Symbiotic specificity, association patterns, and function determine community responses to global changes: defining critical research areas for coral-Symbiodinium symbioses
Abstract
Climate change-driven stressors threaten the persistence of coral reefs worldwide. Symbiotic relationships between scleractinian corals and photosynthetic endosymbionts (genus Symbiodinium) are the foundation of reef ecosystems, and these associations are differentially impacted by stress. Here, we couple empirical data from the coral reefs of Moorea, French Polynesia, and a network theoretic modeling approach to evaluate how patterns in coral-Symbiodinium associations influence community stability under climate change. To introduce the effect of climate perturbations, we simulate local ‘extinctions’ that represent either the loss of coral species or the ability to engage in symbiotic interactions. Community stability is measured by determining the duration and number of species that persist through the simulated extinctions. Our results suggest that four factors greatly increase coral-Symbiodinium community stability in response to global changes: (i) the survival of generalist hosts and symbionts maximizes potential symbiotic unions; (ii) elevated symbiont diversity provides redundant or complementary symbiotic functions; (iii) compatible symbiotic assemblages create the potential for local recolonization; and (iv) the persistence of certain traits associate with symbiotic diversity and redundancy. Symbiodinium may facilitate coral persistence through novel environmental regimes, but this capacity is mediated by symbiotic specificity, association patterns, and the functional performance of the symbionts. Our model-based approach identifies general trends and testable hypotheses in coral-Symbiodinium community responses. Future studies should consider similar methods when community size and/or environmental complexity preclude experimental approaches.
Introduction
Perturbations from climate change are modifying the structure and function of coral-Symbiodinium communities that coral reefs are built upon through differential performance and mortality. Scleractinian corals build and maintain reef structure via their association with Symbiodinium, a diverse genus of photosynthetic, endosymbiotic dinoflagellates (Muscatine & Porter, 1977). The photosynthetic carbon acquired from Symbiodinium is essential for coral survival, but climate change is impairing the functionality of coral-Symbiodinium associations. This symbiosis can be broken down by environmental stress, such as extreme temperatures (Hoegh-Guldberg & Smith, 1989), and reestablishment of the association is essential for coral survival (Douglas, 2003). Novel environmental regimes resulting from global changes are altering the absolute and relative abundances of corals species and Symbiodinium types (Gardner et al., 2003; Baker et al., 2004; Berkelmans & van Oppen, 2006; Jones et al., 2008; LaJeunesse et al., 2009). As a result, coral-Symbiodinium communities may become more depauperate and homogenized as the effects of climate change intensify. Because coral species and Symbiodinium types are limited in their symbiotic partnerships (Baker, 2003; Fabina et al., 2012), the reestablishment of specific coral-Symbiodinium unions in novel environmental regimes is likely to be dependent on community composition and host and symbiont population dynamics. For instance, the establishment of a particular symbiotic union requires the presence of the host and symbiont, as well as sufficient population densities to initiate interactions. However, to our knowledge there are very few studies coupling empirical data and mathematical models to predict how communities of corals and Symbiodinium will respond to a changing climate, despite a wealth of diversity assessments (LaJeunesse, 2002; van Oppen et al., 2005) and species-level modeling efforts (Baskett et al., 2009, 2010; van Woesik et al., 2010; Ortiz et al., 2013).
To address these knowledge gaps, we merge existing coral-Symbiodinium data and network theory to highlight and explore four important and understudied aspects of coral-Symbiodinium community responses to climate change. Our goal is to demonstrate general trends in coral-Symbiodinium community stability using a simple model, while presenting our results and assumptions as opportunities for future empirical and theoretical studies. Specifically, we compare the relative influence of the following on community stability: (i) random vs. nonrandom species loss; (ii) the importance of subdominant or cryptic symbionts; (iii) maintaining species diversity vs. maintaining a diversity of potential associations; and (iv) the importance of mutualistic service vs. thermal tolerance (Fig. 1). We simulated either coral or Symbiodinium removals, observed the resulting extinctions of symbionts or hosts in the community, and quantified community stability by measuring how long biodiversity was maintained. Here, we summarize the importance of each of the four aspects to coral-Symbiodinium community dynamics.
