Basin-scale phenology and effects of climate variability on global timing of initial seaward migration of Atlantic salmon (Salmo salar)
Abstract
Migrations between different habitats are key events in the lives of many organisms. Such movements involve annually recurring travel over long distances usually triggered by seasonal changes in the environment. Often, the migration is associated with travel to or from reproduction areas to regions of growth. Young anadromous Atlantic salmon (Salmo salar) emigrate from freshwater nursery areas during spring and early summer to feed and grow in the North Atlantic Ocean. The transition from the freshwater (‘parr’) stage to the migratory stage where they descend streams and enter salt water (‘smolt’) is characterized by morphological, physiological and behavioural changes where the timing of this parr-smolt transition is cued by photoperiod and water temperature. Environmental conditions in the freshwater habitat control the downstream migration and contribute to within- and among-river variation in migratory timing. Moreover, the timing of the freshwater emigration has likely evolved to meet environmental conditions in the ocean as these affect growth and survival of the post-smolts. Using generalized additive mixed-effects modelling, we analysed spatio-temporal variations in the dates of downstream smolt migration in 67 rivers throughout the North Atlantic during the last five decades and found that migrations were earlier in populations in the east than the west. After accounting for this spatial effect, the initiation of the downstream migration among rivers was positively associated with freshwater temperatures, up to about 10 °C and levelling off at higher values, and with sea-surface temperatures. Earlier migration occurred when river discharge levels were low but increasing. On average, the initiation of the smolt seaward migration has occurred 2.5 days earlier per decade throughout the basin of the North Atlantic. This shift in phenology matches changes in air, river, and ocean temperatures, suggesting that Atlantic salmon emigration is responding to the current global climate changes.
Introduction
Many organisms migrate between different habitats during their life cycle (Dingle, 1996). These movements may occur at different timescales and allow species to (i) take advantage of dietary or reproductive opportunities available in discrete and often distant habitats, and (ii) avoid certain habitats during periods such as winter when conditions may be intolerable. Migrations are usually triggered by seasonal changes in environmental conditions and by internal physiological processes. Habitat shifts may involve annually recurring travel over long distances, such as those undertaken by many species of birds, mammals, reptiles, fishes and insects (Dingle, 1996).
In diadromous fishes, life history strategies and migratory movements between fresh water and the ocean constitute key life history events in the life cycle of these species. Atlantic salmon (Salmo salar) typically emigrate from freshwater in the spring after having reached a growth-dependent size threshold (Økland et al., 1993). Age at emigration is 1–6 years and total length 12–25 cm. Once they reach the ocean, the subsequent growth is compensatory and very rapid (Hogan & Friedland, 2010). After 1–4 years at sea they return with high precision to their natal river to breed, although a small proportion strays to other rivers (Jonsson et al., 2003).
Prior to the seaward migration Atlantic salmon undergo a major transformation often called smolting, which comprises morphological, physiological and behavioural changes. This allows individuals to change from the territorial and relatively sedentary juvenile (‘parr’) stage to the migratory (‘smolt’) stage, during which they move downstream and are able to enter sea water (Hoar, 1976). The parr-smolt transformation is typically associated with increasing temperatures in spring, and is regulated by photoperiod and water temperature through effects on the neuroendocrine system (McCormick et al., 1998). Controlled laboratory studies indicate that photoperiod is the dominant cue of the parr-smolt transformation, with local temperatures playing a subordinate role (McCormick et al., 2002). Once the smolt transformation has been completed there is a short period of time during which the fish are physiologically prepared for seawater entry. Smolt that do not complete their seaward migration within this period desmolt, but may smolt again in the subsequent spring (McCormick et al., 2009). In general, smolt migration occurs in spring or early summer (Thorstad et al., 2012), and the timing of the initiation of the downstream migration differs among rivers.
A number of different environmental factors may trigger the downstream migration. These factors can be river-specific such as water temperature, flow and turbidity (Jonsson & Ruud-Hansen, 1985; McCormick et al., 1998), or related to light conditions in the river (Hansen & Jonsson, 1985; Hvidsten et al., 1995). Also, the presence of other migrants and predators may affect out-migration (McCormick et al., 1998).
Many factors affect post-smolt survival, but the timing of the smolt migration is an important predictor of survival to adulthood (Antonsson et al., 2010). In addition to proximal conditions like river temperature, other mechanisms can also affect survival, including predators, parasites and pathogens, feeding opportunities, and temperatures in the ocean (McCormick et al., 1998, 2009). Each of these factors has the potential to exert selective pressure on the migratory timing, with reduced survival associated with both too early (Kennedy & Crozier, 2010) and delayed migrations (Castro-Santos & Haro, 2003; McCormick et al., 2009). Thus, there exists a critical period of downstream migration (‘environmental smolt-window’) in which fitness is maximized by arrival at the marine environment when conditions are optimal for both survival and growth (McCormick et al., 1998). These are the necessary conditions for stabilizing selection, leading to genetic and phenotypic differentiation among populations of several salmonid species (Stewart et al., 2006; Spence & Hall, 2010).
In fisheries biology, the critical period concept (Cushing, 1990) postulates that survival and recruitment are maximized when there is a temporal match between a predator's phenology and that of its prey. Climate change might, however, alter the patterns of food availability leading to a mismatch if the resource base does not react in a similar way (Durant et al., 2007). Thus, there is evidence that the timing of seasonally recurring biological events (i.e. phenology) is shifting as a result of global increases in temperature (e.g. Parmesan, 2007). However, shifts in phenology appear to vary across taxa (Jonzén et al., 2006; Menzel et al., 2006; Parmesan, 2007; Kauserud et al., 2012), and at different trophic levels (e.g. Edwards & Richardson, 2004), and have important effects on population dynamics and systems ecology (Miller-Rushing et al., 2010); however, the fitness consequences may vary widely (McNamara et al., 2011).
