wright et al., 2010; Westbrook et al., 2011; Katabuchi et al., 2012; Liu et al., 2012); detect directional changes in functional composition (Feeley et al., 2011; Hietz et al., 2011; Swenson et al., 2012; Harrison et al., 2013); improve inventory-based C stock estimates (Martin & Thomas, 2011; Cushman et al., 2014); parameterize models |
| High-precision diameter growth | 28 (32) | Diameter growth is measured weekly to annually using dendrometer bands on a subset of trees. | Understand effects of tree size, species, and environmental conditions on growth; characterize seasonal growth patterns (McMahon & Parker, 2014); estimate the woody stem growth component of aboveground net primary productivity (ANPPwood) |
| Flower & seed production | 24 (33) | Species-level flower & seed production are quantified using weekly to bimonthly censuses of 60–336 0.5-m2 traps. | Quantify reproductive phenology (Zimmerman et al., 2007); infer seed dispersal distances (Muller-Landau et al., 2008); quantify interannual variation and its ecological implications (Wright et al., 1999, 2005; Harms et al., 2000; Usinowicz et al., 2012); detect directional changes (Wright & Calderon, 2006) |
| Seedling performance | 21 (30) | Seedling establishment, growth and survival are quantified annually in three 1-m2 plots associated with each seed trap. | Characterize density- and distance-dependent effects on con- and hetero-specific seedling recruitment (Harms et al., 2000; Comita et al., 2010; Lebrija-Trejos et al., 2013); Understand postdisturbance successional dynamics (Dalling et al., 1998; Dalling & Hubbell, 2002) |
| DNA barcoding | 27 (28) | Short DNA sequences from a standard position within the genome are used to construct phylogenies and distinguish individual species from one another. Can be applied to all tissues of the plants (e.g., roots, pollen, leaves, and bark) or animals. Over 3000 plant species have been barcoded to date. | Build phylogenetic trees of local community relationships and investigate constraints on the assembly of communities (Pei et al., 2011; Swenson et al., 2011; Lebrija-Trejos et al., 2013); identify tree roots to species (Jones et al., 2011); reconstruct networks of feeding, pollination, and parasitism −(Hrcek et al., 2011) |
| Animals | |||
| Arthropods | 5 (13) | A variety of key taxa are monitored 1–4 times annuallycusing a variety of techniques (light traps, Winkler extractors, McPhail traps, butterfly transects, termite transects, and bee baits). | Elucidate the role of arthropods in forest ecosystems (Novotny et al., 2002; Novotny & Basset, 2005); evaluate the impact of global change on the full range of forest trophic levels |
| Vertebrates | 14 (34) | Camera trapping is used to monitor terrestrial mammals. | Elucidate the role of vertebrates in forest ecosystems; detect directional changes |
| Ecosystem & Environmental | |||
| Aboveground biomass | 59 | Ground based: Biomass is estimated from core census data using best available allometries, often in combination with site-specific height and wood density data. | Characterize spatial variation in biomass within sites in relation to environmental gradients and species diversity (Valencia et al., 2009; Chisholm et al., 2013); detect directional changes in C stocks (Chave et al., 2008; Muller-Landau et al., 2014); calibrate and evaluate models of biomass based on airborne LiDAR (Asner et al., 2011; Mascaro et al., 2011; Réjou-Méchain et al., 2014) |
| (15) | Airborne: LiDAR flights (one-time or repeated) provide data on biomass and tree architecture. | ||
| Dead wood/CWD | 21 (25) | Standing dead wood and fallen coarse woody debris are surveyed by transect or comprehensive survey. | Quantify C stocks in dead wood and changes therein |
| Fine root biomass & soil carbon | 16 (32) | Measured to 3 m depth on every hectare, with additional replicates to shallower depths. | Understand the role of associations between plants and mycorrhizal fungi in driving soil carbon storage (Peay et al., 2010; Averill et al., 2014) |
| Soil nutrients | 23 (26) | Extractable soil cations, available N, nitrogen mineralization rates, and extractable phosphorus at 0 to 10-cm depths are measured at high spatial resolution. | Characterize species’ microhabitat associations (Lee et al., 2002; Davies et al., 2003; John et al., 2007; Tan et al., 2009; Baldeck et al., 2013a,b,c; De Oliveira et al., 2014); characterize plant performance in relation to soil nutrients (Russo et al., 2005, 2013) |
| Litterfall | 21 (29) | Litter is collected biweekly to monthly from traps, oven-dried, sorted (to leaves, woody, reproductive, and other), and weighed. | In combination with woody growth data, quantify aboveground net primary productivity (ANPP) and its phenology and environmental drivers |
| Bio-micrometeorology | (13) | Eddy-covariance technique is used to continuously measure CO2, H2O, and energy exchange between ecosystem and the atmosphere. | Understand forest ecophysiology and C cycling on half-hourly to multiannual time scales |
| Meteorology | 5(33) | Some sites have local meteorological stations within 10 km of the plot. | Characterize climatic controls on forest processes such as flower and fruit production, tree growth and mortality, and ecosystem-atmosphere gas exchange (Condit et al., 2004; Wright & Calderon, 2006; Feeley et al., 2007a; Dong et al., 2012; Li et al., 2012) |
- a Numbers indicate sites where measurements have been made or are in progress following a specific CTFS Forest GEO protocol. Numbers in parentheses indicate total number of sites with measurements using any protocol.
