
Intraguild predation between spawning smallmouth bass (Micropterus dolomieu) and nest-raiding crayfish (Orconectes rusticus): implications for bass nesting success
Summary
- Predation on eggs is often assumed to be a primary factor influencing the reproductive success of fish. However, complex interactions between nesting fish and their egg predators may influence reproductive success in unexpected ways. The invasive rusty crayfish (Orconectes rusticus) and smallmouth bass (Micropterus dolomieu) engage in reciprocal predation during the bass spawning season; the net impact on bass reproduction is poorly understood. We hypothesise that the rates of nest abandonment and bass guarding behaviour will be positively related to crayfish abundance and that bass consumption of crayfish will mitigate energy loss due to nest guarding against crayfish.
- We tracked 62 bass nests throughout a spawning season in a north temperate lake with an established population of O. rusticus. Using video surveillance, we observed bass nest-guarding behaviour and crayfish predation on bass nests, reconstructed bass diets using faecal remains and made repeated measurements of mass and length for a subset of the parental bass.
- Results were consistent with all hypotheses. Nest abandonment rate and guarding activity were higher in areas with higher crayfish density. More than 95% of bass consumed crayfish while on the nest and bass condition factor increased during nesting, suggesting that energy consumed exceeded energy expended in defending the nest.
- The lakewide rate of smallmouth bass nest abandonment was 13%, which is lower than most published observations for this species. While high densities of nest-raiding crayfish can reduce nesting success, our limited observations indicate that consumption of crayfish may mitigate rates of nest abandonment by bass. Unexpected outcomes of complex interactions, such as reciprocal predation, should be considered when predicting population trajectories of native and invasive species.
Introduction
Many biotic and abiotic factors influence fish reproductive success, with predation regarded as the primary cause of egg mortality (Bunn, Fox & Webb, 2000). However, the actual impacts observed in the field are variable and the overall effect of predation on egg mortality and reproductive success is poorly understood (Bunn et al., 2000). Complex trophic interactions, such as intraguild predation (IGP; an interaction in which species both prey upon and compete with each other; Polis, Myers & Holt, 1989), between spawning fish and egg predators may give rise to unexpected outcomes. Ontogenetic shifts in diet are commonly responsible for creating IGP interactions; in some cases, the traditional predator–prey roles are reversed and the predator must provide protective parental care against attacking prey (Magalhães et al., 2005). Prey species can benefit from consuming the vulnerable stage of their predator (e.g. eggs or larvae) in two ways: by gaining direct sustenance (Janssen, Willemse & van der Hammen, 2003) and by decreasing future predation pressure via reduction in potential adult predators (Janssen et al., 2002). Here, we examined how IGP interactions influence the net impact of the invasive rusty crayfish (Orconectes rusticus) on the reproductive success of smallmouth bass (Micropterus dolomieu). This is one of only a few studies to assess the impact of crayfish on the reproductive success of fish that actively guard their eggs (but see Rahel, 1989; Dorn & Mittelbach, 2004) and the only such study involving bass (Micropterus spp.).
Rusty crayfish are native to the Ohio River Valley and were introduced to the upper Midwest in the 1960s, probably via anglers' bait buckets (Capelli, 1982). Rusty crayfish can affect all levels of the aquatic food web through direct and indirect interactions (Hobbs & Lodge, 2009). The effects of rusty crayfish are substantial, via competition with congeneric crayfish species (Capelli, 1982; Garvey et al., 2003), herbivory on aquatic plants (Wilson et al., 2004; Rosenthal, Stevens & Lodge, 2006) and predation on macroinvertebrates (Olsen et al., 1991; Kreps, Baldridge & Lodge, 2012). Higher crayfish densities correspond with a lower total biomass of fish (Garvey et al., 2003), which may be the result of predation on their eggs (Dorn & Wojdak, 2004; Jonas et al., 2005), destruction of aquatic plant habitat important for fish (Olsen et al., 1991; Rosenthal et al., 2006; Roth et al., 2007) and competition with fish for macroinvertebrate prey (Dorn & Mittelbach, 1999). However, smallmouth bass do not appear to be affected by higher densities of rusty crayfish (Wilson et al., 2004) and, in some cases, larger smallmouth bass grow faster where rusty crayfish are numerous (Kreps, 2009).