Compared with terrestrial taxa, knowledge of the relationships between the timing of environmental changes and seasonal activities in fishes is sparse (Parmesan, 2007; but see Anderson et al., 2013). Furthermore, despite being a group with numerous species, there is little knowledge of the likely impacts of climate change on the dynamics of migratory fishes (Robinson et al., 2009). In anadromous salmonids some long-term studies have provided evidence that migration from freshwater to saltwater is occurring at earlier dates during a period of environmental warming for both Atlantic (Kennedy & Crozier, 2010) and Pacific (Kovach et al., 2013) species. In any case, productivity of Atlantic salmon has been declining throughout its distribution (Jonsson & Jonsson, 2004), raising major conservation and management concerns (Dempson et al., 2004). This reduction in fish abundance may be due, in part, to an alteration in timing of life history decisions affecting later survival (Hindar et al., 2011). Thus, there is a need to better understand the factors related to the initiation of global seaward migration pattern of Atlantic salmon.
In this article, we analyse large-scale variations in the timing of migration in Atlantic salmon at two migratory audit points (dates of 25 and 50% total smolt emigration) from fresh to salt water. We examine data sampled during 50 years from 1961 to 2010 from 67 North Atlantic rivers. The objective was to study the relationship between the smolt descent and environmental factors in both fresh and salt water while accounting for geographical variability. Furthermore, we tested if there has been a global phenological shift and whether this possible shift can be linked to changes in global and local environmental conditions.
Materials and methods
Study area and smolt sampling
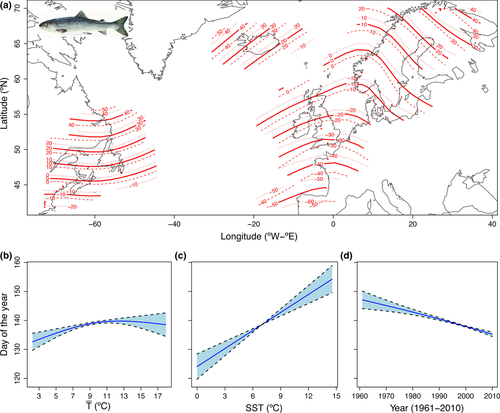
Atlantic salmon are naturally distributed throughout the basin of the North Atlantic Ocean. In the western part of its distribution, they occur from Ungava Bay, Québec, Canada in the north to the Connecticut River, USA, in the south. In the eastern part Atlantic salmon are found from Petchorskaya and the Ural mountains in Russia in the Northeast, along the coast of the European continent south to the River Miño in Spain in addition to Iceland and the British Isles (Jonsson & Jonsson, 2011). Data on timing of smolt downstream migration were obtained from 70 sites on 67 rivers covering most of this east–west and north–south gradient for the period 1961–2010 (Fig. 1a; Table 1). Some sites were situated close to the river mouth, others were in tributaries either close to the confluence with the main river or in the upper reaches, and others were located in the central part of the main stem of a river. Thus, most sites were situated between 1.2 and 34.8 km upstream of the river mouth (Data S1). Downstream migrating smolts were monitored by various methods. In most cases only a fraction of the river width was screened for smolts. However, it is assumed that the sampling schemes provide representative observations of the daily migration pattern and timing. Smolt trapping facilities were typically placed at sites where they could be operated across as broad a spectrum of river discharges as possible. However, such traps may have reduced efficiency during flood events. In some rivers, Wolf traps (Wolf, 1951) spanned the whole width of the river. Most Wolf traps are operated continuously and independent of the discharge. However, others are located on weirs and are subjected to operational constraints related to flows. Video cameras have also been used in some rivers. Cameras were anchored to the riverbed perpendicular to the running water and enabled a sample of migrating smolts to be recorded and subsequently counted. The number of cameras used in each transect depended on river width and water turbidity.
| Country | N° sites | Years (n) | Sampling methods | Comments |
|---|---|---|---|---|
| 1. Canada | 9 | 1970–2010 (192) | Fence, Rotary screw trap | River temperature predicted from air records in three observations. Water flow non-recorded for 41 observations. SST filled in for 16 observations. |
| 2. USA | 7 | 1993–2010 (76) | Rotary screw trap, Inclined screen samplers | River temperature predicted from air records for 24 observations. Water flow non-recorded for 36 observations. |
| 3. Iceland | 3 | 1987–2008 (52) | Fence, Fyke net | River temperature predicted from air records for two observations. Water flow non-recorded for 31 observations. |
| 4. Scotland | 2 | 1975–2010 (48) | Wolf trap, Smolt trap | River temperature predicted from air records for 29 observations. Water flow non-recorded for two observations. SST filled in for seven observations. |
| 5. Ireland | 2 | 1970–2010 (74) | Wolf trap | River temperature predicted from air records for 12 observations. Water flow non-recorded for 41 observations. SST filled in for 16 observations. |
| 6. England | 6 | 1981–2010 (59) | Smolt trap, Rotary screw trap | River temperature predicted from air records for 19 observations. Water flow non-recorded for eight observations. SST filled in for one observation. |
| 7. Wales | 2 | 2000–2010 (17) | Rotary screw trap | River temperature predicted from air records for one observation. |
| 8. France | 2 | 1985–2010 (42) | Smolt trap | Water flow non-recorded for two observations. |
| 9. Spain | 2 | 1999–2009 (21) | Smolt trap | River temperature predicted from air records for four observations. Water flow non-recorded for one observation. |
| 10. Russia | 1 | 1988–1995 (6) | Fence | Water flow non-recorded for eight observations. |
| 11. Finland | 9 | 1972–2009 (90) | Fyke net, Video camera | River temperature predicted from air records for one observation. Water flow non-recorded for 38 observations. SST filled in for eight observations. |
| 12. Norway | 20 | 1976–2010 (203) | Wolf trap, Net trap, Fence, Video camera, River fish lift | River temperature predicted from air records for 32 observations. Water flow non-recorded for 13 observations. SST filled in for six observations. |
| 13. Sweden | 5 | 1961–2010 (23) | Rotary screw trap, Wolf trap, Smolt trap | Water flow non-recorded for five observations. SST filled in for 17 observations. |
Descending smolts were usually monitored throughout the whole migration period. This extended from March to June in southern rivers and from June to August in northern systems (Table S1). The initiation of downstream migration for a given site and year was defined as the day of the year when 25% of the total smolt run had been enumerated (referred to as the onset of the smolt emigration), and the median emigration day was defined as the day of the year when 50% of the total smolt emigration had been counted. These quartiles were chosen because they are standard audit points of the smolt run in Atlantic salmon literature (Antonsson & Gudjonsson, 2002), and describe well the temporal migratory dynamics of each smolt cohort (Kennedy & Crozier, 2010).
