
Metacommunity organisation, spatial extent and dispersal in aquatic systems: patterns, processes and prospects
Summary
- Metacommunity ecology addresses the situation where sets of local communities are connected by the dispersal of a number of potentially interacting species. Aquatic systems (e.g. lentic versus lotic versus marine) differ from each other in connectivity and environmental heterogeneity, suggesting that metacommunity organisation also differs between major aquatic systems. Here, we review findings from observational field studies on metacommunity organisation in aquatic systems.
- Species sorting (i.e. species are ‘filtered’ by environmental factors and occur only at environmentally suitable sites) prevails in aquatic systems, particularly in streams and lakes, but the degree to which dispersal limitation interacts with such environmental control varies among different systems and spatial scales. For example, mainstem rivers and marine coastal systems may be strongly affected by ‘mass effects’ (i.e. where high dispersal rates homogenise communities to some degree at neighbouring localities, irrespective of their abiotic and biotic environmental conditions), whereas isolated lakes and ponds may be structured by dispersal limitation (i.e. some species do not occur at otherwise-suitable localities simply because sites with potential colonists are too far away). Flow directionality in running waters also differs from water movements in other systems, and this difference may also have effects on the role of dispersal in different aquatic systems.
- Dispersal limitation typically increases with increasing spatial distance between sites, mass effects potentially increase in importance with decreasing distance between sites, and the dispersal ability of organisms may determine the spatial extents at which species sorting and dispersal processes are most important.
- A better understanding of the relative roles of species sorting, mass effects and dispersal limitation in affecting aquatic metacommunities requires the following: (i) characterising dispersal rates more directly or adopting better proxies than have been used previously; (ii) considering the nature of aquatic networks; (iii) combining correlative and experimental approaches; (iv) exploring temporal aspects of metacommunity organisation and (v) applying past approaches and statistical methods innovatively for increasing our understanding of metacommunity organisation.
Introduction
The structure of ecological communities varies greatly in space and time, and this biological variation has many causes and consequences. Typically, communities are assembled by a combination of abiotic factors, biotic interactions, priority effects (i.e. competitive dominance given by early colonisation) and dispersal processes (Chase & Leibold, 2003; Leibold et al., 2004). Teasing apart the relative importance of these factors has been a major challenge for understanding the assembly of ecological communities (Holyoak et al., 2005; Hildrew, 2009; Mittelbach, 2012) and managing, conserving and monitoring biodiversity (Brown et al., 2011; Siqueira et al., 2012a; Heino, 2013a). These topics fall within the field of metacommunity ecology, which addresses ‘a set of local communities that are linked by dispersal of multiple potentially interacting species’ (Wilson, 1992). The key terms we use follow the definitions in Leibold et al. (2004), Heino (2011) and Heino, Melo & Bini (2015) and are summarised in Table 1.
| Term | Definition |
|---|---|
| Spatial scale | Spatial scale has two components. Spatial grain refers to the size of the study unit (Wiens, 1989). In aquatic research, spatial grain typically refers to the local arena, such as a stream riffle site, a pond, a lake or a site in a stretch of coastline. Spatial extent refers to the size of the region encompassing all localities in a region unit (Wiens, 1989). Spatial extent can be measured as the distance between the localities situated furthest from each other or as a convex polygon encompassing all localities in a region unit (Heino et al., 2015). It can be a drainage basin in freshwater studies, a defined coastal area or an offshore area in a marine study. |
| Environmental conditions | Environmental features of a locality. Abiotic and biotic environmental factors are responsible for species sorting (Leibold et al., 2004). |
| Community composition | Biological features of a locality. Community composition is made up of species found at a locality at a given point in time (Heino et al., 2015). |
| Species sorting | Species are filtered by environmental factors to occur at environmentally suitable sites. Adequate dispersal rates are necessary so that species can track variation in environmental conditions among localities (Leibold et al., 2004). |
| Mass effects | High dispersal rates homogenise community structure at adjacent localities irrespective of their environmental conditions and, hence, obscure species sorting (Leibold et al., 2004). |
| Dispersal limitation | Some species are precluded from occurring at suitable localities because the nearest occupied sites are too far away. Dispersal limitation prevents perfect species sorting from occurring because species cannot reach all environmentally suitable localities (Heino et al., 2015). |
| Dispersal rate | The rate at which individuals move between two sites. If dispersal rates are high, they may decouple communities from local environmental control (Ng et al., 2009). If dispersal rates are low, they result in non-perfect species sorting because not all species can reach all environmentally suitable sites (Heino & Peckarsky, 2014). |
| Spatial process | Any external process that affects a local community irrespective of local environmental conditions. Spatial processes are typically related to dispersal of species between sites, but no distinction is made whether dispersal rates are high, intermediate or low. The potential importance of spatial processes in a metacommunity can be seen as a variation in community structure explained purely by predictor variables describing the spatial position of a site or distances between sites (but not those describing environmental conditions). |
| Dispersal barrier | Any factor (e.g. geomorphological, hydrological) that prevents species from dispersing to all localities within a region. |
| Variation partitioning | A commonly used approach in regression and constrained ordination analysis to examine how much variation in local community structure is explained by (i) environmental factors; (ii) spatial variables or (iii) their shared effects (Legendre & Legendre, 2012). |
| Spatial structuring | Community composition shows spatially structuring if it is significantly related to the spatial location or varies significantly with increasing spatial distance between sites. |
There are four main metacommunity perspectives (Leibold et al., 2004): neutral, patch dynamics, species sorting and mass effects. In the neutral perspective, random speciation, extinction, migration and immigration determine the community structure (Hubbell, 2001). According to this view, similarity in community structure should decrease with spatial distance between sites, whereas the environment is considered to have no effect on local communities, as species are considered ecologically equivalent. In patch dynamics, there is a colonisation–competition trade-off, with better colonisers dominating in isolated or recently disturbed communities, while better competitors drive them to extinction in less-isolated or mature communities (Holyoak et al., 2005). In species sorting, biotic interactions and abiotic environmental conditions filter the suite of species co-occurring at each locality, provided that there is enough dispersal so that species can track variation in environmental conditions (Leibold et al., 2004; Soininen, 2014). With mass effects, both dispersal and environmental factors are considered important, with high rates of dispersal allowing species to occur in localities with suboptimal environmental conditions (Shmida & Wilson, 1985). Without surplus net dispersal from source sites, the population of a species would go extinct at sink localities (Pulliam, 1988). Although it has also been suggested that the organisation of most metacommunities has imprints of all of these mechanisms (Cottenie, 2005; Gravel et al., 2006; Martiny, Bohannan & Brown, 2006; Logue et al., 2011), testing the relative fit of these perspectives with field data may provide a basis for a better understanding of ecological communities (Cottenie, 2005). However, this approach has been criticised recently by Winegardner et al. (2012), who suggested that researchers should break down these perspectives and go back to basics with regard to the environmental and dispersal effects on metacommunity organisation. In that sense, they also suggested that patch dynamics and mass effects are special cases of species sorting with limiting and homogenising effects of dispersal, respectively (Winegardner et al., 2012). We agree with their reasoning because the focus in metacommunity studies should be on the relative roles of species sorting and dispersal, which are fundamental processes structuring all metacommunities (Lindström & Langenheder, 2012).
The characteristics of aquatic systems present both challenges and opportunities for examining the organisation of metacommunities (Brown et al., 2011; Heino, 2011; Lindström & Langenheder, 2012). In this context, there are various types of freshwater and marine systems that differ fundamentally in environmental heterogeneity, connectivity and spatial extent. For example, consider the differences among marine pelagic systems, marine coastal systems, stream networks, lakes connected by streams, and ponds without stream connections. One can envisage that dispersal rates among localities are higher in offshore and coastal marine systems than among lakes and ponds without stream connections (Jackson, Peres-Neto & Olden, 2001; Shurin, Cottenie & Hillebrand, 2009). However, whether this idea fits real-world metacommunities is likely to depend on the types of organisms inhabiting these systems and their traits in relation to the characteristics of these systems. It is possible, for example, that metacommunity organisation in marine coastal systems is less limited by dispersal and more affected by mass effects and patch availability than in lakes, where metacommunity organisation is more attributable to the combined influences of dispersal limitation and species sorting (Jackson et al., 2001; Olden, Jackson & Peres-Neto, 2001). The validity of these conjectures should depend on the dispersal capacity of organisms, spatial extents and types of study systems (Van der Gucht et al., 2007; Declerck et al., 2011; Lindström & Langenheder, 2012; Moritz et al., 2013).
