Climate change, phenology, and habitat degradation: drivers of gosling body condition and juvenile survival in lesser snow geese
Abstract
Nesting migratory geese are among the dominant herbivores in (sub) arctic environments, which have undergone unprecedented increases in temperatures and plant growing days over the last three decades. Within these regions, the Hudson Bay Lowlands are home to an overabundant breeding population of lesser snow geese that has dramatically damaged the ecosystem, with cascading effects at multiple trophic levels. In some areas the overabundance of geese has led to a drastic reduction in available forage. In addition, warming of this region has widened the gap between goose migration timing and plant green-up, and this ‘mismatch’ between goose and plant phenologies could in turn affect gosling development. The dual effects of climate change and habitat quality on gosling body condition and juvenile survival are not known, but are critical for predicting population growth and related degradation of (sub) arctic ecosystems. To address these issues, we used information on female goslings marked and measured between 1978 and 2005 (4125 individuals). Goslings that developed within and near the traditional center of the breeding colony experienced the effects of long-term habitat degradation: body condition and juvenile survival declined over time. In newly colonized areas, however, we observed the opposite pattern (increase in body condition and juvenile survival). In addition, warmer than average winters and summers resulted in lower gosling body condition and first-year survival. Too few plant ‘growing days’ in the spring relative to hatch led to similar results. Our assessment indicates that geese are recovering from habitat degradation by moving to newly colonized locales. However, a warmer climate could negatively affect snow goose populations in the long-run, but it will depend on which seasons warm the fastest. These antagonistic mechanisms will require further study to help predict snow goose population dynamics and manage the trophic cascade they induce.
Introduction
Polar and subpolar regions have warmed more rapidly than other parts of the globe, which could have dramatic effects on plant and animal communities (Anisimov et al., 2007). Demographic responses to climate change should be investigated more thoroughly because slight changes in climate could have large effects on populations and species interactions (e.g. Yoccoz et al., 2001). Migratory geese are among the dominant herbivores in (sub) arctic environments (Kerbes et al., 1990). Their interaction with plant communities could be particularly sensitive to climate change and its effect on plant phenology relative to goose phenology (Visser & Both, 2005). Worldwide, arctic goose populations are rapidly increasing (e.g. Menu et al., 2002), and it is important to understand how such increases will be mediated by climate change in the future, and how interactions with plant communities will be affected.
Among (sub) arctic geese, these interactions are heavily mediated by environmental conditions. Fluctuations in the availability of forage, along with competition for such forage, can impact gosling growth (e.g. Nicolai et al., 2008; Fondell et al., 2011) and body condition at the time of fledging (e.g. Cooch et al., 1991a,b) resulting in an effect on adult body size (e.g. Black, 1998). A number of studies have shown that individual and annual variation in gosling growth and body condition are directly related to nutrient availability on brood-rearing areas (e.g. Cooch et al., 1993; Lepage et al., 1998; Sedinger et al., 2001). Via demographic responses, these processes can affect population numbers, creating density-dependent feedbacks on forage quantity and quality (Nicolai et al., 2008; Fondell et al., 2011).
Among breeding lesser snow geese (Chen caerulescens caerulescens; hereafter snow geese) at La Pérouse Bay, Manitoba, Canada (henceforth LPB), nutrient availability in brood-rearing habitats is a major environmental driver of developmental body condition in goslings (Cooch et al., 1991a,b). However, snow geese exert both negative and positive impacts on the same resources they need to fledge. Unlike pure grazers, snow geese often kill plants by grubbing their basal and underground portions, but return nutrients to the soil through defecation. This nutrient cycle can in turn enhance production of surviving plants and allow for stable ecosystem dynamics (Walker et al., 2003).
This stability has been disrupted by a 500% increase in snow goose abundance over the past 40 years (Alisauskas et al., 2011) attributed to anthropogenic release from limiting factors on the migration and wintering grounds (Abraham et al., 2005a). There are now more snow geese than the current breeding grounds can support, resulting in heavily degraded coastal saltwater and freshwater marsh along the Hudson Bay Lowlands, especially in the historically used area of LPB (Jefferies, 1997; Jano et al., 1998). The generations of offspring that were reared in such locales have suffered from reduced resource availability and density-related feedbacks (Cooch et al., 1991b). Although habitat degradation has slowed snow goose population growth in some locations, it has not yet negatively affected overall population numbers (Aubry et al., 2010).
Over the last two decades, snow geese have abandoned their strict colonial breeding behavior at LPB. Adults have avoided heavily degraded habitats once used in the vicinity of LPB by dispersing (during nest-site selection and posthatch) to less impacted habitats in the larger Cape Churchill Peninsula region (CCP) and farther inland (Appendix S1; Cooch et al., 1989, 1993; Cooch, 2002; Rockwell et al., 2011). Goslings reared at the edge of this invasion wavefront inland from the original LPB colony or along the CCP benefit from ample per capita foraging opportunities and experience density-independent dynamics (Cooch et al., 1993; Jefferies et al., 2006). The interplay between spatial heterogeneity of forage availability and population density could thus account for large differences in gosling body condition (depending on the quality of brood-rearing habitats) that could consequently affect their probability of juvenile survival after fledging.
