
Changes in Leaf-Litter Chemistry and Microbial Communities Drive Leaf-Litter Decomposition Across River Terrestrial–Aquatic Habitats
Funding: This study was supported by European Union's Horizon 2020, DRYvER, MetaDryNet and FLUFLUX.
ABSTRACT
- River networks are meta-ecosystems in which resources, such as leaf-litter, and their consumers are exchanged across riparian and instream ecosystems. Consumers and leaf-litter quality and decomposition vary depending on riparian land use and instream hydrological conditions, including intermittent drying. However, limited evidence of the mechanisms driving leaf-litter decomposition across aquatic–terrestrial ecosystems hinders our understanding of carbon transfer across river networks.
- We exposed alder (Alnus glutinosa) leaves to seven preconditioning treatments, including different riparian land uses (i.e., coniferous and deciduous forest, cattle-grazed grassland and urban) and instream habitats (i.e., buried in sediments, on a dry riverbed, in an anoxic pool), mimicking environmental conditions litter can be exposed to before entering flowing waters. We determined leaf-litter chemical composition, decomposition rates and microbial community composition in each preconditioning treatment. Then, the same litter was incubated in two flowing rivers with different flow regimes (i.e., perennial and intermittent) to monitor how previous preconditioning affected subsequent microbial succession and decomposition dynamics.
- During preconditioning, leaf chemical diversification, decomposer community composition and leaf-litter decomposition differed among preconditioning treatments and were mostly influenced by the presence of water, with stronger responses observed in instream than in land-use habitats. Preconditioning mediated subsequent decomposition in the flowing rivers through the alteration of leaf chemical composition—for example, depletion of carbon compounds in litter exposed to forested environments—and the turnover of bacterial and fungal taxa, likely driven by priority effects. The effects of preconditioning on aquatic decomposition differed among flow regimes, with changes in microbial community composition explaining a greater proportion of the variance in decomposition rates in the perennial river. Invertebrate-driven decomposition was two to four times faster in the intermittent than in the perennial river, reflecting the context-dependent effects of flow regimes on shredder communities.
- Our results demonstrate how riparian land uses and instream conditions affect river leaf-litter decomposition through cascading effects of leaf preconditioning on microbial communities and their activity. Understanding the dynamics of leaf-litter decomposition across terrestrial–aquatic boundaries is key to better predicting how global changes, including hydrological and land-use changes, may affect ecosystem functioning at the river-network meta-ecosystem scale. As the proportion of intermittent rivers is increasing globally and riparian land use is changing quickly, our conclusions indicate that the transition from perennial to intermittent flow regimes, along with riparian forest loss, could significantly alter carbon cycling across river networks.
1 Introduction
According to the meta-ecosystem concept (Loreau et al. 2003) transfers of resources and organisms connect ecosystems and thus determine their functioning. Across a river network, fluvial and riparian areas are linked by such transfers (e.g., riparian leaf litter inputs which bring terrestrial carbon and nutrients to the stream food webs; Loreau et al. 2003; Gounand et al. 2018). River networks and their biodiversity are increasingly threatened by climate change and anthropogenic activities that modify the organic matter (OM) dynamics across the terrestrial–aquatic interface through changes in riparian habitats, hydrological conditions and ecological connectivity (Dudgeon 2019). Such changes could modify essential inputs of leaf litter to river networks, and the nutritional quality and decomposition of such OM. However, we still know little about the mechanisms controlling the processing of leaf litter between terrestrial and aquatic interfaces within river networks (Sarremejane et al. 2024; del Campo, Foulquier, et al. 2021).
One of the most dynamic exchanges of leaf material across terrestrial and aquatic habitats happens within river networks comprising non-perennial or intermittent rivers (IR, i.e., which regularly experience partial to complete riverbed drying), which represent more than 50% of the global hydrological network (Messager et al. 2021). Decreases in streamflow in non-perennial river networks promote the emergence of a heterogeneous mosaic of terrestrial (e.g., dry channels) and aquatic habitats (e.g., isolated pools). Leaf litter can thus accumulate under contrasting environmental conditions within or outside the river channel before being exposed to flowing water.
Depending on where it falls, leaf litter may undergo various preconditioning processes (i.e., changes in chemical and physical quality; Abelho and Descals 2019; del Campo, Foulquier, et al. 2021; del Campo, Corti, et al. 2021). For example, land use may determine leaf litter preconditioning by influencing physical conditions (e.g., temperature, moisture) and the decomposer communities which initiate decomposition (Drenovsky et al. 2010), yielding contrasting decomposition across forests (Wang et al. 2015), urbanised areas (Hobbie et al. 2014) and croplands (Yuan et al. 2019). For instance, depleted availability of water and nutrients may reduce microbial community colonisation and activity in riparian habitats (Annala et al. 2022; del Campo, Martí, et al. 2021) and on dry riverbeds (Foulquier et al. 2015). On the other hand, the accumulation of leaf litter in residual aquatic habitats from drying reaches (e.g., wet sediments, isolated pools) can increase microbial activity and leaching, resulting in changes in decomposition and leaf chemical composition (Yue et al. 2018; del Campo, Corti, et al. 2021).
After days, months or even years, terrestrially preconditioned leaves may be mobilised into flowing waters through surface runoff during storms and floods, while flow resumption may reconnect isolated instream habitats and initiate the transfer of preconditioned leaves to the water column and downstream habitats. These preconditioned leaves may serve as substrate transporting microbial communities across riparian and instream ecosystems (Ruiz-González et al. 2015). As highlighted by Koivusaari et al. (2019), 65% of the aquatic fungal biomass found on leaves originates from terrestrial habitats. This suggests that competition may occur between early-stage terrestrial microbial decomposers that colonise leaf litter during preconditioning and the subsequent aquatic decomposers, likely leading to important priority effects driving the structure and function of these communities (Debray et al. 2022).