We would expect smallmouth bass to be most vulnerable to negative impacts from rusty crayfish during the spawning season, because of predation by crayfish on fish eggs. Smallmouth bass may abandon nests after most of the offspring have been consumed by predators (Suski et al., 2003; Hanson et al., 2007; cf. Steinhart & Lunn, 2011) or if defending the nest requires too much energy (Steinhart et al., 2005b). Male smallmouth bass provide parental care for their brood from the time that the female deposits her eggs until after the offspring have developed into free-feeding juveniles (29–36 days, Knotek & Orth, 1998). Vigilance from the male is required to keep the developing embryos aerated, to clear the nest of debris and to protect against nest predators (Hinch & Collins, 1991). Providing such care is energetically demanding and can lead to mass loss in the short term and reduce parental survival and fecundity in the long term (Steinhart et al., 2005b), potentially affecting bass population dynamics.
Interactions associated with IGP may influence the population trajectories of both native and invasive species following an introduction (Bampfylde & Lewis, 2007). While the exclusion of one species or another is often predicted by IGP theory, coexistence among species is commonly observed in natural systems due to several mechanisms (Amarasekare, 2007). Smallmouth bass (and other fish species potentially affected by rusty crayfish) are commonly managed to increase populations for harvest by anglers, and effective population management requires a better understanding of the interactions between crayfish and bass (Horan et al., 2011). Previous studies have modelled the coupled population dynamics between rusty crayfish and smallmouth bass (Drury & Lodge, 2009, 2012) and have taken many IGP interactions into account, but the values of some key parameters are poorly known. Predation by bass on crayfish has been well studied (Stein, 1977; Roth, 2001; Garvey et al., 2003), but we know very little about competition between juvenile bass and crayfish, and the impact of predation by crayfish on bass eggs. As a result, the parameters for these interactions have been simplified by necessity. For example, Horan et al. (2011) assumed the impact of crayfish on bass reproduction to be direct and linear and thus combined the egg predation and competition terms. This does not take into account the potential outcomes of reciprocal predation during spawning season, however.
Our goal was to examine the impact of rusty crayfish on the nesting success of smallmouth bass, considering both the costs (e.g. potential energy loss from energy expended on nest protection) and benefits (e.g. potential energy gain from consumption of nest-raiding crayfish) that bass encounter during spawning season. We employed a combination of field surveys, video observation and non-intrusive diet reconstruction to test the following hypotheses: (i) rates of bass nest abandonment are positively related to crayfish abundance, (ii) the frequency of nest-guarding behaviour by bass is positively related to crayfish abundance, and (iii) consumption by bass of nest-raiding crayfish mitigates energy lost to nest guarding, potentially reducing abandonment rates.
Methods
Lake Ottawa is a 218.9-ha lake in Iron Co., Michigan, U.S.A. (46.08°N, 88.77°W), which contains abundant rusty crayfish and smallmouth bass populations. Lake Ottawa has been a catch and release only fishery for smallmouth bass since 2006. Rusty crayfish were first recorded in Lake Ottawa in 1987 and have since reached high densities and become the only observed crayfish species (Rosenthal et al., 2006).
In May-June 2010, seven transects were established around the lake to capture within-lake variations in crayfish density. Transects were selected around natural clusters of bass nests; transect length ranged between 60 and 120 m and each transect contained seven-12 nests (for a lakewide total of 62 nests). This approach allowed for a lakewide estimate of nest abandonment, as well as examination of variation in abandonment rate among transects given the spatial heterogeneity of crayfish. We began marking and tracking nests at the onset of spawning (when water temperature reached 15 °C; Hanson et al., 2008). Initiation of spawning was highly synchronous, with a vast majority of nests beginning within the same 3-day period (19–21 May 2010). We visited each nest every 1–3 days to check for the presence of the male bass and assess progress of offspring development. Observations were made while diving (snorkel or SCUBA) or from the boat, depending on viewing conditions. We scored a nest as ‘successful’ if some of the offspring reached the black fry swim-up stage (Fig. 1b).

