
A manipulative study of macroinvertebrate grazers in Hong Kong streams: do snails compete with insects?
Summary
- Sulcospira hainanensis (Pachychilidae) is the most abundant snail in Hong Kong streams. It constitutes about 1% of total benthic macroinvertebrate abundance but, because of its relatively large size, can comprise over one-third of standing biomass. This snail may have the potential to depress periphytic algae and thereby compete for food with insect grazers.
- Possible competitive interactions were investigated by manipulating snail densities in 4 streams under different shading conditions (2 shaded, 2 unshaded). Since previous work in Hong Kong streams indicated that algal standing stocks were depleted by wet-season spates, manipulations were carried out during both the wet and dry seasons to investigate the extent of variability in interaction intensity.
- Insect colonisation on artificial substrata (tiles) was compared between treatments where wire mesh barriers limited snail access, and under control conditions of natural snail densities. The exclusion treatment reduced snail densities by about 30–50% relative to controls during both seasons. Algal densities were lower during the wet season in two streams, probably reflecting spate-induced disturbance, but snail densities remained relatively stable throughout the study.
- Strong effects of S. hainanensis on algae and insects were observed during the dry season, with higher algal biomass and insect densities (especially mayflies) in snail-exclusion treatments. Differences between treatments during the wet season were generally minor. Snails depressed algae more severely in shaded streams, but this did not result in greater increases in insect abundance in snail-exclusion treatments.
- Evidently snails did exert competitive effects on insects, but spate-induced disturbance during the wet season appeared to override this biotic interaction, in agreement with predictions of the harsh-benign hypothesis. Competition intensity was unaffected by shading conditions, perhaps because other environmental factors moderated interaction intensity in shaded streams.
Introduction
The growth and biomass of periphytic stream algae is typically governed by a combination of bottom-up factors, such as nutrients, light limitation through riparian shading and spate-induced disturbance (Elwood et al., 1981; Hart & Robinson, 1990; Feminella & Hawkins, 1995), plus grazing by herbivores (Peterson et al., 1993; Rosemond, Mulholland & Elwood, 1993; Hill, Ryon & Schilling, 1995). This top-down effect of grazers may be overridden during spates when algal biomass is reduced by washout or scouring (McAuliffe, 1984; Stevenson, 1990; Davies, Bunn & Hamilton, 2008; Yang & Dudgeon, 2010), and this moderation of biotic interactions during times of increased physical (often spate-induced) disturbance in streams is referred to as the harsh-benign hypothesis (Peckarsky, Horn & Statzner, 1990; Boulton et al., 1992). In monsoonal streams, it can lead to the seasonal alteration of abiotic and biotic influences on assemblage structure according to the prevailing flow regime (Dudgeon, 1993; Yang & Dudgeon, 2010).
Depletion of periphytic algae by dominant grazers can lead to interspecific competition for food: for example, snails in temperate streams have been shown to reduce the abundance of invertebrate grazers, especially small sedentary species, by limiting algal availability and ‘bulldozing’ interference (Cuker, 1983; Hawkins & Furnish, 1987; Harvey & Hill, 1991; Hill, 1992). These effects may be moderated or modified by other factors such as riparian shading or streambed sediment heterogeneity, but the interactions between physical and biotic controls on algal biomass, and hence competition intensity, are not fully understood (see reviews by Feminella & Hawkins, 1995; Holomuzki, Feminella & Power, 2010). In monsoonal Hong Kong at least, seasonal variations in algal standing stock (Yang, Tang & Dudgeon, 2009) and grazing intensity (Yang & Dudgeon, 2010) have already been demonstrated, and the extent of riparian shading leads to inter-stream variability in algal biomass (Yang et al., 2009) and grazer abundance (Li & Dudgeon, 2009; Yang & Dudgeon, 2009). We would thus anticipate that the importance of interspecific competition among grazers in Hong Kong streams would vary spatially and temporally.