However, the final effect of preconditioning on aquatic decomposition might vary between perennial reaches (i.e., PR) and IR due to fundamental differences in their decomposer communities (Sarremejane et al. 2024). Flow intermittence can shape the decomposer communities of IR even long after drying events due to the ‘drying legacy’ (Datry et al. 2011a, 2011b). For instance, drying exerts a strong negative impact on macroinvertebrate and shredder-mediated decomposition (Datry et al. 2011a, 2011b). Bacterial and fungal communities developing on leaves are also affected by drying (Schreckinger et al. 2021), but may present drying-resistant strategies, allowing part of the community to remain active if sufficient moisture is preserved on the streambed (Foulquier et al. 2015; Bruder et al. 2016) or buried in the sediment (Burrows et al. 2017).
Despite growing knowledge on the biogeochemical functioning of non-perennial river networks (Datry et al. 2018), little is known about terrestrial–aquatic linkages and how the preconditioning of leaf litter affects decomposition and decomposer communities in rivers. Such information will help identify the environmental conditions (e.g., hydrological and land use) under which ecosystem functioning may be altered across terrestrial–aquatic boundaries. We performed a field experiment, examining (1) how different preconditioning treatments representing different land uses (i.e., urban, forested and grazed lands) and instream conditions (i.e., anoxic pools, dried riverbed and sediments, Figure 1) determined leaf-litter chemical composition, early-stage microbial community and decomposition and (2) how such preconditioning affected subsequent decomposition rates and microbial succession in flowing waters in two rivers with contrasting flow regimes (i.e., intermittent vs. perennial, Figure 1). We expect (H1) that leaf chemical composition and early-stage decomposition differ among preconditioning treatments due to differences in biotic (e.g., early-stage microbial communities) and abiotic (e.g., temperature and moisture) factors among habitats. We also expect (H2) that subsequent leaf litter decomposition and decomposer community in flowing waters differ depending on preconditioning treatments, due to contrasting biotic competition pressures with established early-stage microbial communities, and/or differences in leaf chemical quality. We finally expect that (H3) the effects of preconditioning on the decomposition and decomposer communities in flowing rivers differ depending on the river flow regime, mainly due to differences in decomposer communities and the drying legacy effect.

2 Methods
2.1 Study Area
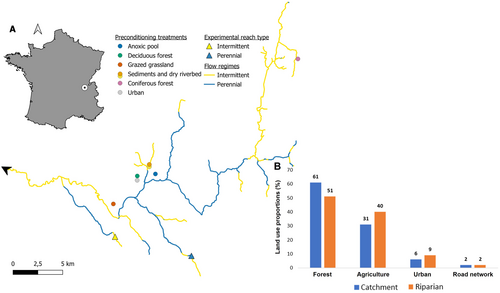
The experiment was conducted in the Albarine River catchment (313 km2; Figure 2), eastern France (Datry et al. 2012). Due to a karstic geology on the uplands and porous alluvial sediments in the valley, the river experiences natural intermittent drying across different sections from its headwaters to its mouth (Figure 2). The Albarine River riparian land use is dominated by deciduous forests in the lowlands and coniferous forests in the uplands (Figure 2). Pastures are the second most common land use while urbanisation (including road networks) is the least common land use of the riparian area (Figure 2).
2.2 Field Decomposition Experiment
Leaves of Alnus glutinosa were collected in autumn 2021 in the riparian area of the Albarine (45.9248587 N, 5.4385315 E). We used A. glutinosa because it is a common riparian species found along intermittent and perennial reaches of the Albarine, which has been used in previous comparable decomposition studies (e.g., Datry et al. 2011a; Foulquier et al. 2015). Leaves were oven-dried (24–48 h, 70°C), weighed into 3.95–4.05 and 1.95–2.05 g portions and enclosed in coarse (CMB; mesh size 1 cm) and fine (FMB; 250 μm mesh size) mesh bags (approximately 10 × 15 cm), respectively. FMB excluded most invertebrates allowing us to estimate the contribution of microbial communities to decomposition, whereas CMB allowed us to measure the decomposition resulting from combined invertebrate and microbial community activity. We recognise that the high temperature used during the drying might have altered the chemical composition of leaf litter (see Bärlocher 2005). However, since all leaves in the experiment were processed uniformly, we anticipate that any effects on the results would be minimal, maintaining the validity of comparisons between treatments.
In February 2022, CMB and FMB were exposed to seven preconditioning treatments, including four different land uses and three instream conditions representative of the common riparian and instream conditions litter may be exposed to in the Albarine catchment (i.e., the preconditioning phase; Figure 1). All land use treatments—grazed grassland (Grass), urban (Urb), deciduous (DecForest) and coniferous (CoForest) forests—were located in the riparian zone (i.e., < 20 m away from the river channel; Figure 2). Instream conditions included the surface (DryRiv) and subsurface (Sed) of a dry riverbed and an anoxic pool (AnPool). In each treatment, 15 CMB and 15 FMB were deployed (total n = 120 per mesh-type). A set of 15 CMB and 15 FMB were prepared in the same way as others but were kept in a thermostatically controlled room for the entirety of the preconditioning phase. These bags were not exposed to any preconditioning and were considered as controls. In each preconditioning treatment, we measured temperature, light, substrate organic matter and moisture content. Preconditioning treatments and their environmental conditions are further described in Appendix S1: Table S1.
Leaf-litter bags were preconditioned for 32 days except for the CoForest treatment, where bags were incubated for 42 days to compensate for the lower and freezing temperatures that may have delayed decomposition there (see Appendix S1). For each preconditioning treatment (i.e., 7 preconditioning conditions + control), 5 CMB and 5 FMB (total n = 40 per mesh-type) were used to assess the effects of preconditioning. The rest was kept cold (~4°C) in a cooler during transport and distributed across a perennial reach (PR; 45.8806450 N, 5.4944304 E) (5 CMB and 5 FMB) and an intermittent reach (IR; 45.9009885 N, 5.3953446 E; 5 CMB and 5 FMB) for in-flowing water incubation (i.e., the flowing river phase; Figure 1 and Table S2). Both rivers were in their flowing phase when the leaf-litter bags were introduced (23rd and 24th March 2022). In each river reach, 40 CMB and 40 FMB (i.e., 5 bags per mesh size and preconditioning treatment) were randomly distributed between 20 locations along a 150 m-long reach. At each location, 2 CMB and 2 FMB were secured to the riverbed using roots and metal bars. Most bags were retrieved and taken to the laboratory after 19–20 days of incubation, except for the CMB in the IR reach. Due to a high activity of decomposer invertebrates in the IR, CMB were removed after 8 days to ensure sufficient leaf mass remained for further analyses. In each flowing river, we measured discharge, flow velocity, depth, water chemical properties and temperature. The environmental conditions of both river reaches are described in Appendix S1.