The dispersal abilities of aquatic organisms, and the distances they travel, are generally poorly known for entire biotas (Bilton, Freeland & Okamura, 2001; Bohonak & Jenkins, 2003). This deficit is a major hindrance to our understanding of dispersal as a regional process structuring ecological communities (Havel & Shurin, 2004; Lindström & Langenheder, 2012). Only for a handful of typically economically valuable species, such as some fish, is there reliable information on dispersal processes, mechanisms and distances (e.g. Matthews, 1998). This is why ecologists need to rely on proxies for dispersal in studies on metacommunity organisation (Jacobson & Peres-Neto, 2010). These proxies include pairwise spatial distances between sites (Landeiro et al., 2011; Grönroos et al., 2013) and various variables related to the isolation of sites (Olden et al., 2001; Jacobson & Peres-Neto, 2010; Altermatt, Seymour & Martinez, 2013). In this vein, an increasingly common strategy consists of dividing species into coarse dispersal ability groups (Thompson & Townsend, 2006; Astorga et al., 2012; Algarte et al., 2014), groups with different body sizes (e.g. Soininen et al., 2011), incidence-based dispersal groups (e.g. Landeiro et al., 2012) or dispersal mode groups (Schulz et al., 2012; Wetzel et al., 2012; Heino, 2013b). Ecologists can then examine the relative effects of spatial location and environmental conditions on the community structure of different dispersal groups, with the premise that spatial effects in terms of dispersal limitation should be more important for weaker than stronger dispersers. Some studies have indeed found support for the use of such proxies for dispersal, and they have found noticeable differences in the magnitude of spatial structuring of community composition among biological groups (Hájek et al., 2011; Maloney & Munguia, 2011; Soininen et al., 2011; Astorga et al., 2012; De Bie et al., 2012; Wetzel et al., 2012). In contrast, other studies have revealed no clear differences among biological groups presumed to differ in dispersal characteristics (Heino et al., 2012; Landeiro et al., 2012; Schulz et al., 2012; Grönroos et al., 2013).
Spatial scale is key to the understanding of various ecological phenomena (Wiens, 1989; Giller, Hildrew & Raffaelli, 1994; Wu & Loucks, 1995), including dispersal (Ng, Carr & Cottenie, 2009; Heino & Peckarsky, 2014; Heino et al., 2015). In the context of metacommunity organisation, one should expect different effects of dispersal on spatial structuring of community composition at different spatial extents (Fig. 1), which may also be related to the dispersal abilities of the organisms (Cottenie, 2005; Heino, 2011; Soininen, 2012). First, for a given biological group, dispersal limitation should increase with increasing spatial distances between sites and spatial extent surveyed (Potapova & Charles, 2002; Mykrä, Heino & Muotka, 2007; Maloney & Munguia, 2011; Soininen, 2012). Second, the spatial distances and spatial extents at which dispersal limitation can be expected to occur are likely to vary among groups of organisms (e.g. bacteria versus fish; Beisner et al., 2006). Third, mass effects should increase in importance with smaller spatial distances between sites and smaller spatial extents surveyed (e.g. Ng et al., 2009). Fourth, the spatial extent at which mass effects occur is likely to be larger for strongly dispersing than weakly dispersing organisms because dispersal abilities and homogenising effects are likely to be more far-reaching in the former group. Thus, it is not surprising that tests of the importance of spatial structuring at different spatial extents and in differently dispersing groups of organisms have received increasing interest from aquatic ecologists (Cottenie, 2005; Mykrä et al., 2007; Shurin et al., 2009; Maloney & Munguia, 2011; Astorga et al., 2012; Alahuhta et al., 2014; LeCraw, Srivastava & Romero, 2014).
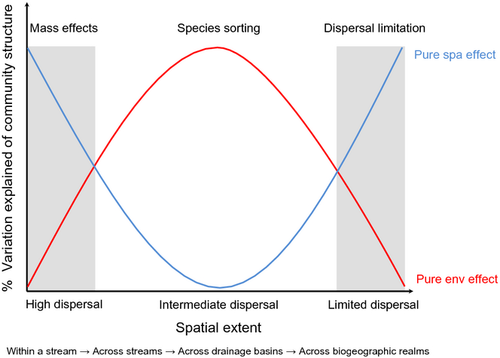
Spatial environmental heterogeneity also constitutes an important part of the theory of metacommunity organisation (Leibold et al., 2004; Martiny et al., 2006). The mass effects and species sorting perspectives incorporate environmental heterogeneity, but assumptions about the effects of dispersal differ between the two (Leibold et al., 2004; Holyoak et al., 2005; Lindström & Langenheder, 2012). Under mass effects, excessive dispersal dampens the influence of local environmental conditions (e.g. Mouquet & Loreau, 2003). On the other hand, sufficient dispersal is necessary for species to track variation in local conditions in the species sorting perspective (e.g. Leibold et al., 2004). A challenge for metacommunity studies is hence to separate the influences of dispersal limitation and intensity from that of species sorting on metacommunity organisation (Ng et al., 2009; Lindström & Langenheder, 2012; Winegardner et al., 2012; Heino et al., 2015).
Here, we consider how well the above conjectures have been supported in empirical field-based metacommunity studies of freshwater and marine systems. We will not provide a formal meta-analysis owing to lack of methods to compare the effect sizes (and associated weights, which are a key aspect of meta-analysis) that are frequently delivered by current metacommunity studies (i.e. fractions estimated by variation partitioning of species data matrices; see Table 1). Also, we will not consider eco-evolutionary dynamics or findings from experimental systems because they have been reviewed recently (Urban et al., 2008; Logue et al., 2011). In addition to recent studies published under the label ‘metacommunity ecology’, we will also consider earlier studies that have tackled the role of scale, dispersal and environmental factors in aquatic systems (see also Giller et al., 1994; Hildrew, 2009). We first consider the spatial extent of a study and how that may affect our understanding of aquatic metacommunities. Second, we describe the role of dispersal and local environmental processes in different types of aquatic systems, ranging from stream networks to marine systems. These systems differ from each other in the characteristics of flow, which potentially have important effects on the dispersal of organisms. Third, we summarise patterns, similarities and differences among different aquatic systems. Finally, we provide a roadmap for further studies, highlighting gaps of knowledge and suggesting fruitful avenues for future research.
Understanding the spatial limits of a local community and a metacommunity
What are the limits of a local community and those of a metacommunity? These are two key issues in metacommunity ecology, because understanding these limits may allow understanding the factors structuring metacommunities and local communities at different scales (Leibold et al., 2004; Holyoak et al., 2005; Carstensen et al., 2013; Heino et al., 2015). While it is relatively easy to delineate a local community for lake- or pond-dwelling organisms, a metacommunity of lakes or ponds can be more difficult to define. In contrast, it is not that straightforward to decipher where a local community ends in streams and even more difficult within continuous aquatic systems, such as marine coastal systems and offshore systems. Hence, researchers have used varying definitions of local communities (Heino et al., 2015). For example, a stream local community has been frequently defined as a riffle site (e.g. Grönroos & Heino, 2012), a stream section including riffles and pools (e.g. Roque et al., 2010) or almost an entire stream (e.g. Lamouroux, Poff & Angermeier, 2002). Similarly, limits to a stream metacommunity have been a single stream (e.g. Grönroos & Heino, 2012), a drainage basin (e.g. Landeiro et al., 2012) or a larger geographical region (e.g. Mykrä et al., 2007). Obviously, these definitions of a local community and a metacommunity affect the conclusions of a study (Heino et al., 2015). Thus, research findings should always be discussed with regard to these spatial definitions and the dispersal capacities of the organisms studied. The latter are as important as the local and regional scales considered. This is because, for efficiently dispersing organisms, the limits of a local community may be the same as the limits of a metacommunity dominated by weakly dispersing organisms. Although most groups of organisms include species that vary in dispersal ability, many studies have compared metacommunity patterns of groups that should, on average, differ widely in dispersal ability (e.g. diatoms versus insects versus fish). At spatial scales large enough, metacommunity ecology merges with biogeography and macroecology (see Martiny et al., 2006; Heino, 2011; Bonada, Dolédec & Statzner, 2012; Passy, 2012; Pinel-Alloul et al., 2013), and we suggest that future studies should focus on how the roles of environmental factors and dispersal vary with changing regional scale (Mykrä et al., 2007; Landeiro et al., 2012; Alahuhta & Heino, 2013; Grönroos et al., 2013).