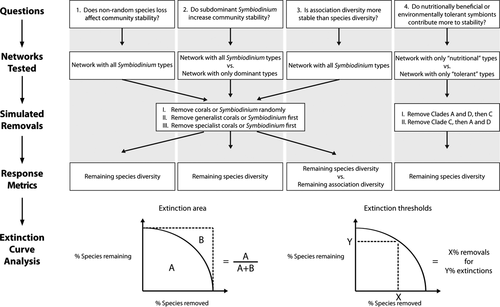
Does nonrandom species loss affect community stability?
Coral-Symbiodinium communities may respond to climate change in a predictable fashion if species loss is nonrandom. Previous work has demonstrated that corals and Symbiodinium vary in their symbiotic partner specificity, ultimately constraining the realized association patterns within a community (Baker, 2003; Fabina et al., 2012). Moreover, corals with more diverse symbiont assemblages (i.e. generalist corals) may be more environmentally sensitive than corals with fewer potential symbiotic associations (i.e. specialist corals; Putnam et al., 2012). Together, the limits on potential coral-Symbiodinium associations and observed patterns of species sensitivity to environmental stress (Loya et al., 2001; van Woesik et al., 2011) influence community stability. Thus, we ask whether nonrandom coral species or Symbiodinium type loss as a result of climate change, for example, is likely to increase or decrease community stability.
Do subdominant Symbiodinium increase community stability?
Dominant Symbiodinium types are often reported via genetic fingerprinting approaches such as DGGE (e.g., LaJeunesse, 2002; Finney et al., 2010), but subdominant or rare symbionts are less frequently identified. In fact, 79% of symbiont samples within a major Symbiodinium database (Franklin et al., 2012) represent only dominant types. More recently, studies have suggested that genetically diverse symbiont assemblages with a variety of subdominant types are the norm, rather than the exception (Baker & Romanski, 2007; Correa & Baker, 2008; Putnam et al., 2012; Silverstein et al., 2012). Previous studies have hypothesized that corals have the potential to switch or shuffle dominant Symbiodinium types in response to environmental disturbances (Buddemeier & Fautin, 1993; Fautin & Buddemeier, 2004), but subdominant Symbiodinium diversity could play a similar role. In numerous systems, subdominant species increase community productivity or the propensity to resist or recover from disturbances, even though dominant species may significantly influence community function and dynamics (Tilman, 1999; Loreau et al., 2001; Elmqvist et al., 2003; Hooper et al., 2005). Thus, we ask whether subdominant Symbiodinium have the potential to increase community stability in a changing physical environment.
Is association diversity more stable than species diversity?
Conservation or management efforts often prioritize species diversity via protection of rare or challenged taxa, but these efforts can also selectively target species with important community-level effects (Hooper et al., 2005). Within the reef environment, the diversity of a coral assemblage may be dependent on the ‘association diversity’ of the symbiont assemblage, which we define as the potential for a host or symbiont to engage in strong interactions with a diverse set of partners (proposed as a stability metric in Kaiser-Bunbury et al., 2010 and adapted from Bascompte et al., 2006; see Methods for the explicit formula). For instance, an assemblage of specialist symbionts with similar host preferences has lower association diversity because they can partner with a less diverse coral assemblage. On the other hand, the same number of generalist symbionts with nonoverlapping host preferences have greater association diversity because they can partner with a more diverse coral assemblage. Essentially, association diversity assigns greater weight to species with more and stronger associations, while species diversity assigns equal weights to all taxa. Thus, we use association diversity to more accurately measure community stability for communities with the same number of extant taxa (species diversity is similar), but distinct association patterns (association diversity is dissimilar). We expect association diversity to be more informative than species diversity when considering the recovery of degraded coral reefs. For example, Symbiodinium assemblages with high association diversity are likely to promote coral recruitment from external sources, and external recruitment can play a major role in coral recovery from environmental disturbances depending on life history and reef connectivity (Hughes et al., 2003). Thus, we ask whether association diversity is more stable than species diversity within coral-Symbiodinium communities.
Do nutritionally beneficial or environmentally tolerant symbionts contribute more to community stability?