2.3 Laboratory Analyses
2.3.1 Decomposition Rates
Leaves from each litter bag were rinsed above a 500 μm sieve to remove biofilm, macroinvertebrate and detritus. Prior to cleaning, 1.5 cm disks were cut off the leaves from FMB using a cork borer (in sterile conditions to prevent DNA cross contamination) and freeze-dried (at −93°C) for subsequent DNA and chemical composition analyses. Leaves were then dried for 48 h at 70°C and then ash-burned (550°C, 3 h) to assess the proportion of mineral matter and subsequently estimate the ash free dry mass (AFDM). Freeze-dried leaf disks mass was added to the final dry mass of FMB, after correction for mineral content estimated from the rest of the sample AFDM.
Decomposition rates on a per degree-day basis (k, /dd) were estimated by fitting data into a negative exponential model (Minshall et al. 1983). Decomposition rates were estimated separately for the preconditioning phase and the flowing river phase using the fraction mass remaining and the cumulative mean daily temperature at the sampling day in each phase. We estimated the dry mass at the start of the flowing phase as a fraction of initial oven-dried mass (before preconditioning) considering the mass remaining after the preconditioning phase for each treatment measured from the CMB and FMB brought back to the laboratory after the preconditioning phase.
2.3.2 Leaf Chemical Composition
To characterise relative changes in the composition of macromolecular organic carbon (i.e., C) compounds in leaves, we measured attenuated total reflectance (ATR) using Fourier Transform Infrared (FTIR) Spectroscopy. FTIR is a common method that allows a quick and semi-quantitative characterisation of the relative proportion of multiple C compounds such as aliphatic acids, polysaccharides, cellulose, lignin, peptides or phenolic compounds (del Campo, Corti, et al. 2021). Peaks and bands within the FTIR spectra are associated with the chemical bonds of the different carbon compounds. The intensity of these peaks and bands can then be used to estimate changes in the relative proportion of the various compounds through multivariate analyses (see Appendix S2 and below). We measured ATR in a VERTEX 70 spectrometer coupled with an automated XYZ-stage and an ATR accessory equipped with a germanium crystal. Such set-up allowed us to measure in direct contact with the sample an integrated area of 32 × 32 μm. We collected spectra in the MIR range of 4000–600 cm−1 with a spectral resolution of 8 cm−1 by averaging 16 scans. We measured one spectrum at a random location on each leaf disk, with 5 CMB leaf disks per treatment (only 4 in the control treatment, total n = 39). A background measurement (i.e., air) was performed before the spectra acquisition and repeated every 2 h. The ATR accessory was cleaned with ethanol after each measurement. Spectra were processed before extracting chemical information. We extracted nine peaks and bands from 800 to 3745 cm−1 range, corresponding to main functional groups of aliphatic acids, polysaccharides, cellulose, lignin, peptides and phenolic compounds (Appendix S2).
2.3.3 Bacterial and Fungal Communities
DNA was extracted from 1 g of lyophilized disk leaves from 3 FMB bags in each treatment, in each experimental phase (i.e., preconditioning and flowing river phase) and river reach (total n = 72) using the FastDNA SPIN Kit for Soil (MP Biomedicals; United States) following the manual instructions. Archaeal, bacterial and fungal biodiversity on leaves was estimated using markers amplifying both Bacteria and Archaea (16S rDNA, Bact03, forward primer: GTGYCAGCMGCCGCGGTAA, reverse primer: GGACTACNVGGGTWTCTAAT; Apprill et al. 2015) and Fungi (ITS1 nuclear rDNA, Fung02, forward primer: GGAAGTAAAAGTCGTAACAAGG, reverse primer: CAAGAGATCCGTTGYTGAAAGT; Epp et al. 2016; Taberlet et al. 2018). Polymerase Chain Reactions (PCR) were performed in four replicates for each DNA extract and each marker (see Appendix S3 for further detail). Sequencing was then performed at Fasteris (Geneva, Switzerland) using the Metafast PCR-free protocol. High-throughput sequencing of the Bact03 and Fung02 marker was performed on an Illumina MiSeq (2 × 250 bp paired-end reads) platform.
Sequencing data were then curated (see Appendix S3) using the OBITools software package (Boyer et al. 2016) and the ‘metabaR’ R package (R Core Team 2020; Zinger et al. 2021). We formed operational taxonomic units (OTUs) by clustering sequences at 97% similarity using the Sumaclust algorithm (Mercier 2013). OTU abundance was defined as the sum of reads sharing these similar sequences. In subsequent analyses, each OTU was represented by its most abundant sequence. Each OTU was assigned a taxonomic clade using the ecotag command (Boyer et al. 2016), and a set of reference databases built with the ecoPCR software from the EMBL database version 136 to refine taxonomic annotations. Taxonomic annotations with > 80% identity were retained. We calculated richness as the total number of OTU for fungi and bacteria separately.
2.3.4 Aquatic Invertebrate Communities
We collected aquatic invertebrates from three randomly chosen CMB from each preconditioning treatment in each flowing river. Aquatic invertebrates were identified in most cases to the genus/family taxonomic level. We calculated richness as the number of taxa and estimated the relative proportion of shredders using Tachet et al. (2010).
2.4 Data Analyses
We summarised leaf chemical composition using a principal component analysis (PCA) based on the FTIR spectra (Appendix S2). We used Principal Coordinates Analysis (PCoA) and Non-Metric Multidimensional Scaling (NMDS) based on Bray–Curtis dissimilarities (i.e., using log-transformed abundance for invertebrates and relative abundance for microorganisms) to summarise and visualise, respectively, invertebrate, fungal and bacterial community composition. Prior to analyses, we rarefied fungal and bacterial community data (n = 10,000 sequences per preconditioning treatment). Rarefied data was used for bacterial but not for fungal communities as responses were influenced by rarefaction for the former but not for the latter.