- b Varies by trait. Number indicates sites with measurements of one or more functional traits.
- c Varies by protocol. See Appendix S1 for details.
Plants
Supplementary measurements on plants include liana abundance and diversity, functional traits, high-precision diameter growth, flower and seed production, seedling performance, and DNA barcoding (Table 3). Liana censuses help to characterize the important role of lianas in forest dynamics. Measurements of functional traits – well-defined, measurable properties of organisms that are strongly associated with ecological performance – provide information on key attributes and ecological roles of the species included in the census. High-precision growth measurements provide fine-scale understanding of temporal and spatial variation in tree growth and forest productivity. Flower, seed and seedling censuses enable study of complete tree life cycles, which are critically important for forest regeneration and long-term species persistence. DNA barcoding provides a powerful means of species identification that allows elucidation of phylogenetic relationships and ecological roles (Dick & Kress, 2009; Kress et al., 2009, 2010).
Animals-
Arthropod and vertebrate initiatives (Table 3) yield understanding of the roles of these taxa in forest dynamics through their roles as herbivores, pollinators, seed dispersers, predators, ecosystem engineers, and vectors of microbial diversity. In a unique effort to monitor multitaxon assemblages in tropical rainforests (Basset et al., 2013; but see Leidner et al., 2010 for long-term monitoring of a single taxon), key arthropod groups are being monitored to better understand how interactions between arthropods and plants affect forest dynamics and to evaluate the impact of global change on the full range of forest trophic levels. Vertebrate monitoring is helping to elucidate how mammals differentially affect tree species and how modification of the fauna may impact the future forest (e.g., Wright et al., 2007; Harrison et al., 2013; see below).
Ecosystem and environmental
Supplementary measurements of ecosystem and environmental variables include major aboveground C stocks and fluxes (aboveground biomass, standing dead wood and coarse woody debris, ANPPwood, litterfall, net ecosystem exchange); soil nutrients, C, and fine root biomass; bio-micrometeorology, and meteorology (Table 3). These measurements provide a basis for understanding environmental and biotic controls on C stocks and fluxes within forest ecosystems and how these may respond to global change. Soils measurements provide a basis for understanding the critical role of soils in determining species composition, forest structure, and primary productivity, as well as their globally significant role as an important C reservoir. Bio-micrometeorological measurements further elucidate the important role of forests in climate regulation through ongoing exchange of CO2, H2O, and energy between the ecosystem and the atmosphere. Meteorological data are critical for understanding how the biotic community and whole ecosystem processes respond to climate variables over half-hourly to multiannual time scales.
Combined applications
In combination, the core tree census and supplementary measurements enable unique analyses of the many interacting components of forest ecosystems, yielding a holistic picture of forest dynamics. For instance, core census data have been combined with data on lianas, vertebrates, seeds, seedlings, and reproductive functional traits to link decreasing populations of seed dispersers to changing patterns of plant reproduction, liana abundance, and tree growth and survival (Wright & Calderon, 2006; Wright et al., 2007; Ingwell et al., 2010; Harrison et al., 2013). Core census, functional trait, and DNA barcoding data have been combined to understand the roles of phylogeny and functional traits in shaping habitat associations and diversity in space and time (Pei et al., 2011; Swenson et al., 2011). The combination of core census data, plant functional traits, ecosystem measurements, soils data, and weather data lend themselves to parameterizing and evaluating ecosystem and earth system models. Thus, the broad suite of standardized measurements at CTFS-ForestGEO sites (Tables 1 and 3) provides opportunities to address a multitude of questions on forest dynamics and their responses to global change pressures.
