Climate warming and agricultural stressors interact to determine stream periphyton community composition
Abstract
Lack of knowledge about how the various drivers of global climate change will interact with multiple stressors already affecting ecosystems is the basis for great uncertainty in projections of future biological change. Despite concerns about the impacts of changes in land use, eutrophication and climate warming in running waters, the interactive effects of these stressors on stream periphyton are largely unknown. We manipulated nutrients (simulating agricultural runoff), deposited fine sediment (simulating agricultural erosion) (two levels each) and water temperature (eight levels, 0–6 °C above ambient) simultaneously in 128 streamside mesocosms. Our aim was to determine the individual and combined effects of the three stressors on the algal and bacterial constituents of the periphyton. All three stressors had pervasive individual effects, but in combination frequently produced synergisms at the population level and antagonisms at the community level. Depending on sediment and nutrient conditions, the effect of raised temperature frequently produced contrasting response patterns, with stronger or opposing effects when one or both stressors were augmented. Thus, warming tended to interact negatively with nutrients or sediment by weakening or reversing positive temperature effects or strengthening negative ones. Five classes of algal growth morphology were all affected in complex ways by raised temperature, suggesting that these measures may prove unreliable in biomonitoring programs in a warming climate. The evenness and diversity of the most abundant bacterial taxa increased with temperature at ambient but not with enriched nutrient levels, indicating that warming coupled with nutrient limitation may lead to a more evenly distributed bacterial community as temperatures rise. Freshwater management decisions that seek to avoid or mitigate the negative effects of agricultural land use on stream periphyton should be informed by knowledge of the interactive effects of multiple stressors in a warming climate.
Introduction
A major challenge facing environmental managers worldwide is how to deal with multiple stressors arising from human activities, whose effects may be further exacerbated by anthropogenic climate change (Ormerod et al., 2010). In streams and rivers draining agricultural catchments, deposited fine sediment and elevated nutrient concentrations represent two important land-use stressors commonly associated with degraded ecological conditions that can interact in complex ways to affect macroinvertebrates (Townsend et al., 2008; Matthaei et al., 2010; Wagenhoff et al., 2011, 2012), benthic algae (Pringle, 1990; Hillebrand & Kahlert, 2002; Wagenhoff et al., 2013) and ecosystem processes (Niyogi et al., 2003; Young et al., 2008). If or how climate-change induced warming will interact with these other stressors is presently unknown, though of considerable interest (Heino et al., 2009). A potential concern is whether increased temperature will interact with existing or impending stressors to create ecological surprises in the form of synergisms (amplified effects) or antagonisms (reduced effects), in comparison to what would be expected based on knowledge of individual effects (Sutherland et al., 2006; Woodward et al., 2010).
In the present study, we use streamside mesocosms to investigate how deposited fine sediment and elevated nutrient concentrations (at levels found in streams draining highly intensified agricultural catchments in New Zealand) interact to affect the algal and bacterial constituents of benthic periphyton (i.e. a matrix of surface attached algae and microorganisms) along a temperature gradient ranging from 0–6 °C above ambient (eight levels). This temperature range encompasses the forecast scale of anthropogenic climate-change induced warming in New Zealand of up to 5.1 °C by 2090 (IPCC, 2007; Ministry for the Environment, 2008). To our knowledge, our experiment provides the first empirical evaluation of the interactive responses of periphyton to multiple agricultural stressors coupled with simulated climatic warming.
The effects of nutrients (nitrogen N and/or phosphorus P) on benthic algae have been subject to extensive research, but the relationship between nutrients and benthic algal community structure is still not well understood, probably because multiple other factors also contribute to the determination of species composition (Borchardt et al., 1996; Liess et al., 2009). In general, it seems that augmentation of nutrients in low-nutrient environments may increase algal primary production by providing subsidies to nutrient-limited taxa, and this may lead to increased species richness and evenness (Biggs et al., 1998). In nutrient-saturated conditions, however, nutrient-tolerant eutrophic species may dominate with prolific nuisance growths that degrade stream health (Biggs, 2000). It has been suggested that the dominant morphological growth form of diatoms at a location may represent a trade-off between nutrient limitation/tolerance and disturbance resistance/susceptibility (Biggs et al., 1998), with ‘low profile’ (adnate or prostrate) forms dominating in low-nutrient environments and ‘high profile’ (erect or stalked) and motile forms dominating where nutrients are abundant (Passy, 2007). Schneck et al. (2011) recently extended this classification to encompass all benthic algae by adding filamentous and metaphyton (algae without a fixation structure) growth forms, thus facilitating the assessment of trait-based responses of the whole algal community.
Deposited fine sediment can transform the substratum inhabited by periphyton and smother a pre-existing community. Increases to substratum instability and habitat heterogeneity when fine sediment is present compared to smooth surfaces may strongly influence the community that establishes, with characteristic algal assemblages, again based on growth form, associated with stone (epilithic) and sediment/sand (epipelic/epipsammic) substrata (Moss, 1977; Burkholder, 1996). Thus, sessile algae may dominate in epilithic habitats, whereas in epipelic/epipsammic habitats motile or mucilaginous stalked forms may be more widespread due to an ability to avoid permanent burial by unstable fine substratum (Pringle, 1990; Burkholder, 1996; Schneck et al., 2011).
In contrast to nutrients and fine sediment, temperature cannot be considered as a ‘useable input’ to stream ecosystems (Webb, 1996). Instead, temperature acts as the principal environmental factor governing biochemical reactions that regulate cellular metabolism, enzymatic activity and specific growth rates (Allan & Castillo, 2007), all of which are constrained by the temperature tolerances of the taxa concerned (Denicola, 1996). In a laboratory study using periphyton from a healthy temperate stream, Cairns (1956) observed a shift in algal dominance from diatoms (<20 °C) to green algae (15–25 °C) to cyanobacteria (>30 °C) as temperature was raised. In a stream subjected to thermal discharge, Patrick (1969) observed that species diversity and biomass increased between 0 and 25 °C, but declined above 30 °C. Warming may also advantage smaller-celled algal taxa due to the higher surface to volume ratios that benefit the acquisition of nutrients relative to the kinetics of metabolism (Winder et al., 2009).
We tested four hypotheses about individual stressor effects on benthic stream algae: (i) nutrient enrichment will have mainly positive effects (increased algal biomass/cell density), but with changes in community composition (increased representation of nutrient-tolerant taxa and declining prevalence of adnate/prostrate growth forms), (ii) fine sediment addition will have positive effects on motile and mucilaginous stalked taxa (represented within the erect/stalked growth form) but negative effects on filamentous and adnate/prostrate forms (as found by Piggott et al., 2012), (iii) increased temperature will have generally positive effects on green algae and cyanobacteria and negative effects on diatoms, though responses may be non-linear and (iv) cell-size composition will shift towards smaller taxa under warmer conditions as large taxa are lost disproportionately (Woodward et al., 2010).