Symbiodinium types differ in their nutritional benefit (e.g., the amount and quality of photosynthetic carbon provided to coral hosts; Stat et al., 2008; Cantin et al., 2009; Baker et al., 2013) and environmental tolerance (e.g., the amount of environmental stress needed to disrupt the symbiosis; Rowan, 2004; Sampayo et al., 2008; Mieog et al., 2009; Baker et al., 2013). Moreover, recent work suggests that there is a physiological trade-off between resource provisioning and thermal tolerance within the symbiosis (Jones & Berkelmans, 2012). The prominence of thermally tolerant Symbiodinium has already increased in response to changing environmental conditions (Baker et al., 2004; Berkelmans & van Oppen, 2006; LaJeunesse et al., 2009) and it is critical to understand the implications of this shift. Although many studies have experimentally compared unique combinations of corals and symbionts (Rowan, 2004; Sampayo et al., 2008; Cantin et al., 2009; Mieog et al., 2009; Jones & Berkelmans, 2012; Baker et al., 2013), we ask whether community stability differs under scenarios where either nutritional benefit or environmental tolerance is required for persistence in a changing climate.
Methods
In this section, we describe (i) data format and network construction; (ii) the simulated extinction algorithm and extinction scenarios; (iii) our method for quantifying stability; and (iv) methods for analyzing the sensitivity of our results to changes in observed data or biological assumptions.
Data and networks
Coral-Symbiodinium data were obtained from previously collected and sequenced Symbiodinium from a set of coral tissue samples collected from fore reef, fringing reef, and lagoon reef habitats surrounding the island of Moorea, French Polynesia (Putnam et al., 2012). A total of 134 tissue samples were collected from 34 coral species within approved Moorea LTER sites. A median number of eight Symbiodinium internal transcribed spacer 2 (ITS2) sequences were obtained per coral sample using Sanger sequencing, with full details found in Putnam et al. (2012).
To assess symbiotic associations, we created coral-Symbiodinium interaction networks (sensu Fabina et al., 2012; Fig. 1). The interaction networks were composed of nodes representing either corals species or Symbiodinium sequences, and links between nodes represented observed associations (Fig. 2). Alternatively, these associations can be displayed as a matrix with coral species and symbiont sequences as rows and columns, respectively, and the matrix cell entries identify an observed association (Fig. S1).
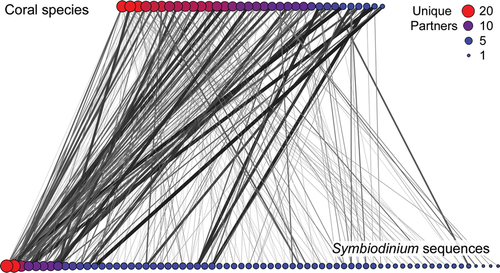
Here, we use the term community to describe the collection of corals and Symbiodinium. We define a Symbiodinium assemblage as the sequences identified within a coral colony, population, species, or community, depending on context, and a coral assemblage as a collection of coral species. We refer to the empirical Symbiodinium ITS2 sequences as ‘sequences’ where possible and use symbiont sequences to describe the constituent components of symbiont assemblages (although ‘species’ is sometimes used as a shorthand to refer to corals and Symbiodinium). The identification of Symbiodinium ‘types’ or ‘species’ is an ongoing area of research and, instead, we assumed that ITS2 sequence diversity is correlated with genetic diversity. In turn, Symbiodinium genetic diversity is likely to be correlated with ecological function, as has been found in other systems (Hughes et al., 2008). In other words, we treat Symbiodinium sequence diversity as a correlate of functional diversity and corals with more symbiont partners have the potential for greater functional diversity or redundancy. Thus, our model describes the consequences of a reduction in coral species diversity or Symbiodinium genetic diversity – we do not assume that Symbiodinium ITS2 sequences are equivalent to species and our model is not meant to simulate the loss of Symbiodinium species diversity.
Simulated extinctions and extinction scenarios
We simulated extinctions using observed coral-Symbiodinium associations to quantify network stability. For simulated extinctions, either corals or symbionts are iteratively removed according to a predefined removal rule (e.g., the most generalist coral species remaining in the network is removed next; Memmott et al., 2004; Dunne & Williams, 2009; Kaiser-Bunbury et al., 2010). When a species (network node) is removed, all associations (network links) involving the removed species are also removed (Fig. 2). Secondary extinctions occur when a species loses its last known partner; e.g., a coral is extinct when all potential symbiont partner sequences are removed. In this stability test, all links are positive because they are necessary for persistence and all links are equivalent because they are equally capable of maintaining species. Thus, a species with more links is more stable within the community because its symbiotic partners add functional diversity or redundancy, and partners are interchangeable. Building up, networks with more observed associations are likely to be more stable because more species removals are necessary to induce secondary extinctions, but this is dependent on the realized association patterns (e.g., partner specificity).