The grazing snail Sulcospira hainanensis (Pachychilidae) is the most abundant and widespread prosobranch snail in Hong Kong streams (Dudgeon, 1982, 1989; Li & Dudgeon, 2009). Mean densities are around 80 ind. m−2, but range from 6 to 210 ind. m−2, according to shading conditions and site characteristics and, typically, make up c. 1% of total macroinvertebrate abundance (Li & Dudgeon, 2009). However, because they are relatively large, with adults (shell aperture width >1 cm) reaching 0.6 g in terms of ash-free dry mass (A. C. Y. Yeung & D. Dudgeon, unpubl. data), they can make up an average of 29% of macroinvertebrate biomass in shaded streams and 41% in unshaded streams, reaching a maximum of 75% in some unshaded sites (Li & Dudgeon, 2009). Stable isotope analyses have shown that S. hainanensis and a variety of insect grazers make extensive use of algae, as deduced from the relative contribution of periphytic algae to assimilated biomass (Li & Dudgeon, 2008; Lau, Leung & Dudgeon, 2009). Given the large size of the snail, it may influence the abundance or assemblage structure of insect grazers through asymmetrical competition (sensu Lawton & Hassell, 1981).
The overall aims of this study were to estimate the effects of interspecific competition between snails and insects in monsoonal Hong Kong streams and determine whether the intensity of competition was influenced by spate-induced disturbance during the wet season, or the degree of riparian shading, which would limit algal growth. We hypothesised that, in accordance with the harsh-benign hypothesis, competition intensity would be higher during stable, low-flow conditions in the dry season, and in more shaded streams where algal biomass would be relatively low. It was anticipated that the results would have implications for our understanding of the relative importance of biotic and abiotic factors in structuring benthic assemblages in monsoonal tropical streams.
Methods
Study sites
All four study sites are located in the mainland New Territories of the Hong Kong Special Administrative Region of the People's Republic of China (Fig. 1), which has a tropical monsoonal climate with two distinct seasons (Dudgeon & Corlett, 2004). The wet season is between May and August, when c. 70% of annual rainfall was received in 2011, and the dry season is from November to February (Hong Kong Observatory, 2012). Stream discharge shows strong seasonality, and frequent wet-season spates have a strong influence on benthic macroinvertebrate standing stocks and community structure (Leung & Dudgeon, 2011; Leung, Li & Dudgeon, 2012). Mean daily air temperature during the wet-season (July–August 2011) and dry-season (February–March 2012) study periods were 29.2 °C (range: 26.5–30.9 °C) and 17.4 °C (12.1–23.9 °C), respectively (Hong Kong Observatory, 2012). Wet-season and dry-season daily water temperature among all the study sites averaged 24.1 °C (22.4–25.9 °C) and 15.9 °C (12.1–20.0 °C), respectively. Total rainfall was 384.4 and 51.6 mm during the wet-season and dry-season study periods, respectively (Hong Kong Observatory, 2012).
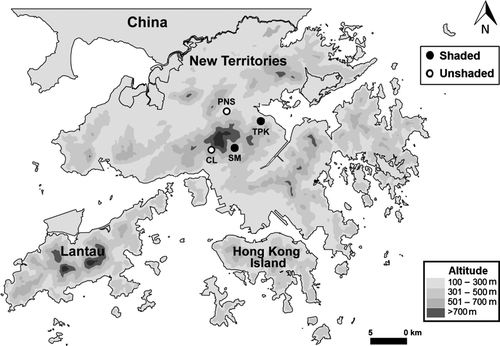
All study sites were second-order streams situated within or immediately adjacent to protected areas/nature reserves within an altitudinal range of 110–330 m above sea level. They had similar granitic parent geology with mixed streambed substrata mainly consisting of boulders and cobbles. Sites were chosen to represent a gradient of shading with two being relatively deeply shaded (>50% canopy closure, see Table 1) and two less shaded (<50% canopy closure; Table 1). Shing Mun Forest stream had a relatively higher discharge than the other streams, whereas Pak Ngau Shek stream was more enriched by nutrients due to market gardens in its catchment. All had well-oxygenated, slightly acidic waters and, with the exceptions noted above, inter-site variations in characteristics were minor (Table 1).