While a number of studies have investigated two-factor interactions involving nutrients, temperature and sediment on benthic stream algae (e.g. nutrients and temperature; Marcarelli & Wurtsbaugh, 2006; Gudmundsdottir et al., 2011; Rasmussen et al., 2011) (or nutrients and sediment; Pringle, 1990; Hillebrand & Kahlert, 2002; Wagenhoff et al., 2013), we are unaware of any study investigating interactions among all three. We therefore tested four hypotheses regarding interactive effects: (i) the effects of nutrient enrichment will strengthen at raised temperature (Gudmundsdottir et al., 2011), (ii) algae abundant on fine sediment will be less affected by raised temperature (Denicola, 1996) or (iii) by nutrient enrichment (Pringle, 1990; Hillebrand & Kahlert, 2002) because sediment dwellers are a specialized flora and (iv) community-level responses will be less susceptible to multiple-stressor effects than population responses because species interactions may dampen and diffuse effects on individual species (Crain et al., 2008). Finally, due to the intimate coupling between algae and bacteria within the periphyton (Carr et al., 2005), we further investigated the bacterial community responses to the manipulated stressors.
Materials and methods
Study site
The experiment was conducted during austral spring/summer from 3 November to 14 December 2009 in circular stream mesocosms installed on the bank of the Kauru River, a third-order stream in the Otago province of New Zealand (170°44.6′ East, 45°6.5′ South, 98 m a.s.l). The Kauru catchment (124 km2) lies in the rain shadow of the Southern Alps, ranges from 55 to 1273 m a. s. l. and receives a mean annual rainfall of 817 mm. Mean annual discharge, measured 300 m upstream of our site, is 1.29 m3 s−1 (ORC, 2003). The vegetation in the catchment consists predominantly of native tussock grass and exotic pasture. Land use is mainly sheep and beef grazing at low stock densities (0.1–3 animals per hectare). The river water is relatively nutrient-poor (see non-enriched nutrient values later), and the river contains diverse and abundant algal (Liess et al., 2009; Lange et al., 2011) and invertebrate communities (Herrmann, 2009).
Experimental design
We manipulated dissolved nutrient levels, fine sediment cover on the bed (inorganic particles <2 mm in diameter; Zweig & Rabeni, 2001) and water temperature in 128 circular flow-through stream mesocosms, with two nutrient treatments (ambient river concentrations or enriched with both N + P), two fine sediment treatments (none added or sediment added) and eight temperature levels in a replicated full factorial design. Temperature treatments were assigned randomly in eight blocks (each consisting of 16 mesocosms). Within each block, nutrient and sediment levels were randomly assigned to the 16 mesocosms, providing four replicates of each treatment combination. The experiment ran for 6 weeks, with a three-week pre-colonization period followed by a 3 week manipulative period (day 0 to day 21) when stressor treatments were in place. Raised temperature and nutrient enrichment began on day 0 and were applied continuously for 21 days. Fine sediment was added on day 0 and remained in sediment-treated mesocosms until day 21.
The experimental set-up (Fig. 1) comprised a 4.1 m high, 20 m long two-level scaffold erected perpendicular to the river, the nearer bank of which was 20 m away. The upper level supported eight 135 l polythene header tanks. Adjacent to the scaffold a 1 m high, 1.2 m wide wooden bench supported the circular stream mesocosms (adapted from Liess et al., 2009), each with an external diameter of 25 cm and an inner outflow ring of 6 cm (volume 3.5l; Microwave Ring Moulds, Interworld, Auckland; Fig. 1b, c).
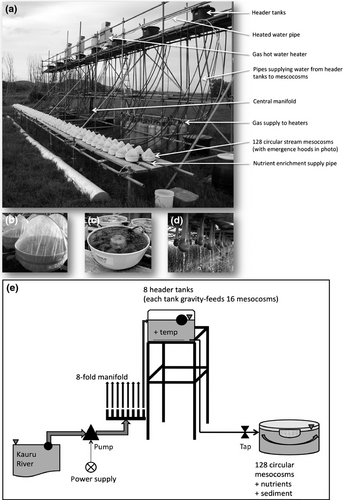
Dual centrifugal pumps (Onga 415, capacity 300 l min−1) supplied the entire set-up with natural stream water (242 l min−1) along dual 20 m long, 50 mm polythene pipes, with dual intakes placed in a run section of the river and protected by cylindrical metal mesh coverings (mesh size 4.5 mm). Dual 80 m long, 50 mm pipes led from the pumps to a central manifold connecting to ten 25 mm polythene pipes. Eight of these pipes supplied stream water directly to the eight header tanks (controlled by ballcocks), while two pipes passed through separate inline filters (0.5 mm) and secondary pressure boosting pumps (model JR.2; LINZ Electric, Italy) to individually supply three gas-fired water heaters per pump (six total; model 16H; Bosch, Germany) mounted along opposite sides of the upper scaffold level. Each heater triplet had a centralized outflow pipe that connected to regulating inflow taps on each header tank. To ensure each header tank received an equal volume of filtered water from the heating system, only one of the two heating supply lines physically heated the water, while the opposite but identical line supplied unheated water in the required proportion.
Each header tank fed stream water by gravity to its block of 16 mesocosms (Fig. 1e) at a constant flow rate of 1.88 ± 0.02 l min−1 per mesocosm (mean ± 1 SE; n = 64), calibrated daily, via a further 4 m of 13 mm polythene piping controlled by a tap regulator and an inflow jet pointing diagonally to create a circular flow (Figure S1). Current velocity in the mesocosms averaged 0.14 ± 0.001 m s−1 (mean ± SE; n = 128; measured once, on day 7) with an average water depth of 45.2 ± 0.3 mm (n = 128; measured once, on day 1).
Water leaving the mesocosms (~1.5 min residence time in mesocosms) flowed over the inner circular opening and out through a nylon net (mesh size 500 μm) to capture drifting invertebrates. Nets were exchanged every third day (Fig. 1d). Polyester hoods (mesh size 500 μm), which permitted air circulation and reduced light by only 5% (measured on a cloudless day using a L1-250 Light Meter; Li-Cor Inc., Lincoln, NE, USA), were used to capture emerging adult insects. The hoods covered each mesocosm, supported vertically by a wire cross pole and held in place by an elastic band around the outside wall, and were exchanged every three days (Fig. 1b). The invertebrate data were collected for a separate study.
Nutrients (nitrate and phosphate as NaNO3 and KH2PO4) were supplied to half the mesocosms in solution by individually attached pressure compensating drippers (model RXLD2SC; RX Plastics, New Zealand) at a rate of 2 l h−1 from a 13 mm pipe. The pipe ran the length of the wooden bench beside the mesocosms and was connected to the central manifold via an inline filter (mesh size 0.5 mm). A highly concentrated nutrient solution was continuously injected into this pipe by a fluid metering pump (FMI CERAMPUMP® Lab Pump Model QBG, Fluid Metering Inc., Syosset, NY, USA) from an adjacent 300 L supply barrel (nutrient concentrations described later).