For each scenario, we separately tested the removal of Symbiodinium sequences and coral species. We first removed Symbiodinium sequences and recorded secondary coral extinctions to simulate conditions where symbionts became nonviable mutualistic partners (e.g., temperature rise disrupts photosynthetic capabilities; Rowan, 2004). Second, we removed coral species and recorded Symbiodinium secondary extinctions to simulate conditions in which host species were lost (e.g., corals cannot persist under novel environmental or competitive regimes (e.g., hurricanes or macroalgal overgrowth; Hughes et al., 2003). We simulated extinctions for four scenarios with different predefined removal rules or stability metrics, corresponding to the four proposed questions in the introduction (Fig. 1 and see below).
Scenario 1: Does nonrandom species loss affect community stability?
We tested whether coral-Symbiodinium networks were more sensitive to the loss of generalists or specialists, given that generalist corals may be more environmentally sensitive (Putnam et al., 2012). For example, in the full dataset coral specificity ranges from the generalist Pocillopora meandrina (15 partners) to the specialist Porites irregularis (2 partners) (Fig. S1). To test random and nonrandom species loss, we (i) removed species randomly as a baseline; (ii) removed species from most generalist to most specialist; and (iii) removed species from most specialist to most generalist. One thousand extinction simulations were run for each group (corals removed vs. symbionts removed) and removal rule (random, generalists first, specialists first) to break ties in specificity.
Scenario 2: Do subdominant Symbiodinium increase community stability?
We tested whether subdominant symbiont sequences increased the topological stability of coral-Symbiodinium networks. The extinction curves in Scenario 1 were generated using all observed coral-Symbiodinium associations. For Scenario 2, we simulated extinctions using a second dataset of only dominant symbiont sequences (Fig. S2). The ‘dominants-only’ dataset included only the most abundant sequence (or sequences in the case of ties) found in a coral sample. Thus, subdominant associations were excluded from the simulation. Fewer sequences were present in the ‘dominants-only’ dataset, so we normalized our results by the number of Symbiodinium sequences initially present in the community to enable relative comparisons. To address the potential for subdominant Symbiodinium diversity to increase the stability of coral assemblages we report symbiont removal in our results (coral removal is reported in Table S1).
Scenario 3: Is association diversity more stable than species diversity?
To test whether association diversity was more stable than species diversity, we used species strength (Bascompte et al., 2006), rather than species diversity (Scenario 1), as a response variable. In short, greater Symbiodinium species strengths indicate a symbiont sequence has many partners or frequently serves as a primary symbiont for its hosts; weaker species strengths indicate a symbiont sequence has few partners or frequently serves as an alternative symbiont. Thus, species strength may be a more accurate measure of community health (Kaiser-Bunbury et al., 2010) if Symbiodinium with greater species strengths can support a more diverse coral assemblage and facilitate community recovery. Importantly, the term ‘species strength’ is applied to Symbiodinium sequences to be consistent with previous studies, despite symbiont ITS2 sequences not being equivalent to species.
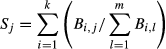
The extinction curves in Scenario 1 used the percentage of species diversity remaining as the response variable. The extinction curves for Scenario 3 followed the same removal rules, but tracked association diversity as defined as the percentage of species strength remaining. Specifically, we initially summed all species strengths and subtracted a species' strength when a secondary extinction occurred. To address the potential for depauperate Symbiodinium assemblages to retain association diversity and to facilitate recolonization of locally extirpated coral species, we report the results for simulated coral removal (the results for simulated symbiont removal are reported in Table S2).
Scenario 4: Do nutritionally beneficial or environmentally tolerant symbionts contribute more to community stability?