| Chuen Lung | Pak Ngau Shek | Tai Po Kau | Shing Mun | |||||
|---|---|---|---|---|---|---|---|---|
| Wet | Dry | Wet | Dry | Wet | Dry | Wet | Dry | |
| Site abbreviation | CL | PNS | TPK | SM | ||||
| Riparian canopy closure (%) | ||||||||
| Mid-channel | 48 | 52 | 38 | 40 | 93 | 92 | 79 | 75 |
| Bankside | 78 | 62 | 56 | 46 | 99 | 93 | 95 | 88 |
| Universal Transverse Mercator (UTM) grid reference |
50Q KK 026 796 |
50Q KK 036 841 |
50Q KK 097 823 |
50Q KK 065 796 |
||||
| Sampling/Survey date | 20/07/2011 | 16/03/2012 | 21/07/2011 | 18/03/2012 | 21/07/2011 | 18/03/2012 | 20/07/2011 | 16/03/2012 |
| Altitude of study reach (m) | 330 | 110 | 180 | 300 | ||||
| Stream order | 2nd | 2nd | 2nd | 2nd | ||||
| Wet width (m) Mean | 3.11 (1.77–5.15) | 1.51 (0.80–2.70) | 2.43 (1.50–4.82) | 1.63 (0.6–2.80) | 3.40 (0.90–5.78) | 2.71 (0.5–6.50) | 7.70 (3.60–10.47) | 6.30 (2.95–9.00) |
| Depth (m) Mean | 0.23 (0.07–0.40) | 0.14 (0.04–0.28) | 0.19 (0.09–0.45) | 0.14 (0.03–0.28) | 0.30 (0.10–0.48) | 0.23 (0.06–0.40) | 0.25 (0.07–0.43) | 0.16 (0.10–0.25) |
| Current velocity in riffles (m s−1) Mean | 0.22 (0.16–0.32) | 0.25 (0.18–0.37) | 0.26 (0.25–0.31) | 0.29 (0.27–0.31) | 0.28 (0.20–0.39) | 0.26 (0.16–0.30) | 0.37 (0.27–0.64) | 0.31 (0.26–0.39) |
| Discharge (L s−1) Mean | 43.68 (28.73–58.63) | 2.55 (1.38–3.33) | 10.62 (5.20–20.40) | 3.94 (3.03–4.46) | 20.46 (12.92–31.84) | 5.65 (1.95–9.60) | 219.45 (135.12–303.78) | 29.65 (22.40–36.60) |
| Substratum composition (%) | ||||||||
| Bedrock | 10 | 20 | 10 | 10 | ||||
| Boulder (>256 mm) | 30 | 20 | 30 | 40 | ||||
| Cobble (64–256 mm) | 30 | 35 | 30 | 25 | ||||
| Gravel (2–64 mm) | 20 | 20 | 20 | 15 | ||||
| Sand (<2 mm) | 10 | 5 | 10 | 10 | ||||
|
Mean daily stream temperature (°C) Mean |
23.8 (22.5–24.8) | 16.3 (12.6–19.6) | 24.5 (23.5–25.9) | 16.6 (13.0–20.0) | 23.7 (22.4–24.5) | 15.0 (12.1–18.1) | 24.4 (23.3–25.3) | 15.5 (12.5–18.8) |
| Conductivity (μS cm−1) | 29.6 | 51.1 | 63.4 | 83.8 | 37.9 | 45.0 | 27.3 | 38.2 |
| Dissolved oxygen (mg L−1) | 8.8 | 8.8 | 8.7 | 8.8 | 8.7 | 9.0 | 8.9 | 8.9 |
| pH | 6.8 | 6.5 | 7.2 | 6.9 | 7.0 | 6.5 | 6.6 | 6.6 |
| Ammonia N (μg L−1) Mean | 0.00 (0.00–0.00) | 17.96 (16.31–19.61) | 13.67 (11.90–16.39) | 4.51 (3.66–5.34) | ||||
| Nitrite N (μg L−1) Mean | 3.71 (3.62–3.80) | 5.73 (5.48–5.88) | 3.38 (3.32–3.41) | 3.69 (3.65–3.91) | ||||
| Nitrate N (μg L−1) Mean | 184.59 (182.66–188.66) | 850.54 (848.37–853.89) | 80.18 (79.62–80.75) | 49.51 (49.06–49.83) | ||||
| Phosphate P (μg L−1) Mean | 36.19 (34.42–39.63) | 74.61 (72.52–76.65) | 22.4 (20.47–25.19) | 21.41 (20.46–22.00) | ||||
| Land use | Shrubland, agricultural land | Plantation, agricultural land | Secondary forest | Secondary forest | ||||
- Mean values are presented (with ranges in parentheses) for other parameters.