Concomitant with changes in habitat, climatologists have recently shown that the Hudson Bay region has undergone warming, in part associated with the Arctic Oscillation Index (Hochheim & Barber, 2010). Climate change at regional and local scales is affecting the phenology of many plant and animal species, especially in Polar regions (e.g. Parmesan & Yohe, 2003; Root et al., 2003). The snow goose life history is sensitive to climatic variation, which has been shown to have strong effects on the timing of nest initiation, clutch size, gosling development, and reproductive success (e.g. Dickey et al., 2008). Changes in climate and plant phenology (e.g. growing degree days) could help explain variability in forage emergence, availability, and quality, which likely affect gosling body condition.
Snow geese have thus far been extremely successful at plastically adapting to environmental change by invading unexploited habitat as their abundance rapidly increases (Cooch et al., 1991b). However, the gap between goose migration timing (driven primarily by photoperiod; e.g. Gwinner, 1996) and plant green-up at the breeding grounds upon arrival (affected by the timing of snow melt and growing degree days; Strong & Trost, 1994) has widened, and this mismatch between goose and plant phenologies could in turn affect gosling development (e.g. Mainguy et al., 2006). Pioneering generations of goslings developing at the habitat invasion wavefront might benefit from ample forage quantity, but climate change and the associated phenological mismatch between snow geese and forage plants could negatively affect forage quality during gosling development, and affect their body condition and future survival. The net impact of climate change and habitat quality on gosling body condition and consequent chances of survival after fledging are not known, but are critical for predicting how snow goose population growth and habitat degradation will be affected in the future. In this study, our aim was to (1) identify the intrinsic and extrinsic drivers of change in snow goose gosling body condition over space and time and (2) assess how such changes affect juvenile survival.
Materials and methods
Study area and data collection
We used data on gosling captures from 1978 to 2005 for available years and information on subsequent recaptures and hunter recoveries from 1978 to 2008, as we wanted to estimate the survival for known-age individuals (i.e. individuals marked as goslings). During this time period, we banded and measured morphological variables for 8086 goslings (4125 females and 3961 males). Because sex differences in gosling development are significant (Cooch et al., 1996, 1997), we decided to restrict all analyses to females because they are the philopatric sex for which the effect of variability in gosling body condition on juvenile survival later in life can be assessed (only 5.2% of the males banded, measured, and released as goslings were recovered dead). Thorough descriptions of the study site, field methods, and data collection protocols are provided by Davies et al. (1988) and Cooke et al. (1995). A complete description of the study area and data used in this study are moreover provided in Appendix S1.
Drivers of variation in gosling body condition
In snow geese, gosling growth is linear for structural metrics for the period of time when goslings are measured (Cooch et al., 1999). To attain an index of body condition (BC) for goslings, we therefore conducted a linear regression of body mass on tarsus length (‘TL’, known as the best indicator of structural size in snow geese, Cooke et al., 1995) while also controlling for Developmental Age (DA) at the time of capture (Cooch et al., 1991a), such as: YBC = slope + βTL*XTL + βDA*XDA, where βs are the regression predictors and Xs are the variables of interest (see also Cooch et al., 1991a). We then used the residuals from this regression as an index of individual BC. A gosling's developmental age at the time of capture was approximated as DA = BD − HD + 1, where BD is the date at which a gosling was captured and banded (ranging from calendar day 202 to 224) and HD is the average date of hatching within a given year (ranging from calendar day 164 to 188 across years). For more details on these measurements, the accuracy of the DA approximation (assessed on a subsample of known-age gosling), and the robustness of our results to these assumptions, see Appendix S2. Temporal trends and variation in all of the following drivers of gosling body condition described below can be found in Appendix S3.
Habitat degradation
To determine whether or not habitat degradation led to deterioration in gosling body condition over time, we created a variable that divided the brood-rearing landscape into four areas representative of the progressive expansion and cumulative damage of the breeding colony over time (see Jano et al., 1998 and Appendix S1). Zone 1 corresponds to the original nesting colony in and around LPB; goslings exclusively used the band of coastal saltwater marsh within LPB; zone 2 encompasses the inland zone of freshwater marsh adjacent to zone 1, which was not used for nesting or brood-rearing until the 1990s; zone 3 is located in the vicinity of Cape Churchill and south to the White Whale River; and zone 4 extends south of zone 3 (zones 3 and 4 include both coastal saltwater marsh and inland freshwater marsh). This geographically expanded snow goose nesting area is what is now referred to as the Cape Churchill Peninsula and includes the historic La Pérouse Bay colony (Appendix S1). Based on where each gosling was captured and banded, it was assigned to one of these habitat zones. We compared a division between all four zones to alternatives where we pooled the zones into two or three categories (e.g. pooling zone 1 with zone 2 and zone 3 with zone 4, etc.; see ‘model selection’ below).