Global change pressures at CTFS-ForestGEO sites
All ecosystems on Earth – including CTFS-ForestGEO's relatively intact forests – are affected by anthropogenic influences (Fig. 3). Human appropriation of land and water for agriculture and other purposes; emission of extraneous compounds to the atmosphere (e.g., CO2, CH4, N2O, NOy, NHx, SO2) and water (e.g., NO3−, PO43−); extraction of food, fuel, and fiber from natural ecosystems; and transport of species around the globe has so pervasively influenced Earth's climate, hydrology, biogeochemistry, land cover, and species diversity as to warrant classification of a new geologic period in Earth's history – the Anthropocene (Schlesinger, 2012; Vitousek et al., 1997a; Zalasiewicz et al., 2010, 2011).
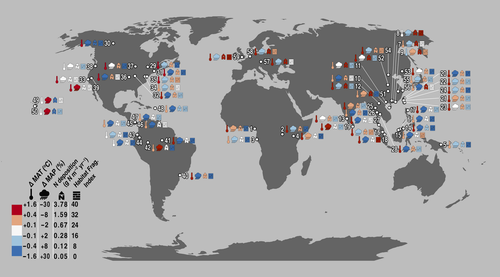
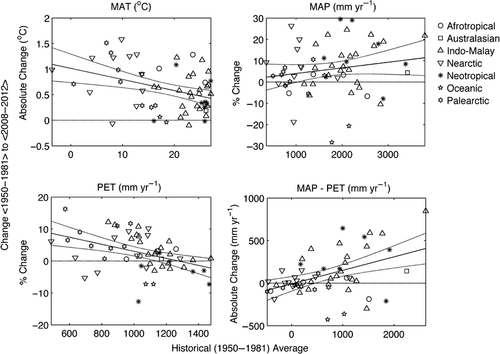
Over the lifetime of the CTFS-ForestGEO network, atmospheric CO2 has increased 16%, from 340 ppm in 1981 to 396 ppm in 2013 (Tans & Keeling, 2014), with variable effects on climate globally. Over a similar time frame, temperatures have increased across the network by an average of 0.61 °C, with greater increases at colder sites (Figs 3 and 4; Table S3; details on data and analysis in Appendix S1). On both annual and daily time scales, minimum temperatures have increased more than maximum temperatures, leading to decreases in the diurnal temperature range. Frost-day frequency has decreased at sites that experience frost. Potential evapotranspiration (PET) has increased slightly on average (+2.5%) – particularly at low-PET sites. A tendency for increased cloud cover has offset the increases in PET that would be expected based on temperature increases alone, and high-PET sites have therefore experienced little change in PET on average (Fig. 4). Changes in mean annual precipitation (MAP) and wet-day frequency have been variable, with an overall tendency toward increases (averaging 6.0% and 2.7%, respectively) – particularly at high-precipitation sites (Fig. 4). Changes to the difference between annual MAP and PET have also been variable, with a tendency for wet sites (high MAP-PET) to become wetter – particularly in the Neotropical and Indo-Malay biogeographic zones – and low MAP-PET sites to become drier (Fig. 4). Changes in seasonality and the number of months with precipitation<PET have been variable across the network. In summary, CTFS-ForestGEO sites have experienced warming and variable changes in precipitation and aridity.
Ongoing climate change is inevitable, with its course dependent upon future greenhouse gas emissions and land use patterns (IPCC, 2013). The IPCC AR5 examines four representative concentration pathways (RCP's), the most optimistic of which has greenhouse gas emissions going to zero before 2100 (RCP 2.6) and the most pessimistic of which denotes continuously increasing emissions leading to a radiative forcing of 8.5 W m−2 by 2100 (RCP 8.5; IPCC, 2013). Across this range of future scenarios, the HADGEM2-ES model predicts MAT increases averaging 2.0 °C under RCP 2.6 (range: 1.2–3.6 °C) to 3.0 °C under RCP 8.5 (range: 1.9–5.7 °C) across the CTFS-ForestGEO sites (Fig. 2; Table S4). This warming will push some tropical forests into climates with no current analog (Fig. 2). Predicted changes in annual precipitation at these sites range from −8.6% to +19.0% under RCP 2.6 and −13.6% to +7.3% under RCP 8.5 (Fig. 2; Table S4). When coupled with predicted warming and associated increases in potential evapotranspiration, constant or decreasing precipitation – which is predicted for approximately half the sites (Fig. 2, Table S4) – implies that conditions will become more arid. At most CTFS-ForestGEO sites, soil moisture and relative humidity are predicted to decline in the near-term (i.e., 2016–2035), even under a modest emissions scenario (Kirtman et al., 2013; Sherwood & Fu, 2014).