To simulate the natural substratum of small sheep/beef farmland streams in the Otago region (Matthaei et al., 2006; Townsend et al., 2008), mesocosms were filled with 500 ml of 2–20 mm (b diameter) gravels from the dry floodplain, washed and sieved to remove particles smaller than 2 mm in diameter, on top of which 16 randomly selected flat surface stones (b diameter 30 mm) were placed. The resulting substratum had a depth of 20–30 mm with a benthic habitat surface area of c. 0.045 m2 prior to addition of fine sediment.
Flow began on 3 November 2009 (day -21), with mesocosms being left for 21 days for natural colonization and equilibration by drifting invertebrates and algae (Lange et al., 2011; Wagenhoff et al., 2012). Drifting invertebrate colonization of these mesocosms has been shown to be highly effective (~1000 individuals per 100 m3 into each mesocosm per 49-h period; Wagenhoff et al., 2012). On 19 November (day -4), natural colonization in each mesocosm was augmented by adding one standard load of invertebrates (including taxa underrepresented in the drift), in the form of an eighth of a kick-net sample (3 min duration; frame 60 × 40 cm; mesh size 200 μm) obtained from the adjacent river in bed patches of 0.36 m2 (comparable to the habitat surface area of eight mesocosms) and divided using an automated subsampler (Waters, 1969). Kick-net samples were collected sequentially from downstream to upstream in a uniform riffle-run area of riverbed and sample eighths assigned randomly to mesocosms.
The manipulative period began on 23 November (day 0). Manipulated water temperatures were simulated to encompass the upper (5.1 °C) and lower (0.7 °C) climate change warming projections for New Zealand by 2090 (IPCC, 2007; Ministry for the Environment, 2008). Temperature treatments were achieved using the gas-fired heating system (described earlier) and covered a gradient of 0 to 6 °C (eight levels, means achieved in °C ± 1 SD; 0.7 ± 0.15, 1.8 ± 0.19, 2.6 ± 0.14, 3.6 ± 0.22, 4.2 ± 0.20, 5.1 ± 0.59, 6.0 ± 0.46) in evenly spaced increments (0.85 ± 0.06 °C; mean ± 1 SE) above the ambient temperature treatment (16.1 ± 0.03 °C; mean ± 1 SE, 21.7 °C maximum, 11.6 °C minimum during the manipulative period; Figure S2). Water temperatures were measured at 5 min intervals from day 0–21 (Hobo Water Temp Pro; Onset Computer Corporation, Bourne, MA, USA). Temperatures in unheated mesocosms were virtually identical to the incoming river water (0.05 ± 0.01°C cooler; mean ± 1 SE).
Nutrient addition to half the mesocosms increased concentrations to levels in the upper range of those regularly recorded in intensively farmed New Zealand streams (Hamill & Mcbride, 2003; Buck et al., 2004; Wilcock et al., 2006; Monaghan et al., 2007). Means achieved in the 64 mesocosms with nutrient enrichment, measured on days 1, 7, 13 and 19 using standard methods (APHA, 1998), were 3035 ± 46 μg l−1 for nitrate-N and 247 ± 4 μg l−1 for soluble reactive phosphorus-P (n = 256). Means in the 64 non-enriched mesocosms (ambient) were 28.9 ± 0.5 μg l−1 and 4.2 ± 0.1 μg l−1 for N and P, respectively. Nutrient ratios in both ambient and enriched mesocosms were generally within the range of co-limitation (N : P between 7 : 1 and 15 : 1), based on the New Zealand classification of Mcdowell et al. (2009).
Fine river sand sourced from the floodplain of the Taieri River (grain size 0.2 mm; Matthaei et al., 2006) was added on day 0 to half the mesocosms, resulting in deposited sediment values equivalent to those occurring in intensively farmed New Zealand streams (Townsend et al., 2008; Clapcott et al., 2011; Wagenhoff, 2011). The fine sediment had been stored without contact to river water for many years and thus contained no attached freshwater algae and was colonized by terrestrial microbes, similar to fine sediment reaching real streams in agricultural catchments. The sediment was also very low in total organic carbon (0.29 ± 0.03% of dry mass; mean ± SE; n = 5), nitrogen (0.009 ± 0.002%; n = 2) and phosphorus (0.045 ± 0.004%; n = 2). Means achieved on day 1 in the 64 mesocosms with added sediment were 90.1 ± 0.9% streambed cover and 12.6 ± 0.5 mm sediment depth (n = 64; measured once, on day 1). This compares with zero values for the 64 mesocosms without sediment (see also Figure S1).
Periphyton sampling and response variables
On day 20 of the manipulative period, benthic algae were sampled using one of two methods depending on the substratum present. With the flow in each mesocosm temporarily stopped, three randomly selected surface stones (b diameter 30 mm) were sampled from mesocosms without added sediment and placed in sealed plastic containers, whereas in mesocosms with added sediment a 3.5 cm inner diameter ring was placed at three random locations on the sediment surface and the material within (algae and top 2 mm of sediment) sucked up with a pipette into a 100 ml Astraline bottle. When standardized by surface area these sampling methods have been shown not to systematically influence measures of algal biomass or cell densities (Magbanua, 2012). An additional sample for analysis of the bacterial community was collected from all channels by pipetting the periphyton from around the base of the inner outflow ring into individual sterile 50 ml Falcon tubes. All samples were placed on ice in the dark during transportation and then frozen at −18 °C until processing. According to Biggs & Kilroy (2000), freezing is the only practical method for studies such as ours where periphyton samples are analysed for algal biomass and abundances of diatoms as well as soft-bodied taxa such as green algae and cyanobacteria.
After thawing in the laboratory, stone samples were scrubbed for 20 s over their entire surface and attached algae rinsed into a 100 ml Astraline bottle. Each stone's surface area was obtained using the formula: stone surface area (cm2) = 1.59 + 0.811 (xy + yz + xz), where x, y, and z are the lengths of the three main axes of the stone in centimetres; the formula includes an adjustment for the area of the stone normally protruding into the water that is colonisable by periphyton (~ 65% of the total surface area; Biggs & Kilroy, 2000). Algal samples (excluding bacterial community samples) were homogenized for 1 min with a blender (Omni Mixer, Ivan Sorval Inc., Newton, MA, USA) to separate clumps of organic material. Then two 20 ml aliquots were subsampled from the slurry, one for algal biomass as chlorophyll a (determined spectrophotometrically using standard methods; Biggs & Kilroy, 2000) and the second for taxonomic analysis following the protocol in Biggs & Kilroy (2000). This protocol includes specific recommendations for working with frozen algal samples including, for example, how to deal with cellular/chloroplast deformities. Approximately 300 cells were identified at 400x magnification to the lowest practical taxonomic level using an inverted microscope (Zeiss Axiovert 25, Jena, Germany). As a consequence of this approach, some species-level responses may reflect aggregates of closely related taxa (e.g. Gomphonema parvulum). Where algal filaments were present and cells were difficult to discern, 10 μm increments were counted as ‘cells’ (Biggs & Kilroy, 2000).