Environmental data
River conditions
To test for association between time of emigration and relevant environmental conditions in freshwater we estimated the mean temperature ( in °C) and the mean discharge (
in °C) and the mean discharge (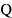 in m3 s−1) for the 10-day period preceding the 25 and 50% smolt descent dates. Furthermore, we estimated the relative change in discharge as the discharge-day relationship (slope, ΔQ) for that period. Such levels or changes in environmental conditions may act as triggers initiating the downstream migration. Discharge in each site was highly skewed thus it was ln-transformed before analysis. Temperature and discharge were mostly recorded using data loggers at the smolt counting station, or as close to this as possible (Data S1).
in m3 s−1) for the 10-day period preceding the 25 and 50% smolt descent dates. Furthermore, we estimated the relative change in discharge as the discharge-day relationship (slope, ΔQ) for that period. Such levels or changes in environmental conditions may act as triggers initiating the downstream migration. Discharge in each site was highly skewed thus it was ln-transformed before analysis. Temperature and discharge were mostly recorded using data loggers at the smolt counting station, or as close to this as possible (Data S1).
Sea surface temperature
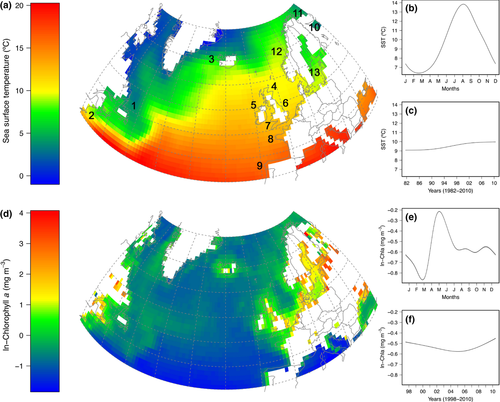
Optimum Interpolation sea surface temperature (NOAA_OI_SSTV2) data available at weekly 1° latitude × 1° longitude grid resolution from a combination of satellite and in situ measurements (Reynolds et al., 2002) were obtained from the NOAA Earth System Research Laboratory (http://www.esrl.noaa.gov/psd/) for the period 1982–2010 (Fig. 1a–c). To evaluate the potential association between downstream migration dates and sea surface temperature (SST in °C) at sea entry we used the average SST for the 7-day period preceding the date of 25 and 50% descent for those cells whose centres were located nearest to the ocean entry point of a given river. In eleven rivers where smolt sampling started before the availability of the satellite data set, SST was obtained from different sources (Data S1). Sampling sites were located at various distances from the river mouth. The time (25 and 50% dates) for smolts to reach the ocean was adjusted for this variation using the distance from the sampling station to the river mouth and an average migration speed of about 32 km d−1 obtained from measurements recorded in various rivers (Table S2).
Chlorophyll a
Phytoplankton concentration is important for defining suitable pelagic habitats and might be a surrogate for oceanic feeding conditions (e.g. Bi et al., 2007). To test if timing of sea entry is adjusted to a period of sufficient primary production, we compiled data on satellite-derived chlorophyll a concentration (8-day composites on surface concentration, Chla, in mg m−3) from the Sea-viewing Wide Field-of-View Sensor (SeaWiFS) at 1° latitude × 1° longitude grid resolution for the period 1998–2010 (Fig. 1d–f). For the same SST coastal cells, we used the ln-transformed (to make the distribution more symmetrical) concentration of chlorophyll a from the 8-day composite previous to the 25 and 50% downstream dates (Data S1).
Air temperature
Water temperature was for most rivers obtained daily during the smolt migration period and not all year round. Moreover, many rivers were sampled during a few years only; accordingly, the length of the water temperature time series was shorter than 10 years in many cases. This makes it difficult to reliably estimate global trends in freshwater conditions. Thus, air temperature, which correlates with river temperature (Fig. S14) was used as a surrogate to generate a global description of the thermal environment faced by each sampled river. Therefore, we collected data on daily mean air temperatures (Table S3) using the ‘WeatherData’ function (http://reference.wolfram.com/mathematica/ref/WeatherData.html) in Mathematica 8.04 (Wolfram Research, Inc., 2010) (Data S1).