Further, with a large increase in the spatial extent of a metacommunity study, the species data matrix will probably contain different regional species pools, which are phylogenetically structured and thus moulded by evolutionary factors (Peres-Neto, Leibold & Dray, 2012). A strong relationship between species and environmental factors, however, can no longer be interpreted unequivocally as reflecting species sorting processes. This is because other feasible explanations would include recent speciation events and the effects of historical barriers (Leibold, Economo & Peres-Neto, 2010). Disentangling the role of history from current processes shaping local communities sampled across biogeographical scales is therefore a major challenge for metacommunity studies. Although a number of studies have examined genetic differences between populations of aquatic species (Wilcock, Hildrew & Nichols, 2001; Hughes, 2007; Wilcock et al., 2007; Hughes, Schmidt & Finn, 2009), they have mostly focussed on a single or a few species at the same time and have not directly tackled the issue of phylogenetic structure of local communities and entire metacommunities.
Spatial and environmental processes in aquatic systems
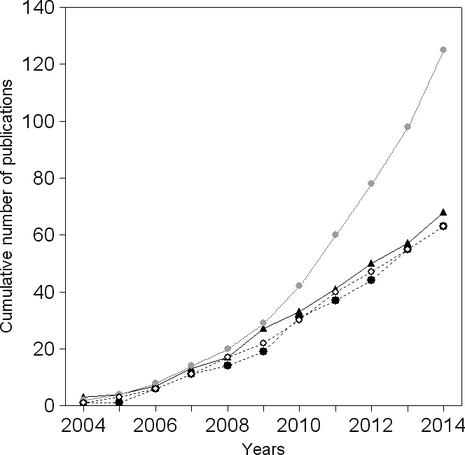
To evaluate research on the metacommunity ecology of different types of aquatic systems, we searched for the following combination of keywords in the Web of Science database (timespan = 1 January 2004–29 October 2014; field = topic): (1) metacommunit*AND (stream* OR river*), (2) metacommunit* AND (lake*) NOT pond*, (3) metacommunit* AND (pond*) NOT lake* and (4) metacommunit* AND (marine* OR estuar*). We found that most aquatic studies on metacommunity ecology have been carried out in freshwater systems, with emphasis on lentic ecosystems (after summing the amount of studies carried out in lakes and ponds; Fig. 2). It is also noteworthy that after 2009, the number of studies on lotic systems increased more rapidly than those on lentic and marine systems. Although Logue et al. (2011) also found that most studies on metacommunity ecology were carried out in aquatic systems, our new results clearly indicate a relative deficit of research on marine systems. This is particularly true for studies examining the spatial and environmental processes governing variation in community structure.
Evidence from stream networks
Stream ecosystems are structured as networks, in which small streams coalesce to form progressively larger rivers. In general, most of the stream network consists of small headwater streams, at least with regard to their overall length (Downing et al., 2012; Altermatt, 2013). Thus, in stream networks, headwater streams represent ‘typical’ habitats in the sense that they are more common in the landscapes than larger rivers. Yet, many headwaters streams are relatively isolated. As a consequence, organisms inhabiting headwater streams need to cross the landscape when dispersing to other headwater streams, whether using fully aquatic or terrestrial pathways (Clarke et al., 2008; Brown et al., 2011; Miyasono & Taylor, 2013). Not only does the distance between headwater streams promote isolation (Olden et al., 2001), but also the resistance of flow to upstream dispersal may affect relative isolation (Baguette et al., 2013). Additionally, first-order streams being in the periphery of stream networks presumably receive most migrants from downstream, at least those dispersing via watercourses (Auerbach & Poff, 2011). Thus, the importance of dispersal for metacommunity organisation may vary in different parts of the stream network (Brown & Swan, 2010; Brown et al., 2011; Altermatt et al., 2013).
The dispersal of stream organisms occurs primarily along the network itself in strictly aquatic organisms or overland, upstream and downstream in organisms with winged adults (Fagan, 2002; Urban et al., 2006; Grant, Lowe & Fagan, 2007; Brown et al., 2011). There are various modes of dispersal in stream organisms, however, ranging from aquatic passive dispersal with stream flow (e.g. drift of larval insects), passive aquatic dispersal via animal vectors (e.g. mussels, microalgae), active aquatic dispersal within streams (e.g. fish, shrimps), overland passive dispersal with wind (e.g. microalgae, bacteria), overland passive dispersal via animal vectors (e.g. snails, mites, microalgae, bacteria) and overland active dispersal (e.g. winged adult insects). Even among aquatic insects alone, the effective dispersal distances of adults vary among species, although our knowledge is rudimentary in this regard (Bilton et al., 2001; Altermatt, 2013; Heino & Peckarsky, 2014).
The maximum dispersal distances of stream organisms are likely to vary considerably, ranging from short in some fish (Matthews, 1998) to potentially large in some microorganisms because they are so numerous that a few cells may reach distant sites (Fenchel & Finlay, 2004). Inferring dispersal distances for freshwater organisms is problematic, however, as dispersal distances may vary remarkably among species within a given biological group (e.g. fish). For example, among stream fish, some species disperse large distances, of which anadromous salmonids or catadromous eels are perhaps the best examples, while some species move very short distances during an individual's life (Matthews, 1998) or are restricted to a few streams or small networks, as in cave-dwelling fish (Buhay et al., 2007). Thus, if there is such vast variation within a well-known biological group, one can only imagine the true variation in dispersal distances among species in groups of microorganisms, macrophytes and aquatic insects, to name a few (Lindström & Langenheder, 2012; Heino & Peckarsky, 2014). For example, there is huge variation in body size among aquatic microorganisms alone, ranging from the smallest bacteria or viruses to the largest diatoms with a cell length up to 500 μm. Such variation in body size is likely to have important consequences for dispersal ability, mechanisms and distances (Jenkins et al., 2007; Rundle, Bilton & Foggo, 2007; Hildrew, 2009). It is thus important to consider the variation in dispersal-related traits at finer resolutions within a given group of aquatic organisms (Fenchel, 1993; Heino & Soininen, 2006).
Most stream studies have found that environmental control (e.g. in terms of stream size, velocity, acidity, nutrients) prevails over spatial (e.g. dispersal) constraints (Potapova & Charles, 2002; Mykrä et al., 2007; Heino & Mykrä, 2008; Landeiro et al., 2011; Siqueira et al., 2012b; Göthe, Angeler & Sandin, 2013a). However, within the same set of sites and, therefore, the same spatial extent, some studies have found that differently dispersing organisms show different spatial structuring and environmental control of community structure (Thompson & Townsend, 2006; Maloney & Munguia, 2011; Astorga et al., 2012). Astorga et al. (2012) found that diatoms (efficient passive dispersers) showed stronger environmental control and weaker spatial structuring than bryophytes (passive dispersers with intermediate dispersal ability) and invertebrates (inefficient active dispersers). Furthermore, within invertebrates, weaker dispersers showed lower environmental control and higher spatial structuring than stronger dispersers. In contrast, Heino et al. (2012) did not find clear differences among diatoms, bryophytes and invertebrates in this regard. Rather, independent of the biological group, environmental factors (e.g. stream width, current velocity, total phosphorus) accounted for most of the explained variation in community structure. These different findings are probably attributable to the difference in the spatial extent of these two studies (Heino et al., 2015): in the former, the largest between-site geographical distance was about 800 km (Astorga et al., 2012), while the latter was restricted to small areas in two separate drainage basins with the largest distances between sites being less than 100 km (Heino et al., 2012). Therefore, it can be assumed that the relative importance of mass effects, species sorting and dispersal limitation varies with regard to the spatial extent encompassing the sampling sites (Fig. 1).
The effects of spatial extent on metacommunity organisation should also be evident in other contexts. For example, the distribution of microorganisms may not be limited by dispersal, with species occurring wherever the environment is suitable (Fenchel & Finlay 2004; Logue & Lindström, 2008). Nevertheless, while some studies have indeed found little spatial structuring in microbial community composition attributable to dispersal limitation, others have found that communities of microorganisms are to some degree dispersal limited at large spatial scales (Lindström & Langenheder, 2012; Soininen, 2012). For example, recent research suggests that assemblages of lotic diatoms are both spatially structured and environmentally controlled, although some unknown environmental factor(s) could also account for part of the apparent ‘spatial structure’ (Table 1) in community composition (Soininen, Paavola & Muotka, 2004; Heino et al., 2010; Wetzel et al., 2012; Göthe et al., 2013b).