To assess for differences in microbial community composition and leaf-litter chemical composition among preconditioning treatments at the end of the preconditioning phase, we performed Analyses of Variance (ANOVA) with preconditioning treatment as a grouping factor and the first two axes of the chemical composition PCA and the fungal and bacterial OTU richness as response variables. To assess for differences in decomposition among preconditioning treatments, we used a two-way ANOVA on the decomposition rates (k) of the bags retrieved after the preconditioning phase. FMB and CMB were analysed in a single analysis and preconditioning treatment; bag mesh size and their interactions were used as grouping factors. When the ANOVA assumption of normality and heteroscedasticity was not met, we used two-way ordinal regressions. If the ANOVA was significant, we performed a Tukey HSD test to identify pairwise differences.
To assess if preconditioning affected decomposition rates during the flowing river phase, we used a linear mixed effect model (LMM) with k from CMB and FMB as response variables and the interaction between preconditioning treatment and flow regime (i.e., PR vs. IR) as fixed factors. Bag location within each flow regime was used as a random intercept. We used the same LMM specifications to assess for differences in invertebrate, fungal and bacterial OTU richness among flow regimes and preconditioning treatments.
We performed permutational ANOVA (PERMANOVA; Anderson 2017) to test for differences in invertebrate, fungal and bacterial community composition among preconditioning treatments and flow regime. To identify the invertebrate taxa contributing most to community differences among flow regime and preconditioning treatment, we used a multi-level pattern analyses (indicspecies package; Cáceres and Legendre 2009).
For each preconditioning treatment and flow regime, we determined fungal and bacterial community turnover (i.e., beta diversity) between the preconditioning and the flowing river phases as the distances between each preconditioning treatment PCoA's centroids of the preconditioning and flowing river phases. Similarly, we calculated the percentage of overlapping OTUs between each preconditioning treatment and the preconditioning and flowing river communities. We also calculated the percentage of overlap and centroid distances between the control and other preconditioning treatments during the preconditioning phase to assess the extent of microbial colonisation at each preconditioning treatment compared to the controlled laboratory environment.
We used linear models to determine the drivers of CMB and FMB decomposition during the flowing river phase. For all models, we averaged each response (decomposition rates) and predictor variables per preconditioning treatment and flow regime. We built two sets of models to determine if (i) the preconditioning phase chemical conditioning and microbial communities or if (ii) the communities of the flowing rivers had the most effects on river decomposition. For (i) we included the two first axes of the chemical composition PCA and the fungal and bacterial community composition PCoA coordinates, and fungal and bacterial richness from the preconditioning phase as predictors of river decomposition. For (ii) we included fungal and bacterial PCoA axes and richness from the flowing river phase as predictors of river decomposition. In a third model (iii) we included the two first axes of the PCoA and the richness of invertebrate as predictors of instream CMB only. In all models, flow regime and its interactions with every other predictor were included to assess for differences in responses between flow regimes. To limit the number of predictors per model and avoid overfitting, fungal, bacterial and invertebrate community metrics were used as predictors in separate models. For each model, we selected the most influential set of predictors using a model-averaging approach (Harrison et al. 2018). As such, all predictor combinations were assessed, but only parameters of models with corrected Akaike's information criteria (ΔAICc) < 2 were selected, and their estimates averaged. We identified which of model i, ii, or iii had the lowest AICc; models with ΔAICc < 2 were considered equally good.
All analyses were conducted in R studio software (version 4.0.2; 2020-06-22). Packages lme (Bates et al. 2015) and MuMIn (Bartoń 2019) were used for modelling and vegan (Oksanen 2020) was used for NMDS, PERMANOVA and calculation of turnover metrics.
3 Results
3.1 Preconditioning Phase
3.1.1 Leaf Chemical Composition
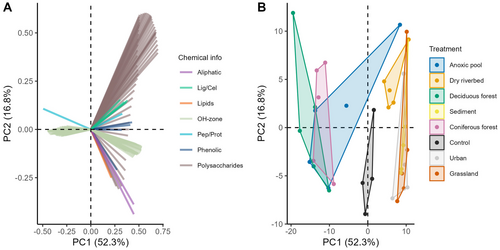
The first two PCA axes explained 69.1% of the variation in leaf chemical composition (Figure 3). PC1 represented mainly a concentration gradient of various C compounds, mainly polysaccharides, aliphatic and phenolic compounds. PC1 differed among preconditioning treatments (ANOVA, p < 0.001), indicating that DecForest and CoForest had lower PC1 scores compared to the control, whereas Urb, Grass and Sed had higher ones (Figure 3, Appendix S2: Table S3). This indicated that leaves in DecForest and CoForest treatments were depleted in C compounds from aliphatic compounds, lipids, lignin, cellulose and other polysaccharides. In contrast, the Urb, Grass and Sed treatments still contained a high concentration of these C compounds (Appendix S2: Figure S1). PC2 represented instead a transition in the complexity of the C fraction, from more simple aliphatic acids and lipids (negative scores) to more complex compounds including cellulose or lignin (positive scores). On average, control, Urb and Grass had lower PC2 scores than other treatments, but no significant differences were found (Figure S1).
3.1.2 Bacterial and Fungal Community Composition
Bacterial OTU richness differed among preconditioning treatments (ANOVA, p < 0.01; Figure S2A). It was higher in Sed than in AnPool, CoForest, DecForest, DryRiv and control (post hoc test; all p < 0.05; Figure S2A). Bacterial communities were dominated by Proteobacteria in land use (i.e., Urb, Grass, DecForest and CoForest) and control preconditioning treatments, whereas AnPool was characterised by a high proportion of Firmicutes and the absence of Actinobacteria during the preconditioning phase (Figure 4A). Bacterial OTU turnover between control and other preconditioning treatments was higher in the Sed and AnPool treatments than in land use treatments (e.g., DecForest, Urb; Figure 5A, Table S4).