The following algal community response variables were calculated: (i) total algal cell density, (ii) algal biomass (as chlorophyll a), (iii) algal taxon richness, (iv) Simpson's diversity index (D), (v) Pielou's evenness index (J'), (vi) densities of cells (based on the longest axis of the average cell size for each taxon) to which we assigned the size classes small (<30 μm), medium (30–90 μm) and large algae (>90 μm) and (viii) cell densities of the broad taxonomic groups of filamentous green algae, non-filamentous green algae, cyanobacteria and diatoms. Further, algae were classified according to growth form after Schneck et al. (2011) as (i) adnate or prostate, (ii) erect or with mucilaginous stalks, (iii) motile, (iv) filamentous and (v) metaphyton (refer to Piggott et al., 2012 for taxa classifications). Population-level response variables included densities of the 19 most common algal taxa (those present in at least half of all mesocosms or comprising more that 0.5% of the total community), which together made up 97% of the total community.
The bacterial component of the periphyton was characterized using Automated Ribosomal Intergenic Spacer Analysis (ARISA), a DNA molecular-fingerprinting technique that profiles bacterial community structure without taxonomic identification of individual organisms (Lear et al., 2008). Detailed descriptions of the DNA extraction method, ARISA and quantitative methods used to determine bacterial community composition can be found in Lear & Lewis (2009). This method measures the proportion of different intergenic spacer fragment lengths in the 16S–23S region of the bacterial rRNA gene. The length of this intergenic spacer region varies among bacterial species and thus each fragment length can be tentatively assumed to be associated with a single taxon (Lear & Lewis, 2009). It is important to note that such methods grossly underestimate the diversity of complex environmental communities, excluding rare bacteria in particular (Bent et al., 2007). However, DNA fingerprinting methodologies remain the method of choice for the community-based assessment of large sample numbers and have been shown to produce results that are broadly consistent with more sophisticated methods (e.g. DNA sequencing; Shade et al., 2012) and the total number of different fragment lengths detected per sample (220 ± 3.2, mean ± SE; n = 120) was used as an index of bacterial taxon richness, which was in turn used to calculate Pielou's evenness and Simpson's diversity measures for each sample.
Data analysis
General Linear Model (GLM) analyses were conducted in PASW Statistics 18.0 (SPSS: An IBM Company, Chicago, USA). Nutrients and sediment were fixed categorical factors, whereas temperature was a continuous predictor variable with eight levels. A quadratic term for temperature was included to test for non-linear temperature effects. To avoid potential collinearity problems the continuous predictor temperature was centred (Quinn & Keough, 2002). The resulting model was intercept (d.f. 1) + nutrients (1) + sediment (1) + temperature (1) + temperature × temperature (1) + nutrients × temperature (1) + sediment × temperature (1) + nutrients × sediment (1) + nutrients × sediment × temperature + error (120, n = 128).
To assess effects on algal community composition, we performed GLMM (i.e. the multivariate equivalent of the model above) and examined the between-subjects effects for each individual taxon.
- An additive effect (i.e. no significant interaction in anova) represents the sum of the individual effects that may arise from double positive individual effects (e.g. +1 + 1 = 2 for an effect of 1 for each stressor), double negative individual effects (−1 + −1 = −2), or opposing individual effects (−1 + 1 = 0).
- An interaction that deviates from additive (i.e. a significant two-factor interaction term in anova) and is less than the sum of the individual effects or less-than-or-equal-to any individual effect in the same direction is positive antagonistic (+A; less positive than predicted additively) when +1 + 1 = 0 < (+A) <2 or −1 + 1 = −1 ≤ (+A) <0, or negative antagonistic (−A; less negative than predicted additively) when −1 + −1 = −2 < (−A)<0 or −1 + 1 = 0 < (−A) ≤1.
- A deviation from additive that is greater than the sum of individual effects and greater than any individual effect in the same direction or has an interaction effect that is greater than both in absolute terms is positive synergistic (+S; more positive than predicted additively) when +1 + 1 = (+S) >2 or −1 + −1 = (+S) >0 or −1 + 1 = (+S) >1, or negative synergistic (−S; more negative than predicted additively) when +1 + 1 = (−S)<0 or −1 + −1 = (−S) < −2 or −1 + 1 = (−S) <−1.
| Dependent variable | % | Nutrients | N | Sediment | S | Temperature | T | Temperature × Temperature | Nutrients × Temperature | C | Sediment × temperature | C | Sediment × nutrients | C | Nutrients × Sediment × Temperature |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Algal cell density | <0.001 (0.3) | + | <0.001 (0.58) | + | 0.1 (0.02) | 0.13 | 0.06 (0.03) | 0.002 (0.08) | -> | 0.83 | AD | 0.18 | |||
| Algal biomass | <0.001 (0.49) | + | <0.001 (0.12) | + | 0.42 | 0.06 (0.03) | 0.61 | 0.47 | 0.08 (0.02) | AD | 0.99 | ||||
| Algal taxon richness | 0.01 (0.05) | × | <0.001 (0.47) | + | 0.46 | 0.92 | 0.07 (0.03) | 0.004 (0.07) | -< | <0.001 (0.19) | +A | 0.36 | |||
| Algal diversity (Simpson's) | 0.24 | <0.001 (0.45) | + | <0.001 (0.17) | − | 0 (0.08) | 0.31 | 0.006 (0.06) | × | <0.001 (0.37) | +A | <0.001 (0.11) | |||
| Algal evenness (Pielou's) | 0.55 | <0.001 (0.46) | + | <0.001 (0.14) | − | <0.001 (0.1) | 0.049 (0.03) | × | 0.004 (0.07) | × | <0.001 (0.33) | +A | <0.001 (0.14) | ||
| Small (<30 μm) algae | 56.2 | <0.001 (0.09) | × | <0.001 (0.22) | + | 0.09 (0.02) | 0.19 | 0.18 | <0.001 (0.17) | -> | <0.001 (0.12) | +A | 0.99 | ||
| Medium (30–90 μm) algae | 41.4 | <0.001 (0.44) | + | <0.001 (0.69) | + | 0.39 | 0.2 | 0.049 −0.03 | × | 0.97 | <0.001 (0.23) | +S | 0.01 (0.06) | ||
| Large (>90 μm) algae | 2.5 | 0.03 (0.04) | × | <0.001 (0.42) | + | 0.047 (0.03) | − | 0.88 | 0.37 | 0.65 | <0.001 (0.14) | +A | 0.95 | ||
| Filamentous greens | 7.3 | 0.003 (0.07) | + | 0.001 (0.08) | − | 0.06 (0.03) | 0.6 | 0.11 | 0.09 (0.02) | 0 (0.07) | +A | 0.14 | |||
| Non-filamentous greens | 29.2 | <0.001 (0.54) | + | <0.001 (0.55) | + | 0.2 | 0.98 | 0.004 (0.07) | × | 0.01 (0.05) | × | <0.001 (0.35) | +S | <0.001 (0.1) | |
| Cyanobacteria | 3.5 | 0.07 (0.03) | 0.04 (0.04) | × | 0.65 | 0.01 (0.06) | 0.02 (0.04) | ± | 0.86 | 0.02 (0.05) | +A | 0.47 | |||
| Diatoms | 60.0 | 0.81 | <0.001 (0.69) | + | <0.001 (0.19) | × | 0.02 (0.04) | 0.84 | <0.001 (0.23) | -> | 0.03 (0.04) | +A | 0.48 | ||
| Filamentous % | 9.7 | <0.001 (0.15) | × | <0.001 (0.37) | − | 0.002 (0.13) | + | 0.93 | 0.29 | 0.01 (0.09) | +< | <0.001 (0.2) | +A | 0.2 | |
| Erect or stalked % | 24.3 | <0.001 (0.52) | − | <0.001 (0.82) | + | <0.001 (0.32) | − | 0.12 | 0.78 | 0.02 (0.04) | -> | <0.001 (0.41) | +A | 0.14 | |
| Adnate orprostrate % | 33.9 | <0.001 (0.39) | − | <0.001 (0.71) | − | 0.03 (0.04) | × | 0.02 (0.04) | 0.003 (0.07) | +< | 0.05 (0.03) | <0.001 (0.35) | −A | 0.34 | |
| Motile % | 4.8 | 0.03 (0.04) | × | <0.001 (0.55) | + | 0.01 (0.06) | + | 0.76 | 0.11 | 0.01 (0.06) | +> | 0.03 (0.04) | × | 0.01 (0.06) | |
| Metaphyton % | 27.3 | <0.001 (0.55) | + | 0.002 (0.08) | × | 0.26 | 0.43 | 0.04 (0.04) | × | <0.001 (0.26) | ± | <0.001 (0.21) | +S | 0.02 (0.05) | |
| Bacterial taxon richness | 0.25 | 0.29 | 0.4 | 0.44 | 0.13 | 0.97 | 0.68 | O | 0.96 | ||||||
| Bacterial evenness (Pielou's) | 0.17 | <0.001 (0.59) | + | 0.07 (0.03) | 0.56 | 0.02 (0.05) | +< | 0.19 | 0.06 (0.03) | AD | 0.99 | ||||
| Bacterial diversity (Simpson's) | 0.27 | <0.001 (0.37) | + | 0.02 (0.05) | × | 0.61 | 0.01 (0.06) | +< | 0.9 | 0.05 (0.04) | +A | 0.41 |
- c, combined.