The biogeochemistry of these sites has also been modified by human activities. The global nitrogen (N) cycle has been dramatically transformed by human activities (Schlesinger, 2012; Vitousek et al., 1997a; Galloway et al., 2008; Canfield et al., 2010). Atmospheric deposition of reactive N can fertilize forests that are N limited (Magnani et al., 2007; Yu et al., 2014), and can also impair ecosystem function through soil acidification and N saturation (Aber et al., 1989; Schlesinger, 2012; Vitousek et al., 1997b). At CTFS-ForestGEO sites, current N deposition has a median value of 0.9 g N m−2 yr−1 and ranges from 0.05 (Scotty Creek) to 3.8 g N m−2 yr−1 (Badagongshan, China; Fig. 3), implying that N deposition at many sites may exceed critical loads for soil acidification (Bouwman et al., 2002). In addition, sulfuric acid deposition reduces soil fertility (e.g., Likens et al., 1996) and increases tree mortality (Dietze & Moorcroft, 2011). Across the network, annual SOx deposition has a median value of 0.5 g S m−2 yr−1 (range 0.08 g S m−2 yr−1 at Mpala, Kenya to 3.1 g S m−2 yr−1 at Tiantongshan, China; Table S5; data from Dentener et al., 2006; see Appendix S1 for details). Nitrogen and sulfur deposition is predicted to continue to increase in the future (Dentener et al., 2006).
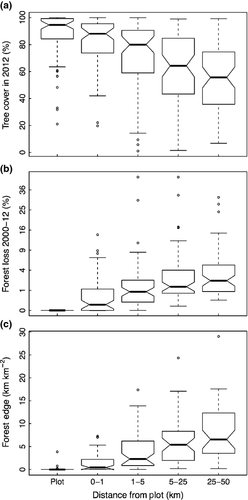
At the local level, CTFS-ForestGEO sites have also been directly exposed to a range of past and ongoing anthropogenic perturbations. Some sites and their surrounding areas were partially to fully logged in the past, and in some cases the land was used for farming or pasture (Table S5). Historical and contemporary forest loss (through deforestation or natural stand-clearing disturbance) in surrounding areas has exposed some sites to severe habitat fragmentation, whereas others are surrounded by vast expanses of near-pristine forest (Figs 3 and 5; Table S5). By the year 2012, 27 sites (primarily in Europe, North America, and Asia) had tree cover within a 5 km radius reduced by more than 10% relative to tree cover in the plot, and seven sites even had reductions >40%. Generally speaking, percent tree cover on the landscape decreases with distance from the site, while recent (2000–2012) forest loss rates and forest fragmentation increase (Fig. 5; data from Hansen et al., 2013; see Appendix S1 for details). In addition to forest loss in the surrounding landscapes, the majority of sites have been exposed to past and/or ongoing extraction of timber or nontimber forest products, hunting, or invasive species (Table S5). A few sites are have high human population density in the surrounding areas and are affected by urbanization.
Forest responses to global change
As described above, all CTFS-ForestGEO sites are experiencing multifaceted global change pressures (Fig. 3). With spatially explicit dynamic tree data for large forest dynamics plots and the additional measurements summarized above (Table 2), the network is poised to advance mechanistic understanding of the impact of global and environmental change on the world's forests.
Are forests changing?
Change is the natural condition of forests (e.g., Baker et al., 2005; Laurance et al., 2009), which makes it challenging to detect and attribute directional responses to global change pressures. A key finding from the network is that forests generally, and in particular tropical forests, are highly dynamic; for instance, in the first 18 years of monitoring at BCI, >40% of trees ≥1 cm DBH (or 34% ≥10 cm DBH) turned over, and 75% of all species changed in abundance by >10% (Leigh et al., 2004). Superimposed upon this dynamism, forests are responding to global change pressures. Data from the network reveal some generalities and long-term trends of change in forests worldwide.