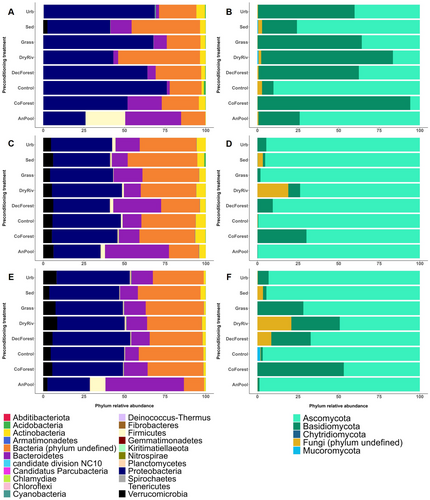
Fungal OTU richness differed across the preconditioning treatments (ANOVA, p < 0.001; Figure S2C). It was higher in Sed than in Grass and DryRiv, with the latter also having lower richness than AnPool (post hoc test; all p < 0.05; Figure S2C). Fungal communities were dominated by Ascomycota in Sed, Control and AnPool and by Basidiomycota in other treatments (Figure 4B). Fungal community turnover between Control and other treatments was higher in CoForest, DryRiv and DecForest and lower in AnPool (Figure 5B, Table S4).
3.1.3 Leaf Litter Decomposition
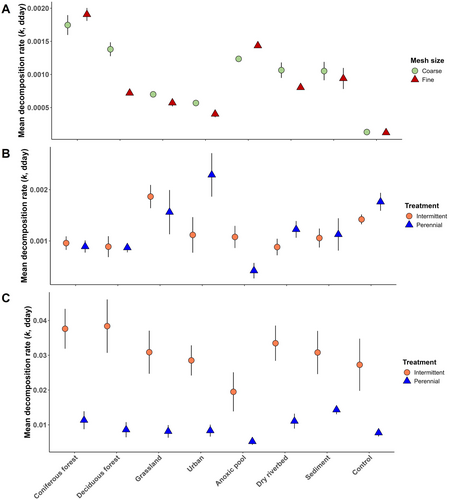
Decomposition rates varied across preconditioning treatments (two-way ANOVA, p < 0.001), mesh size (p < 0.01) and their interaction (p < 0.001). For instance, in FMB, decomposition rates were higher in the CoForest (mean 81.4% AFDM remaining) and AnPool treatments (mean 75.1% AFDM remaining) than in any other treatment (mean 87.1% AFDM remaining Tukey HSD; p < 0.05; Figure 6A, Table S5). CMB decomposition rates were also higher in the CoForest than in any other treatments, and Urb, Grass and control treatments had the lowest decomposition rates with 82.2%, 84% and 91.6% of mean AFDM remaining, respectively (Figure 6A, Table S6). Decomposition rate was higher in CMB (75% AFDM remaining) than in FMB (86% AFDM remaining) in DecForest only (p < 0.001; Figure 6), suggesting a significant effect of terrestrial invertebrates on decomposition there. DryRiv and Sed showed intermediate decomposition rates for both FMB and CMB.
3.2 Flowing River Phase
3.2.1 Bacterial and Fungal Communities
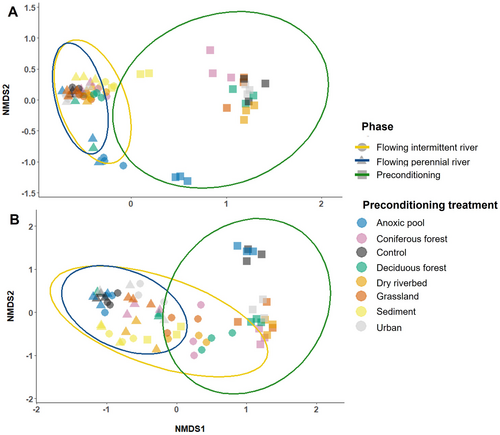
There was no difference in bacterial OTU richness between preconditioning treatments (LMM, p = 0.335) or flow regime (p = 0.135) at the end of the flowing river phase. Bacterial communities did differ between the PR and the IR, and also from the preconditioning phase bacterial communities (PERMANOVA; all p < 0.01; Figure 5A). River bacterial communities were dominated by Proteobacteria, Bacteroidetes and Verrucomicrobia (Figure 4C,E). Bacterial communities also differed between preconditioning treatments (PERMANOVA; all p < 0.001); AnPool was the most homogenous community across the preconditioning and flowing river phases (PERMANOVA; all p < 0.05; Figure 5A). On average 23% (±SD = 12%) of the bacterial OTU found at the end of the preconditioning phase were still present at the end of the flowing river phase (Table 1). These overlapping taxa represented 91% (±6%) of the sequences found at the end of the preconditioning phase (Table 1) and were in most cases, still dominant after the flowing river phase, representing 65% (±19%) of the communities (Table 1). Bacterial community turnover between the two phases was minimal for the Sed and AnPool treatments. The most significant shifts in bacterial community composition between the two phases were observed in the Control and Urb treatments (Table 1, Figure 5A). Bacterial community turnover was generally higher in PR than in IR (Table 1, Figure 5A).