Statistical analyses
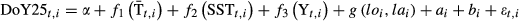 (1)
(1) (river temperature), SST (sea surface temperature), Y (year), and site location at longitude lo and latitude la. We assumed that including a function of longitude and latitude would act as a ‘catch-all’ proxy for factors that vary spatially such as photoperiod and seasonal freshets. The smoothing functions were fit by penalized cubic regression splines and a thin plate regression spline with 3 and 15 knots for the one- and two-dimensional functions respectively (Wood, 2006). If any of the nonparametric relationships are essentially linear, those covariates can be modelled as parametric terms within the GAMM formulation. For instance, the relationship with SST is linear (see below), thus that term in eqn 1 becomes β1 × SSTt,i where β1 is a coefficient that describes the change in the date of emigration for a unit change in SST. ai is a random intercept allowing for variation between sites, and bi is a random slope allowing, for instance, the relationship with SST to differ by site. Random effects are assumed to be normally distributed with mean 0 and variances
(river temperature), SST (sea surface temperature), Y (year), and site location at longitude lo and latitude la. We assumed that including a function of longitude and latitude would act as a ‘catch-all’ proxy for factors that vary spatially such as photoperiod and seasonal freshets. The smoothing functions were fit by penalized cubic regression splines and a thin plate regression spline with 3 and 15 knots for the one- and two-dimensional functions respectively (Wood, 2006). If any of the nonparametric relationships are essentially linear, those covariates can be modelled as parametric terms within the GAMM formulation. For instance, the relationship with SST is linear (see below), thus that term in eqn 1 becomes β1 × SSTt,i where β1 is a coefficient that describes the change in the date of emigration for a unit change in SST. ai is a random intercept allowing for variation between sites, and bi is a random slope allowing, for instance, the relationship with SST to differ by site. Random effects are assumed to be normally distributed with mean 0 and variances 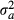 and
and  . The residuals ɛt,i are a normally distributed random error with mean 0 representing the within-site variation. Given the sequential nature of the data a residual correlation structure was added to the model. An autoregressive correlation of order 1 is suitable for regular spaced data. Because our data were commonly irregularly spaced in time, we tested if including a linear spatial correlation structure (Pinheiro & Bates, 2000), that can accommodate the imbalance in time, improved the model fitting. In addition, the variance in residual dates (σ2) was further modelled as a function of possible covariates included in eqn 1, for instance,
. The residuals ɛt,i are a normally distributed random error with mean 0 representing the within-site variation. Given the sequential nature of the data a residual correlation structure was added to the model. An autoregressive correlation of order 1 is suitable for regular spaced data. Because our data were commonly irregularly spaced in time, we tested if including a linear spatial correlation structure (Pinheiro & Bates, 2000), that can accommodate the imbalance in time, improved the model fitting. In addition, the variance in residual dates (σ2) was further modelled as a function of possible covariates included in eqn 1, for instance,
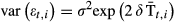 (2)
(2)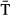 . This model of the residual variance was compared with other variance structures through selection criteria.
. This model of the residual variance was compared with other variance structures through selection criteria.The difference between river temperature ( ) and SST can be related to the onset of migration (Kennedy & Crozier, 2010). Therefore, we explored this potential effect running a separate model that included the thermal difference (TDif) between both environments as a covariate. Water flow records were unavailable for numerous site-year combinations (Table 1), thus substantially reducing the migration information. Therefore, discharge was not used in eqn 1. It was, however, included in a separate model that contained only those sites with sufficient data. The same happened with chlorophyll a that was available only from 1998 to 2010.
) and SST can be related to the onset of migration (Kennedy & Crozier, 2010). Therefore, we explored this potential effect running a separate model that included the thermal difference (TDif) between both environments as a covariate. Water flow records were unavailable for numerous site-year combinations (Table 1), thus substantially reducing the migration information. Therefore, discharge was not used in eqn 1. It was, however, included in a separate model that contained only those sites with sufficient data. The same happened with chlorophyll a that was available only from 1998 to 2010.
For any equation, model selection was performed iteratively. First, with all fixed effects included in the model, appropriate random effects and residual correlation structure were selected using the Bayesian Information Criterion that puts a heavier penalty on models with more parameters. Model parameters were estimated by means of restricted maximum likelihood (REML). Second, the variance models were selected. Third, the optimal fixed effects were determined by means of maximum likelihood (ML) parameter estimation. Finally, with the optimal fixed structure in place the random effects were reassessed and model parameters presented were estimated by REML (Zuur et al., 2009). The same procedure was used to model the median of downstream migration (i.e. DoY50).
For each river, air and sea surface temperature time series, individually average warming rates and changes in the timing of seasonal warming were computed (Data S1).
All analyses and treatment of data were performed with R 2.15.0 language (R Development Core Team, 2012) and using the ‘mgcv 1.7–13’ (Wood, 2006) and ‘nlme 3.1–103’ (Pinheiro & Bates, 2000) packages.
Results
Summary of smolt migration patterns
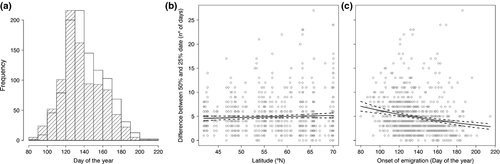
Downstream migration timing varied among and within sites. The earliest onset of emigration occurred in the Tea River (Spain) where 25% descent was recorded on the 20th March in 2000, whereas the latest onset date was recorded on the third August 1995 in the Vesturdalsa River (northern Iceland) (Table S1). Collectively, the observations of time of 25 and 50% descent (river and year combinations) showed that ca. 75% were within a 30-day period between the beginning of May (ca. day 120 s) and the beginning of June (ca. day 150 s) (Fig. 2a). In addition, the difference between the 50 and 25% emigration date occurred within a narrow time window with 75% of the observations (river and year combinations) extending over a period of less than 6 days, though a maximum difference of 27 days was recorded in Vesturdalsa River in 1998. The variability of this difference was not related to latitude (Fig. 2b). However, on average, time between 50 and 25% of emigration dates appeared to be shorter when the onset of emigration occurred later (Fig. 2c).
Onset of emigration
Model selection favoured a random intercept indicating high variability in emigration dates from site to site, and also a random slope allowing the relationship with SST to differ by site (Table S4). Within-site residual correlation structures did not improve the model fitting (Table S4). The optimal model of the onset of emigration timing revealed a strong spatial trend showing a clear west–east and south–north gradient (Fig. 3a). The average onset of downstream migration was about the 18th May (day 138) (Table 2) at 45°N in the western Atlantic, whereas in the eastern Atlantic this date occurred approximately at 63°N (isopleth zero in Fig. 3a). This resulted in the mean onset of emigration date in Northern Norway, Finland and Russia occurring about 90 days later than in Spain, at the southern limit of the species distribution in Europe (Fig. 3a). After accounting for the spatial trend, river temperature ( ) had a slight non-linear effect on the among-river onset of emigration reaching a plateau at about 10 °C (Fig. 3b). In addition, there was evidence for changes in the spread of the onset of emigration related to
) had a slight non-linear effect on the among-river onset of emigration reaching a plateau at about 10 °C (Fig. 3b). In addition, there was evidence for changes in the spread of the onset of emigration related to  (Table 2 and Table S5). The estimated exponential variance parameter corresponds to a 14.9% increase in variance with a 2 °C warming in river temperature.