Riparian canopy closure in mid-channel and at bankside was determined using a densiometer (Model A spherical densiometer; Forest Suppliers, Inc., Jackson, MS, U.S.A.), and five measurements were taken at equal spacing along the 20-m study reach in each stream. Altitude of each site was estimated from 1 : 20 000 topographic maps of Hong Kong. Stream water temperatures (1-h interval) were measured continuously by temperature loggers (Thermochron iButton DS1923-F5; Maxim Integrated Products, Sunnyvale, CA, U.S.A.) during the study period. Conductivity and dissolved oxygen were measured using an YSI Model 85 m (YSI Incorporated, Yellow Springs, OH, U.S.A.), and pH measured using an YSI pH 100 m (Yellow Springs Instrument Co., Yellow Springs, OH, U.S.A.). Current velocity was measured in riffles by a Swoffer Model 2100 current velocity meter (Swoffer Instruments Inc., Seattle, WA, U.S.A.). Discharge was calculated using the velocity-wetted-area method (Gordon et al., 2004) and was measured at three equally spaced cross sections along the study reach in each stream. Ammonia, nitrite, nitrate and phosphate concentrations of water samples were measured using a QuikChem 8000 flow injection analyser (Lachat Instruments, Inc., Milwaukee, WI, U.S.A.).
Experimental design
A snail inclusion–exclusion experiment was designed to investigate the effects of S. hainanensis on aquatic insects and periphyton during the wet season in 2011 and dry season in 2012. The experiments were conducted at all sites from July to August (wet) and February to March 2012 (dry). Twenty-five unglazed tiles (10.0 × 10.0 × 1.65 cm) used as standardised algal colonisation substrata (Lamberti & Resh, 1983; Sturt, Jansen & Harrison, 2011) were cleaned, ashed at 550 °C for 3 h in a muffle furnace (Thermolyne Largest Tabletop Muffle Furnace, Item 30400; Thermo Fisher Scientific Inc., Waltham, MA, U.S.A.) and then placed in each stream. Algal ‘conditioning’ of tiles was allowed to proceed for 4 weeks prior to each experiment (Lei, Lam & Hu, 2011). Twenty of these tiles were chosen randomly, and 10 of each were assigned to the snail-exclusion and snail-inclusion (control) treatments.
The exclusion treatment comprised a tile rack to hold conditioned tiles in place on top of an elevated (by 3.6 cm) platform made of stainless steel wire mesh (mesh size: 60 × 60 mm), with the rack tied to the platform by twist ties. The platform was orientated parallel to the stream, and cobbles were positioned at the upstream and downstream ends of the platform to hold it in position. One ‘conditioned’ tile was placed on the rack, on top of an ‘unconditioned’ base tile, so as to provide a substratum for insects beneath the ‘conditioned’ tile. The upper surface of the conditioned tile was thus raised 6.9 cm (i.e. 3.60 + 1.65 + 1.65 cm) above the steam bed (Fig. 2). The wire mesh platform acted as a barrier limiting snail access to the upper tile, reducing their density by about 80% in laboratory trials relative to controls. The ‘control’ snail-inclusion treatment consisted of a tile rack with a conditioned tile atop a base tile, but no elevated platform, allowing snails to crawl upon the upper tile surface. Each replicate of the inclusion treatment was positioned on the streambed about 20 cm adjacent to an exclusion replicate in a pairwise arrangement. Ten pairs were placed randomly within runs and pools (five in each microhabitat) along a 20-m reach in each stream. Deployment of the snail-inclusion member of the pair made use of natural streambed topography (Lamberti & Feminella, 2006), or repositioning of cobbles and other substrata as necessary, in order to ensure that the upper tile surfaces of each treatment were elevated to the same degree (and were equidistance from the stream surface). Each pair of tiles was positioned in locations with similar current velocities (±5 cm s−1 as measured by the Swoffer 2100 meter), so as to minimise any potential flow differences between treatments.
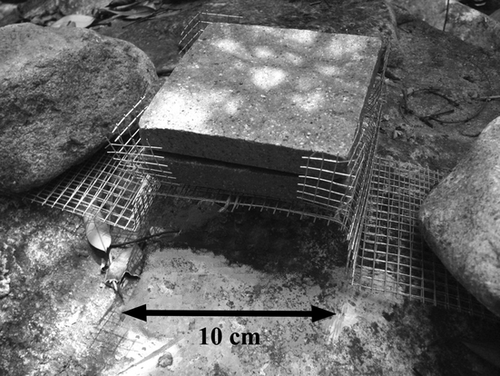
The upper ‘conditioned’ tiles were retrieved after 30 days of grazing in both seasons. During retrieval, a fine-meshed (250 μm) hand net was placed immediately downstream of the tiles to collect dislodged animals when the tile was lifted from the water. All animals on the surface and undersides of the tile and those previously dislodged into the net were washed onto a 212-μm sieve and preserved in 70% ethanol in the field. The cases of sessile insects, such as chironomid midges and net-spinning caddisflies, were removed by forceps and became part of the animal sample and were thus not included in the subsequent periphyton analysis (Lamberti & Feminella, 2006). Tiles were placed in separate, clean plastic bags and stored in a dark cool-box. All samples were taken to the laboratory within 2 h.