Headwater streams are usually isolated because there is no direct source of colonist (via fully aquatic pathways) available upstream, and they will thus receive migrants from downstream reaches in strictly aquatic organisms (e.g. fish), as well from other headwater streams in organisms with flying adults (e.g. insects). The high degree of isolation in comparison with mainstem rivers is compounded by the small population sizes of organisms, such as fish, inhabiting the small habitats available in a headwater stream. Accordingly, one could expect high extinction rates and low community persistence in headwater streams when compared to large streams in the central parts of a drainage basin. In fact, such findings have been observed for stream fish (Gotelli & Taylor, 1999; Miyasono & Taylor, 2013). A study on stream invertebrates showed, however, the opposite pattern, with greatest persistence of community composition in environmentally stable upstream sites (Townsend, Hildrew & Schofield, 1987). Also, the restricted ability to receive migrants may cause high spatial turnover in species composition among headwater streams (Clarke et al., 2008; Miyasono & Taylor, 2013), which is enhanced by high variation in environmental conditions among small streams (Clarke et al., 2008). This restriction may have important implications for metacommunity studies aimed at partitioning variation in species composition between environmental and spatial factors. That is, stochastic factors (e.g. rare events of colonisation and extinction) may lead to a distinct local community composition that, based on snapshot sampling, may not be easily predicted by local environmental conditions (Heino & Mykrä, 2008; Erős et al., 2012a). This may result in rather low amounts of variation in local community structure explained by environmental and spatial variables, as has been exemplified by recent studies using modern statistical methods (Heino et al., 2012; Landeiro et al., 2012; Göthe et al., 2013a; Grönroos et al., 2013).
A metacommunity approach that takes into account the dendritic nature of stream network structure may provide interesting insights for stream research (Altermatt, 2013; Altermatt et al., 2013). Brown & Swan (2010) hypothesised that isolated headwater streams receive few migrants and thus local factors should be the preponderant force controlling local communities, as predicted by the species sorting view. However, large streams should receive many migrants from tributaries, as well as from downstream, and the importance of high dispersal rates should thus be pronounced (Brown & Swan, 2010; Göthe et al., 2013b; but see Milesi & Melo, 2014). At the periphery of the drainage basin, low-order streams usually coalesce with others of a similar size, whereas they can flow into either similar-sized or much larger streams in central parts of the drainage basin. This spatial arrangement may provide opportunities for natural experiments with stream metacommunities, as the importance of regional dispersal is presumably distinct in different parts of the network. For instance, small streams coalescing with streams of a similar size should be more frequently colonised by species well adapted to the conditions than small streams only connected directly to the mainstem; Grant et al., 2007). In the latter, however, mass effects may allow some species to occur in unsuitable environments through source–sink dynamics (Pulliam, 1988).
Evidence from lakes
Lakes are typically defined as waterbodies of more than two hectares in area, whereas smaller standing waters are considered ponds (Biggs et al., 2005). The distinction is important, as the communities of lakes may be structured by different factors from those of ponds (Wellborn, Skelly & Werner, 1996; Oertli et al., 2005). For example, lakes are usually more permanent than ponds, which typically go through severe reductions in area or even dry out periodically. Deep lakes are also typically thermally stratified, while shallow lakes and most ponds exhibit constant mixing (Wetzel, 2000). One could thus envisage that ecological communities in lakes are more controlled by deterministic processes, while those in ponds are more influenced by stochastic forces at ecological time scales (Chase, 2007).
Studies on community patterns across sets of lakes have considered fish (Rodriguez & Lewis, 1997; Magnuson et al., 1998; Olden et al., 2001; Beisner et al., 2006; Mehner, Emmerich & Hartwig, 2014), plankton (Pinel-Alloul, Niyonsenga & Legendre, 1995; Cottenie et al., 2003; Cottenie & De Meester, 2004; Beisner et al., 2006; Ptacnik et al., 2010; Soininen et al., 2011; Lopes, Bini & DeClerck, 2014), molluscs (Aho, 1978; Lewis & Magnuson, 2000; Heino & Muotka, 2005), macroinvertebrates (e.g. Heino, 2013b), macrophytes (Capers, Selsky & Bugbee, 2010; Alahuhta & Heino, 2013; Alahuhta et al., 2014) and bacteria (Beisner et al., 2006; Van der Gucht et al., 2007). Early studies on fish in lakes addressed questions very similar to those of modern metacommunity ecology, showing that regional fish communities are structured by extinction and colonisation dynamics (Tonn & Magnuson, 1982; Rahel, 1984). These studies revealed that extinction events (e.g. due to low pH, low oxygen and high predation pressure) dominate in temperate and boreal lakes, while the colonisation of waterbodies is generally slow, varying between lakes connected to the drainage network by streams and isolated lakes (Magnuson et al., 1998; Jackson et al., 2001). Furthermore, the size of the stream and potential dispersal barriers, such as waterfalls and dams, may also be related to the structuring of fish communities in drainage lakes (Jackson et al., 2001; Olden et al., 2001). Thus, metacommunity organisation in lacustrine fish is contingent on the connectedness of the lake and, secondly, on how environmental factors and biotic interactions determine the subset of species from the regional pool that occur in each lake.
As in the studies on fish, research on lake molluscs has shown that their assemblages are structured by factors related to dispersal, lake size and local environmental factors. Early studies on snail assemblages in lakes can be considered as metacommunity studies, although they were mostly intended to test the predictions of the theory of island biogeography (MacArthur & Wilson, 1967). Aho (1978) pioneered this field of study in lakes, showing that both isolation by distance to a large lake (the source) and local environmental conditions jointly determined species richness. Further studies based on the data collected by Aho (1966) have also shown that: (i) snail and clam assemblages in small lakes are a subset of the fauna in a large source lake; (ii) distance to the source is also related to species composition and (iii) the effects of local environmental conditions and spatial location of small lakes cannot be fully separated due to spatially structured variation in lake environmental conditions (Heino & Muotka, 2006). Essentially, the same questions have been studied in North America, where environmental factors (e.g. lake size, hydroperiod, pH, fish predation) were generally found to be more important than isolation and dispersal in determining the assemblage structure and diversity of snails (Lewis & Magnuson, 2000; Hoverman et al., 2011).
Tests of the effects of dispersal on metacommunity structuring in lake systems have typically been based on proxies for dispersal ability (for recent examples, see De Bie et al., 2012; Padial, Ceschin & Declerck, 2014). Beisner et al. (2006) examined fish, zooplankton, phytoplankton and bacteria in a set of connected lakes and found that distance along watercourses between sites explained some variation in fish assemblages, whereas for the other three groups, neither intervening aquatic nor overland (terrestrial) distances between sites generally explained assemblage structure. However, overland distance explained a similar amount of variation in zooplankton abundance data as environmental variables. These findings are not unexpected because, within a small drainage basin, the efficient passive overland dispersal of these small organisms should distribute species over most lakes (Beisner et al., 2006), whereas fish that are restricted in movements to the stream network itself should be more affected by the spatial location of a lake (Olden et al., 2001).
Following the seminal paper by Beisner et al. (2006), other researchers have tackled similar questions for lakes in other regions. Soininen et al. (2007) found that both environmental and spatial variables were important in structuring zooplankton and phytoplankton communities, but spatial effects were stronger for the former than the latter, possibly due to differences in body size and consequent dispersal abilities. Heino (2013b) found that the relative roles of spatial structuring and local lake environmental variables varied among four macroinvertebrate groups with differing dispersal ability, albeit with roughly similar body size. These dispersal ability groups ranged from those with no winged adult (e.g. snails, crustaceans) to those with strong aerial dispersal (e.g. diving beetles, dragonflies). In general, taking into account the proportions of variation in community structure explained, weak dispersers showed least environmental control and strongest spatial structuring, while strong dispersers showed greatest environmental control and no significant spatial structuring (see also Padial et al., 2014). Thus, studies examining metacommunity organisation across the same set of lakes for several biological groups have suggested that dispersal limitation matters. However, examples of null results, with no significant spatial and environmental structuring in lake communities, can also be found (Nabout et al., 2009).
Metacommunity organisation apparent in lakes may be strongly affected by the spatial extent of the region encompassed, but very few studies have directly examined this issue. Soininen et al. (2011) did not find significant spatial structuring across 20 lakes within a drainage basin, but found significant spatial structuring across five drainage basins at the spatial extent of about 600 km. Their study considered very small organisms, including bacterioplankton, phytoplankton and zooplankton, which are thought to be controlled mostly by local environmental conditions (Van der Gucht et al., 2007). In contrast, Alahuhta & Heino (2013) found no positive relationship between spatial structuring and spatial extent in lake macrophytes, although the degree of spatial structuring was specific to a study region. However, it seems that at least some effects of spatial processes are important for lake communities at extents up to several hundred kilometres, although environmental factors still overcome the effects of dispersal limitation (Van der Gucht et al., 2007; Alahuhta et al., 2014).