Symbiodinium types differ in their nutritional benefit (Stat et al., 2008; Cantin et al., 2009; Baker et al., 2013) and environmental tolerance (Rowan, 2004; Sampayo et al., 2008; Mieog et al., 2009; Baker et al., 2013), and there is evidence for a physiological trade-off between resource provisioning and thermal tolerance within the symbiosis (Jones & Berkelmans, 2012). We follow an initial trait-based approach to ask whether communities composed of only nutritionally beneficial symbionts are more stable than communities composed of only environmentally tolerant symbionts. In the first case, we assume that high nutritional benefit under historical reef conditions is critical for coral persistence and, in the second, we assume that environmental tolerance to current and projected extreme conditions is critical.
As a starting point, we assume that clade C symbionts have higher nutritional benefit (Stat et al., 2008; Cantin et al., 2009; Baker et al., 2013) and that clades D and A are more thermally tolerant or opportunistic (Rowan, 2004; Sampayo et al., 2008; Mieog et al., 2009; Baker et al., 2013). We apply this frequently used cladal generalization as a first step to understanding how the stability of a coral assemblage is influenced by nutritional and environmental requirements (see Baker et al., 2004; Little et al., 2004; Rowan, 2004; Stat et al., 2008; Ortiz et al., 2013). We acknowledge that Symbiodinium functional characteristics can vary within clade (Sampayo et al., 2008; Fitt et al., 2009; Mieog et al., 2009; Brading et al., 2011) but, to the best of our knowledge, there is not enough physiological data available to account for differences in photosynthetic capabilities and environmental optima for the diversity of symbionts in our dataset. Notably, our results are not sensitive to the introduction of intracladal variance in Symbiodinium characteristics (see Sensitivity Analyses below).
Quantifying network stability
We used the secondary extinction data from each of the four scenarios to calculate community stability. Specifically, we analyzed the percentage of species remaining as a function of the percentage of species removals having occurred. Percentages were calculated by separately averaging outcomes for the 24 sets of 1000 simulations. The 24 sets of simulations are derived from the four scenarios and three removal rules (random, generalists first, specialists first) for the two cases of simulated removals (coral or Symbiodinium; Fig. 1).
We quantified network stability using two measures: the proportion of area underneath the extinction curve and the percentage of removals needed to achieve a secondary extinction threshold (25, 50, and 75% secondary extinctions occurred; defined as robustness in Dunne & Williams, 2009; Fig. 1). The extinction area is calculated as the proportion of area subsumed by the extinction curve divided by the total possible area under the extinction curve (i.e. if no extinctions were to occur until the last removal; Fig. 1). We modified this metric for bipartite mutualistic networks as its original use was to analyze unipartite food webs (Allesina & Pascual, 2009). The extinction area reaches a maximum of 1 when all species survive until the last removal and a minimum close to zero when all but one species goes extinct after the first removal, but the actual minimum is dependent on the number of species in the community. More stable networks have greater extinction area and needed more removals to reach the extinction thresholds. Although extinction area and percentage of removals needed to reach an extinction threshold are correlated, each measure is informative. For comparative purposes, we show the linear loss line in our figures, which corresponds to one percentage point in secondary extinctions for one percentage point removal.
Sensitivity Analyses
We additionally conducted extensive sensitivity analyses to determine whether our quantitative results or qualitative trends were sensitive to changes in observed data or biological assumptions (see Supplemental Information). For our sensitivity analyses, we first modified the dataset or classification of symbionts and repeated the above analyses. Specifically, we either (i) removed rare Symbiodinium sequences from the dataset to simulate observational noise or lack of functional importance; (ii) added associations between corals and already observed Symbiodinium to simulate insufficient sampling; or (iii) reclassified the benefit and tolerance of symbionts within clades to test variance at the subclade or type level. In summary, our model results were insensitive to random changes in the observed associations or assumptions, suggesting that the following results are general trends for structurally similar coral-Symbiodinium communities.