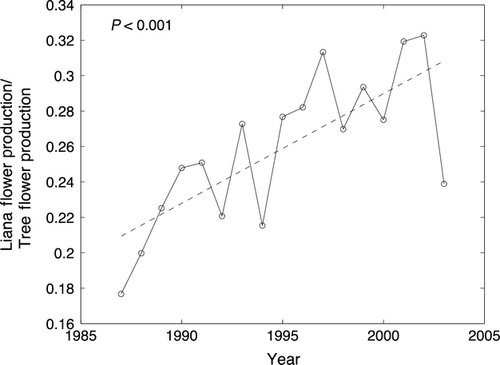
Forest composition in terms of species and functional groups has changed at multiple sites across the network, in different directions at different sites (Condit et al., 1996; Chave et al., 2008; Feeley et al., 2011; Makana et al., 2011; Swenson et al., 2011). An analysis of data from twelve CTFS-ForestGEO sites reveals that environmental variability – as opposed to demographic stochasticity – is the most important factor driving tree population dynamics on decadal time scales (Chisholm et al., 2014). Across relatively undisturbed tropical forests, the dominance of slow-growing species increased at nine of ten sites analyzed (significantly so at five sites), indicating that these forests may be recovering from past disturbances, even as they are impacted by a variety of global change pressures (Chave et al., 2008). In addition, at six tropical sites monitored over more than 10 years, there have been long term increases in the proportions of flowers and seeds produced by lianas (Fig. 6; Wright & Calderon, 2006; Wright, unpublished analysis) – a trend that corresponds with long term increases in the abundance of lianas observed on BCI (Panama) and elsewhere in the tropics (Ingwell et al., 2010; Schnitzer & Bongers, 2011). While community change appears to be the rule rather than the exception across the network, and while there have been some instances of rapid change in forest composition (e.g., Condit et al., 1995; Chave et al., 2008), there have not been any hugely dramatic changes such as a forest die-off affecting the majority of large trees at the network sites.
Trends in various components of aboveground net primary productivity (ANPP) have also been monitored at some sites. Across the network, the woody component of NPP (ANPPwood) has increased or decreased, as a function of both climate change and succession. Forests globally are mixed in terms of their productivity trends (Laurance et al., 2004; Clark et al., 2010; Gedalof & Berg, 2010; Wright, 2010). For instance, decreases in ANPPwood were observed in tropical forests in Panama (BCI) during 1981–2005 and Malaysia (Pasoh) during 1990–2000 (Feeley et al., 2007b) and increases in ANPPwood were observed in secondary forests in Maryland, USA (SERC; McMahon et al., 2010). Notably lacking is evidence of consistent increases in ANPP, as might be expected based solely on increasing atmospheric CO2 concentration (e.g., Norby et al., 2005). In the tropics, allocation of NPP to reproduction appears to have shifted; at five of six tropical sites where flower and seed production has been monitored for more than 10 years, there has been a long-term increase in flower production but not seed production (Wright & Calderon, 2006; Wright, unpublished analysis). Ongoing monitoring of NPP and flower and seed production will be vital to characterizing trends in productivity and C allocation.
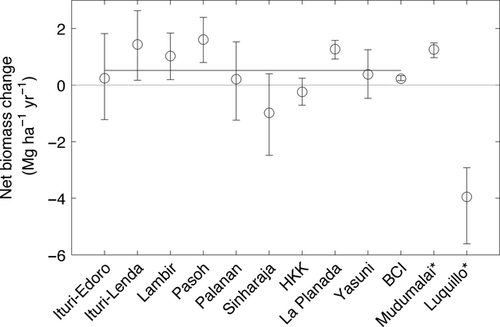
Finally, changes in standing biomass over time have been detected. Across ten relatively undisturbed tropical forests, highly resolved estimates of net biomass change show that aboveground biomass increased on average 0.24 ± 0.16 Mg C ha−1 yr−1 (Fig. 7; Chave et al., 2008). This value is comparable to (though slightly lower than) values recorded for networks of small forest plots in Amazonia (0.62 ± 0.23 Mg C ha−1 yr−1; Baker et al., 2004), and Africa (0.63 ± 0.36 Mg C ha−1 yr−1; Lewis et al., 2009a). Combining published data for the CTFS-ForestGEO, RAINFOR, and AfriTRON tropical forest sites leads to an overall average of 0.34 ± 0.11 Mg C ha−1 yr−1 based on a total of 8243 ha-years of monitoring (Muller-Landau et al., 2014). Ongoing monitoring will be important for quantifying trends in biomass in the global forests represented by CTFS-ForestGEO.
What are the mechanisms by which global change impacts forests?
While data from the CTFS-ForestGEO network add to abundant evidence that forests globally are changing (e.g., Soja et al., 2007; Lewis et al., 2009b; Allen et al., 2010; Wright, 2010), it is difficult to identify the mechanisms underlying such changes given ubiquitous and simultaneous changes in multiple global change drivers (Fig. 3). The information-rich nature of CTFS-ForestGEO sites has yielded insights into the mechanisms of response to global change pressures.