| Dependent variable | % | GF | SC | Nutrients | N | Sediment | S | Temperature | T | Temperature × Temperature | Nutrients × Temperature | C | Sediment × temperature | C | Sediment × nutrients | C | Nutrients × Sediment × Temperature |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GLMM Common Taxa | 97.1 | <0.001 (0.77) | <0.001 (0.9) | <0.001 (0.65) | 0.01 (0.27) | <0.001 (0.4) | <0.001 (0.56) | <0.001 (0.72) | <0.001 (0.36) | ||||||||
| Scenedesmus spp. | 23.7 | ME | M | <0.001 (0.57) | + | <0.001 (0.56) | + | 0.04 (0.03) | × | 0.68 | 0.003 (0.07) | × | 0.005 (0.07) | × | <0.001 (0.41) | +S | <0.001 (0.11) |
| Gomphonema minutum | 13.5 | AP | S | 0.006 (0.06) | + | <0.001 (0.27) | − | 0.08 (0.03) | 0.75 | 0.76 | <0.001 (0.16) | ± | 0.07 (0.03) | AD | 0.61 | ||
| Fragilaria vaucheriae | 10.2 | ES | S | 1 | <0.001 (0.71) | + | <0.001 (0.19) | − | 0.01 (0.05) | 0.11 | <0.001 (0.15) | -> | 0.52 | AD | 0.03 (0.04) | ||
| Encyonema minutum | 9.4 | ES | S | <0.001 (0.21) | × | <0.001 (0.62) | + | <0.001 (0.28) | − | 0.47 | 0.28 | <0.001 (0.19) | -> | <0.001 (0.25) | +A | 0.1 (0.02) | |
| Cymbella kappii | 8.9 | ES | M | 0.14 | <0.001 (0.46) | + | <0.001 (0.29) | − | 0.001 (0.08) | 0.97 | <0.001 (0.25) | -> | 0.06 (0.03) | AD | 0.7 | ||
| Stigeoclonium spp. | 6.6 | F | S | 0.002 (0.08) | + | <0.001 (0.09) | − | 0.046 (0.03) | + | 0.66 | 0.09 (0.02) | 0.06 (0.03) | 0.003 (0.07) | +A | 0.11 | ||
| Gomphonema parvulum | 5.3 | AP | S | <0.001 (0.4) | + | <0.001 (0.69) | + | 0.53 | 0.31 | 1 | 0.36 | <0.001 (0.34) | +S | 0.7 | |||
| Ankistrodesmus sp. | 5.2 | ME | S | 0.01 (0.05) | + | <0.001 (0.13) | + | 0.006 (0.06) | − | 0.32 | 0.48 | 0.81 | 0.7 | AD | 0.19 | ||
| Navicula cryptocephala | 4.2 | MO | M | <0.001 (0.16) | + | <0.001 (0.47) | + | 0.21 | 0.14 | 0.75 | 0.26 | 0.03 (0.04) | +S | 0.74 | |||
| Aphanocapsa spp. | 2.1 | ME | S | 0.02 (0.05) | + | 0.007 (0.06) | − | 0.3 | 0.01 (0.05) | 0.05 (0.03) | 0.56 | 0.09 (0.02) | AD | 0.94 | |||
| Rossithidium spp. | 1.5 | AP | S | 0.21 | <0.001 (0.27) | + | <0.001 (0.11) | − | 0.002 (0.08) | 0.02 (0.05) | -> | 0.13 | 0.98 | AD | 0.45 | ||
| Phormidium spp. | 1.3 | F | S | 0.72 | 0.6 | 0.3 | 0.23 | 0.22 | 0.55 | 0.08 (0.02) | O | 0.31 | |||||
| Cocconeis placentula | 1.2 | AP | L | 0.01 (0.05) | × | <0.001 (0.23) | + | 0.002 (0.08) | − | 0.66 | 0.98 | 0.05 (0.03) | <0.001 (0.16) | +A | 0.61 | ||
| Fragilaria ungeriana | 1.0 | ES | M | 0.06 (0.03) | <0.001 (0.25) | + | <0.001 (0.12) | + | 0.3 | 0.09 (0.02) | <0.001 (0.12) | +> | 0.06 (0.03) | AD | 0.09 (0.02) | ||
| Nitzschia palea | 0.9 | MO | M | <0.001 (0.11) | + | <0.001 (0.38) | + | <0.001 (0.09) | − | 0.16 | 0.4 | 0.01 (0.05) | -> | 0.04 (0.04) | +S | 0.7 | |
| Achnanthidium minutissimum | 0.6 | AP | S | <0.001 (0.15) | − | <0.001 (0.31) | + | 0.01 (0.05) | × | 0.35 | 0.01 (0.05) | × | 0.02 (0.04) | × | <0.001 (0.15) | +A | 0.003 (0.07) |
| Navicula cryptotenella | 0.6 | MO | M | 0.04 (0.03) | × | <0.001 (0.33) | + | 0.001 (0.08) | + | 0.53 | 0.04 (0.04) | × | 0.004 (0.07) | +> | 0.009 (0.06) | +A | 0.01 (0.05) |
| Rhoicosphenia abbreviata | 0.5 | AP | M | 0.23 | <0.001 (0.15) | + | 0.23 | 0.32 | 0.58 | 0.57 | 0.05 (0.03) | AD | 0.83 | ||||
| Gomphoneis minuta var. cassiae | 0.4 | ES | L | 0.47 | <0.001 (0.19) | + | <0.001 (0.09) | − | 0.4 | 0.59 | 0.53 | 0.03 (0.04) | +A | 0.22 |
- c, combined.