Regional winter climate
We considered the indirect effects of the winter Arctic Oscillation Index (AOI) on gosling body condition. At latitudes above 45 degrees north, atmospheric pressure fluctuates between positive and negative phases. Positive AOI values generally indicate warming in northern latitudes, whereas negative values are associated with frigid winter air that extends from Arctic regions into the middle of North America (cooling; Thompson & Wallace, 1998). Indeed, both the North Atlantic Oscillation and Arctic Oscillation indices show that the Hudson Bay area has recently undergone a climate regime shift, which has resulted in a significant reduction in sea ice during the freeze-up period (Hochheim & Barber, 2010). Warm temperatures in late winter and early spring can increase the frequency of freeze-thaw cycles that are associated with microbial die-off and nutrient flushes (Edwards et al., 2006), which cannot be utilized by some plant species because these events occur well before their growing season (Edwards & Jefferies, 2010). In addition, winter warming results in loss of insulating snow and the loss of freeze tolerance in plants, which can cause severe damage to vegetation through freezing, winter desiccation, or ice encasement by refreezing snow, and delayed budburst or flower production (e.g. Andrews, 1996). Climate change is expected to increase the frequency of freeze-thaw cycle (Edwards & Jefferies, 2010) and increase winter warming (Thompson & Wallace, 1998), thus we averaged AOI values from the 1st of January, February, or March up to the 1st adjusted growing day (AGD; see below) to account for the potential impact of changing freeze-thaw episodes on plant production in the spring. If warming during that time interval reduced nutrient availability and in turn affected plant production in early spring, then forage quantity and quality is expected to be reduced later in the year (Edwards & Jefferies, 2010). We thus explored the possibility that this phenomenon could in turn affect gosling body condition prior to fledging.
Spring plant phenology
The number of Growing Degree Days (GDD) is often a very good predictor of the day a flower will bloom or when a crop will reach maturity (McMaster & Wilhelm, 1997); a correlate of plant phenology. We defined a slightly different measurement of green-up adapted to what we thought would matter to plant phenology in this particular ecosystem. We calculated six variants of AGD, or the Adjusted cumulative number of Growing Days where the mean daily temperature was above 0, 1, 2, 3, 4, or 5 °C (AGD0, AGD1, AGD2, AGD3, AGD4, AGD5) after snow melt (i.e. when snow depth was persistently less <5 cm). This temperature window seemed plausible for green-up to occur in most plants within the subarctic plant community. Each AGD measurement was accumulated up to the mean hatch date each year, because we wanted to account for forage phenology ‘relative’ to that of the goslings. We compared these six measurements of AGD (i.e. AGD0, 1, 2, 3, 4, or 5) to assess which minimal temperature for calculation of AGD best explained variability in gosling BC as a function of plant phenology. We accumulated AGDs after snow melt because a number of subarctic tundra plant species have been found to be sensitive to change in snow melt during early phases of development (i.e. early phenophase, Wipf, 2010).
Local summer climate
At the local scale, we considered the effects of various temperature variables (averaged daily high temperature (AHT), averaged daily mean temperature (AMT), averaged daily low temperature (ALT) in °C) and precipitation (i.e. total rainfall (TRF), and total precipitation (TPP) in mm) during the gosling developmental time period on body condition just before fledging (Dickey et al., 2008). We looked at a number of temperature and precipitation variables as we had little a priori knowledge about which variable could explain more variability in gosling body condition. First, we averaged each climate variable for the time period between the mean hatch and mean banding dates within a given year (e.g. AHT, ALT, TPP). Second, we compared each measure to an average measure between mean hatching date and 1 week after hatch (e.g. AHTw, ALTw, TPPw) because early development is a critical period when goslings can be particularly vulnerable to abiotic conditions (Sedinger et al., 1992), and may explain more variability in gosling body condition than AHT, ALT, and TPP.