(Table 2 and Table S5). The estimated exponential variance parameter corresponds to a 14.9% increase in variance with a 2 °C warming in river temperature.
 ), (b) and sea surface temperature (SST), (c) on the onset of seaward migration. The long-term trend during the last five decades is shown in (d). See Fig. S15 for a plot showing the distribution of the data in (b–d). Smolt drawing credits in panel (a): © Atlantic Salmon Federation (www.asf.ca)/J.O. Pennanen.
), (b) and sea surface temperature (SST), (c) on the onset of seaward migration. The long-term trend during the last five decades is shown in (d). See Fig. S15 for a plot showing the distribution of the data in (b–d). Smolt drawing credits in panel (a): © Atlantic Salmon Federation (www.asf.ca)/J.O. Pennanen.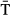 = Mean River Temperature; SST = Sea Surface Temperature; Y = Year. These covariates were centred by subtracting 9 and 7 °C, and year 1986, respectively, before inclusion in the model. lo = longitude; la = latitude; SD = standard deviation; SE = standard error; CI = 95% confidence interval; Edf = estimated degrees of freedom. Note that exploratory generalized additive models revealed a linear relationship with SST (edf =1), thus this term was modelled as a parametric term.
= Mean River Temperature; SST = Sea Surface Temperature; Y = Year. These covariates were centred by subtracting 9 and 7 °C, and year 1986, respectively, before inclusion in the model. lo = longitude; la = latitude; SD = standard deviation; SE = standard error; CI = 95% confidence interval; Edf = estimated degrees of freedom. Note that exploratory generalized additive models revealed a linear relationship with SST (edf =1), thus this term was modelled as a parametric term.| Parameter | Estimate | SE | CI | t-value | Edf | F-value | P-value |
|---|---|---|---|---|---|---|---|
| Fixed effects | |||||||
| Intercept | 138.60 | 0.85 | 163.41 | <0.0001 | |||

|
1.86 | 9.23 | <0.0001 | ||||
| SST | 2.07 | 0.32 | 6.55 | <0.0001 | |||
| Y | 1.26 | 69.92 | <0.0001 | ||||
| lo,la | 10.81 | 61.63 | <0.0001 | ||||
| Random effects (SD) | |||||||
| σ a | 5.81 | 4.68; 7.21 | |||||
| σ b | 1.28 | 0.80; 2.06 | |||||
| σ | 6.01 | 5.72; 6.32 | |||||
| Variance function | |||||||
| δ | 0.035 | 0.016; 0.053 | |||||
When the smolt migrated later the SST at the oceanic entry point was warmer (Fig. 3c). This effect resulted in an estimated average (±SE) increase of 2.1 ± 0.3 days in onset of emigration per 1 °C increase in SST. Furthermore, the model also revealed a slight non-linear shift towards earlier onset of emigration timing during the last 50 years (Fig. 3d). Modelling the long-term trend as a parametric component resulted in an earlier downstream migration of, on average, 2.5 ± 0.3 days per decade, which means that, over the entire 50 years studied, the data showed an earlier onset out-migration of 12.7 ± 1.4 days.
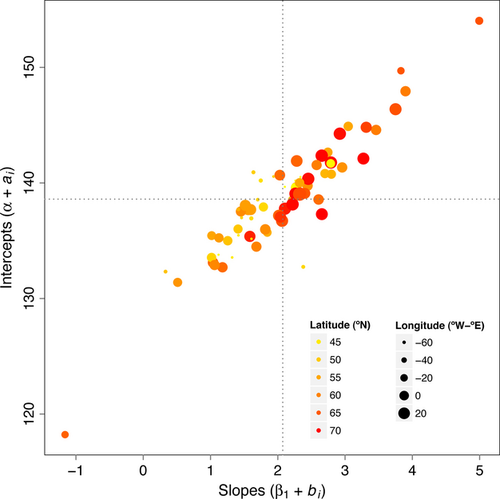
There were variability in mean emigration onset dates from site to site, and also the relationship with SST differed by site (Table 2). Furthermore, there was a positive correlation between the random effects indicating that sites that had larger intercepts (i.e. later emigration) also had larger slopes (i.e. a stronger relationship with SST) (Fig. 4). Moreover, this pattern slightly varied with geography with larger intercepts and slopes occurring at higher latitudes.
Finally, residuals of the optimal model did not show any apparent heterogeneity or major departures from normality (Fig. S16–S17). The estimated random effects were also reasonably normal (Fig. S18). Moreover, there was no spatial pattern in residuals; there was no clear clustering of positive (or negative) averaged residuals per sampling site (Fig. S19).
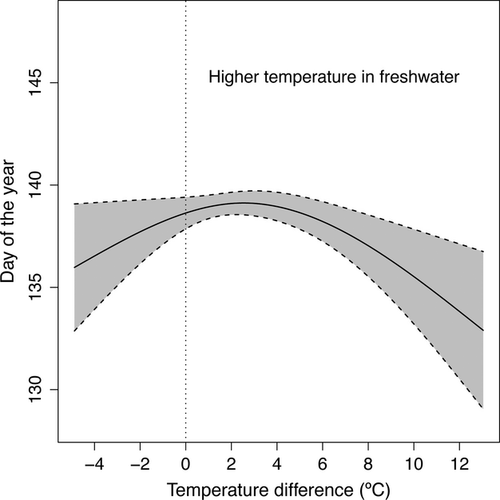
Further examination of the combined effects of temperature in both fresh- and saltwater habitats showed a nonlinear relationship between the among-river onset of migration and the thermal difference between both environments (Table S7). This mostly revealed that when freshwater temperature was 3 °C warmer than the ocean SST the onset of smolt emigration occurred earlier (Fig. 5).