Results
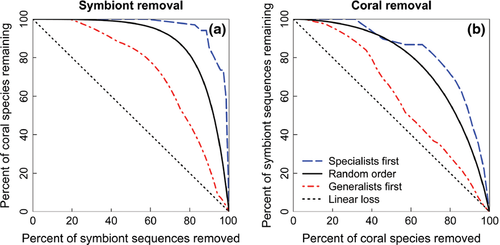
Coral-Symbiodinium communities lose biodiversity more quickly when generalists are more sensitive to a changing climate
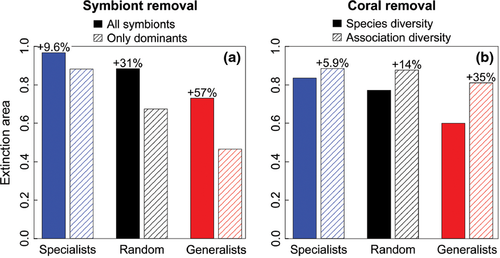
Coral-Symbiodinium communities were most sensitive to loss of generalists according to both extinction area and removal thresholds (Fig. 3, Tables S1 and S2). Communities were most stable when specialists were removed first. For instance, coral removals had 0.84 extinction area when specialists were removed first, but only 0.60 extinction area when generalists were removed first. In general, coral assemblages were more stable when symbionts were removed than Symbiodinium assemblages were when corals were removed. For example, 63% of Symbiodinium needed to be removed to cause 25% of corals to go extinct, but removing only 41% of corals caused 25% of symbionts to go extinct (for generalist-first removal). Simulated extinction curves were above the linear loss line, such that proportionally more species needed to be removed to induce a single extinction when the community was intact, but that a single removal led to several secondary extinctions when the community was highly degraded. In terms of network topology, this occurred because both specialist corals and symbionts are most often connected to generalists within the opposite guild, rather than other specialists.
Subdominant symbionts have the potential to greatly increase the stability of coral-Symbiodinium communities
In particular, coral assemblages were much more stable if subdominant Symbiodinium are functionally important (Fig. 4a, Tables S1 and S2). This effect was most pronounced when generalist symbionts were removed first. The dominants-only network had only 0.47 extinction area with generalist symbiont removal, while the original network (including subdominants) had 0.73 extinction area (a 57% increase). Underscoring the instability of dominants-only networks, only 27% of dominant Symbiodinium needed to be removed to cause 25% of corals to go extinct. In comparison, 63% of symbiont sequences needed to be removed from the full network to cause the same percent of coral extinctions – more than double the percentage for the dominants-only network. The nearly linear loss of species in dominants-only networks occurred because a few Symbiodinium (i.e. ITS2 C1, C1b, C3) had many observed associations, while most other symbionts were relatively specialists.
Even depauperate coral assemblages can support diverse symbiont assemblages (and vice versa), indicating that impoverished communities have the potential to recover from environmental disturbances.
Association diversity was much more stable than species diversity under all removal rules (Fig. 4b, Tables S1 and S2). Specifically, coral species can be removed from the community and symbiont sequences are lost, but the remaining symbionts retain the potential to interact with a diverse coral assemblage. This effect signals the potential for depauperate Symbiodinium assemblages to facilitate recolonization of locally extirpated coral species, provided those corals can recruit to the local area. When generalist corals are removed, the extinction area for association diversity is 35% greater than for species diversity. The same pattern is displayed by the extinction curves; 25% of symbiont diversity is lost after only 41% of corals are removed, but 74% of corals need to be removed to eliminate 25% of association diversity.
Coral-Symbiodinium communities will be less stable if symbionts are selected for thermal tolerance rather than nutritional benefit
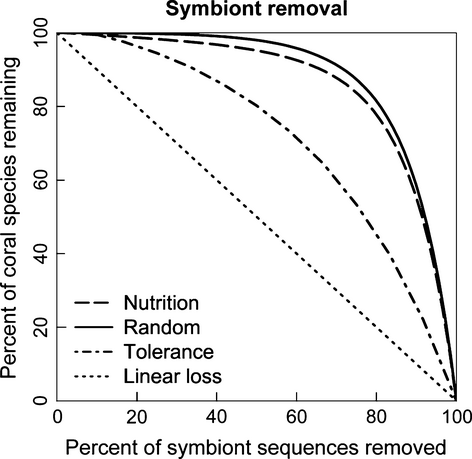
The relative importance of symbionts providing nutritional benefit in comparison to symbionts with environmental tolerance had implications for the proportion of viable corals in the long-term and the stability of those communities (Fig. 5). Over 94% of corals species associated with a symbiont providing greater nutritional benefit, while only 44% of corals associated with an environmentally tolerant symbiont. Thus, environmental stress would make over 55% of the corals examined here unsuccessful in the long term in the absence of other compensatory responses. The effect was even more critical if both characteristics were essential: only 21% of corals interact with at least one symbiont from clade C and clades D or A. Coral-Symbiodinium communities composed of only symbionts providing greater nutritional benefit were 23% more stable than communities composed of environmentally tolerant symbionts (Fig. 5). Communities composed of corals and clade C Symbiodinium collapse less quickly because these associations have greater redundancy; coral species are likely to have many clade C partners and the removal of a single symbiont is unlikely to result in a secondary extinction until the community is significantly degraded. In contrast, associations between corals and clade D and A Symbiodinium collapse more quickly because associations have less redundancy. In this case, the removal of a single clade D or A symbiont is likely to cause a secondary extinction, even when the community is relatively intact. This test also demonstrates that Symbiodinium from clades D and A do not add much topological stability to the community because the extinction curve for clade C is nearly identical to the extinction curve for the whole community (Fig. 5).