Overall, studies conducted in lakes point to the joint importance of spatial processes and local environmental factors as determinants of community structure (Jackson et al., 2001; Olden et al., 2001; Capers et al., 2010; Alahuhta & Heino, 2013). The relative effect of these two groups of predictors is likely to depend on the dispersal abilities of the biological groups, spatial extent and environmental gradients (Cottenie, 2005; Heino, 2013b; but see Van der Gucht et al., 2007). First, the better the dispersal ability, the more should environmental conditions explain variation in community structure (e.g. Heino, 2013b). Second, the greater the spatial extent, the greater the role of spatial factors related to dispersal limitations (e.g. Soininen et al., 2011). Third, the larger the environmental gradient, the greater the role of environmental factors (e.g. Jackson et al., 2001).
Evidence from ponds
Dispersal limitation should be very important for ecological communities living in isolated ponds (De Bie et al., 2012; Baguette et al., 2013). Furthermore, in such isolated systems, good dispersal ability should be an important trait for organisms, due to the fact that small ponds are also typically ephemeral (Wellborn et al., 1996). Ponds may dry out either seasonally or for longer. In temporary ponds, environmental control may not necessarily be strong. Rather, one can expect strong ‘small island effects’ (Oertli et al., 2005) and a role for ‘elements of chance’ (Jeffries, 1988) in community structure. Thus, temporary ponds might demonstrate the role of spatial processes, such as dispersal limitation, in affecting community structure. However, due to stochasticity and high heterogeneity, it may not be easy to explain the effects of such dispersal-related processes using spatial predictors (Jeffries, 2005; Gioria, Bacaro & Feehan, 2010). For example, Florencio et al. (2014) found that variation in macroinvertebrate assemblages was driven by high environmental heterogeneity and large spatial distances in a set of temporary ponds. High environmental heterogeneity was also the likely explanation for the greater importance of species turnover than nestedness (resultant from species richness differences; Baselga, 2012) in driving beta diversity. Despite the difficulty of predicting species richness and composition, species inhabiting temporary ponds should share some adaptations to habitat dynamism, such as high dispersal, production of resting eggs, fast development and resistance to desiccation and associated conditions (Wellborn et al., 1996; Both et al., 2011; Pellowe-Wagstaff & Simonis, 2014). Dynamism is also characteristic of floodplain ponds, where the cycle of drying and wetting may alternate the importance of local and regional factors in shaping community structure (Medley & Havel, 2007). Local biotic interactions may be important in ponds during the drying phase, whereas dispersal processes are more influential during the wetting phase due to high dispersal rates.
Permanent ponds show higher habitat stability and community persistence (Wellborn et al., 1996). Accordingly, local abiotic and biotic factors may be more important in determining community structure. Permanent ponds are usually deeper, and thus likely to have high habitat heterogeneity, than temporary ponds. This heterogeneity increases possibilities for species sorting through habitat partitioning. For instance, Both et al. (2011) studied tadpole assemblages in tropical ponds and found that water permanence was important in determining variation among species in two guilds (based on habitat use) of tadpoles, with nektonic species being associated with deep ponds and benthic species with shallow ponds (i.e. water depth was a surrogate for desiccation stress). Also, predation pressure by large predators, such as fish and dragonfly larvae, should strongly affect assemblage structure in ponds, although their effects may differ markedly between temporary and permanent ponds (Wellborn et al., 1996). Again, in contrast to temporary ponds, permanent ponds may be constantly interconnected, facilitating species sorting among preferred habitats (Cottenie et al., 2003).
Regional factors, such as dispersal and distance between sites, and local environmental conditions, such as hydroperiod and water chemistry, jointly determine the distribution of single species of pond insects (Jeffries, 2005). This finding also suggests that there should be no strong dichotomy between dispersal and species sorting in determining pond community structure, and both should be important, albeit their relative importance is probably scale dependent (Declerck et al., 2011). Declerck et al. (2011) found that environmental factors were the most important predictors of zooplankton community composition within wetlands. At the largest spatial extent, encompassing ponds in a number of wetlands in different valleys, variation in community composition could not be entirely explained by environmental variables. Their study exemplifies a situation where environmental control (species sorting) may be the dominant process structuring a metacommunity at the smallest spatial extent, whereas dispersal limitation is the most likely structuring force at the largest spatial extent. However, it would be interesting to determine whether regional control through dispersal is more important in temporary than permanent ponds, although testing this issue may be difficult using survey data and is more likely to be answered using experimental ponds.
Evidence from marine systems
Communities of marine organisms, be they composed of macrophytes, invertebrates, fish or plankton, have traditionally been studied within different habitats types. Benthic soft sediments or pelagic systems represent some of the most common habitats on earth, covering c. 70% of the planet (Snelgrove, 1999). Coastal systems, in turn, are critical habitats for many of these species, linking the sea with land and freshwater habitats (Levin et al., 2001; Cowen & Sponaugle, 2009). Other traditional divisions of study include coral reefs, rocky subtidal and intertidal systems, mangroves, seagrass meadows and salt marshes (Bertness, Hay & Gaines, 2001). An important consideration is that a habitat or a study unit is often embedded within larger landscapes that collectively represent a continuum of environmental gradients and may share a suite of common species. For example, seagrass meadows can be viewed as more or less discrete habitat patches (Boström, Jackson & Simenstad, 2006), but they may also influence and be influenced by adjacent soft sediments. Fish may also show cross-habitat connections between mangrove and coral reef systems (Mumby, Edwards & Arias-Gonsales, 2004). Dispersal between non-isolated communities in marine systems has the potential to influence patterns of marine diversity across multiple spatial and temporal scales (Whitlatch et al., 1998; Grantham, Eckert & Shanks, 2003; Cornell & Harrison, 2013).
In marine systems, it is especially important to consider at what scale a metacommunity is studied (Giller et al., 1994; Ellis & Schneider, 2008; Hewitt et al., 2010; Cornell & Harrison, 2013). Moreover, what we define as ‘local’ or ‘regional’ in marine systems will depend on the spatial and temporal scales of the study and the specific goals of the researcher. Although spatial scale has been considered a core theme in marine ecology for a long time (see several chapters in Giller et al., 1994), very few marine studies have considered the issues of spatial scale and simultaneously applied the typical approaches of modern metacommunity ecology (rock pools, Pandit, Kolasa & Cottenie, 2009; rocky intertidal, Okuda et al., 2010; deep sea, McClain, Stegen & Hurlbert, 2012; soft sediment, Moritz et al., 2013). Okuda et al. (2010) studied macroalgal, sessile invertebrate and mobile molluscan metacommunities across a set of rocky intertidal sites. They found that environmental factors accounted for more variation in community composition than spatial location, but the relative roles of environmental and spatial processes seemed to vary between the biological groups. Both spatial and environmental processes were obviously important for macroalgal and molluscan metacommunities, but environmental factors dominated in structuring the metacommunity of sessile invertebrates. In a similar study on polychaetes, Moritz et al. (2009) found that environmental effects dominated in metacommunity structuring at a large spatial extent, suggesting the importance of species sorting. However, at smaller spatial extents and along more homogeneous environmental gradients, both flow connectivity and geographical distance were also important in metacommunity structuring, suggesting the role of mass effects. Marine metacommunities thus seem to be controlled by a combination of species sorting and mass effects, the relative importance of which are related to life-history traits, dispersal modes and dispersal capacities that may vary between different habitat types (Grantham et al., 2003).
Dispersal between sites in marine systems differs in a few fundamental ways from that in other aquatic systems. It is a process by which the spatial distribution of individuals changes by movement in the water column, on the bottom substratum or within the sediments. Dispersal may involve spatial scales ranging from a few centimetres to thousands of kilometres, and temporal frequencies ranging from seconds to seasons (Palmer, 1988; Armonies, 1994; Palmer, Allan & Butman, 1996). The propensity to disperse depends on species traits. For example, a species may be planktotrophic, lecithotrophic, it may have direct development, or it may rely on internal or external brooding (Thorson, 1950; Pedersen et al., 2008; Pappalardo & Fernándes, 2013). Also, a species may be free-living, infaunal, epifaunal or a burrow dweller (Grantham et al., 2003; Valanko, Norkko & Norkko, 2010). Dispersal-related life-history modes of marine species can, in general, be divided into a larval dispersal phase, which occurs before initial settlement, and a post-larval or adult dispersal phase, which occurs after initial settlement (Whitlatch et al., 1998).