Warming is expected to alter forest dynamics, but predicting effects at the ecosystem scale remains a major scientific challenge (e.g., U.S. DOE, 2012). Monitoring, physiological measurements, and nearby warming experiments combine to yield insights into how warming may impact forest dynamics. The effects of warming are perhaps most dramatic at Scotty Creek, Canada – the highest latitude site, which is experiencing rapid warming (Figs 2 and 3; Tables S3 and S4) – where accelerating permafrost thaw is resulting in tree functional decline near forested plateau edges (i.e., reduced sap flow, radial growth, and leaf area) and driving loss of forest area at a rate of 0.5% yr−1 (Baltzer et al., 2014). At another Canadian site (Haliburton Forest), a heat wave event during spring leaf-out in 2010 resulted in a >50% decline in leaf area of the dominant tree species (Filewod & Thomas, 2014), and a large net ecosystem carbon loss in the same year (Geddes et al., 2014). However, at most temperate and tropical sites, the impacts of warming are less obvious and tend to be confounded by other aspects of global change (Fig. 3). Data from four tropical forest sites (BCI, Huai Kha Khaeng, Lambir, and Pasoh) indicate that tree growth rate correlates negatively with nighttime temperature, as expected from increased respiration rates causing a reduced carbon balance (Feeley et al., 2007a; Dong et al., 2012) – a trend that has also been observed at an external site in Costa Rica (Clark et al., 2010). In contrast, warming experiments associated with two of the sites reveal that warming may also directly or indirectly increase woody productivity; specifically, soil warming at Harvard Forest has increased tree growth through increased N mineralization (Melillo et al., 2011), and chamber warming experiments in Panama revealed that increased nighttime temperatures increased seedling growth rates (Cheesman & Winter, 2013). Ongoing monitoring, experimentation, and modeling will be necessary to disentangle the diverse productivity responses of forests to warming. Warming may also shift C allocation to reproduction; flower production at BCI, Panama has increased with increasing temperature (Pau et al., 2013). Future warming (Fig. 2) will inevitably impact forests, and ongoing monitoring at CTFS-ForestGEO sites should help to document and explain these changes.
Changes in aridity and drought severity have the potential to impact forests worldwide, including those in wet climates (Allen et al., 2010; Choat et al., 2012). Across the tropics, increases in aridity or the occurrence of severe droughts have led to forest “browning”, mortality episodes, or fires (Van Nieuwstadt & Sheil, 2005; Lewis et al., 2011; Zhou et al., 2014), and there is concern that potential future increases in aridity in some parts of the tropics could result in severe tropical forest dieback (e.g., U.S. DOE, 2012). Research across the CTFS-ForestGEO network has yielded insights into the role of aridity in shaping tropical forest dynamics. Droughts in Panama (BCI, San Lorenzo, and Cocoli) and Malaysia (Lambir) have revealed differential drought sensitivity by size class, microhabitat association, and functional type (Condit et al., 1995, 2004; Potts, 2003). In Panama, mild or even fairly strong drought increased both woody productivity and production of flowers and seeds – presumably because of increased solar radiation (Condit et al., 2004; Wright & Calderon, 2006). At a tropical dry forest in India (Mudumalai), drought increased mortality rate, but with a 2–3 year lag for larger trees (Suresh et al., 2010). These findings yield insight into how moist tropical forests may respond to predicted changes in aridity (Fig. 2; Table S4; IPCC, 2013).
Beyond climate, impacts of other global change drivers have been observed across the CTFS-ForestGEO network. Nitrogen deposition (Fig. 1; Table S5) has altered forest biogeochemistry across the globe. Temperate forests are typically N limited; however, high N deposition at Haliburton Forest, Canada, has caused a shift from N to P limitation (Gradowski & Thomas, 2006, 2008), providing evidence of constraints on increases in temperate forest productivity driven by elevated CO2 and/or nitrogen deposition. Because tropical forests are typically limited by elements other than N, N deposition is not expected to increase the productivity of these forests (Matson et al., 1999). At the two tropical CTFS-ForestGEO sites where relevant measurements have been made, increased 15N concentrations in plant tissues suggests substantial N deposition and altered N cycles (Hietz et al., 2011). Specifically, on BCI, leaf N and δ15N in recent (2007) samples were elevated relative to herbarium samples (~1968) (Hietz et al., 2011). These changes have been mechanistically linked to increased N availability through a nearby fertilization experiment, which increased foliar N concentrations and δ15N by similar amounts but did not affect productivity (Wright et al., 2011; Mayor et al., 2014a,b). A similar increase in δ15N was observed in wood from Huai Kha Khaeng, Thailand (Hietz et al., 2011). These results imply that, in tropical forests, N deposition is accelerating N cycling without increasing productivity, and reduced cation availability resulting from N deposition may be one potential explanation for observed declines in tree growth rates at some tropical sites (see above; Matson et al., 1999).