Discussion
We have shown that simulations of coral-Symbiodinium community stability (i) are more sensitive to nonrandom loss of generalists; (ii) are supported by the inclusion of subdominant or cryptic symbionts; (iii) indicate that degraded communities have the potential to recover; and (iv) are negatively impacted by maintaining environmental tolerance at the cost of nutritional benefit (Fig. 1). Although these four areas of coral-Symbiodinium research are essential to predict community responses to environmental stress and future climate change, accurate projections have not been available given the current state of knowledge. For instance, recent efforts have attempted to categorize corals according to their life-history strategies or symbiotic associations (Darling et al., 2012; Fabina et al., 2012; Putnam et al., 2012) and connect coral categories with trends in population sizes or coral cover (Loya et al., 2001; Knowlton & Rohwer, 2003; Fabricius et al., 2011; van Woesik et al., 2011). However, these efforts can be stymied due to limited phenotypic information and/or time-series data. To this end, the simulated extinctions used here are a simple model for species loss and highlight how topology affects community stability.
Coral-Symbiodinium community stability may be even more critical to explore if coral individuals, populations, or species have the potential to exchange Symbiodinium partners. There is already extensive evidence that Symbiodinium can vary from specialist to generalist at various spatial scales (Baker, 2003; Fabina et al., 2012). For example, within the community studied in this article, Symbiodinium D1a was relatively specialist by associating with only five coral hosts, while C1 and C3 were relatively generalist by associating with 20 coral hosts each. Symbiodinium populations with multiple coral hosts have the potential to be shared among symbiotic partners. Again, within our dataset, Acropora valida, Pavona cactus, Pocillopora meandrina, and at least 17 other coral taxa were simultaneously associating with Symbiodinium from the C1 population. If the C1 population is connected across multiple symbiotic and environmental habitats, the ecological and evolutionary dynamics of coral hosts can be influenced via shared associations with C1. For instance, the abundance and distribution of C1 within Acropora valida hosts may be dependent on the population dynamics of C1 within Pavona cactus, Pocillopora meandrina, and other symbiotic and environmental habitats. The coevolution between Acropora valida and C1 may be influenced by evolution between C1 and other coral hosts, as well as C1 adaptation to environmental habitats (this is likely to be more important for Symbiodinium types for which environmental habitats are not dead ends). Thus, given the potential for ecological and evolutionary coupling between hosts and symbionts within a community – not just within individuals – community stability becomes a much more complicated and necessary topic to study.
Our results demonstrate similarities to other symbiotic systems where diversity, association, and function are important contributors to community stability and dynamics. For instance, the diversity of plant symbionts, such as arbuscular mycorrhizal fungi, can influence host plant phenotype, fitness, interactions, and diversity (Koch et al., 2006, 2012; reviewed in Kernaghan, 2005; Johnson et al., 2012). Moreover, these effects are more pronounced when symbiont communities are genetically diverse (Koch et al., 2006). Our results demonstrate that the full complement of Symbiodinium genetic diversity may determine whole community responses to novel environmental regimes. In support of this, concurrent work has demonstrated correlations between subdominant Symbiodinium diversity and coral-Symbiodinium association patterns (Fabina et al., in review). For instance, the analysis of subdominant Symbiodinium diversity highlights the relationship between symbiont transmission mode and host–symbiont specificity: corals that transmit Symbiodinium vertically, from parent to offspring, have more specialist symbionts. The strength of these patterns increases when subdominant Symbiodinium are considered, which suggests that symbiont diversity is an influential component of host ecology and evolution. Despite these advances, there is much to learn about the relationship between coral diversity and Symbiodinium diversity, as well as the functional implications of particular host–symbiont unions. These present two promising lines of inquiry for future research.