 (1)
(1) (2)
(2)To observe bass and crayfish interactions in greater detail, we monitored a group of three nests in close proximity on each of three transects using video. The three transects were chosen to represent a wide range of ambient crayfish abundance. Each video monitoring station consisted of one submersible night vision camera with 3.6 mm lens (CVS1000; Lorex Technology, Inc., Markham, Ontario, Canada) and separate LED spotlight (IR045; Clover Electronics, Cerritos, California, U.S.A.) per nest. Footage from the cameras was stored on a digital video recorder (GS2001; Gadspot Inc., City of Industry, California, U.S.A.). Nests were recorded by night and day with the camera view focussed primarily on the nest proper (Fig. 1a). The camera was kept on the nest until black fry developed. In the event of nest abandonment, the camera was moved to the closest available nest. For each nest, we observed footage from three evenings (2000 h until the following 0600 h) during the earliest stage of nest development (i.e. when offspring were either eggs or clear fry), because this is the stage when nests are most prone to abandonment (Steinhart et al., 2008), and crayfish are most active at night (Hill & Lodge, 1994). We reviewed 2-min segments every 15 min and noted (i) the number of times the bass rushed off the nest and the orientation of the rush (upward, horizontal or downward), (ii) the number of crayfish viewed in or around the nest and (iii) the number of attacks made by bass on crayfish. A rush was characterised by a sudden acceleration away from the nest; slow departures were not counted. The orientation of the rush was used to imply the motivation for leaving the nest (Steinhart et al., 2005b). A downward dive at the substratum within or surrounding the nest was assumed to represent a threat from a benthic predator; acceleration off of the nest along a horizontal or upward plane suggests a pelagic predator.
 (3)
(3)To assess crayfish density, we conducted quadrat surveys using 10–15 0.25 m2 quadrats haphazardly placed along each transect. Once, early in the nesting period, SCUBA divers recorded the number of crayfish in each size category (carapace length 10–20 mm, 20–30 mm, >30 mm) based on visual estimates. We counted only crayfish with carapace length >10 mm because crayfish under this size are unlikely to be raiding bass nests (personal obs. from video). For analyses focussed at the transect scale, we used the mean crayfish density from all quadrats for a given transect. For analyses at the video station scale, we used the mean densities for the five quadrats closest to the cluster of video-recorded nests.
Statistical analysis
At the transect scale, we tested the relationship between crayfish density and nest abandonment rate by fitting a suite of linear and nonlinear functional forms to the data. We used nonlinear least squares to determine our model parameters and determined the best model using Akaike's information criterion (AIC). To account for low sample size (n = 7), we applied the AICc correction (Burnham & Anderson, 2002). The data met the assumptions of normality and equal variance according to Shapiro–Wilk tests and visual inspection of residual plots. At the video station scale, we used Pearson's correlation tests to examine the relationships between crayfish density and two metrics of bass behaviour for the video-recorded nests.
We conducted independent sample and paired t-tests to detect changes in adult male bass condition factor between early (egg or clear fry) and late (black fry) stages of offspring development. For bass that were measured more than once, we selected at random either an early or late value for condition factor when doing the independent sample t-test. We used Pearson's correlation tests to examine the relationships between bass condition factor and (i) daily consumption of crayfish and (ii) crayfish density. To test how consumption of crayfish influenced nest abandonment, we used logistic regression of nest fate as a function of daily crayfish biomass consumed by the guarding bass. All analyses were conducted using the R Statistics Package (v 2.15.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
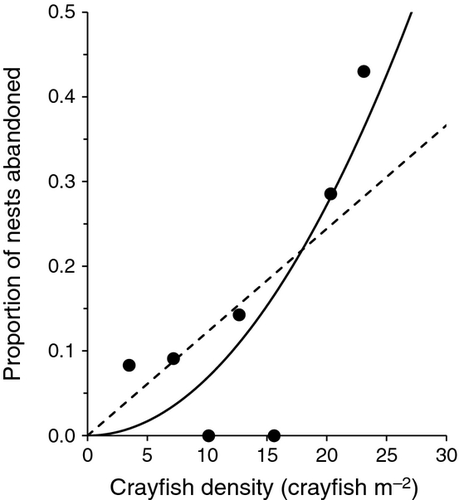
The lakewide rate of nest abandonment by bass was 13%, although rates differed greatly among the seven transects (range: 0–43%). As predicted, nest abandonment was higher along transects with higher densities of rusty crayfish; the relationship was best described by an exponential function with no intercept (r2 = 0.70), and the next best model was linear with no intercept (r2 = 0.49; Table 1). Abandonment rate increased most strongly when crayfish density exceeded 15 crayfish m−2 (Fig. 2).