Habitat fragmentation (Fig. 5) and faunal degradation have also been linked to altered dynamics at CTFS-ForestGEO sites. The CTFS-ForestGEO site near Manaus, Brazil, is part of the Biological Dynamics of Forest Fragments Project (BDFFP), which has revealed that forest fragmentation rapidly and profoundly alters tree, arthropod, bird, and primate communities, reducing species diversity and shifting composition toward dominance of more disturbance-adapted species (Laurance et al., 2006). Across the network, more highly fragmented sites (e.g., Witham Woods, UK; Bukit Timah, Singapore; Lambir, Malaysia; Heishiding, China; Fig. 3; Table S5) tend to have degraded faunas, as indicated by the absence of apex predators and larger vertebrates that were present historically, whereas faunal communities tend to remain more intact in unfragmented forests such as Yasuni (Ecuador), Rabi (Gabon), and Scotty Creek (Canada) (Turner & T Corlett, 1996; LaFrankie et al., 2005; Laurance et al., 2012; Harrison et al., 2013;W.F. Laurance, personal communication). As detailed below, faunal degradation – whether caused by habitat fragmentation, hunting, or other pressures – has strong impacts on forest structure and dynamics.
The strong influence of fauna on forest composition and dynamics (e.g., Wright, 2010; Estes et al., 2011; Schmitz et al., 2013) has been documented at several CTFS-ForestGEO sites. At Mpala, Kenya, an experiment excluding herbivores of different sizes and replicated across a rainfall gradient revealed that herbivores of different sizes influence the biomass and growth rates of trees and understory plants, plant community composition, and small mammal communities (Goheen et al., 2013). At Mudumalai, elephants (Elephas maximus) cause high mortality among the small- to medium-sized stems, particularly in a few favored forage species (Sukumar et al., 2005). At SCBI (Virginia, USA), where white-tailed deer (Odocoileus virginianus) populations greatly exceed their historical levels, 20 years of deer exclusion from a 4-ha subsection of the CTFS-ForestGEO plot has resulted in a >4-fold increase in sapling abundance relative to heavily browsed forest outside the exclosure (McGarvey et al., 2013). Large impacts of mammalian herbivores have also been found in an exclosure study adjacent to the Pasoh plot site in Malaysia (Ickes et al., 2001), where native pigs (Sus scrofa) have a dramatic effect on tree recruitment. In Panama, comparison of forest plots protected from bushmeat hunting with those exposed to poachers revealed that by reducing the abundance of frugivores and seed dispersers, hunting decreases the abundance of plant species with seeds dispersed by these animals while increasing the abundance of species with seeds dispersed by bats, small birds, or mechanical means (Wright et al., 2007). The latter includes lianas whose seeds are much more likely to be dispersed by wind (60% of liana species vs. 25% of canopy trees and <10% of midstory and understory trees and shrubs). Lianas have thus increased disproportionately in abundance where hunters remove the frugivores that disperse the seeds of most tree species, hence hunting may have unforeseen consequences for carbon sequestration (Jansen et al., 2010). Directional change in tree communities driven by faunal degradation has also been demonstrated. At Lambir, where populations of large mammals and birds have been severely impacted by hunting, tree community dynamics changed profoundly from 1992 to 2008 (Harrison et al., 2013). Specifically, sapling densities increased and regeneration of tree species with animal-dispersed seeds decreased and became more spatially clustered (Harrison et al., 2013). Thus, ongoing faunal degradation due to hunting and habitat fragmentation in many forests globally is expected to alter forest community composition, tree dispersal and regeneration, species diversity, forest structure, and carbon cycling.
CTFS-ForestGEO research has also shed light on community interactions that will act to either magnify or buffer forest responses to global change. Species are linked to one another through complex webs of interaction. For example, mapping of quantitative trophic foodwebs at Wanang (Papua New Guinea) and current efforts to document tritrophic foodwebs of seeds, seed predators and parasitoids at this same location, at Khao Chong (Thailand) and Barro Colorado Island (Panama) demonstrates the complexity of ecological interactions in forest ecosystems (Novotny et al., 2010). Studies of seed dispersal and seedling recruitment demonstrate the critical role of vertebrates and insects in tree reproduction and the composition of future forests (e.g., Wright et al., 2007; Harrison et al., 2013). It is therefore unsurprising that global change impacts on one group cascade through the ecosystem. For example, as described above, dramatic reduction in large mammal and bird populations at Lambir, Malaysia has altered the dynamics of tree dispersal and regeneration (Harrison et al., 2013). Similarly, in the light-limited moist tropical forests of Panama, El Niño events bring relatively cloud free, sunny conditions that enhance fruit production while subsequent La Niña events bring rainy, cloudy conditions, and lower levels of fruit production that can lead to famines, particularly among terrestrial frugivores and granivores (Wright et al., 1999; Wright & Calderon, 2006). Climate change is bringing changes in cloud cover and atmospheric transmissivity to PAR (Table S3) with cascading effects on frugivores, granivores, and the plant species with which they interact.