Early studies on dispersal in marine systems have emphasised supply-side ecology (Gaines, Brown & Roughgarden, 1985), where dispersal is limited to episodic long-distance dispersal by larvae (Lundquist et al., 2006; Pineda, Reyns & Starcsak, 2009; Cowen & Sponaugle, 2009). In supply-side ecology, dispersal was assumed to be a physical process, whereby larvae from a well-mixed larval pool disperse and will settle into local populations (i.e. demographically open, over >1000 km). In this view, a stochastic supply of larvae determines how limited by dispersal a local site is or, conversely, how ‘connected’ the site is. However, in contrast to passive particles, larval behaviour may distort settlement estimates based on supply alone (Pineda et al., 2010). For example, upon release, larvae can also be retained within very close proximity (Osman & Whitlatch, 1998). Larval dispersal can also be very costly, incurring high rates of mortality (Pedersen et al., 2008). In addition, it has been shown that larval density may diminish rapidly with distance and time owing to advective and diffusive properties of the mixing and stirring of currents (e.g. 20–30 km; Becker et al., 2007).
It is now widely recognised that, in addition to initial larval recruitment, many marine species also continue to disperse as post-larvae and as adults (Whitlatch et al., 1998). These post-larval processes can thus act to buffer spatiotemporal variation in larval supply (Caro, Navarrete & Castilla, 2010). In shallow soft-sediment habitats, rates of post-larval dispersal can be especially high, often due to increased waves and currents, and species not being permanently attached to the substratum (Armonies, 1994; Hewitt et al., 1997; Norkko et al., 2001). It has been shown that this is not a purely passive process because many species are able to burrow deeper or actively emerge, thus regulating their subsequent transport along the bottom in the bedload (Armonies, 1994; Lundquist et al., 2006). In tidal systems, many post-larval benthic invertebrates, even those lacking a planktonic larval stage (up to 40–60% of taxa; Grantham et al., 2003), have been observed to be passively transported or actively swim higher up in the water column (Martel & Chia, 1991; Armonies, 1994; Valanko et al., 2010). In contrast to a stochastic supply of individuals from a common species pool, frequent small-scale active and passive dispersal can thus lead to a metacommunity structure in which habitat conditions select particular sets of species (Hewitt et al., 1997; Valanko, Norkko & Norkko, ). In such systems, post-larval dispersal may be relatively more important in maintaining community composition than site-to-site variation in initial larval recruitment, which can be considered to be largely independent of local adult abundances (Pedersen et al., 2008; Pineda et al., 2009).
Community composition in marine systems also varies relatively predictably, depending on the niche requirements of species along dominant local environmental gradients, such as substratum grain size (e.g. Gray, 1974) or food supply (e.g. Pearson & Rosenberg, 1978). Deep-sea benthic diversity has, for example, been correlated with the diversity of substratum particle sizes (Levin et al., 2001; Etter & Grassle, 1992). Many of the environmental variables affecting marine species have also synergetic effects (e.g. pH, oxygen, depth, organic content), and they may often operate over different spatial and temporal scales in the assembly of species at sites (Sandman et al., 2013; Villnäs et al., 2013). Local species richness can also be enhanced by small-scale biogenic structures, which increase environmental heterogeneity (e.g. Hewitt et al., 2005). One of the best-known examples is the habitat heterogeneity provided by coral reefs or other reef structures, which support a significantly higher diversity than adjacent areas (Connolly et al., 2005; Dornelas, Connolly & Hughes, 2006; Messmer et al., 2011). Environmental heterogeneity increases when the number of habitat types increases and is paralleled by an increase in beta diversity (Valentine, 2009). This can be the case when, for example, comparing a straight coastline to a coastline with an archipelago that supports a variety of habitat types (e.g. rocky shores, soft-sediment bays and lagoons).
While spatially synchronous recruitment events are mostly driven by seasonal larval recruitment, many species will also continue to disperse at a smaller scale after settlement throughout the year as post-larvae and adults (Whitlatch et al., 1998; Grantham et al., 2003; Valanko et al., 2010). It can thus be expected that across smaller spatial distances, species sorting can be maintained more effectively and, if rates of dispersal are high, local species richness may also increase beyond expectations based solely on species niche requirements because of high rates of dispersal. Marine systems echo the general suggestion by Winegardner et al. (2012) in that simultaneous consideration of dispersal and underlying environmental heterogeneity plus species niche requirements are important. However, dispersal may be either limiting (Whitlatch et al., 1998) or partly homogenising community structure (Moritz et al., 2013) in marine systems, thereby potentially dampening the effects of species sorting (Lindström & Langenheder, 2012).
Synthesis
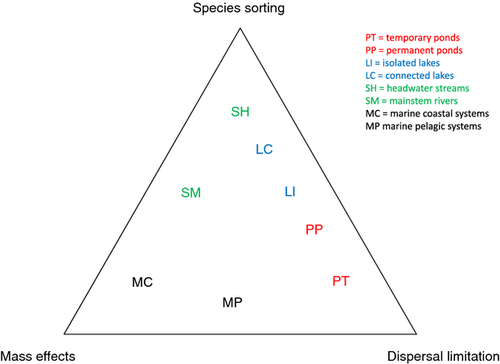
Although more studies are needed to confirm or challenge the generality of existing findings, the following system-specific evidence can be used partially to summarise what we know about metacommunity organisation in aquatic systems and to guide further studies (Fig. 3).
Streams
Studies from streams suggest that species sorting is mostly responsible for metacommunity organisation. This finding seems to be true at least within small drainage basins (Heino et al., 2012; Landeiro et al., 2012; Göthe et al., 2013a; Grönroos et al., 2013), but stronger spatial structuring attributable to dispersal limitation is likely to be found across a number of drainage basins (Mykrä et al., 2007; Astorga et al., 2012). The importance of high dispersal rates in structuring stream metacommunities is uncertain, but their role may be related to the specific features of a study system (Brown et al., 2011; Heino & Peckarsky, 2014). For example, in well-connected mainstem rivers, mass effects may be more important than in more isolated headwaters (Brown & Swan, 2010; Göthe et al., 2013a; but see Milesi & Melo, 2014), although this idea requires further testing. Finally, in comparison with many other systems, stream communities may show a relatively high degree of randomness due to frequent floods and droughts that prevent species sorting from organising metacommunities. This idea requires further testing. However, recent stream research using modern statistical methods has shown that what we have previously considered as local communities fitting well with the prevailing environmental conditions may actually be relatively poorly predicted by local environmental variables (Landeiro et al., 2012; Göthe et al., 2013a2013b). Rather, local community organisation may also be interfered by stochastic extinction–colonisation dynamics, resulting in temporally variable community–environment relationships (Heino & Mykrä, 2008; Erős et al., 2012b).
Lakes
Studies from lakes, at least relatively isolated ones, suggest that both species sorting and dispersal limitation are important factors structuring metacommunities (Tonn & Magnuson, 1982; Magnuson et al., 1998; Jackson et al., 2001; Olden et al., 2001). However, as in streams, the importance of dispersal limitation may increase with increasing spatial extent (Soininen et al., 2011; Mehner et al., 2014). In comparison with other aquatic systems, there appears to be more definite environmental control in connected lakes, as well as clearer dispersal limitation in some biological groups in non-connected lakes (Jackson et al., 2001; Olden et al., 2001). This reasoning is also suggested by the relatively greater variation in metacommunity structure being explained in some analyses of lakes than streams in the same drainage basin (Heino et al., 2012; Heino, 2013b).
Ponds
Ponds are characterised by a high degrees of randomness in both environmental conditions and biota (Jeffries, 1988; Chase, 2007). This randomness may lead to less definite environmental control in ponds than in lakes, for example. The temporal variability of ponds also suggests that mass effects (at small spatial extents), priority effects (at small spatial extents) and dispersal limitation (at large spatial extents) may be important in these systems, although proving their importance with field survey data may be challenging (see also Declerck et al., 2011).
Marine systems
In marine systems, the importance of mass effects and species sorting for metacommunity organisation is apparently high. This is because these systems have strong environmental gradients and are rather open to dispersal, yet perhaps not so open that mass effects prevent true species sorting from occurring. However, surprisingly few studies have assessed metacommunity organisation in marine systems based on survey data (Okuda et al., 2010; Moritz et al., 2013), thereby precluding a stronger synthesis. Across larger scales, the mixing of water masses means that the difference between a local community and a metacommunity is unclear. Earlier reviews of dispersal (Palmer et al., 1996) have suggested, for example, a common dispersal pool from which recruitment occurs. Moreover, in coastal systems where continued post-larval dispersal is common, the application of metacommunity ecology can help to interpret how dispersal and local environmental gradients work together to produce regional patterns of alpha, beta and gamma diversity.
Avenues for further studies
There are several issues that need to be considered in further studies on metacommunity organisation in aquatic systems. These issues are pragmatic, practical and methodological, and a combination of all three. We highlight five topics that provide useful ideas for further studies and interesting new insights into aquatic metacommunities.