| Model | k | r 2 | AICc | ΔAICc | Rank |
|---|---|---|---|---|---|
| β0 + ε | 2 | 0 | 0.04 | 8.37 | 5 |
| β1*density + ε | 2 | 0.49 | −4.73 | 3.60 | 2 |
| β2*density2 + ε | 2 | 0.70 | −8.33 | 0.00 | BEST |
| β0 + β1*density + ε | 3 | 0.54 | 1.57 | 9.91 | 6 |
| β0 + β 2*density2 + ε | 3 | 0.70 | −1.33 | 7.00 | 4 |
| β1*density + β2*density2 + ε | 3 | 0.72 | −1.82 | 6.51 | 3 |
| β0 + β1*density + β2*density2 + ε | 4 | 0.84 | 8.11 | 16.45 | 7 |
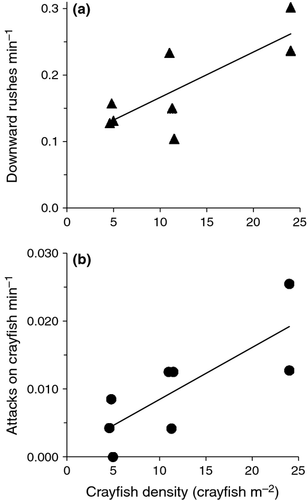
Among the successful nests that we video-recorded, there were, as expected, significant, positive linear relationships between crayfish density and: (i) downward rushes by bass per minute (r = 0.80; P = 0.017; Fig. 3a) and (ii) bass attacks on crayfish (r = 0.79; P = 0.021; Fig. 3b). Only two of the video-recorded nests were abandoned (one of which has no footage from the two evenings leading up to abandonment), thus preventing any meaningful statistical comparisons of guarding behaviour between abandoned and successful nests.
In all video segments for all nests, the only potential egg predators that we observed challenging guarded nests were rusty crayfish. Following abandonment, rusty crayfish (carapace length: range = 10–50 mm, mean ± SD = 28.3 ± 9.7 mm) filled the nest and consumed all bass eggs (Fig. 1d). Other fish species observed consuming eggs after abandonment included yellow perch (Perca flavescens), smallmouth bass and white suckers (Catostomus commersonii), although to a much lesser degree than crayfish.
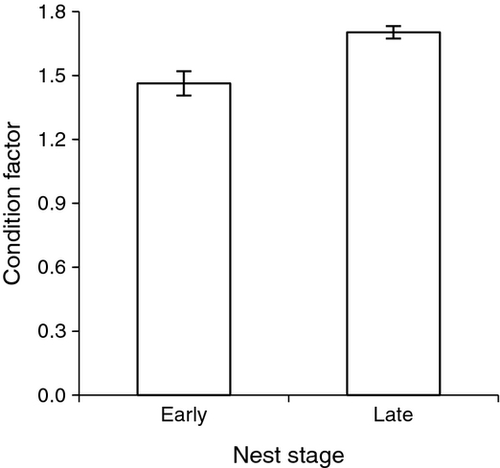
Guarding bass had a mean total length of 397 ± 35.8 (SD) mm and weighed relatively more during the later stage of nest development, as indicated by significantly higher condition factors (t-test; t15 = −3.746, P = 0.003; Fig. 4). This trend was confirmed by the subset of bass that were weighed and measured during both the early and late nest stages (one-tailed paired t-test; t5 = −2.38, P = 0.032). Mean mass gain for these repeatedly measured bass was 68.3 ± 72.8 (SD) g, or ~7%. Total length was similar for bass that abandoned nests (n = 4, mean = 387 mm, range = 328–460 mm) and successful bass (n = 17, mean = 399 mm, range 335–440 mm), but low sample size for bass that abandoned their nest rendered meaningful statistical comparison impossible. Neither daily consumption of crayfish (r = −0.41, P = 0.10) nor crayfish density (r = −0.22, P = 0.40) was significantly correlated with bass condition factor.