At the same time, the diversity and complexity of forest communities may serve to provide some resilience to global change. A diversity of tree physiological strategies implies a wide range of responses to global change that helps to provide ecosystem resilience (e.g., Isbell et al., 2011; Mori et al., 2013). For example, Panamanian tree species have displayed a wide range of physiological responses to temperature variation (Cheesman & Winter, 2013; Slot et al., 2014), and trees of different species have generally responded differently to experimental manipulation of CO2, temperature, or precipitation globally (Anderson-Teixeira et al., 2013). The resilience enabled by species diversity may be exemplified by the stability of biomass, size structure, and functional composition of the BCI forest (Chave et al., 2008; Swenson et al., 2012) despite severe droughts that impacted drought-sensitive species (Condit et al., 1995, 1996). In addition, in the tropics, pervasive negative density dependence – i.e., elevated mortality of a plant species in areas where it is abundant – may buffer change because as a species becomes rare, it will suffer less from negative density dependence (Comita et al., 2010). Thus, accounting for biodiversity in ecosystem models will be important for predicting forest responses to climate change. While such complexity makes it challenging to predict forest responses to global change, it may serve to partially buffer forest response to global change, which might otherwise be more dramatic.
Conclusions
The CTFS-ForestGEO forest dynamics sites are representative of the world's more intact forests, covering a diversity of geographical, climatic, edaphic, topographic, and biotic environments (Figs 1 and 2; Table 2). Yet, even this selection of the world's more intact forests is being impacted by multifaceted global change drivers (Figs 2-5). Because many interacting species and processes are simultaneously being affected by a variety of global change pressures, extracting a mechanistic understanding of observed forest changes is challenging, requiring a holistic understanding of the abiotic setting, site history, demography for all tree life stages, trophic interactions, and ecosystem-level processes. The broad suite of measurements made at CTFS-ForestGEO sites (Tables 1 and 3) makes it possible to understand the complex ways in which global change is impacting forest dynamics.
Ongoing research across the CTFS-ForestGEO network is yielding insights into how and why the forests are changing. As global change pressures inevitably intensify (Fig. 2; IPCC, 2013), ongoing monitoring across the network should prove valuable for documenting and understanding multifaceted forest responses and feedbacks to the climate system. To project into the future, broad suite of variables measured at CTFS-ForestGEO sites (Tables 1 and 3) will be invaluable for parameterizing and evaluating ecosystem and earth system models, particularly those that characterize forest demography and differences among species or functional groups (e.g., Moorcroft et al., 2001; Medvigy et al., 2009). Together, CTFS-ForestGEO's unique standardized core census (Table 1) and supplementary measurements (Table 3), applied across all of the world's major forest biomes (Fig. 1; Table 1), will provide mechanistic insight as forests change in the 21st century.
Acknowledgements
We thank everyone involved in the collection of the vast quantity of data and information in the CTFS-ForestGEO network; to F. Dentener and W. Laurance for providing data; E. Leigh, Y. Lin, J. McGarvey and A. Miller for helpful comments; E. Aikens, L. Gonzalez and M. Azimi for help with analysis and figures. Study on this manuscript was funded in part by a Smithsonian Competitive Grants Program for Science award to KJAT. The CTFS-ForestGEO network has received major support from the Smithsonian Institution – particularly the Smithsonian Tropical Research Institute, the Arnold Arboretum of Harvard University, the National Science Foundation (multiple grants), the Rockefeller Foundation, the John Merck Fund, the John D. and Catherine T. MacArthur Foundation, the Andrew W. Mellon Foundation, the Frank Levinson Family Foundation, the HSBC Climate Partnership, the Bromley Charitable Trust, John Swire & Sons Pty Ltd, Celerity, F.H. Levinson Fund, Small World Institute Fund and Jennifer and Greg Johnson. Site-specific support is listed in Table S8.