Upon return to the laboratory, periphyton attached on the upper surface of each tile was removed during 3 min of scouring with a hard-bristled toothbrush. Any remaining insects were removed using forceps after brushing. The resulting algal slurry was vacuum-filtered through a glass-fibre filter (Advantec© GC50, 0.5-μm pore size, Toyo Kaisha Ltd., Tokyo, Japan), and the phytopigments were extracted with 90% acetone at 4 °C in the dark for 24 h, and chlorophyll a concentrations (mg m−2, normalised for tile surface area) were calculated from the extract optical densities measured with a spectrophotometer (SMART Spectro™ Spectrophotometer; LaMotte Company, Chestertown, MD, U.S.A.). Measurements were taken prior to and after the addition of 0.1 N HCl to estimate and correct for phaeophytin a (procedures followed Axler & Owen, 1994; APHA, 1998; Thompson, Norton & Hawkins, 2004).
Insects and snails from each tile were sorted under a Leica MZ8 stereomicroscope (Leica Microsystems, Wetzlar, Germany), at 32× magnification, identified to species or morphospecies (or chironomid subfamily) and counted. Morphospecies counts were used to calculate the densities and relative abundance of functional feeding groups (FFGs) on each tile. The assignment of FFGs was based on the studies by Dudgeon (1999) and Li & Dudgeon (2008). To provide an indication of the biomass of major taxa, S. hainanensis, Ephemeroptera, non-tanypodinae chironomids, predators and ‘all other insects’ on each tile were oven-dried at 70 °C to constant weight, cooled in a desiccator, weighed using an electronic balance (Analytical balance, Item HA-202M; A&D Company Limited, Tokyo, Japan) to yield dry mass (±0.1 mg) and then ashed at 550 °C in for 3 h in a muffle furnace and reweighed to calculate ash-free dry mass (±0.1 mg).
Data analyses
To better explain the spatiotemporal variability of competition intensity, differences in algal biomass, total insect and scraper densities on snail-inclusion treatments among the four study streams were investigated by four-way nested analysis of variance (anova). Treatment, shading conditions (shaded versus unshaded), seasons (dry versus wet) and microhabitats (run versus pool) were fixed factors, while stream identity (CL, PNS, TPK and SM) was a random factor nested within shading conditions (unshaded: CL and PNS; shaded: TPK and SM). anova was followed by multiple comparisons of seasons, shading conditions and microhabitats with Student–Newman–Keuls (SNK) tests. In cases where the numbers of snail-inclusion treatments retrieved from each site were different, resulting in an unbalanced design, a Satterthwaite correction was used to compute the correct denominator degrees of freedom (d.f.), yielding non-integer d.f. in anovas.
The effectiveness of snail exclusion was measured by the natural logarithm of the ratio of S. hainanensis densities on snail-inclusion to snail-exclusion treatments. A one-way t-test (one-tailed) was used to determine whether the response ratio was significantly smaller than zero for each site, which indicated an effective exclusion of snails. The grazing and competitive effect of S. hainanensis was measured by the natural logarithm of the ratio of algal biomass (grazing), and insect taxon richness, densities and biomass (competitive) on snail-exclusion to snail-inclusion treatment. These logarithms are hereafter referred to as the ‘Grazer Impact’ index (GI) and ‘Competitor Impact’ index (CI) and are equivalent to the ‘Predator Impact’ index of Cooper, Walde & Peckarsky (1990). A GI or CI >0 indicates a grazing/competitive effect of S. hainanensis. Generalised linear models (GLiMs), using normal distributions and log-link functions, were constructed to compare the response ratio of the following dependent variables across each pair of tiles: algal biomass, insect taxon richness, and abundances and AFDM of FFGs and major taxa. Treatment, shading conditions, seasons, microhabitats (fixed factors) and stream identity (random factor nested within shading), as used in the anova, were treated as explanatory terms in the GLiMs, and the global model included them and their interactions. Each second-order interaction among factors was then tested individually in a model that included only the main effects. Interactions with P < 0.10 were included in the process of model selection. Subsequently, a backward elimination of non-significant factors (P > 0.05) was performed beginning with the global model. Selected model sets incorporated all the main factors unless such models did not have a significantly better fit to the data than an intercept-only (null) model, as inferred from an omnibus test (O'Connell, 2006). Backward elimination of factors continued until the omnibus test for a simpler model was passed (Burnham & Anderson, 2002). The Wald chi-square statistic was used to determine the significance of each explanatory term in the model. A more rigorous α = 0.01 was used to reduce the likelihood of a Type-I error, in view of the large number of tests performed. Whenever GLiMs revealed significant main effects for dependent variables, Fisher's least significant difference (LSD) multiple comparison tests were undertaken. The estimated marginal means (EMM) and associated 95% Wald confidence intervals from the top-ranked model were generated for each level of the main factors. When the lower confidence interval exceeded 0, EMM was positive and significant; when the upper confidence interval was below 0, then EMM was negative and significant. All parametric tests were undertaken with spss 17.0 (SPSS Inc., Chicago, IL, U.S.A.) at α = 0.05, unless otherwise specified.