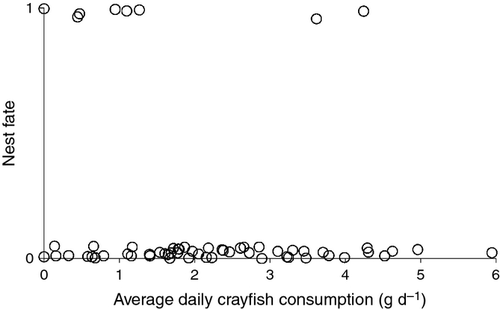
Mean daily consumption of crayfish did not influence the fate of smallmouth bass nests (logistic regression; P = 0.15; Fig. 5). Pooling all nests together across the lake, the mean daily consumption of crayfish by bass was 2.2 g (range: 0–5.9 g, SD = 1.4), equivalent to about one crayfish with 19 mm carapace length. The average size crayfish consumed was 22.8 ± 4.2 (SD) mm. We did not observe remains from any species other than crayfish in the faecal samples.
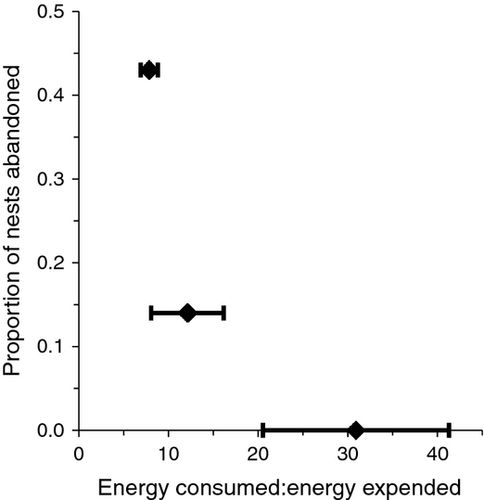
There was a negative relationship between abandonment rate and the ratio of our indices for energy consumed (i.e. daily crayfish biomass) and energy expended (i.e. number of downward rushes off nest min−1 from Fig. 3) for the three video-monitored transects (Fig. 6). Because we have only three data points for this relationship, statistical analysis of this trend is impossible, but results were consistent with the expectation that abandonment would be lower in areas with greater consumption relative to energy expended guarding the nest.
Discussion
All of our hypotheses were supported by the results, revealing that counteracting forces between reciprocal predators are at play during spawning by smallmouth bass. We demonstrated that the density of nest predators can influence the rate of nest abandonment within a single lake and provide the first assessment of smallmouth bass nesting activity and success in relation to crayfish density. The guarding bass exhibited consistently higher condition factors during the late stage of offspring development relative to early in the nest-guarding period. Thus, the bass were achieving a net positive intake of energy, despite the energetic demands of nest defence. Supplemental feeding has been shown to increase nesting success for bass (Ridgway & Shuter, 1994), and we propose that consumption of crayfish is playing this role in Lake Ottawa. The bass, which do not usually forage away from the nest while guarding, eat the potentially predatory crayfish. This energy supplement is widespread, since 95% of the nesting bass showed evidence of having taken crayfish.
Comparisons between our indices of energy consumed and energy expended by parental bass reveal that higher consumption relative to energy expended is associated with decreased rates of nest abandonment. The video-recorded transect with zero abandonment had a high level of consumption (in the top 20% compared with the rest of the guarding bass in the lake), which offsets a moderate level of energy expended. Bass from another transect with zero abandonment (not video-recorded) and in an area of moderate crayfish abundance also had relatively high levels of crayfish consumption. When nest abandonment was considered against consumption alone, the lakewide pattern was not significant. However, the bass from the two abandoned nests that exhibited higher rates of daily consumption also had higher guarding demands (as confirmed by video footage). Thus, it is important to consider both energy expended and energy consumed simultaneously. Because most bass in Lake Ottawa were consuming crayfish, we could not examine the consequence of not consuming nest predators. Thus, we looked to other published studies to make some broad comparisons.