Our simulation provides evidence that coral-Symbiodinium communities may be most sensitive to loss of generalist species. Simulated extinctions have been used in other systems to demonstrate that sensitivity to loss of generalist species tends to be a general result, including plant-pollinator communities (Memmott et al., 2004; Kaiser-Bunbury et al., 2010) and food webs (Dunne & Williams, 2009). Within the above ecological communities and others, specialists are most likely to be connected to generalists. Thus, the removal of a single generalist has the potential to cause the secondary extinctions of many specialists, while the removal of a specialist is likely to leave generalists with alternative partners (Bascompte et al., 2003; Memmott et al., 2004; Dunne & Williams, 2009; Kaiser-Bunbury et al., 2010). This structural characteristic is thought to increase the stability of communities. For instance, plant-pollinator communities are likely to be relatively stable because core generalists are less sensitive than specialists to changes in species composition or environmental conditions, and variance in interactions across space and time may buffer the effects of disturbances (Olesen et al., 2008; Petanidou et al., 2008; Kaiser-Bunbury et al., 2010; Sabatino et al., 2010; Burkle & Alarcón, 2011). However, unlike plant-pollinator communities, coral-Symbiodinium communities may be relatively unstable because generalist corals may be most sensitive to environmental changes and local association networks may be more compartmentalized (e.g., Fig. S1; Fabina et al., 2012; Putnam et al., 2012). Additional community studies and taxonomic resolution will help to shed light on these hypotheses.
Simulated extinctions are a simple model for community responses to disturbances. These simulations often do not incorporate important real world dynamics like population dynamics or the relative benefits and costs of interactions. However, simulated extinctions are used to understand general trends and generate hypotheses for how communities may respond to disturbances, not to make absolute predictions for specific communities. Notably, the general trends identified via application of simulated extinctions to plant-pollinator communities have been recently supported by community observations over a 120-year window (Burkle et al., 2013). In addition, plant-pollinator simulated extinction studies have generated hypotheses for further studies, including research on the potential for partner switching to increase community stability (Kaiser-Bunbury et al., 2010), the relationship between increased habitat size and increased community stability (Aizen et al., 2012), and how phenotypic similarity and phylogenetic relationships may structure community associations (Rezende et al., 2007). Similarly, we present the current model and results as a first step for coral-Symbiodinium communities. The major theme of this article is that the differential responses of coral reef taxa to environmental changes (Pandolfi et al., 2011; van Woesik et al., 2011) has implications for coral-Symbiodinium community stability and a more detailed understanding of the causes and consequences is necessary.
Biological realities make it difficult to experimentally study coral reef responses to novel environmental regimes. Coral-Symbiodinium communities harbor large amounts of species and genetic diversity, with hundreds of coral species and Symbiodinium types, as well as numerous potential unions. Comprehensive empirical characterization is impossible for all host–symbiont combinations and global changes are proceeding rapidly relative to coral generation times. We believe that coral-Symbiodinium models have the potential to highlight general trends and generate experimentally testable hypotheses (Baskett et al., 2009, 2010; van Woesik et al., 2010; Ortiz et al., 2013). We suggest that a combination of theoretical approaches and comparisons to other mutualistic and/or symbiotic systems can provide valuable insights, and openly available coral-Symbiodinium data can facilitate the merging of theory and empiricism (Franklin et al., 2012). Indeed, as reef environments continue to change, interdisciplinary collaborations will be essential to understanding future reef ecosystems.
Acknowledgements
The authors thank the editors and three anonymous reviewers, as well as Sebastian Schreiber, Julia Moore, Shahla Farzan, for their help in improving the manuscript. Funding support was provided by the US EPA (FP917199, FP917096), US NSF (OCE-0752604, OCE1041673, EF-0928987, 04-17412), the AIMS-CSIRO-UWA collaborative agreement, the ARCS foundation, and the UCD CPB. This is HIMB contribution 1557 and SOEST contribution 8964.s