Regarding water flow, the onset of emigration occurred later at higher average discharge ( ) (Fig. 6a). This relationship implied that a one percent increase in
) (Fig. 6a). This relationship implied that a one percent increase in  would result in a 0.011 ± 0.003 day delay in the average onset date of emigration. Furthermore, the model also revealed a nonlinear relation between the onset date and the change in water flow (ΔQ), with earlier emigration when the rate of change in discharge tended to increase (Fig. 6b).
would result in a 0.011 ± 0.003 day delay in the average onset date of emigration. Furthermore, the model also revealed a nonlinear relation between the onset date and the change in water flow (ΔQ), with earlier emigration when the rate of change in discharge tended to increase (Fig. 6b).
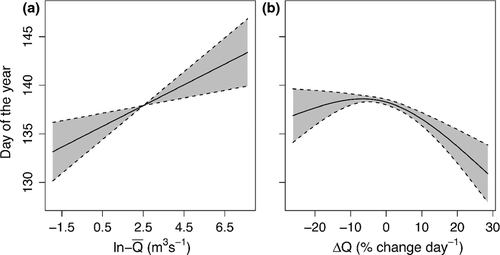
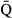 ) (a), and the discharge-day slope for the 10-day period preceding the 25% smolt descent dates (ΔQ) (b) on the onset of seaward migration. See full results of this model in Table S8 and Fig. S21.
) (a), and the discharge-day slope for the 10-day period preceding the 25% smolt descent dates (ΔQ) (b) on the onset of seaward migration. See full results of this model in Table S8 and Fig. S21.Chlorophyll a was not correlated with the onset of emigration. Running a model from 1998 to 2010 (n = 443) that included surface concentrations of chlorophyll a (Fig. 1d) at the oceanic entry point as a new covariate, revealed no association (P > 0.1).
The onset and median emigration dates were correlated (r2 = 0.97), thus the modelling yielded similar results (Table S9 and Fig. S22).
Trends and seasonal shifts in air, river and sea surface temperature
Overall, the analysis of temperature trends in rivers with at least 10 years of data revealed an increase in water temperature at an average rate (±SE) of 0.36 ± 0.06 °C per decade (Fig. 7a). This warming corresponded well with air temperature records at stations close to the smolt sampling locations, for which the mean increase was 0.25 ± 0.03 °C per decade (Fig. 7b). The SST at the ocean entry points warmed at a mean rate of 0.33 ± 0.02 °C per decade (Fig. 7c). Furthermore, it was observed that seasonal warming (air temperature stations and the coastal cells) generally occurred earlier in the year. These shifts revealed an advance of seasonal warming in air temperatures at an average rate of 2.70 ± 0.34 days per decade (Fig. 7d), and an earlier arrival of seasonal warming in SST at an average rate of 5.02 ± 0.49 days per decade (Fig. 7e). Finally, the among-river date of onset of emigration was related to the date of seasonal warming in air temperatures (Fig. 7f).
Discussion
In this work, we examined the geographical pattern of the initial timing of the downstream migration of young anadromous Atlantic salmon (smolts) throughout its natural distribution and found that timing of downstream migration varies strongly among rivers. This variation is probably a response to selection driven by prevailing regional conditions (Thorstad et al., 2011), and thus we could expect large-scale patterns that reflected these spatial environmental differences. Results showed that – in addition to the latitudinal cline with southern populations migrating earlier than northern ones (Hvidsten et al., 1998) – the timing of out-migration differed strongly between the East and West Atlantic, with western populations migrating to sea at later dates than eastern populations at corresponding latitudes.
What may be the selective agent leading to this geographical pattern in downstream migration? Geographical variation in timing is most probably driven by the spatial pattern of average SST (compare isotherms in Fig. 1a with isopleths in Fig. 3a). In particular, there is large variation in SST between the East and West part of the Atlantic, which is the result of both the organization of atmospheric circulation forcing and oceanic current systems (Deser et al., 2010; Fig. S23). The latitudinal variation in SST is also well known. However, even if SST is the selective force leading to differences in phenology, salmon smolts cannot use it as a cue for initiating the downstream migration. A cue that might be associated with SST may be used. Consequently, the latitudinal patterns in phenology are most probably cued by the geographical variation in photoperiod (Fig. S24). Photoperiodism is widespread across multiple taxa (Bradshaw & Holzapfel, 2007), and Atlantic salmon are shown to assess and use day length to initiate the physiological changes associated with smolting. However, the response to photoperiod may be adjusted by variation in local environmental factors such as river temperatures (McCormick et al., 2002). Long-term selection may then lead to changes in how salmon populations respond to these cues.
After accounting for this geographical variation, among-river migratory patterns of Atlantic salmon were related to freshwater conditions. The onset of the smolt emigration was positively associated with river temperatures up to about 10 °C, levelling off or potentially decreasing at higher temperatures. This result indicates that the onset of the freshwater emigration does not occur beyond a given day of the year (or temperature) despite continued temperature increase. In rivers and years with the latest onset dates the temperature at smolt descent ranged from 10 to 17 °C, values that are close to the seasonal peak temperature for those rivers. This agrees well with previous knowledge about the secondary role of river temperature in impairment of the fishes tolerance of saltwater (McCormick et al., 2002, 2009). In addition, emigration dates were more heterogeneous in rivers and years experiencing elevated temperatures, though this effect might be a result of scarce data at high temperatures.
Water temperature has been already identified as a primary environmental factor cuing downstream migration (Thorstad et al., 2012). Some studies suggest that the initiation of the smolt run require passing a certain temperature threshold (e.g. 10 °C) for wild (Antonsson & Gudjonsson, 2002) and hatchery reared smolt (Jutila et al., 2005). However, when pooling rivers across the distribution of Atlantic salmon there was no clearly defined lower temperature limit associated with the commencement of the smolt migration, and the lower thermal threshold appears river-specific (McCormick et al., 2002). At high water temperatures smolt characters (e.g. salinity tolerance) are lost sooner and quicker (McCormick et al., 2009). Consequently, it is important for the smolts to emigrate from freshwater well before reaching very high water temperatures. Our results suggest that also the upper thermal limit is river specific.