Model selection
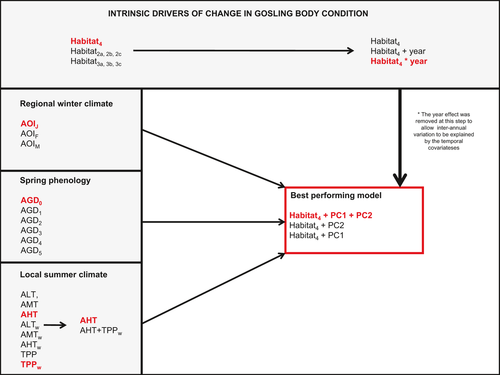
We used generalized linear models (with a normal distribution and identity link) to test for the effects of the above-mentioned covariates on gosling body condition (‘glm’ function in the ‘base’ R library, R Development Core team, 2011; version 2.14.1). We began our analysis by investigating the effects of habitat degradation, regional winter climate (indexed by AOI), spring plant phenology (indexed by AGD), and local summer climate (indexed by precipitation and temperature variables) on gosling body condition in separate sets of analyses to maintain a reasonable number of models under consideration. Within each covariate family (i.e. intrinsic drivers, regional winter climate, spring phenology, and local summer climate), respectively, we compared the different variant of each set of variables to assess which best explained variability in body condition. For example, within the ‘regional winter climate’ family, either AOIJ, AOIF, or AOIM could be selected. This progressive model selection procedure based on AICc was used to avoid multicolinearity within variables that belonged to the same ‘family’. Once we identified the top-performing habitat, phenology, and climate variables, we performed a principal component analysis (PCA, princomp' function in the ‘stats’ R library, R Development Core team, 2011; version 2.14.1) to create uncorrelated synthetic covariates of climate and plant phenology (Jolliffe, 2002) and tested their effects on gosling body condition. This method efficiently prevented multicolinearity in our analyses (correlation coefficients among climate and phenology covariates were <0.5). We only retained the principal components that explained >10% of the global variation among all climate and plant phenology covariates (Fig. 1). All generalized linear models were compared using Akaike's Information Criterion adjusted for sample size (AICc, Akaike, 1973; Burnham & Anderson, 2002).
Consequences of variation in gosling body condition on juvenile survival
We used Burnham models (Burnham, 1993) to study the consequences of variability in gosling body condition on first-year survival (obj. 2). Hunter band recoveries were provided by the USGS Bird Banding Laboratory (BBL). We used joint information on live and dead (hunter) encounters to estimate: pi = recapture probability; ri = probability of being recovered and reported conditional upon death; Seber, 1970; Fi = fidelity (i.e. 1 − Fi reflects the probability of permanent emigration but does not include temporary emigration); and Si = survival probability. Once we identified the best performing model for parameters F, r, and p (see Appendix S4 for additional details), we considered the effects of BC on juvenile survival (Sj). We included interactions between standardized BC (treated as a continuous variable) and Hatch Date groups noted as ‘HD’ (a categorical variable) to test for differences in juvenile survival as a function of variation in mean hatch dates and BC. We classified each year as ‘early’ (i.e. mean hatch ‘HD1’ between Julian day 164 and 171), ‘average’ (i.e. ‘HD2’ between days 172 and 178), and ‘late’ (i.e. ‘HD3’ past day 178). We also accounted for an effect of habitat quality (i.e. either four zones denoted as ‘habitat4’, or a grouping of zones 1 and 2, 3 and 4 denoted as ‘habitat2’), and interactions between BC, HD, and habitat quality as habitat degradation might affect juvenile survival independently of body condition (e.g. developmental immunity, parasitism). We used AICc to compare alternative survival models (Burnham & Anderson, 2002). All Burnham models were implemented using the RMARK package (Laake & Rexstad, 2008) in R 2.14.1 (R Development Core team, 2011 version 2.14.1). A full description of the Burnham analysis and model selection can be found in Appendix S4.
Results
Drivers of variation in gosling body condition
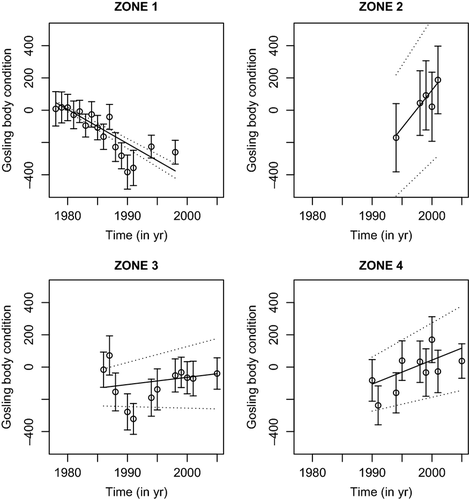
Following the sequential order of model selection presented in Fig. 1, we found that a model accounting for habitat variation in BC treated as a 4-level factor (habitat4; four zones presented in Appendix S1) outperformed any other parameterization (e.g. habitat divided in to two or three zones; Fig. 1, ∆AICc > 64.5, left side of top panel). A model accounting for an interaction between habitat4 and a year trend outperformed an additive model (i.e. habitat4 + year), as well as a model that did not account for annual variation in gosling BC (∆AICc > 77.95; Fig. 1, right side of top panel). In zone 1, gosling body condition declined throughout the 1980s and 1990s as the habitat became degraded (Fig. 2). Following that time period, adults generally moved from zone 1 toward zones 2 and 3, which improved gosling body condition over time in zone 2, where improvements in BC were the highest (Fig. 2, 1994 to 2001, improvement from −200 up to 200) also in zone 3 (Fig. 2, 1986 to 2005, improvement from ~ −200 to 0 on a standardized scale of residuals). Expansion south of the CCP along the coastline also resulted in a similar improvement in zone 4, but over a longer time period (Fig. 2, improvement from −200 to 200 on a standardized scale of residuals between 1990 and 2005).