Two-way interactions involving temperature were coded in a manner equivalent to the simple effects contrasts for the sediment by nutrient interaction; however, for this continuous predictor variable the codes were determined from the GLM regression coefficients (slope change ± 95% confidence intervals). These codes were then classified directionally (+ or −) based on whether the effect of temperature was stronger (>) or weaker (<) when the second stressor was present (i.e. combined). Therefore, a significant two-way interaction with temperature is not equivalent to a synergism or antagonism in absolute terms, but is an indication that the relative presence, direction or strength of the temperature effect changed in the presence of a second stressor. Lastly, a significant three-way interaction implies that the presence, direction or strength of a two-way sediment by nutrient interaction (additive, synergistic or antagonistic) changed along the temperature gradient. In other words, this interaction type indicates that the response to increased temperature depends on the nutrient and sediment conditions present.
Where significant higher-order interactions are present, interpretation of the lower-order interactions or main effects of the experimental factors concerned must be done with care. We followed the recommendation of Quinn & Keough (2002) that lower-order interactions and main effects should be interpreted only where the effect size of the higher-order interaction is smaller than the size of the corresponding lower-order interactions or main effects. All exceptions to this rule are identified in Tables 1 and 2. Where significant two-way interactions were present, results are described for significant simple effects contrasts rather than for overall main effects that may or may not be significant.
Results
Algal community
Total algal cell density and algal biomass increased significantly with nutrient enrichment and fine sediment addition (Table 1, Fig. 2) while increased temperature reduced algal cell density in mesocosms with added sediment (sediment × temperature interaction). Algal taxon richness increased with added sediment but this response weakened with enriched nutrients (positive antagonistic sediment × nutrients interaction); richness also decreased with increased temperature in mesocosms without added sediment (sediment × temperature interaction). Both algal diversity and evenness increased with added sediment or enriched nutrients individually but with a combined effect similar to that of nutrient enrichment alone (positive antagonistic sediment × nutrients interaction); raised temperature decreased diversity and evenness (with a non-linear decline - less marked at the four highest levels), except in mesocosms with added sediment at ambient nutrients where the temperature effect was positive (nutrients × sediment × temperature interaction).
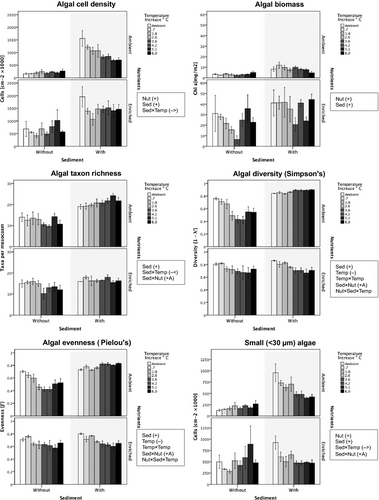
Algal size classes
Cell densities of small-sized (<30 μm) algal taxa (see Table 2 for the common taxa in each size class) increased significantly with added sediment or nutrient enrichment but with a combined effect comparable to the individual effect of either stressor (positive antagonistic sediment × nutrients interaction) (Table 1, Fig. 2), while increased temperature had a negative effect in mesocosms with added sediment (sediment × temperature interaction). Densities of medium-sized (30–90 μm) algal taxa (Table 1, Fig. 3) increased with both added sediment and nutrient enrichment, with an amplified positive effect when combined (positive synergistic sediment × nutrients interaction), and a negative effect of increased temperature, except in mesocosms with added sediment with enriched nutrients (three-way interaction). Densities of large-sized (>90 μm) algal taxa increased with added sediment but this effect weakened with enriched nutrients (positive antagonistic sediment × nutrients interaction); these algae also declined with increased temperature.
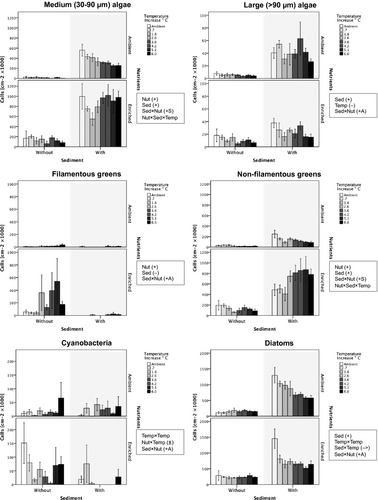
Taxonomic groups
Cell densities of both filamentous green algae and cyanobacteria increased with nutrient enrichment alone but not when combined with added sediment (positive antagonistic sediment × nutrients interactions) (Table 1, Fig. 3). Further, cyanobacteria showed a non-linear response to increased temperature that tended positive at ambient nutrient concentrations but negative at enriched nutrient concentrations (nutrients × temperature interaction). Densities of non-filamentous green algae increased with added sediment and nutrient enrichment, with an amplified positive effect when combined (positive synergistic sediment × nutrients interaction), whereas raised temperature had a predominantly negative effect that only became positive when added sediment and enriched nutrients were combined (three-way interaction). Diatom densities increased strongly with added sediment, but this effect was weakened with enriched nutrients (positive antagonistic sediment × nutrients interaction), while raised temperature had a negative effect in mesocosms with added sediment (sediment × temperature interaction).
Algal growth forms
Representation (as relative abundance) of the filamentous algal growth form increased with enriched nutrients and decreased with added sediment, with a combined effect similar to that of added sediment alone (positive antagonistic sediment × nutrient interaction; Table 1, Fig. 4) (see Table 2 for the common taxa in each growth form). Further, this growth form became more prevalent with raised temperature in mesocosms without sediment (sediment × temperature interaction). The erect/stalked growth form increased strongly with added sediment but this effect was weakened when combined with enriched nutrients (positive antagonistic sediment × nutrient interaction), and there was a negative effect of raised temperature that was stronger with added sediment (sediment × temperature interaction). The adnate/prostrate growth form decreased strongly with added sediment and decreased moderately with enriched nutrients with a combined effect similar to that of added sediment alone (negative antagonistic sediment × nutrient interaction), and with a non-linear, unimodal temperature effect when sediment was absent (nutrient × temperature interaction). The motile growth form increased strongly with added sediment and moderately with nutrient enrichment. Raised temperature had a positive overall effect on this growth form that was stronger with added sediment (sediment × temperature interaction), but was weakly negative in mesocosms without sediment at ambient nutrients (three-way interaction). The metaphyton growth form increased with nutrient enrichment, with an amplified positive effect when combined with added sediment (positive synergistic sediment × nutrient interaction). This growth form also showed a negative effect of raised temperature in mesocosms without sediment, a neutral temperature effect with sediment alone, and a positive effect when sediment and nutrients were combined (sediment × temperature and three-way interactions).