A better quantification of dispersal
Metacommunity studies rely heavily on proxies of dispersal, which has been quantified by indirect methods in the vast majority of empirical studies (Jacobson & Peres-Neto, 2010; Heino & Peckarsky, 2014). Among the indirect methods, eigenfunction spatial analyses (Peres-Neto & Legendre, 2010; Landeiro et al., 2011), in combination with variation partitioning analysis (Peres-Neto et al., 2006 Legendre & Legendre, 2012), have commonly been used to infer the role of dispersal in community structuring. However, recent simulation studies have demonstrated that the typical interpretation of the role of dispersal, which can be derived from the use of spatial variables in variation partitioning analysis, is to some degree flawed (Tuomisto, Ruokolainen & Ruokolainen, 2012). For instance, Gilbert & Bennett (2010) found that multivariate models based on eigenfunction analysis often produced overestimated coefficients of determination due to overfitting the spatial component or poor modelling of the environmental component. Smith & Lundholm (2010) used simulated data to show that the strength of ‘dispersal limitation contributes to both the pure environmental and pure spatial variance partitions’, which are the quantities usually used to assess the relative roles of environmental and spatial structuring. These problems complicate examining the effects of dispersal limitation on metacommunity organisation at scales pertinent to natural ecological systems.
Different types of approaches for field-based metacommunity studies are hence sorely needed. These include population genetic methods, DNA barcoding of entire assemblages, mark–recapture of various species in a metacommunity and direct quantification of the dispersal of multiple species (Jacobson & Peres-Neto, 2010; Altermatt, 2013). Also, a variety of trap types have been used to quantify rates of dispersal (Armonies, 1994; Petersen et al., 1999; Lundquist et al., 2006; Valanko et al., 2010; Gray & Arnott, 2011, 2012). However, large numbers of traps are difficult to replicate across space, and they often only provide a short-time snapshot of community assembly. It is important to bear in mind that in nature, many species will differ in their mode of dispersal and can have high rates of mortality throughout the dispersal process. Hence, future methods should attempt to incorporate more effectively longer temporal and larger spatial scales in the measurement and quantification of dispersal, for instance, using genetic methods. Genetic methods have been employed previously to study genetic differences, gene flow and potential dispersal limitation between populations of one or a few species (Hughes, 2007; Hughes et al., 2009; Hildrew, 2009; Finn et al., 2011). Although genetic methods are certainly superior to coarse proxies for dispersal, they are typically limited by the focus on a few species at the same time, whereas most metacommunities comprise dozens to hundreds of species, most of which are difficult to study due to their pronounced rarity. The above goals are thus not easy to accomplish, present severe challenges and are again at best approximations of the importance of dispersal in structuring an entire metacommunity.
A related issue is how best to evaluate the common assumption that the species making up the assemblages are homogeneous in their dispersal ability. For instance, an assemblage of ‘stream macroinvertebrates’ includes species that have aerial adults (i.e. most insects), while others remain their entire lives in the water (e.g. crustaceans, flatworms). Further, an assemblage of ‘stream fish’ may include both long-range migrants and species in which individuals are confined to a few sections of a single stream (Matthews, 1998). Accordingly, the limited variation explained by multivariate models, particularly regarding the spatial component of variation, may result from the mixing of species with different dispersal capability. A promising approach is to group species based on dispersal traits and perform a number of analyses (Algarte et al., 2014; Heino & Peckarsky, 2014). This is not to say that multiple analyses will make it easier to find something ‘significant’, as distinct hypotheses can be raised for each dispersal trait group of organisms. An important difficulty with this approach is the lack of knowledge on the natural history of aquatic species, although recent studies have provided some basis for future research (Poff et al., 2006; Vieira et al., 2006; Passy, 2007; Verberk, Van Noorwijk & Hildrew, 2013). There is thus an urgent need to increase our knowledge of the natural history of aquatic species. Despite the importance of natural history for general and applied ecology, such research is unfortunately not well funded.
Implications of the spatial arrangement of aquatic networks
Although all aquatic systems are connected by dispersal to some extent, network effects on the dispersal process are most visible in stream networks and drainage lakes (Olden et al., 2001; Fagan, 2002; Altermatt, 2013). Stream networks are formed by the junction of streams of various sizes. Peripheral areas of drainage basins are usually composed of small streams, whereas central parts include both small and large streams. Also, streams in peripheral areas usually are located in steep areas which, in turn, causes high flow velocity and produces a coarse substratum. These characteristics have profound effects on the biota. The classical river continuum concept (RCC) envisages stream metacommunities as determined by a continuum of environmental conditions and resources from headwater streams to lowland rivers (Vannote et al., 1980). Recent research has, however, indicated a number of problems with the RCC paradigm. For instance, the RCC ignores the distinct habitats created at stream confluences as well as the response of the organisms to these features (Statzner & Higler, 1985, 1986; Rice, Greenwood & Joyce, 2001; Rice, Ferguson & Hoey, 2006). Accordingly, recent theories have conceived lotic systems as networks, a view that should provide interesting insights into metacommunities of stream systems and drainage lakes (Fagan, 2002; Poole, 2002; Benda et al., 2004; Kiffney et al., 2006; Brown et al., 2011; Erős, Schmera & Schick, 2011; Milesi & Melo, 2014). Further studies should indicate how the network structure affects the relative roles of dispersal limitation, species sorting and mass effect for local communities in different parts of the stream network.
There is clearly a need to refine the use of tools related to spatial network information in metacommunity studies. Those tools include network analysis, which model network shape and modularity, and may utilise undirected and directed graphs (Dale & Fortin, 2010; Erős et al., 2011). The recent applications of such approaches to stream networks and drainage lakes have been promising in modelling species distributions (Erős et al., 2012b) and may show considerable success in the field of metacommunity ecology. Similarly, modelled water flow-related connectivity has been used along with geographical and environmental predictors in marine metacommunity studies (Moritz et al., 2013).
The issues related to networks should also be investigated in the context of aquatic biomonitoring, as they have great potential to bring new insights over existing approaches (Heino, 2013a; Siqueira, Durães & Roque, 2014). Predictive modelling, for example, is mainly based on the idea of species sorting, and the existing approaches, such as RIVPACS-type predictive models (Wright et al., 1996; Clarke, Wright & Furse (2003), do not take into account spatial processes, such as dispersal, or the spatial structure of the aquatic network well enough. For example, mass effects may hinder the detection of the effect of a local environmental alteration (e.g. organic pollution), as dispersal from a neighbouring unaltered site may allow occurrence of a pollution-intolerant species at the polluted site (Siqueira et al., 2014). Thus, the spatial structure of the aquatic network, specifically the spatial arrangement of monitored sites, may have important implications for the construction of predictive models. Such refined predictive models can also complement, for example, assessments based on time-series data (e.g. Levin et al., 2014) to help identify environmental gradients over which biodiversity increases or declines. Despite the fact that predictive models have been highly successful as biomonitoring tools (Wright et al., 1996; Furse et al., 2003), further improvements of these models may be obtained if variables describing processes other than species sorting are also included. This idea is important not only in the context of bioassessment, but also in monitoring the effect of restoration on aquatic ecosystems (Brown et al., 2011; Tonkin et al., 2014).
Using both correlative and experimental approaches
A typical problem with correlational survey-based studies is a large, shared explained variance between spatial and environmental effects on community structure (Pinel-Alloul et al., 1995; Cottenie, 2005; Mykrä et al., 2007; Landeiro et al., 2012; Al-Shami et al., 2013; Heino et al., 2014b). It is very difficult to infer whether the shared effects relate to dispersal processes or species sorting. There are at least two potential solutions to infer the relative importance of dispersal effects: (i) small-scale experiments (e.g. in replicated ponds; see Carrara et al., 2014) and (ii) natural experiments (e.g. selection of a set of study sites that are independent in terms of spatial location and environmental factors). However, these two approaches are not without problems. Unnaturally, low spatial distances among sites, for example, may plague small-scale experiments, and then, the inferences may not be necessarily applicable to most situations in nature. Natural experiments may be plagued by ‘hidden environmental effects’, as researchers may not know a priori all the environmental factors that vary among the selected study sites. Despite these potential problems, we urge researchers to use both approaches when attempting to unravel the structuring of aquatic metacommunities.