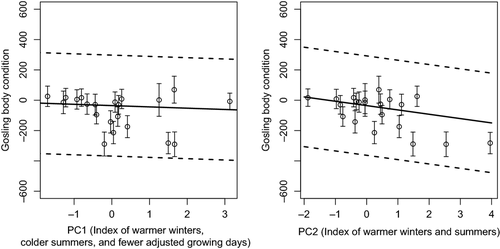
When examining the effects of regional winter climate on gosling body condition in subsequent seasons, we found that AOI averaged from the 1st of January up to the 1st growing day (AOIJ) outperformed other parameterizations beginning in February (AOIF) or March (AOIM) (∆AICc > 15.51; Fig. 1, ‘Regional Winter Climate’ panel). We did not detect any trends in AOIJ, however, this index varied greatly over the course of the study (Appendix S3, Coefficient of Variation ‘CV’ = 66.13%) and warmer winters were detrimental to body condition (Fig. 3). In models that accounted for the effects of plant phenology (via AGD) on gosling development, we found that an effect of AGD0 (i.e. when growing days above 0 °C were summed up) was far superior to any other AGD parameterization (∆AICc > 16.64; Fig. 1, Spring Phenology panel). Even though there was no trend in AGD0 over time, it varied moderately over the course of the study (Appendix S3, CV = 23.2%) and had a positive effect on gosling body condition as part of PC1, alongside AHT and AOIJ (Fig. 3). In addition, temperature highs averaged from mean hatch up to banding (AHT) outperformed all other temperature covariates (∆AICc > 22.79; Fig. 1, top of Local Climate panel), as well as models with combinations of temperature and precipitation (∆AICc for a model with AHT + TPP was 1.93, making precipitation an uninformative parameter). AHT weakly fluctuated over time (Appendix S3, CV = 12.5%) and along with AOIJ, it negatively impacted gosling body condition as a part of PC1 and PC2 (Fig. 3).
After combining the best performing climate and plant phenology covariates in the PCA, we found that PC1 accounted for 52% of the variation among the covariates (Table 1). AOIJ was positively correlated with PC1 (loading of 0.55), and AGD0 and AHT loaded negatively (Table 1: −0.76 and −0.34, respectively). Hence, we considered positive values of PC1 as an overall index of warm winter, cold summer, and shortage of adjusted growing days prehatch. PC2 explained 41% of the variation among climate and plant phenology covariates (Table 1). AOIJ and AHT were positively correlated with PC2 (Table 1, PC2: loadings of 0.61 and 0.79, respectively), but AGD0 had a null loading. Thus, we considered positive values of PC2 as an index of warm winter and summer conditions. PC3 explained only 7% of the variation among covariates, and thus we did not consider it in body condition analyses (Table 1).
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| AOIJ | 0.55 | 0.61 | 0.57 |
| AGD0 | −0.76 | 0.00 | 0.65 |
| AHT | −0.34 | 0.79 | −0.51 |
| Proportion of variance explained | 0.52 | 0.41 | 0.07 |
The best performing model that combined habitat degradation, winter climate, spring plant phenology, and summer climate included habitat4 and the first two principal components from the PCA analysis (∆AICc = 6.54; Table 2, Fig. 1). According to this model, gosling body condition was on average the highest in zones 2 and 4, and lowest in zones 1 and 3 (Table 2). Moreover, PC1 had a slightly negative effect on gosling body condition (Table 2; Fig. 3) indicating that warm winters, cold summers, and few adjusted growing days had a negative impact. Yet, PC2 had a much stronger negative impact on gosling body condition, indicating that warm winters and summers led to the worst possible climatic conditions for gosling development (Table 2; Fig. 3; the impact of summer climate in PC2 trumped that in PC1).
| β | se | 95% lower CL | 95% upper CL | P-value | |
|---|---|---|---|---|---|
| Zone 1 | −55.49 | 3.21 | −61.79 | −49.20 | <0.001 |
| Zone 2 | 131.93 | 10.40 | 111.54 | 152.32 | <0.001 |
| Zone 3 | −11.96 | 8.03 | −27.71 | 3.79 | 0.137 |
| Zone 4 | 72.40 | 7.11 | 58.45 | 86.35 | <0.001 |
| PC1 | −5.98 | 2.05 | −9.99 | −1.97 | 0.003 |
| PC2 | −38.91 | 2.42 | −43.66 | −34.17 | <0.001 |
- se, standard error; CL, confidence limits.