| Treatment | NOTU preconditioning | Intermittent reach | Perennial reach | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NOTU | %OTU | %Seq | Turn | NOTU | %OTU | %Seq | Turn | ||
| Bacterial communities | |||||||||
| Anoxic pool | 402 | 520 | 25.7 | 94.2 | 0.562 | 609 | 24.5 | 95.6 | 0.637 |
| Coniferous forest | 411 | 619 | 20.5 | 95.5 | 0.670 | 635 | 16.5 | 83.4 | 0.779 |
| Control | 368 | 394 | 15.7 | 90.9 | 0.865 | 570 | 11.2 | 88.9 | 0.879 |
| Deciduous forest | 345 | 579 | 19.5 | 94.6 | 0.763 | 790 | 13.4 | 92.8 | 0.841 |
| Dry riverbed | 295 | 481 | 20.4 | 95.3 | 0.811 | 836 | 15.7 | 96.9 | 0.828 |
| Grassland | 595 | 413 | 20.3 | 81.1 | 0.803 | 538 | 19.0 | 75.2 | 0.867 |
| Sediment | 735 | 701 | 59.9 | 97.9 | 0.312 | 825 | 42.4 | 94.7 | 0.443 |
| Urban | 510 | 641 | 17.2 | 89.9 | 0.898 | 674 | 20.2 | 93.1 | 0.881 |
| Fungal communities | |||||||||
| Anoxic pool | 426 | 520 | 33.7 | 94.6 | 0.906 | 222 | 25.7 | 72.8 | 0.959 |
| Coniferous forest | 221 | 201 | 40.3 | 97.9 | 0.538 | 367 | 27.8 | 97.5 | 0.696 |
| Control | 225 | 425 | 20.0 | 91.1 | 0.933 | 193 | 15.8 | 76.9 | 0.965 |
| Deciduous forest | 241 | 152 | 38.2 | 93.9 | 0.576 | 217 | 31.3 | 92.7 | 0.758 |
| Dry riverbed | 172 | 154 | 29.9 | 91.1 | 0.659 | 255 | 18.4 | 95.1 | 0.886 |
| Grassland | 243 | 226 | 41.2 | 93.6 | 0.600 | 231 | 32.0 | 89.4 | 0.827 |
| Sediment | 457 | 312 | 64.4 | 90.7 | 0.425 | 280 | 63.2 | 81.3 | 0.499 |
| Urban | 235 | 395 | 28.6 | 96.2 | 0.787 | 224 | 27.7 | 90.9 | 0.816 |
- Abbreviations: %OTU = percentage of OTU present during the flowing river phase that were present during the preconditioning phase; %Seq = percentage of sequences that the overlapping taxa represented in the preconditioning community; NOTU = total number of OTU; Turn = turnover calculated as the distance between community centroids.
Fungal OTU richness was lower in the PR than the IR (LMM, p = 0.043) and differed among preconditioning treatments (p = 0.023) in the IR only (interaction term, p = 0.003). Fungal richness in the AnPool treatment was higher than in the DryRiv, CoForest, DecForest and Grass treatments (post hoc test; all p < 0.05; Figure S2D). Fungal communities were dominated by Ascomycota, which composed > 97% of the communities in Control and AnPool across both PR and IR. Larger proportion of Basidiomycota were identified in the IR than in the PR (Figure 4D,F). Fungal community composition differed both among flow regimes and preconditioning treatments (PERMANOVA p < 0.01; Figure 5B). On average, 34% (±14%) of the fungal OTU found at the end of the preconditioning phase were still present after incubation in both rivers (Table 1). These overlapping taxa represented 90% (±7%) of the sequences found after the preconditioning phase (Table 1) and were in most cases still dominant at the end of the flowing river phase, representing 50% (±30%) of the communities across both rivers (Table 1). Fungal community turnover between the two phases was the lowest for the Sed treatment and the highest for the Control and AnPool treatments (Table 1, Figure 4B). Communities overlap between the two phases was generally higher in IR than PR (Table 1, Figure 5B).
3.2.2 Invertebrate Communities
Invertebrate richness was higher in the IR than in the PR (ANOVA; all p < 0.001) but there were no differences among preconditioning treatments. Instream invertebrate communities were composed on average by 50% (±17%; SD) of shredders but their proportion did not differ with flow regime or preconditioning treatments. Macroinvertebrate communities differed between PR and IR reaches (PERMANOVA, p < 0.001) but not among preconditioning treatments. Identified indicator taxa of the PR comprised Gammarus sp., Sericostoma sp. and Atherix sp. whereas several Trichoptera (e.g., Stenophylax sp., Micropterna sp.), Ephemeroptera (e.g., Baetis sp., Electrogena sp.) and Plecoptera (e.g., Nemoura sp., Isoperla sp.) taxa characterised the IR invertebrate community (Table S8).
3.2.3 River Decomposition
River FMB decomposition rates differed among preconditioning treatments (LMM, p < 0.001; Figure 6B) and among flow regimes for Urb and AnPool only (interaction term p < 0.05) (Figure 6B). Leaves preconditioned in AnPool, CoForest and DecForest were decomposed more slowly (69.8%, 69% and 74.1% mean AFDM remaining, respectively) than those from the Urb treatment (59.3% mean AFDM remaining) in the PR only (LMM post hoc; all p < 0.05). CMB decomposition rates only differed between flow regimes (LMM; p < 0.001). Except for the AnPool and Sed, CMB decomposition rates were higher in the IR (13.9% mean AFDM remaining) than in the PR (69.2% mean AFDM remaining; post hoc; all p < 0.05; Figure 6C).
3.2.4 Drivers of River Decomposition
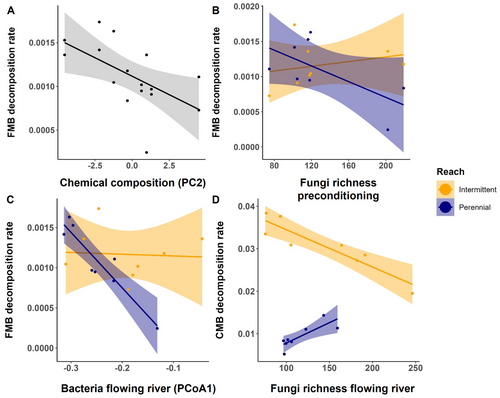
The FMB decomposition rates of the flowing river phase were equally explained by the bacterial communities of that flowing river phase and the fungal and bacterial community and leaf chemical quality from the preconditioning phase (Table S7). The decrease in decomposition rates in FMB was associated with changes in leaf chemical composition, specifically an increase in PC2 scores, which reflected a relative gain of larger, more recalcitrant C compounds in leaf litter over more labile ones (Figure 3A, Figure 7A). Decomposition rates in FMB also decreased in treatments with higher fungal richness during the preconditioning phase (only in PR; Figure 7B, Table S7), while increasing with bacterial richness during the preconditioning phase (Table S7). FMB decomposition was also correlated to the bacterial community PCoA1 from the flowing river phase, particularly in the PR (Figure 7C). CMB decomposition rates increased with fungal richness from the flowing river phase (best model: AIC = −138.4) in the PR but decreased in the IR (Figure 7D). Preconditioning phase bacterial and fungal communities and leaf chemical composition also had significant effects on CMB, but models were generally less performant (e.g., AIC = −128.8; Figure S3, Table S7). Invertebrate communities did not explain variability in CMB decomposition rates among preconditioning treatments but drove strong differences among flow regimes (Table S7).