Downstream migration timing has frequently been related to water flow (McCormick et al., 1998) showing that migration of Atlantic salmon smolts can be initiated by increased water discharge during spring freshets (Hvidsten et al., 1995) albeit this correlation may be highly variable (Jonsson & Jonsson, 2009). We found that earlier migration among rivers occurred at lower average water flow and at a higher positive change (increase) in flow. This might indicate that smaller rivers (low average flow) with an increase in the rate of change in discharge are more unstable in their hydrology and thus emptying the smolts out earlier. Alternatively, because rivers with larger discharge are usually longer some of the observed relationship could be due to longer migration distances to saltwater from multiple headwater streams.
Among-river variation in downstream migration was associated with oceanic thermal conditions at the sea entry point with later migrants finding higher sea temperatures. This relationship also varied from site to site, with sites that had later emigration also had a stronger relationship with SST. Several studies have reported the thermal regime experienced by smolts during the initial marine migration. For instance, Antonsson & Gudjonsson (2002) showed that smolts leaving northern Icelandic rivers would enter seawater at 5 °C, Hvidsten et al. (1998) reported a consistent SST of ca. 8 °C for smolts emigrating from five rivers in Norway, and Kennedy & Crozier (2010) showed that smolt in Northern Ireland would experience a thermal regime ranging from 7 to 12 °C. Our analysis shows that among-river variation in smolt emigration was associated with a range of SST of about 0 to 15 °C, and this further suggests that populations would be adapted to emigrate from the rivers and enter salt water at a particular and prevailing regional sea temperature. The specific sea temperature at which each population reaches the ocean environment should be consequently connected with a specific value of photoperiod (Fig S24c), the main cue used by the salmon to initiate smolting.
Year-to-year variability in the timing of the smolt run within rivers has often been related to variation in water temperature, resulting in delayed migration in cooler springs (Jonsson & Ruud-Hansen, 1985; Jensen et al., 2012). We found that the timing of migration for the whole set of rivers and years was related to the thermal difference between fresh and salt water. When temperature in fresh water was ca. 3 °C warmer than in the sea outside the river mouth, the migration occurred earlier. Earlier onset of migration at an increased temperature contrast between fresh and saltwater was previously shown for the River Bush, Northern Ireland (Kennedy & Crozier, 2010). Therefore, we conclude that smolts migrate earlier in warm river years, and that river temperature influences the timing of the smolt run, but selection has regulated the fish's ability to use photoperiod as a priming mechanism for the migration. This is consistent with laboratory studies that have shown clear physiological linkages between the photoperiod and the physiological preparation for smolting with local temperature serving a subordinate role (McCormick et al., 2002). The onset of migration, although accompanied and mediated by physiological changes, is a behavioural response. As such, priming mechanisms prepare animals for migration, and tend to be synchronized with long-term average conditions that are associated with selective drivers of migration. Releasing mechanisms are often de-coupled from these priming mechanisms, however, and allow animals to fine-tune behavioural responses to maximize their ability to take advantage of variable conditions (Dingle, 1996). Our data are consistent with this interpretation: salmon in each site would use specific day length to initiate smolting and enter the saltwater at a particular sea surface temperature. Photoperiod as a priming mechanism would tend to stabilize dates of migratory onset, but local temperatures and flow would be responsible for annual variation.
Time of ocean entry of Atlantic salmon influences post-smolt survival (Hansen & Jonsson, 1989; Antonsson et al., 2010) as has also been shown for several Pacific salmon species (Scheuerell et al., 2009). Therefore, natural selection would favour migration at a time when conditions are favourable (Hansen & Jonsson, 1989, 1991). During this time window the ionoregulatory ability of the fish may be optimal, with smolts that migrate too early or too late experiencing physiological stress (Handeland et al., 1998) and increased mortality (Antonsson et al., 2010). Increased mortality might be related to predation and its interaction with the physiological status of the smolts (Handeland et al., 1996), to food availability (Hvidsten et al., 2009), or to other stressors (Thorstad et al., 2012). Matching the sea temperature that is optimal for iono-regulation and antipredator behaviour, and the link with resource peaks that favour rapid growth is crucial for survival. For instance, Jutila et al. (2005) showed that for hatchery reared salmon smolt survival in the northern Baltic Sea was related to SST in June during the smolt emigration, and this relationship followed a dome-shaped pattern with optimal survival at 9–12 °C. Furthermore, warmer sea temperatures at the time of ocean entry increase subsequent catches of salmon that have spent one winter at sea (Otero et al., 2011).
Various factors including food availability affect marine survival (Beaugrand & Reid, 2012). We therefore used data-rich satellite information on chlorophyll a as a proxy for productivity. We found no support for a positive association between migration timing and chlorophyll a concentration at sea entry, and chlorophyll a concentration did not track SST or was connected with photoperiod. This suggests that smolt emigration is probably not adjusted to chlorophyll peaks, and that phytoplankton abundance is a poor indicator for early post-smolt feeding conditions. Successful initial feeding might be better represented by the abundance of fish larvae, large crustaceans and nekton (Hvidsten et al., 2009; Renkawitz & Sheehan, 2011) and various other prey groups not available at the scale of this study. Information on these prey types would probably allow evaluation of the temporal connection of marine resources with migratory cues. Nevertheless, successful feeding for early post-smolts is crucial to enhance growth and avoid predators (Rikardsen & Dempson, 2011). In addition, a ‘correct’ migration timing should ensure that post-smolts arrive at distant water feeding grounds during periods of high prey abundance. Distance from the river to the feeding area in the North Atlantic increases with decreasing latitude notably for the south European populations and it is important to be present in the north at the start of the growth season (Friedland et al., 2013). Moreover, the importance of a precise timing at ocean entry is further emphasized by the fact that smolts entering seawater outside the narrow migration window stray more to other rivers when returning to spawn (Hansen & Jonsson, 1991).