A comparison of Lake Ottawa with lakes Erie and Opeongo (Steinhart et al., 2005b) reveals large differences in predator density, changes in the body mass of bass, and nest abandonment rate. Smallmouth bass in Lake Erie were confronted by abundant nest-raiding round gobies (Neogobius melanostomus) that were rarely consumed by bass; the energetic demands of nest guarding were apparent in the estimated 12% loss in body mass exhibited by the guarding bass (Steinhart et al., 2005b). Lake Erie bass had relatively high rates of abandonment, at 59–79%. Lake Opeongo bass had low nest predation pressure and consumed a significantly higher biomass of prey than bass from Lake Erie (Steinhart et al., 2005b). Nevertheless, there was no significant change in bass body mass and overall nest abandonment was 50% (Steinhart et al., 2005b). In Lake Ottawa, where the bass were readily consuming the abundant nest predators, the abandonment rate was considerably lower at 13%. That Lake Ottawa bass gained 7% body mass on average indicates that energy consumed exceeded energy expended. The abandonment rate in Lake Ottawa is lower than 22 of the 27 published estimates for smallmouth bass nesting success compiled by Steinhart et al. (2005a). While this comparison among studies does not take other important factors into account (e.g. storm events, angling pressure), it does demonstrate that it is possible for a lake with abundant nest predators to still have high overall nesting success.
Several previous studies found a positive relationship between bass size and nesting success (Suski & Ridgway, 2007; Steinhart et al., 2008; Gingerich & Suski, 2011). Our data did not suggest such a trend among Lake Ottawa bass, although Lake Ottawa spawning males (total length = 328–460 mm) fall in the upper end of size ranges reported in other studies (c. 275–455 mm and 280–430 mm, Steinhart et al., 2005b; 234–464 mm, Gingerich & Suski, 2011). We would expect smaller bass to be more likely to abandon their nests under the conditions in Lake Ottawa, but we did not have the opportunity to test this. However, even among these larger Lake Ottawa bass with presumably higher thresholds for abandonment, we found that abandonment rate increased with crayfish density and that higher consumption relative to activity level was associated with lower abandonment.
We believe our video surveillance methods and passive estimation of consumption provided accurate indices of parental response to natural predators. It is well documented that more intrusive methods present too many experimental artefacts that could interfere with the natural reactions of bass (Cooke et al., 2008). For example, excessive handling of nesting bass can influence abandonment (Philipp et al., 1997); hence, we relied upon faecal remains, rather than gut contents collected by gastric lavage, as a proxy for biomass consumed. Doing so introduces a conservative bias to our consumption estimates because we were unlikely to collect all faeces expelled. Under normal conditions, smallmouth bass typically consume a daily prey ration representing about 3% of their body mass (Vigg et al., 1991), but feeding during nest guarding is greatly suppressed (Hinch & Collins, 1991). Our conservative estimates from faecal remains indicate that Lake Ottawa bass are consuming 2.2 g crayfish d−1 (between 0.1 and 0.5% of bass body mass). This estimate is much lower than values based on stomach contents of nest guarding (Steinhart et al., 2005b) and non-nest guarding (Roth, 2001) adult smallmouth bass, but we believe that this is largely an artefact of our sampling method. Therefore, our reconstruction of diets based on faecal analysis is more appropriate for comparisons among individuals within this population rather than for estimating the absolute biomass consumed.
This study provides additional insights into how bass respond to natural variations in predation pressure. Previous work has considered extreme differences in nest predation pressure between two lakes (Steinhart et al., 2005b) and a gradient of predation pressure across six north temperate lakes (Gravel & Cooke, 2009). The heterogeneous distribution of a nest predator within a single lake allowed us to examine the impact of predation on both bass activity and nesting success without introducing variation from other exogenous factors. In areas of high crayfish density in Lake Ottawa, the bass exhibited more defensive behaviours, such as downward rushes at the substratum and attacks on crayfish, that resulted in physical removal from the nest or consumption. This pattern is consistent with among-lake differences in smallmouth bass anti-predatory behaviour documented by Gravel & Cooke (2009). In Lake Ottawa, the number of downward rushes min−1 in areas of the highest crayfish density was twice that of areas of low crayfish density. Bass in Lake Erie, which contains an abundance of nest-raiding round gobies, had an activity level about 2.5 times higher than bass in a lake without round gobies (Steinhart et al., 2005b). These quantifications of bass response to predation pressure bring us closer to making links between individual behaviour and community interactions (Gravel & Cooke, 2009), including the IGP interactions modelled by Horan et al. (2011).