Consequences of variation in gosling body condition on juvenile survival
The best performing Burnham model testing for the effects of habitat and gosling body condition on juvenile female survival indicated that an additive effect between BC (corrected for hatch date) and habitat performed better than any other parameterization (Table 3; all ∆AICc > 16.43). We then compared alternative parameterizations of pooled habitat zones (e.g. habitat2: zones 1 and 2 were pooled together, as well as zones 3 and 4) and hatch groups (e.g. HD1 relative to HD2 and HD3 pooled together) on juvenile survival. The exploratory model that accounted for an effect of habitat2 and two hatch groups was 192.98 AICc units better than a similar model with four habitat zones and three hatch groups (Table 3).
| Model parameterization for survival | Np | AICc | ΔAICc |
|---|---|---|---|
| juv : habitat2 + juv : BC : HD1 + juv : BC : HD2 + ad | 14 | 4503.48 | 0.00 |
| juv : habitat2 : BC : HD1 + juv : habitat2 : BC : HD2 + ad | 14 | 4519.91 | 16.43 |
| juv : habitat2 + ad | 12 | 4522.57 | 19.08 |
| juv : habitat4 + juv : BC : HD1 + juv : BC : HD2 + juv : BC : HD3 + ad | 13 | 4696.46 | 192.98 |
| juv : habitat4 + ad | 10 | 4707.91 | 204.43 |
| juv : BC : HD1 + juv : BC : HD2 + juv : BC : HD3 + ad | 9 | 4746.70 | 243.22 |
| juv : BC : HD1 : habitat4 + juv : BC : HD2 : habitat4 + juv : BC : HD3 : habitat4 + ad | 18 | 4755.12 | 251.63 |
- HD was divided into 3 groups: HD1 (i.e. hatched in an early year), HD2 (i.e. hatched in an average year), and HD3 (i.e. hatched in a late year), with the option of pulling HD2 and HD3 together. Habitat was either divided into four zones 1, 2, 3, and 4 (i.e. habitat4) or two zones (i.e. habitat2 defined by two grouped zones where zones 1 and 2, and 3 and 4 were merged). Age class allowed testing for difference between juvenile ‘juv’ and adult ‘ad’ female survival. Full interactions between model covariates are denoted by ×, partial interactions (denoted by :) are only fit for non-zero levels of discrete factors (e.g. when HD1 = 1). Np denotes the number of estimated parameters in a model. Fidelity, recovery, and recapture parameterizations are described in the methods section and Appendix S4.
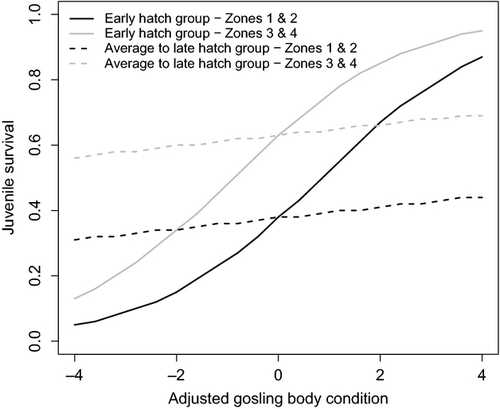
Independent of the direct effects of gosling body condition, additional factors (e.g. parasite burden) caused juvenile survival to, on average, be lower in zones 1 and 2 (pooled together) compared to zones 3 and 4 (Table 4; Fig. 4). We also found that an increase in gosling BC always resulted in an improvement in first-year survival (Table 4). Moreover, juvenile survival for goslings hatched in an early year was more heavily mediated by BC relative to goslings hatched in an average or late year. As such, goslings that hatch an average or late year showed the greatest potential for high juvenile survival, provided that they were in good condition (Fig. 4).
| Parameter description | Β | se | 95% lower CL | 95% upper CL |
|---|---|---|---|---|
| Sjuv: habitat (zones 1 and 2) | −1.616 | 0.261 | −1.104 | −1.104 |
| Sjuv: habitat (zones 3 and 4) | −0.546 | 0.297 | 0.036 | 0.036 |
| Sjuv: BC2 : HD1 (early hatch year) | 0.679 | 0.149 | 0.972 | 0.972 |
| Sjuv: BC2 : HD2 (average-late hatch year) | 0.317 | 0.140 | 0.592 | 0.592 |
| Sad | 1.539 | 0.099 | 1.732 | 1.732 |
| pintercept | −3.572 | 0.282 | −3.019 | −3.019 |
| page4 | 0.663 | 0.102 | 0.864 | 0.864 |
| rjuv | −2.769 | 0.212 | −2.354 | −2.354 |
| rad | −1.274 | 0.478 | −0.337 | −0.337 |
| rtime | −0.144 | 0.059 | −0.029 | −0.029 |
| rtime2 | 0.005 | 0.003 | 0.010 | 0.010 |
| rad :time | 0.014 | 0.089 | 0.189 | 0.189 |
| rad : time2 | −0.001 | 0.003 | 0.005 | 0.005 |
| Fjuv | −0.538 | 0.328 | 0.104 | 0.104 |
| Fad | Adult female fidelity fixed to 1 | |||
- se, standard error; CL, confidence limits.