We found a shift towards earlier onset of downstream migration for the Atlantic salmon smolts during the last five decades. For diadromous fishes, habitat shifts are key life history events subject to environmental variation. The downstream migration of species seems to be population-specific (Crozier et al., 2008; Jensen et al., 2012), but often with a trend towards earlier timing in recent years as noted for Atlantic salmon (Kennedy & Crozier, 2010), and a number of Pacific salmon species (Kovach et al., 2013). Nevertheless, our combined data set estimated an overall out-migration advancement across the North Atlantic of 2.5 days per decade in the initial time of migration. This value parallels current mean estimates of global shifts of phenological responses of 2.8 days per decade in spring across the northern hemisphere for multiple taxonomic groups (Parmesan, 2007). Our estimate is, however, slightly faster than the 1.5 days shift per decade observed for a number of Pacific salmon species in an Alaskan river (Kovach et al., 2013). It is generally hypothesized that earlier phenology might be associated with the impacts of current climatic changes, and especially related to warming. However, different organisms respond differently, even when experiencing similar climatic trends (Parmesan, 2007). Further, the patterns of climatic changes are highly heterogeneous across Earth, thus very different responses are expected among species. However, despite the differences in sensitivity to temperature, organisms should maintain their thermal niches by tracking temperature patterns (Cleland et al., 2012).
Recent global analyses show that, despite spatial and seasonal heterogeneity, oceanic and terrestrial ecosystems (Burrows et al., 2011) and coastal regions (Lima & Wethey, 2012) have experienced significant increases in temperature since the middle of the last century. In addition, seasonal shifts in temperature towards earlier spring arrival have been identified. However, Burrows et al. (2011) concluded that despite land temperatures warming three times faster than the ocean, the seasonal shifts were generally greater in the sea than on land. At the scale of our analysis, we found similar trends. First, we observed comparable average warming rates of freshwater, air, and sea surface temperatures at the coastal entry points. Second, we also observed earlier seasonal warming of air and sea surface temperatures, and an association between the onset of emigration and the timing of seasonal warming in air temperature. Increasing water temperature results in earlier migration (Jonsson & Ruud-Hansen, 1985). This effect has been described also for other salmonids such as brown trout (Salmo trutta) (Jonsson & Jonsson, 2011), and Arctic charr (Salvelinus alpinus) (Jensen et al., 2012). Thus, it is plausible that global downstream smolt migrations have advanced due to increased river temperatures as changes in climate (Burrows et al., 2011), together with hydrological changes, are driving the current river temperature trends (van Vliet et al., 2011), which ultimately might have multiple implications for salmonid resources (Isaak et al., 2012). Thus, global warming could lead to a greater disconnect between cues for migration (photoperiod that is insensitive to climatic changes and water temperature) and the environmental conditions in the receiving marine environment with potential implications for fitness and productivity (Piou & Prévost, 2013). This effect might point to further long-term stock depression because it would no longer be possible to optimize survival if the cues are disconnected from the environment. This mis-timing would have a survival cost; however, population dynamics could still be quite stable due to compensatory dynamics (Reed et al., 2011).
Whether this change in migration timing of Atlantic salmon smolts is due to phenotypic plasticity or has a genetic basis remains to be understood. Despite evidence that adaptive microevolution can occur rapidly in many populations, separating the contribution of genetic adaptation and phenotypic plasticity is difficult (Hoffmann & Sgrò, 2011). For salmonids, recent studies have shown that evolutionary and plastic responses can explain a phenotypic trend towards earlier migration into freshwater of sockeye salmon (Oncorhynchus nerka) (Crozier et al., 2011). Similarly, a genetically based change towards earlier upstream migration of pink salmon (O. gorbuscha) has been documented (Kovach et al., 2012). Nonetheless, our analysis cannot distinguish between the two responses and additional research is needed.
Timing of downstream migration may vary depending on smolt age and size. Slower growing and older smolts tend to migrate earlier in spring within a river (Jonsson et al., 1990; Jutila & Jokikokko, 2008; but see Jensen et al., 2012). Recent analyses suggest that juvenile salmon now tend to grow faster and migrate to sea at younger ages and smaller sizes (Jonsson & Jonsson, 2005), but with large variation among rivers (Russell et al., 2012). The cause of this change in size and age may partially be ascribed to the hydrological regime and to an increase in temperature that regulate parr growth and age at smolting. Unfortunately, it was not possible to assess the influence of smolt age on emigration timing since information on smolt age was lacking for most rivers and years.
We conclude that downstream migration timing of Atlantic salmon is strongly spatially structured as a result of photoperiodicity. Photoperiod would be linked with the spatial pattern of sea surface temperature at the time of ocean entry and would then be a priming mechanism differentiating the latitudinal among-river initiation of the smolt emigration. An overall trend towards earlier smolt migration was evident and probably associated with observed warming trends in the freshwater habitat. Global warming could lead to a reduced connection between the cues for migration and the environmental conditions in the receiving marine environment with potential implications for salmon survival through a mismatch with seawater conditions affecting population fitness and productivity. Declining survival would probably be associated with suboptimal ionoregulatory conditions causing an altered antipredator behaviour of the early post-smolts. Moreover, growth opportunities might be reduced if emigration timing does not match with the production of prey items that are experiencing changes in their own phenology inducing further food web alterations.
Acknowledgements
This study is part of the Norwegian Research Council project no 183989/S30. J.O. acknowledges additional funding from Norwegian Water and Energy Directorate (NVE). We are grateful to all those people who manned the smolt counting facilities in all countries. This study would not be possible without their work. Earth Observation data were provided by the NERC EO Data Acquisition and Analysis Service, Plymouth. Dr. Alex Haro and four reviewers provided helpful comments and suggestions that greatly improved earlier versions of the manuscript.