Non-metric multidimensional scaling ordination was used to investigate insect assemblage composition between treatments in both seasons at all sites. Permutational multivariate anova (permanova) was undertaken to describe differences in benthic assemblage structure attributable to the presence of snails using the Bray–Curtis similarity matrix with five fixed factors: snail inclusion versus exclusion, seasons, shading conditions, microhabitats and sites (nested within shading conditions). Log(x + 1) transformations were applied to down-weight the effects of abundant taxa on the analyses. All the ordinations and associated multivariate analyses were performed using primer v6 with permanova + extension (PRIMER-E Ltd., Plymouth, U.K.).
Results
Overall spatiotemporal variability in algal biomass, insect abundance and biomass
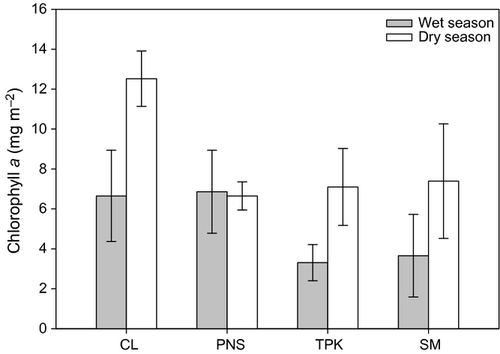
Comparisons of ‘background’ spatiotemporal variability of algal and insect abundance on the snail-inclusion tiles (only) revealed that, overall, algal biomass was similar among seasons (Table 2), but was approximately double during the dry season at two sites (CL: 90% higher; TPK: 110% higher; Fig. 3), as shown by two-sample t-tests (CL: t = 2.94, d.f. = 15, P < 0.05; TPK: t = 2.30; d.f. = 16, P < 0.05). Algal biomass differed significantly across shading conditions (Table 2) and was more than 70% higher at the two unshaded sites (SNK test, P < 0.05; Fig. 3). Total insect abundance and biomass and scraper abundance were unaffected by season and shading conditions (Table 2).
| Algal biomass (mg m−2) | Insect abundance (ind. m−2) | Scraper abundance (ind. m−2) | Insect biomass (mg AFDM m−2) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | d.f. | P | F | d.f. | P | F | d.f. | P | F | d.f. | P | |
| Season | 3.86 | 1, 2.001 | 0.189 | 14.95 | 1, 2.001 | 0.061 | 10.95 | 1, 2.002 | 0.080 | 3.75 | 1, 2.001 | 0.193 |
| Shading condition | 42.27 | 1, 2.006 | < 0.05 | 8.43 | 1, 2.001 | 0.101 | 1.95 | 1, 2.000 | 0.298 | 0.20 | 1, 2.000 | 0.700 |
- Other main effects (stream identity nested within shading condition and microhabitat) were insignificant and thus have not been presented.
Effects of snails on algae
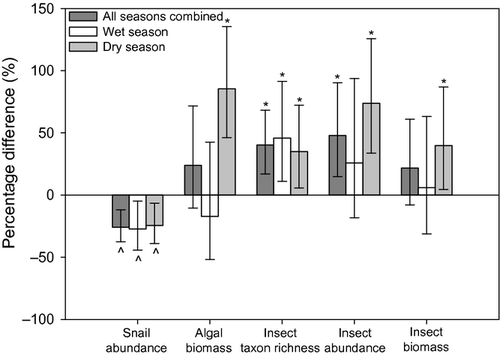
Despite some spate-induced disturbance of one or more of the tiles in each pair at some sites (especially during the wet season), there were at least six complete replicate pairs at any site in every season. One-sample t-tests confirmed that snail densities were significantly lower (28–53% reduction) in snail-exclusion treatments at all sites during both seasons, except at SM during the wet season where the reduction (17%) was insignificant. Algal biomass combined across all sites was about 85% greater in exclusion treatments during the dry season, but snails had no effect on algae during the wet season (Fig. 4). Overall, the grazing effects of snails were only evident in shaded streams (Wald chi-square: 10.40; d.f. = 1; P < 0.01) where algal biomass was about 80% higher on exclusion treatments.