Discussion
We have established that goslings growing within and near the traditional center of the snow goose colony experienced the effects of long-term degradation of habitat conditions and suffered a decrease in body condition over time that also led to decreased juvenile survival. In newly colonized areas, however, gosling body condition increased over time, which in turn led to an increase in juvenile survival. Concomitant with foraging-induced changes in habitat quality, warmer than average winters and summers led to the worst possible climatic conditions for gosling development and chances of survival to the first birthday. In addition, gosling body condition and first-year survival were diminished when hatch occurred early relative to plant phenology (i.e. accrual of too few plant ‘growing days’).
Habitat degradation and early life fitness
Goslings that developed near the traditional center of the colony experienced ever-degrading habitat conditions leading to a long-term decline in gosling body condition. In turn, this led to lower juvenile survival. The underlying cause of the deterioration in gosling habitat and early life fitness is grubbing by a progressively overabundant snow goose population during the prebreeding season (see Alisauskas et al., 2011). On its own, this would lead to density-dependent regulation of the local population. Few snow geese, however, rear their goslings in this area today, and most families are able to avoid their own density-related habitat destruction by exploiting new nesting and brood-rearing habitats (see also Cooch et al., 1993). Much like suburban sprawl, snow geese have largely moved to zones 3 and 4 for brood-rearing, the goslings benefit, and the local snow goose population continues to grow (Aubry et al., 2010).
However, snow geese did not immediately benefit by moving into new grazing habitats. The grazing optimization hypothesis predicts that ungrazed swards of graminoids should be less nutritious than moderately grazed swards because grazing can facilitate rates of plant tissue production, nutrient concentration, decreased cover of dead biomass, and denser sward (e.g. Van Der Graaf et al., 2005). Experimental studies strongly suggest that intermediate grazing improves graminoid nutrition for developing snow geese at our nitrogen-limited study area (Cargill & Jefferies 1984a,b, Hik & Jefferies 1990, Zellmer et al. 1993). Thus, the increase in gosling body condition from initially low levels in zones 2–4 was likely facilitated by the geese themselves. Unless snow goose numbers decrease, however, they will likely eat themselves out of the local seed corn and continue their outward movement from the original colony; further exacerbating the problem of habitat destruction across the landscape (Jano et al., 1998).
Climate and early life fitness
Large-scale climate indices have previously been shown to affect the body condition of Arctic waterbirds (Descamps et al., 2010), but underlying mechanisms for these relationships are not known. We found that AO signals indicative of warm winters resulted in lower gosling body condition, which could be associated with freeze-thaw cycling or snow depth and the timing of snow melt. Warm temperatures in late winter and early spring can increase the frequency of freeze-thaw cycles that are associated with microbial die-off and nutrient flushes (Edwards et al., 2006), which cannot be utilized by some plant species because of the events occurring well before their growing season (Edwards & Jefferies, 2010). Extreme winter warming also results in loss of insulating snow and the loss of freeze tolerance in plants, which can cause severe damage to vegetation through freezing, winter desiccation, or ice encasement by refreezing snow, and delayed budburst or flower production (Andrews, 1996). Cold conditions buffer against these events. Further research will be required to identify the specific mechanisms determining how generally cool vs. warm winters affect the plants that goslings prefer.
Based on PC2, warm summers (daily high temperatures averaged from mean hatch up to banding) were also detrimental to gosling body condition. In (sub) Arctic goose species, goslings are most susceptible to weather in the first days of life due to their small size, incompletely developed thermoregulatory ability, and low energy reserves (Sedinger et al., 1992). We hypothesize that high summer temperatures could simply lead to overheating due to a lack of ability to thermoregulate in goslings. High summer temperatures could also affect forage digestibility, which could in turn diminish gosling body condition. Finally, above average heat might dry out freshwater ponds making it difficult for goslings to hydrate, especially when already weakened by overheating.
One last hypothesis could have some weight in explaining habitat and climate-specific differences in gosling body condition: a variety of parasites affect goslings at our study sites (Gomis et al., 1996). Cecal nematodes (Trichostrongylus spp.), primarily found in goslings that were raised along the coastline and to a lesser extent in goslings raised more inland (Mellor & Rockwell, 2006), are known to affect gosling kidney function with consequences for gosling body condition and weight, even survival chances in some cases (Gomis et al., 1996). These effects could be exacerbated by warmer temperatures in the summer, when water balance and hydration become an issue for goslings during development. This underlying mechanism could explain the reason for decreased gosling body condition in years where summer days frequently reached higher than average maximum daily temperatures.