Exploring temporal aspects of metacommunity dynamics
Metacommunity studies have usually been conducted using a data set (i.e. species, environmental and spatial variables × sites) gathered at a single point in time (i.e. based on a snapshot survey). However, in systems with high variation in the levels of connectivity among the sampling units, one can expect temporal changes in the relative importance for community structure of spatial and environmental variables (Erős et al., 2012a; Fernandes et al., 2014). For instance, beta diversity is predicted to be higher in floodplain lakes during periods of low than high water level (Thomaz, Bini & Bozelli, 2007). Thus, ecologists may draw inconsistent conclusions about metacommunity organisation if a study is based on only a single point in time (Heino & Mykrä, 2008; Erős et al., 2012a). For example, in a study of rock pools on the coast of the Baltic Sea, sampled on 11 occasions over one year, Langenheder et al. (2012) found that the effects of environmental variables on a bacterial metacommunity were more frequent than those of spatial variables. However, during three periods of low beta diversity, spatial factors increased in importance. Due to the paucity of studies combining biological, spatial and environmental information from the same set of sites collected at a number of times, any generalisations would be premature. Finally, although statistical methods able to handle such complex data sets (i.e. species, environmental and spatial variables × sites at multiple times) have already been developed (Anderson & Gribble, 1998; Legendre & Legendre, 2012), surprisingly few studies have actually examined temporal changes in the roles of environmental control and spatial structuring of metacommunity composition (Heino & Mykrä, 2008; Erős et al., 2012a; Fernandes et al., 2014; Padial et al., 2014). This is certainly an area of research that deserves further studies. Such studies might borrow ideas from palaeolimnological research (e.g. Mergeay et al., 2007), and we believe that integrating approaches used in palaeolimnology with metacommunity ecology might result in fruitful new findings with implications for both basic and applied research.
Combining successful past approaches
Previous evidence and theory suggest that a promising approach to tease apart the roles of environmental and spatial processes on community organisation using survey data would consist of the following steps.
- The importance of ‘separating apples from oranges’. Instead of analysing the whole species data matrix (e.g. when using variation partitioning in the context of constrained ordination), we think that ecologists would benefit from considering a deconstructive approach based on dispersal ability-related traits (Grönroos et al., 2013; Algarte et al., 2014), traits related to habitat specialisation (Pandit et al., 2009; Székely & Langenheder, 2014), rarity (Siqueira et al., 2012a; Swan & Brown, 2014) or traits related to oviposition behaviour in insects (McCreadie & Adler, 2012; Heino & Peckarsky, 2014). Thus, different response matrices would be created based on a given trait.
- The importance of specifying different hypotheses about spatial relationship between sites. In addition to an overland distance matrix, there are other possibilities for specifying the spatial relationship among sites, and ecologists should also consider distances along the river network (i.e. purely aquatic pathways; Landeiro et al., 2012; Altermatt et al., 2013; Grönroos et al., 2013; Meier & Soininen, 2014; Soininen & Meier, 2014) and, even more promising, directional processes (Blanchet, Legendre & Borcard, 2008; Blanchet et al., 2011; Göthe et al., 2013a,b; Liu et al., 2013) when using eigenfunction spatial analyses to create spatial variables.
- The importance of identifying the nature of the pure spatial fraction in statistical models. Ecologists are aware that relevant environmental predictors are probably missing in their studies, no matter how comprehensive the list of these predictors is. Thus, a high coefficient of determination associated with spatial variables cannot be easily interpreted as indicating the role of dispersal processes (Peres-Neto & Legendre, 2010). The first step in reducing uncertainty regarding the nature of the pure spatial fraction consists, of course, in allowing for a well-chosen list of environmental predictors (Chang et al., 2013), based on previous evidences and theoretical reasoning. Even so, a high pure spatial fraction can be detected (Fig. 1) and, in this case, the use of the method developed by Diniz-Filho et al. (2012) could be useful. This is based on the analytical protocol first proposed by Sokal & Oden (1978a,b) to infer which evolutionary processes were more likely to generate spatial patterns in genetic data. Under a neutral process, allelic frequencies should be uncorrelated; however, these frequencies should exhibit similar patterns of spatial autocorrelation (Sokal & Wartenberg, 1983). Thus, Diniz-Filho et al. (2012) adapted this protocol to infer the nature of a pure spatial effect obtained with metacommunity data. Accordingly, to infer a predominant role for neutral processes, the correlations between the abundances of the species (estimated by a pure spatial model) should be (i) low, (ii) these abundances should exhibit similar patterns of spatial autocorrelation (which can be measured by calculating Manhattan distances between the spatial correlograms), and (iii) the relationship between a matrix containing the correlations between the abundances and a matrix containing the similarity between the correlograms (based on these abundances) should be non-significant.
We emphasise that the gain in understanding of a study system will depend on the combined implementation of the three recommendations outlined above [See 5.5 (i) – (iii)].
For instance, stronger insights into the role of dispersal would be reached by the following combination of results: (i) a higher spatial fraction for weaker dispersers than for stronger dispersers (see recommendation 1), (ii) especially when the spatial network is taken into account (see recommendation 2) and (iii) when the abundances of the species predicted by a spatial model exhibited similar spatial autocorrelation patterns but are unrelated to each other (see recommendation 3).
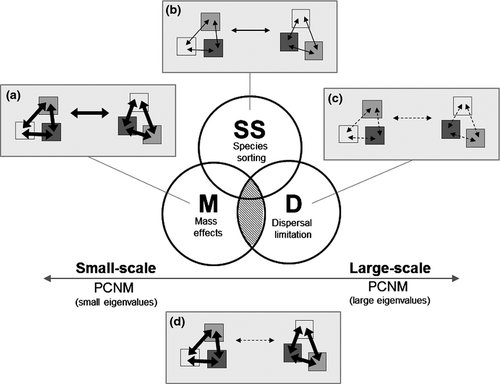
Furthermore, a potential means of inferring the importance of species sorting, mass effect and dispersal limitation for metacommunity organisation (Fig. 4) is to apply eigenfunction spatial analysis and subsequent variation partitioning in constrained ordination analysis (Borcard & Legendre, 2002; Legendre & Legendre, 2012). For example, in such an approach, the spatial eigenvectors from principal coordinates of neighbour matrices (PCNM; Borcard & Legendre, 2002) or Moran's eigenvector maps (MEM; Dray, Legendre & Peres-Neto, 2006) are related to various spatial scales. Such spatial variables at different spatial scales could be used for interpreting different spatial processes, for example, if (i) small-scale spatial variables (i.e. those with small eigenvalues; more likely to pertain to mass effects) are more important than (ii) large-scale spatial variables (i.e. those with large eigenvalues; more likely to be related to dispersal limitation), or vice versa. However, such interpretations of the relative importance of mass effects versus dispersal limitation should always be considered with regard to the spatial extent of the study and dispersal distances of the organisms in question.
Where are we now and where should we go in the future?
Metacommunity ecology in aquatic systems has progressed rapidly in recent years (Fig. 2). The largest numbers of recent true metacommunity studies have dealt with freshwater systems, whereas marine systems have thus far been studied less frequently. Lakes were also considered in the 1970s if we count studies addressing the theory of island biogeography. In contrast, empirical studies on marine metacommunities are in their infancy, and studies using similar statistical techniques to those used in studies of inland waters should also be used to increase our understanding of marine metacommunities. Although we acknowledge that data are still limited, we tentatively conclude that: (i) environmental effects in terms of species sorting prevail, especially in streams and lakes, whereas mass effects may increase in importance in open systems, such as coastal and offshore marine systems; (ii) dispersal limitation increases with increasing isolation, spatial distance between sites and spatial extent surveyed; (iii) mass effects increase in importance with decreasing distances between sites and small spatial extents surveyed and (iv) the dispersal characteristics of organisms may determine the spatial extents at which mass effects, species sorting and dispersal limitation are most effective.
We suggest that further studies on metacommunities in aquatic systems should concentrate on the following issues: (i) direct quantification of dispersal rather than using proxied; (ii) taking the spatial arrangement of aquatic networks into consideration; (iii) using various correlative and experimental approaches to disentangle the effects of mass effects, species sorting and dispersal limitation on metacommunity organisation; (iv) exploring temporal aspects of metacommunity dynamics rather than basing conclusions on a single snapshot survey and (v) applying existing approaches and statistical methods innovatively. Future studies should thus answer the question: What are the relative effects of abiotic factors, biotic interactions, priority effects and regional dispersal on metacommunity organisation? We strongly believe that beyond increasing basic ecological knowledge, future metacommunity studies will be of paramount importance to inform policymakers on how to minimise biodiversity loss and monitor water quality.
Acknowledgments
Our research on community ecology has been continuously supported by grants from the Academy of Finland, Maj and Tor Nessling Foundation and Brazilian National Council of Technological and Scientific Development. This work is part of the project ‘Scaling biodiversity in tropical and boreal streams: implications for diversity mapping and environmental assessment’ funded by grants from the Academy of Finland (no: 273557 and no: 273560) and São Paulo Research Foundation (FAPESP; no: 2013/50424-1). We appreciate comments by Alan Hildrew and two anonymous reviewers who greatly helped us to improve this paper.