Effects of snails on benthic insects
Taxon richness of insects was generally over one-third higher in snail-exclusion treatments (Fig. 4). However, although significant, the difference in the mean number of taxa between treatments at any one site (1.9–4.4) was quite small. There was an overall tendency for total insect densities, densities of FFGs and major taxa to be higher in exclusion treatments, reflected in the positive values of their respective CIs (Table S1). These increases were significant for mayflies (about 60% higher), including baetids during both seasons (40–90% higher), heptageniids (40% higher) during the wet season and leptophlebiids (Choroterpes sp. and Habrophlebiodes gilliesi: about 30% higher) during the dry season. Mean densities of non-tanypodinae chironomids on exclusion treatments increased by about 90% in the dry season, but did not differ from those on inclusion treatments in the wet season, and these animals had the highest range of variation of CI of any group (Table S1). There were also higher densities and biomasses of predatory insects on exclusion treatments during the wet season (Table S1), mainly attributable to tanypodinae chironomids at CL and PNS, and damselflies (Rhinocypha perforata and Euphaea decorata) at TPK.
Total insect biomass was about 40% higher on exclusion treatments during the dry season, and the extent of increases in mayfly and non-tanypodinae chironomid biomass (20%) was less than those in their respective densities (Table S1). The effects of snails on the biomass of all insects and major taxa did not differ significantly among shading conditions and microhabitats.
Effects of snails on benthic insect assemblage structure
Five-way permanova results showed that benthic insect assemblage structure differed significantly among seasons (Pseudo-F1,104 = 9.30, P < 0.01), shading conditions (Pseudo-F1,104 = 15.40, P < 0.01) and sites nested within shading conditions (Pseudo-F2,104 = 2.84, P < 0.01). However no significant differences in assemblage structure were detected between snail-exclusion and snail-inclusion treatments (Pseudo-F1,104 = 0.64, P = 0.67; Fig. S1a). Treatment effects were also insignificant when 4-way permanova was performed for insect assemblages in each season (Fig. S1b,c).
Discussion
Algal biomass showed significant seasonal variation in only two of the study streams, where it was approximately double that during the wet season. This was contrary to the anticipation of a consistent wet-season-low and dry-season-high pattern of algal standing stocks driven by spate-induced disturbance (McAuliffe, 1984; Stevenson, 1990; Davies et al., 2008; Yang & Dudgeon, 2010) although, as predicted, algal abundance was higher at unshaded sites. However, snail and scraper densities, and hence grazing pressure, were similar between seasons at all sites. These background data suggest that the intensity of competition between snails and insects for algae in the study streams was less likely to be influenced by seasonal changes in competitor abundance and largely driven by the effects of spates and/or the site-specific extent of shading and light limitation on algae.
Our experimental results indicated that S. hainanensis exerted overall strong effects on algae and benthic insects during the dry season. A 70% increase in insect densities, mainly mayflies and non-tanypodinae chironomids, associated with a 40% increase in biomass, was observed on snail-exclusion treatments in the dry season, but the differences between treatments in the wet season were minor, as were variations in CI across shading conditions and microhabitats. The ordination analysis indicated that, irrespective of dry-season increases in insect abundance in snail-exclusion treatments, snails had negligible effects on assemblage structure, when compared with those caused by the variability of seasons, shading conditions and sites. This may have been attributable, in part, to the efficacy of snail-exclusion treatments, which generally reduced snail densities by no more than half. This is less than some other studies demonstrating strong competitive effects, where densities of snails were reduced by 70–100% in exclusion treatments (Hawkins & Furnish, 1987; Harvey & Hill, 1991).