Phenology and early life fitness
A shortage of plant growing days at hatch resulted in lower gosling body condition, with further consequences on juvenile survival. Phenological mismatches between trophic levels are not a new phenomenon, and the concept dates back to the ‘Critical Period Hypothesis’ (Hjort, 1914). The match-mismatch hypothesis states that ‘if the most energetically expensive part of the consumer's breeding cycle occurs at the same time as the peak availability of resources, then recruitment will be enhanced' (paraphrased from Durant et al., 2007). In light of global warming, trends toward earlier phenology and addition of plant growing degree days are usually consistent with a warming climate, which has been found to be the case in a survey of 677 species of plants and animals, where 62% demonstrated such a relationship (Parmesan & Yohe, 2003). In a similar study of arctic-nesting greater snow geese (C. caerulescens atlanticus), Dickey et al. (2008) found that gosling mass and size were reduced following warmer springs because of a reduction in the availability of high quality forage likely resulting from a mismatch between gosling and plant phenologies. At our study area, however, early springs and accrual of more growing days before hatch seems to have always been beneficial to goslings. Additional data on plant phenology will nevertheless be needed to determine if plant phenology will eventually advance too fast for CCP snow goose phenology, creating a situation where geese could mismatch the phenology of preferred plants in either direction of an optimal number of plant growing days.
Conclusions
Herbivory by geese in saltmarshes results in some of the highest levels of primary production removed for any system (Crawley, 1983). Geese are almost entirely herbivorous and goslings have a very limited time period in which they have to grow large enough to migrate; as a result, survival and growth rates of goslings are strongly limited by food quality (e.g. Cooch et al., 1993; Sedinger et al., 1998, 2001). Goslings are highly selective grazers, preferring plants high in N, P, and Ca, and low in fiber and silica (Sedinger & Raveling, 1984; Gadallah & Jefferies, 1995). Forage quality during the first 2 weeks following hatch is particularly critical at LPB, since snow goose goslings can increase their weight five to sixfold over this time period (Gadallah & Jefferies, 1995). Since nutrient content of forage decreases rapidly over the course of the growth season, geese that hatch early generally benefit from high nutrient availability in new forage (Sedinger and Raveling 1986; Cooch et al., 1991a; Sedinger & Flint, 1991). Over the past four decades, snow geese at LPB have advanced their date of hatch at a rate of 0.16 days per year (Rockwell et al., 2011), perhaps in an attempt to track the phenology of preferred plant species. Future research will evaluate the extent to which phenology of preferred forage species can explain gosling growth responses and gauge how climate change will affect goslings via changes in plant phenology.
Our assessment also indicates that geese are recovering from habitat degradation by moving to new locales, therefore contributing to continued population growth and expansion. Like other native invasive species, snow geese pose a threat to biodiversity and create a number of concerns. High densities of migrating snow geese cause substantial damage to cereal crops in the central U.S.; create a large reservoir for avian cholera, which is easily transmitted to other bird species (Johnson, 1997); and damage coastal ecosystems in (sub) arctic regions. The invasion and spread of arctic and subarctic breeding colonies into new areas threatens the abundance and diversity of plants, insects, and avifauna through dramatic modification of the landscape and the trophic cascade that ensues (e.g. Rockwell et al., 2003; Abraham et al., 2005a; Jefferies et al., 2006). However, warmer winters and summers could negatively affect the population in the long run, and advancing plant phenology could potentially have similar detrimental effects in the future (Dickey et al., 2008). These mechanisms will deserve continued attention to help predict snow goose population dynamics and guide future management to mitigate the cascading trophic effects that overabundant snow geese have on subarctic biodiversity.
Acknowledgements
Financial support for data collection and analyses were provided in part by the Arctic Goose Joint Venture, the Canadian Wildlife Service, the Central and Mississippi Flyway Councils, the U.S. Fish and Wildlife Service, and Wapusk National Park (Parks Canada). L. M. Aubry was supported by NSF (DEB 1019613) and the Berryman Institute at Utah State University. On-ground research at La Pérouse Bay was done in cooperation with and under permits from Wapusk National Park and the Canadian Wildlife Service (CWS permit 10653). We acknowledge the volunteers and scientists who contributed to data collection at LPB during the last 43 years. We thank the late RL Jefferies for his contributions to our understanding of goose-plant interactions. We also thank participants in the USGS Circumpolar Assessment of the Ecological Mismatch Between Avian Herbivores and Plant Phenology workshop for discussions related to these topics. Danny Bystrak at the USGS BBL lab was especially helpful with providing hunter recovery data, and a special thanks to Pat Terletzky for her assistance with GIS map creation.