4 Discussion
This study aimed to assess the habitat-specific biotic characteristics driving leaf-litter processing across terrestrial–aquatic boundaries. Although a few studies have looked at decomposition across terrestrial–aquatic habitats (e.g., Foulquier et al. 2015; Abelho and Descals 2019; Sarremejane et al. 2024), very little is known about the mechanisms through which leaf microbial communities and chemical changes determine cross-habitat decomposition. We observed differences in leaf chemical and microbial community composition and decomposition across preconditioning treatments, evidencing the effects of riparian land use and instream conditions on microbial communities, leaf-litter quality and decomposition (H1). Partially validating our hypothesis (H2), we found evidence of the effects of terrestrial microbial communities and leaf chemical transformation on decomposition in flowing rivers. These results suggest that the microbial community developed in each preconditioning habitat may have a legacy effect on aquatic microbial community colonisation and subsequent leaf decomposition. In line with hypothesis (H3) we identified differences in the effects of preconditioning on microbial communities and decomposition between IR and PR, suggesting that the effects of preconditioning on organic matter processing may be affected by a river flow regime.
4.1 Effects of Preconditioning on Early-Stage Community Colonisation and Decomposition
Leaf-litter decomposition varies depending on environmental conditions, with the presence–absence of water constituting a major driver of leaf chemical diversification (del Campo, Corti, et al. 2021) and decomposer community composition and activity (Datry et al. 2011a, 2011b). Higher decomposition in forested riparian habitats and the anoxic pool during the preconditioning phase were associated with higher chemical differentiation of those leaves (i.e., impoverishment of C compounds, especially labile forms as aliphatic acids or lipids). Such high decomposition and diversification are likely associated with high microbial activity (del Campo, Martí, et al. 2021), which enhances decomposition efficiency when coupled with photochemical and thermal degradation (Gliksman et al. 2017). During the preconditioning phase, bacterial community richness and turnover from control were highest in the anoxic pool and inside the riverbed sediment, whereas fungal turnover from control was higher in the forested riparian habitats (i.e., DecForest and CoForest). This suggests that bacterial and fungal colonisation may differ and have different contributions to decomposition depending on habitats (Hieber and Gessner 2002; Pascoal and Cássio 2004). In anoxic pools and sediments, water and fine sediment particles provide a matrix with three-dimensional colonisation pathways (i.e., lateral, vertical and horizontal), representing a larger contact area for colonisation and preserving relatively constant moisture and temperature which may have enhanced microbial colonisation (Danger et al. 2012) and leaching (Dieter et al. 2013; del Campo, Corti, et al. 2021). In addition, leaf leaching and anoxia may have favoured bacterial development by depressing fungal activity and colonisation. Anoxic pool bacterial communities were characterised by a high proportion of Firmicutes, which are often found in extreme environments, including anoxic conditions (Wagner et al. 2022). Decomposition was highest in the coniferous forest treatment during the preconditioning phase despite lower microbial richness. Optimal humidity levels under the snow cover could have enhanced colonisation and decomposition by fungal communities (Wu 2020). This rich forest soil may also have harboured a high fungal biomass. Alternatively, the succession in freeze–thaw cycles may have affected the physical integrity of leaf-litter, enhancing mass loss during the preconditioning phase (Wu et al. 2010). In the coniferous and deciduous forests, the Home-Field Advantage (i.e., HFA) could also have supported high decomposition rates observed as microbial communities on forested soil may be particularly adapted to leaf material decomposition (Yuan et al. 2019). Community turnover (compared to control) was generally lower in grassland and urban habitats potentially due to limited colonisation pathways (particularly on impervious urban surfaces disconnecting the leaves from the soil communities), low humidity, high temperature and prolonged exposure to solar radiation in those treatments (Hobbie et al. 2014; del Campo, Corti, et al. 2021). As found in previous studies, a large proportion of the microbial community (24%–66%) was already present on the leaves (control), suggesting that at least part of the communities on senescent leaves could contribute to early decomposition (Koivusaari et al. 2019; Hayer et al. 2022). Finally, although we cannot entirely rule out an influence of the slightly longer preconditioning period in the coniferous treatment compared to others, we believe that the strong differences in environmental conditions among preconditioning treatments—rather than their duration—were the primary drivers of the chemical transformation of leaf litter and its microbial colonisation.
4.2 Effects of Preconditioning on Subsequent River Decomposition Dynamics
Preconditioning in terrestrial environments affects river decomposition dynamics (Pastor et al. 2014; del Campo, Corti, et al. 2021) but little is known about the role of initial decomposer communities and their succession in driving river decomposition (Sridhar et al. 2009). We found that communities and leaf chemical changes from the preconditioning phase influenced later decomposition in flowing water. Such results indicate that microbial communities and chemical changes during the preconditioning phase may play an important role in determining subsequent river community composition and decomposition activity (Koivusaari et al. 2019). We observed a stronger bacterial and fungal turnover between the preconditioning and flowing river phases for leaves that were preconditioned in riparian than in instream environments. Microbial colonisation during preconditioning may give established communities competitive advantage as they enter the lotic environment (Sridhar et al. 2009). The community replacement between the preconditioning phase and the end of the flowing river phase was lowest for the leaves exposed to the anoxic pool (bacteria only) and sediment treatments (fungi and bacteria), indicating that the microbial communities already established (1) may have priority over instream communities found during the flowing phase or (2) are already adapted to the aquatic environment and similar in composition to instream communities (Hayer et al. 2022). However, such priority effect did not enhance river decomposition in those treatments. In the anoxic pool, the combined action of decomposition by anaerobic microorganisms and leaching during the preconditioning phase may have induced depletion in labile compounds (e.g., proteins, dissolved organic carbon) available for instream communities, potentially explaining low river decomposition for this treatment (Dieter et al. 2013; del Campo, Corti, et al. 2021). Community turnover between the two phases was higher for riparian land-use treatments, probably because terrestrial communities are more easily outcompeted by aquatic communities in flowing waters (Ruiz-González et al. 2015). The preconditioning treatments that were little decomposed during the preconditioning phase (e.g., urban and control) experienced higher microbial decomposition instream, at least in the perennial reach, likely due to higher proportions of labile compounds (e.g., aliphatic compounds, fatty acids, simple polysaccharides, and so forth) available for instream decomposers and reduced competition with early-stage communities on leaves. Similarly, Santschi et al. (2018) showed that leaf-litter quality and the instream microbial communities complexity played a key role in determining river decomposition rates. However, such patterns could differ when taking into account different leaf species with contrasted initial chemical composition and palatability (del Campo et al. 2025).