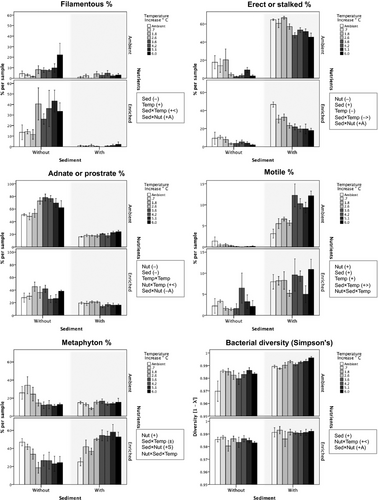
Bacterial community
Bacterial taxon richness showed no response to any of the manipulated stressors (Table 1). Bacterial evenness (Table 1) and diversity (Table 1, Fig. 4) both increased with added sediment, with a positive effect of raised temperature in mesocosms with ambient nutrients (nutrient × temperature interactions). Further, for bacterial diversity, the positive effect of added sediment was weaker with enriched nutrients (positive antagonistic sediment × nutrient interaction).
Algal common taxa
The algal common taxa GLMM revealed that community composition differed across nutrient, sediment and temperature treatments and all two and three-way interaction terms were significant (Table 2). Further, all but one of the 19 commonest algal taxa responded significantly to at least one of the experimental factors (Table 2). Individual taxon response patterns are described in the Figures S3–S5.
Discussion
The three stressors compared
As interpretable main factor effects (i.e. where the main effect size was larger than higher-order interaction effect sizes; Quinn & Keough, 2002) nutrients, sediment and temperature respectively affected 45%, 90% and 45% of all response variables. As predicted (hypothesis 1), nutrient enrichment had mainly positive main effects (14/17 significant univariate cases), increasing algal cell density and biomass, densities of medium-sized algae, filamentous and non-filamentous green algae and representation of the metaphytic growth form, and densities of eight of the 19 common taxa. The latter included both pollution-tolerant taxa (Stigeoclonium spp., Gomphonema parvulum, Nitzschia palea and Navicula cryptocephala) and those commonly associated with moderate (Scenedesmus spp., Ankistrodesmus spp.) or low-nutrient conditions (Gomphonema minutum) (Biggs & Kilroy, 2000). In full support of hypothesis 1, and as predicted by Passy (2007), the relative abundance of the adnate/prostrate growth form decreased with nutrient enrichment; this pattern was driven by declines in Achnanthidium minutissimum, a taxon commonly associated with low-nutrient conditions (Biggs & Kilroy, 2000).
Fine sediment addition proved the strongest (based on its mean partial eta squared main effect size) and most pervasive stressor, having mostly positive main effects (29/35 significant univariate cases) for the majority of algal and bacterial response variables, as found in an earlier study (Piggott et al., 2012). Our predictions (hypothesis 2) that fine sediment addition would have positive effects on motile and mucilaginous stalked taxa (represented in the erect/stalked growth form) but negative effects on filamentous and adnate/prostrate forms was fully supported at the community level for growth form relative abundance patterns, but only partially supported at the population level by declines in cell densities of Gomphonema minutum (the dominant adnate/prostrate representative), while the remaining adnate/prostrate taxa increased with added sediment, reflecting higher total algal cell density with added sediment, which we attribute to increased habitat heterogeneity (Pringle, 1990; Schneck et al., 2011; Piggott et al., 2012).
Raised temperature had mainly negative main effects (12/17 significant univariate cases) on algal response variables. Our prediction (hypothesis 3) of generally positive effects on green algae and cyanobacteria and negative effects on diatoms was not supported at the community level but received partial support at the population level with the filamentous green alga Stigeoclonium spp. increasing with raised temperature whereas eight diatom taxa declined (Fragilaria vaucheriae, Encyonema minutum, Cymbella kappii, Rossithidium, Cocconeis placentula, Nitzschia palea, Gomphoneis minuta var. cassia). That Fragilaria ungeriana and Navicula cryptotenella increased with raised temperature while the non-filamentous green alga Ankistrodesmus declined most likely reflects the specific thermal preferences of these taxa as opposed to the general tendencies of their respective taxonomic groups (Denicola, 1996). The observed decline in Cocconeis placentula with raised temperature contrasts with the view that species of this genus generally prefer warmer conditions (Patrick, 1971; Klarer & Hickman, 1975; Squires et al., 1979). Our prediction (hypothesis 3) that responses to raised temperature would be non-linear was supported in nine cases, with examples in all response categories (population, growth form, community) for both algal and bacterial measures, highlighting the complexity of responses to increased temperature (Denicola, 1996). As further predicted (hypothesis 4), large-celled algal taxa declined with increased temperature, indicating that cell-size composition may shift towards smaller-celled taxa under warmer conditions as large-celled taxa are lost disproportionately (Petchey et al., 1999; Woodward et al., 2010).
Three-way interactions
Interactions amongst the manipulated stressors affected 34 of the 40 algal and bacterial response variables. The most complex of these were three-way nutrients by sediment by temperature interactions, which affected 28% of all response variables. In these, increasing temperature caused three distinct response patterns: (i) a stronger response when sediment (Achnanthidium minutissimum, Navicula cryptotenella and Motile %) or sediment and nutrients (Fragilaria vaucheriae) were in operation, (ii) reversal or suppression of a prevailing temperature effect when sediment (diversity, evenness) or nutrients and sediment were in operation (medium-sized algae, non-filamentous greens and Scenedesmus spp.) or (iii) suppression with just sediment and reversal when combined (Metaphyton %). These interactions indicate clearly that raised temperature can have markedly different effects, which may worsen, improve or remain the same, depending on the sediment and/or nutrient conditions present. Our results support the contention of Denicola (1996) that the interactive effects of temperature with other stressors can be expected to be complex and difficult to untangle except by careful experimental manipulation.
Two-way interactions involving temperature
Nutrients by temperature interactions affected 18% of all response variables, with nutrient enrichment modifying the effect of increased temperature by enhancing its negative effect (one case: Rossithidium spp.), reducing its positive effect (three cases: adnate/prostrate, bacterial evenness and diversity) or causing opposing effects (one case: Cyanobacteria). Thus, increased temperature tended to interact negatively with enriched nutrients by weakening or reversing positive temperature effects or by strengthening negative ones. We had predicted (hypothesis 3) that Cyanobacteria would respond positively to raised temperature, but this was only the case with ambient nutrients, most likely due to the competitive advantage conferred by the capacity of some Cyanobacteria to fix nitrogen in low-nutrient environments as temperatures rise being lost under enriched nutrient conditions (Borchardt et al., 1996). Similarly, the fact that bacterial community evenness and diversity increased with temperature at ambient but not with enriched nutrients indicates that increased temperature coupled with nutrient limitation may lead to a more evenly distributed bacterial community as temperatures rise, while under enriched conditions nutrient-tolerant taxa remain dominant across the temperature range. For the adnate/prostrate algal growth form, the weakened positive effect of raised temperature with enriched nutrients suggests that a competitive advantage of this growth form in low-nutrient conditions and on smooth substrata (Pringle, 1990; Schneck et al., 2011) may also extend to raised temperatures.