The outcome of IGP interactions will depend upon the characteristics of the species involved. The virile crayfish (Orconectes virilis) caused nearly total reproductive failure among nesting bluegill sunfish (Lepomis macrochirus) in experimental ponds (Dorn & Mittelbach, 2004). Although sunfish are capable of consuming young-of-the-year crayfish (Roth et al., 2007), the parental fish would have been unlikely to be able to defend against, let alone consume, the adult crayfish challenging the nests. In contrast, smallmouth bass are adept predators of crayfish and can forcibly remove individuals too big to eat. This difference in vulnerability to crayfish predation could explain the greater negative effect of rusty crayfish invasion on sunfish populations relative to smallmouth bass that was observed by Wilson et al. (2004). Other factors, such as crayfish species and crayfish size, influence feeding rate on substratum-bound fish eggs (Morse, Baldridge & Sargent, 2013). The intensity of IGP therefore depends not only on the exact species involved, but also the size structures of the populations.
Counter predation by prey can lead to direct and dramatic reductions in predator density, as demonstrated between thrips and predatory mites (Janssen et al., 2002). However, for rusty crayfish and smallmouth bass, the connection between predation on bass eggs and bass recruitment remains poorly described because of the stochastic events that influence survival of juvenile bass. The rate of survival from egg to juvenile fish is highly variable, and the number of eggs is often not an accurate predictor of total young-of-the-year production in the autumn of the same year (Gillooly et al., 2000). Juvenile growth and survival may depend more on temperature and food availability (Serns, 1982). The impact of nest predation is most likely to be realised only if the number of successful spawners falls below a minimum threshold (Garvey, Wright & Marschall, 2009), as demonstrated by dense populations of bluegill sunfish limiting recruitment of predatory largemouth bass by consuming bass eggs and fry (Flickinger, Bulow & Willis, 1999). This threshold has yet to be determined for smallmouth bass, although it is likely to be very low based on evidence that a small number of spawning males can produce the majority of the young-of-the-year cohort (Gross & Kapuscinski, 1997).
Smallmouth bass are an important game fish throughout the upper Midwest and a common target for management actions to increase population size for harvesting by anglers. Removal of rusty crayfish is often motivated by the desire to reduce predation pressure on game fish, including smallmouth bass (Horan et al., 2011). Previous modelling efforts on this bass-crayfish system (Drury & Lodge, 2009; Horan et al., 2011) combine predation of crayfish on bass (via consumption of eggs) with the competition parameter, assuming the relationship between crayfish density and the impact on bass to be linear. Our results indicate rather that nest abandonment increases exponentially as crayfish density increases. Furthermore, our results suggest that consumption of crayfish at least partially mitigates the energy spent on nest defence in areas with low to medium densities of crayfish, but that the energetic demands of nest protection against the highest densities of crayfish may exceed what many bass can expend. Thus, previous realisations of the bass-crayfish IGP model (Drury & Lodge, 2009), which were acknowledged to be without good parameter estimates, may have overestimated the negative impact of crayfish on bass reproductive success. On the other hand, until estimates of the impact of competition for invertebrate food between crayfish and bass are available, the net outcome of the IGP interactions between crayfish and bass remains unknown.
Much of the previous work on smallmouth bass nesting success aims to predict the impact of endogenous and exogenous forces on rates of abandonment (e.g., Suski et al., 2003; Steinhart et al., 2008; Lunn & Steinhart, 2010). For future studies on the reproductive success of nesting fish, we recommend that complex interactions with nest predators be considered as an important biotic factor.
Acknowledgments
We are grateful to K Drury for discussions of the bass-crayfish IGP system; A Deines and M Wittmann and two anonymous reviewers for editorial comments; E Grey and C Jerde for consultation on statistical analyses; and J Bergner, E Elgin, J Morse, A Ruohomaki and L Sargent for assistance in the field. All work reported here was conducted in agreement with the University of Notre Dame's Institutional Animal Care and Use Committee protocol #11–043 and permits from the Michigan Department of Natural Resources and United States Forest Service. This work was supported by the National Science Foundation under Grant #NSF-DGE-0504495 to the GLOBES interdisciplinary training program at the University of Notre Dame, Sigma Xi Grants-in-Aid of Research program and NOAA's CSCOR program.