There were at least six replicates per site per season in this manipulative experiment, and so the statistical power (sensu Self & Mauritsen, 1988) for analyses of CI was reasonably high (usually above 0.8) in both seasons; thus, the seasonal differences in reduction in the competitive effect of snails appears to be ‘real’ result. Taxon-specific decreases in insect densities, especially mayflies and non-tanypodinae chironomids, were probably due to exploitative competition for algae with snails. Taxon-specific changes in grazers to the manipulation of primary consumer densities have been widely reported (Hawkins & Furnish, 1987; Harvey & Hill, 1991; Kohler, 1992), with effects that include mobile mayflies moving to locations with higher algal densities where competitors may be absent (Hart, 1985; Kohler, 1992). Positive responses to snail reductions in the present study were also detected for predatory tanypod chironomids, which may have been a result of them tracking increases in their chironomid prey, as suggested by studies of chironomid populations (Fahy, 1975; Vodopich & Cowell, 1984). The lack of any difference in algal biomass between treatments in the wet season indicated that exploitative competition was not apparent, and a lack of any effect of snail exclusion on sessile tube-dwelling chironomids suggests that competition for space (via ‘bulldozing’ interference competition) did not occur, although it has been implicated in other studies (cf. Cuker, 1983; Hawkins & Furnish, 1987).
Strong top-down control of algae by S. hainanensis grazing was comparable to that reported for grazers in temperate streams (Lamberti et al., 1987; Steinman et al., 1987; Rosemond et al., 1993) where such effects can persist throughout the year (caddisflies: Kohler, 1992; snails: Rosemond, 1994). However, these studies were conducted in hydrologically stable conditions, and wet-season spates in Hong Kong appeared to cause a reduction in grazing and competition intensity in CL and TPK. We do not know why the effects of spates were apparently site specific, but other abiotic factors, such as higher nutrient availability (in PNS) and stream temperature (in PNS and SM), might have enhanced algal productivity (DeNicola, 1996; Dubé, Culp & Scrimgeour, 1997) and modulated the effects of snail grazing at these sites. Nonetheless, similar effects of spates over-riding grazing pressure have been observed for algivorous fish in Hong Kong and elsewhere (Pringle & Hamazaki, 1997; Yang & Dudgeon, 2010). The competitive effects of snails on insects were also suppressed during the wet season, in accordance with the harsh-benign hypothesis that predicts a reduction in the significance of biotic interactions (i.e. competition) when conditions are disturbed (Peckarsky et al., 1990). Lowered grazing pressure and reduced competition for algae might also be attributed to increased habitat availability, as indicated by greater wetted width and depth of streams during the wet season and a consequential reduction of localised crowding effects (Cattaneo & Mousseau, 1995; Covich, Crowl & Scatena, 2003; Leberfinger, Bohman & Herrmann, 2010). However, competitor density did not change between seasons, and thus, any reduction in the influence of snails on algae and insects during the wet season was unlikely to be a result of increased habitat availability since it did not lead to lower population densities of consumers.
Although S. hainanensis depleted dry-season biomass of algae more substantially in shaded streams than at unshaded sites, this did not lead to any increase in competition effects on insects, which contradicts our initial hypothesis. Similarly, competition effects of the prosobranch snail Juga silicula on most insects in U.S. streams tended to be more apparent under unshaded conditions, and abiotic disturbance was proposed to have overridden the effects of shade on competition intensity (Hawkins & Furnish, 1987). Apparently, food limitation resulting from heavy shade may not necessarily predispose herbivores to more intense competition in streams that experience variability in disturbance regime or other abiotic factors.
The present study addressed the spatiotemporal variability of snail-insect competition in tropical Hong Kong streams. The top-down control by S. hainanensis of algae and its competitive effects on insects were significant only during the dry season, when spate-induced disturbance was minimal. This accords with the predictions of the harsh-benign hypothesis (Peckarsky et al., 1990) and agrees with studies showing increases in the intensity of predation and grazing effects by fish during the dry season in Hong Kong streams (Dudgeon, 1993; Yang & Dudgeon, 2010). Our results demonstrated the importance of snail-insect competition during base-flow periods during the dry season, despite the absence of any marked influence of snails upon assemblage structure, and reflected spatiotemporal variability in disturbance and other site-specific abiotic factors such as shading.
Acknowledgments
The authors thank John Bacon-Shone for his advice on the statistical analyses and Colin Townsend and two anonymous reviewers for helpful comments on the manuscript. We are also grateful to Lily Ng for her technical assistance, Jessie Lai for her help in water analysis, and to Hiromi Uno, Ivan Tse, Bill Ho, Veta Wang and Samuel Wang for their field support. Alex C.Y. Yeung was supported by a postgraduate studentship from the University of Hong Kong.