Our prediction (hypothesis 5) that the effects of nutrient enrichment would strengthen with raised temperature received no systematic support. Rather, in the few instances where such a pattern was apparent (the filamentous growth form including Stigeoclonium spp. and the metaphytic growth form including Scenedesmus spp.), the positive additive effect or positive synergistic effect of nutrient enrichment and raised temperature were conditional on the absence or presence of sediment, respectively.
Sediment by temperature interactions affected 40% of all response variables, with sediment addition modifying the effect of raised temperature by enhancing its negative effect in eight cases (algal cell density, small-sized algae, diatoms, Fragilaria vaucheriae, Encyonema minutum, Cymbella kappii, Nitzschia palea and erect or stalked %) or its positive effect in three cases (Fragilaria ungeriana, Navicula cryptotenella and motile %), by reducing its negative (algal taxon richness) or positive (filamentous %) effect in one case each or with opposing effects in two cases (Gomphonema minutum and metaphyton %). These results imply that the effect of raised temperature was generally stronger when added sediment was present and tended to interact with negative effects. These interactive patterns run counter to our hypothesis 6, which predicted algal communities on fine sediment substrata would be less affected by raised temperature (Denicola, 1996). In fact, they indicate that algal communities on fine sediment substrata may be more susceptible to the negative effects of raised temperature than their epilithic counterparts, with diatom communities disproportionately affected. These results also provide partial support for our hypothesis 3 that diatoms would decline at raised temperatures (Cairns, 1956).
Interactions between sediment and nutrients
Two-way interactions between nutrients and sediment affected 65% of all algal response variables. Nutrient enrichment without added sediment had no significant effect in 11 cases, a positive effect in 13 cases and a negative effect in just one case. Conversely, added sediment at ambient nutrients had no significant effect in four cases, a positive effect in 19 cases and a negative effect in two cases. When both were combined (enriched nutrients with added sediment), however, there were positive antagonistic interactions (less positive than predicted) in 17 cases, a negative antagonistic interaction (less negative than predicted) in a single case and positive synergistic interactions (more positive than predicted) in seven cases. The two-way sediment by nutrient interaction for the motile growth form could not be classified because of the stronger higher-order three-way interactive effect, such that nutrients and sediment interacted synergistically at ambient temperature, additively at mid-temperature levels and antagonistically at higher levels. In the remaining 11 cases, the combined effect was not significantly different from an additive pattern (i.e. no interaction present). Notwithstanding the interactive effects of raised temperature that strengthened or weakened the sediment by nutrient interaction, these findings suggest that the combined effects of augmented nutrients and deposited fine sediment generally resulted in antagonistic outcomes that were less positive than predicted additively. This supports our hypothesis 7, which predicted algae on sediment would be less affected by nutrient enrichment because of their status as a specialized flora adapted to less favourable physical conditions, or to reduced access to water-column nutrients, in fine sediment habitat (Pringle, 1990; Hillebrand & Kahlert, 2002). In the seven instances where synergisms occurred in our experiment, four (medium-sized algae, non-filamentous greens and metaphyton %) reflected the strong positive synergistic response of Scenedesmus spp., which as the dominant taxon accounted for over 23% of the total algal community. The pattern is indicative of the ability of this colonial green alga to proliferate under nutrient-rich conditions (Biggs & Kilroy, 2000) and in fine sediment habitats (our results), with a further synergistic effect of raised temperature (three-way interaction). Similarly, the positive synergistic effects for Gomphonema parvulum, Navicula cryptocephala and Nitzschia palea reflect the capacities of these pollution-tolerant diatoms (Biggs & Kilroy, 2000) to proliferate when elevated nutrients and fine sediment are combined.
Our final hypothesis (8) predicted that community-level responses would be less susceptible to multiple-stressor effects than population responses. This prediction was based on a meta-analysis of marine multiple-stressor studies, conducted by Crain et al. (2008), which found that community level two-stressor responses tended to be antagonistic, whereas population-level responses tended to be synergistic. Based on the shape of the sediment by nutrient interactions in our experiment, this prediction was supported at the algal community level (for taxon richness, diversity and evenness) where the two stressors interacted with mainly antagonistic effects. At the algal population level, however, only four taxa showed synergistic effects (see above), and effects were more often additive (eight taxa) or antagonistic (six taxa). Nonetheless, the fact that synergisms were reasonably common at the population level, but never present at the community level, lends some weight to the idea that community-level variables may be generally less susceptible to multiple-stressor effects because species interactions may dampen and diffuse the effects on individual species (Crain et al., 2008). This being said, complex three-way interactions affected a higher proportion of the algal community-level variables (40%) than population-level variables (21%) in our experiment, indicating that the sediment by nutrient interaction was modified by temperature.
Management implications
Our mesocosm experiment has demonstrated how the effects of simulated climate-induced warming on stream periphyton communities may interact in complex ways with the individual and combined effects of elevated levels of dissolved nutrients and deposited fine sediment. Extrapolation of our results to field conditions should be done with care due to the restricted spatial (a single location) and temporal scales (a single 6-week period) of the study. Consequently, our study fails to capture successional responses in the periphyton (e.g. sloughing and regrowth cycles, shifting dominance patterns) that can be expected to change with time and by season. In addition, our mesocosm approach makes no allowance for likely shifts in geographic ranges and species compositions of algae and bacteria in real streams in response to increased temperatures (Hellmann et al., 2012) or to the potential of the species present to evolve in a way that changes their temperature optima (Skelly et al., 2007). Notwithstanding these limitations, to our knowledge this study provides the first empirical evaluation of the interactive responses of benthic algae and bacteria to multiple agricultural stressors coupled with simulated climatic warming, and of the complexity of responses that may arise. While informative in their own right, these responses may also provide insights into the bottom-up mechanistic pathways for effects observed at higher trophic levels (Dodds, 2007).
Of greatest significance amongst our findings is the fact that increased temperature often had markedly different effects on stream periphyton communities depending on the sediment and nutrient conditions present, with stronger or opposing effects when one or both stressors were augmented. The fact that all five studied algal growth forms displayed complex two or three-way interactive effects among the manipulated agricultural stressors and raised temperature suggests such measures may prove unreliable in biomonitoring programs (Passy, 2007) in a future climate scenario. Thus, freshwater management decisions that seek to avoid or mitigate the negative effects of agricultural land use on stream periphyton should be informed by knowledge of the interactive effects of multiple stressors in a warming climate.
Acknowledgements
We thank Nicky McHugh, Greg Stanley, Lauren Leicester and Katharina Lange for their help with field and/or laboratory work. We also thank Jan and Clyde Douglas for access to the study site. Funding was provided by New Zealand's Ministry of Business, Innovation and Employment contract C01X1005.