4.3 Differences in Communities and Decomposition Among River Types
We found clear differences in community composition among rivers and lower fungal and bacterial community replacement in the IR than in the PR. Differences in community composition and functioning among flow regimes are likely resulting from the overarching effects of drying, as taxa are sorted depending on their drying sensitivity along drying duration, frequency and magnitude gradients (Colls et al. 2019; Simões et al. 2021; Arias-Real et al. 2023; Foulquier et al. 2024). Such results may indicate that the intermittent river microbial communities were composed of (1) poor competitors, which were less efficient at replacing communities from preconditioning and/or (2) of similar taxa than those found on preconditioned leaves. Basidiomycetes were more common on leaves from forest, dry riverbed and grassland treatments in the IR than in the PR, suggesting that some taxa from this typically terrestrial phylum may persist even when submerged (Koivusaari et al. 2019), particularly in IR. We found a higher proportion of Basidiomycota than expected in dry riverbed sediments (Foulquier et al. 2024). Some Basidiomycetes yeasts can easily metabolise recalcitrant compounds (Sampaio et al. 2007), making them a better competitor for leaf litter decomposition than aquatic hyphomycete communities. Although microbial decomposition did not differ among flow regimes, the effect of fungal and bacterial communities on decomposition differed, with often stronger relationships in PR. Such results could indicate a stronger biodiversity-ecosystem functioning relationship in perennial than intermittent rivers as environmental stability (perennial flow) may promote niche selection and species sorting to their favoured resource in the former (Leibold et al. 2017). Also, invertebrate-driven decomposition increased and decreased with fungal diversity in PR and IR, respectively, potentially reflecting the effects of invertebrate grazing pressure, reducing fungi richness as decomposition increases in IR (e.g., Mora-Gómez et al. 2016). Another mechanism explaining the link between fungal community composition and invertebrates feeding activities could be the selective feeding of shredders on leaves colonised by specific fungal communities (Arsuffi and Suberkropp 1989).
Invertebrate-driven decomposition was two to four times faster in the IR than in the PR. Such results contradict previous observation of slower decomposition due to negative effects of drying on shredders in IR (e.g., Datry et al. 2011a, 2011b; Gruppuso et al. 2022). Higher macroinvertebrate diversity in the IR may explain the faster decomposition rate we observed if taxa feed on different parts of the leaves (Jonsson et al. 2002), reducing interspecific competition and promoting leaf breakdown. As suggested in García-Palacios et al. (2016) decomposer complexity could also have played a key role in instream litter processing through strong impacts on litter C loss. However, the dominance of intermittent-specialist taxa such as Stenophylax sp. (Ruiz-García and Ferreras-Romero 2007)—which complete their entire life cycle before the river dries—may better explain the high consumption rates observed in IR since shredder identity and dominance can have stronger impacts on decomposition than diversity (Dangles and Malmqvist 2004; Tolkkinen et al. 2013). Our results thus suggest that intermittent rivers can contribute equally, if not more, to river-network scale organic matter processing (see e.g., Sarremejane et al. 2024), to the conservation of distinct decomposer biodiversity (see e.g., Santos and Stevenson 2011) and biogeochemical cycles (Datry et al. 2018). However, our observations based on two reaches have to be taken with caution, and further research could test if our results stand across multiple sites and leaf species within or among non-perennial river networks.
5 Conclusion
Our results demonstrate how riparian land uses and instream conditions may determine litter processing in rivers through cascading effects of leaf preconditioning on microbial community establishment. Understanding the dynamics of OM decomposition across terrestrial–aquatic boundaries is an undermining challenge to better predict how global changes, including hydrological and land use changes, may affect ecosystem functioning at the river-network meta-ecosystem scale (Sarremejane et al. 2024). Further research, integrating multiple rivers and leaf species, could help better understand the processing of OM in complex perennial–intermittent river networks and predict their carbon budget.
Author Contributions
Conceptualisation and developing methods: M.J., R.S., T.D., A.F., R.C. and G.S. Conducting the research: M.J., R.S., E.E., C.L., L.G. and F.B. Data analysis and data interpretation: M.J., R.S., C.L., R.C. and E.E. Preparing figures and tables: M.J., R.S., R.C., C.L. and E.E. Writing: M.J., R.S., T.D., A.F., G.S., E.E., C.L., R.C., L.G. and F.B.
Acknowledgements
This research was supported by the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement no. 891090 (MetaDryNet), the DRYvER (Datry et al. 2021; grant agreement no. 869226) and the FLUFLUX (ERC-STG 716196) projects. We thank Angelique Arbaretaz, Teresa Silverthorn, Amélie Truchy and the technical team of the EcoFlowS and EMA research groups (INRAE), including Bertrand Launay, Maxence Forcellini, Abdel Azougui, Guillaume Le Goff, Bernard Motte and Bernadette Volat for their help in the field and the lab. We also thank the landowners (including Didier Cristini) on the Albarine catchment, who provided access to their properties.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data will be made available upon acceptance of the Dryad Digital Repository.




