
Strong spatial differentiation of N and P deficiency, primary productivity and community composition between Nyanza Gulf and Lake Victoria (Kenya, East Africa) and the implications for nutrient management
Summary
- Study of phytoplankton nutrient status, biomass, productivity and species composition was carried out between March 2005 and March 2006, along a transect between north-eastern open Lake Victoria and the large, shallow Nyanza Gulf in order to examine how the terrestrial run-off can influence phytoplankton community and nutrient status and determine whether nutrient management of catchment run-off has the potential to control the algal blooms in the gulf.
- Hydrological and nutrient differences between the open lake and the gulf create a transition from P deficiency for phytoplankton within the gulf to nitrogen deficiency in open lake. The shallow and turbid gulf was continuously dominated by non-nitrogen-fixing filamentous and chroococcale colonial cyanobacteria, but seasonal stratification and deeper mixing depth in the open lake favoured diazotrophic cyanobacteria and diatoms.
- Seston ratios and metabolic nutrient assays indicated the gulf to be sufficiently phosphorus deficient to impose P limitation on phytoplankton growth and biomass. In contrast, the open lake is not P deficient and is more likely to experience N deficiency that favours diazotrophic cyanobacteria. Because of high turbidity in the gulf, the euphotic zone is very shallow, limiting integral primary productivity compared to the less turbid open lake; high PAR extinction may also favour Microcystis blooms in the gulf.
- Increased P loading into the gulf may translate to higher algal biomass, mainly of the bloom-forming and potentially toxic cyanobacteria, and therefore, reduction in P loading into the gulf should be a management priority. However, a review of historical data indicates that the greatest change in water quality in the gulf is increased turbidity that reduces light availability and may limit algal growth more than P deficiency in years of high rainfall and river discharge.
Introduction
Lake Victoria has experienced major ecological changes in the past century, including increases to phytoplankton biomass and integral photosynthesis (Mugidde, 1993; Silsbe et al., 2006), increases in total phosphorus (P) concentrations accompanied by depletion in dissolved Si (required for diatom growth) (Hecky, 1993; Verschuren et al., 2002), changes in phytoplankton assemblages with the bloom-forming diazotrophic cyanobacteria and thin needle-like diatoms of the genus Nitzschia replacing the more heavily silicified chain-forming centric diatom Aulacoseira as the dominant taxa in the phytoplankton (Kling, Mugidde & Hecky, 2001; Guildford et al., 2003), and changes in the consumer community including a major loss of endemic species as well as a dramatic increase in fish yields (Kolding et al., 2008). Hypothesised causes of changes in the phytoplankton community include increased nutrient (mainly phosphorus) loading from surface run-off and atmospheric deposition resulting from increased human population and associated land use for agriculture (Verschuren et al., 2002; Tamatamah, Hecky & Duthie, 2005; Hecky et al., 2010), increased water column stability due to regional warming with consequent deoxygenation of deep waters (Hecky et al., 1994; Kolding et al., 2008) and increased internal phosphorus loading as hypolimnetic deoxygenation caused increased P release from the sediments and also enhanced denitrification (Hecky et al., 1996, 2010). These physical and chemical changes were accompanied by silica depletion (Hecky et al., 2010), and together, these changes have favoured the proliferation of nitrogen-fixing cyanobacterial species (Kling et al., 2001), especially in the open Lake Victoria (Hecky et al., 2010).
The most complete historical set of phytoplankton and nutrient observations on Lake Victoria are for the open lake (Talling, 1965, 1966). These open lake data are invaluable for assessing changes in this great lake over time, but observations of only the open lake can make the identification of drivers of change difficult because the open lake phytoplankton responses integrate all nutrient and physical changes to the lake (e.g. P concentrations may change because of increased internal loading associated with increased anoxia or because external inputs have increased or both). Nyanza Gulf (also known as Winam or Kavirondo Gulf) in Kenya is a large, shallow, nearly completely enclosed gulf that receives riverine inputs from some of the most highly populated and intensively cultivated land in the Lake Victoria basin, including large tea and coffee plantations as well as smaller family farms. These high inputs to the gulf have previously raised concern, not only about the impacts on Nyanza Gulf but also on Lake Victoria proper (Calamari, Akech & Ochumba, 1995). Nyanza Gulf provides a very distinct limnological environment (Gikuma-Njuru & Hecky, 2005), which has experienced the same enhanced terrestrial inputs that the open lake has received but, because of its shallow depths, the gulf has not suffered the changes in stratification and prolonged deep water anoxia observed in the open lake (Hecky et al., 1994). Nyanza Gulf therefore provides a unique opportunity to examine how increased loading, primarily from terrestrial run-off, can influence the phytoplankton community and primary productivity of receiving waters.
Gikuma-Njuru et al. (2013) have previously described and modelled nutrient exchange between the gulf and the open lake, demonstrating strong differences in nutrient concentrations and relative availability of nutrients. The objective of the current study was to examine how the terrestrial run-off that dominates nutrient loading in the Nyanza Gulf can influence nutrient and physical conditions and affect phytoplankton nutrient status, taxonomic composition, biomass and productivity in Nyanza Gulf compared with Lake Victoria proper. This contrast between the gulf and open lake is based on a combination of physical and nutrient conditions and offers the opportunity to test hypotheses on the role of increased nutrient availability and water column stability in the phytoplankton changes that have occurred in Lake Victoria as well as Nyanza Gulf. A second objective was to determine whether nutrient management of catchment run-off has the potential to control the algal blooms, which are now a feature of the gulf (Ochumba & Kibaara, 1989), including blooms of toxic cyanobacteria (Sitoki, Kurmayer & Rott, 2012).
Methods
Study area
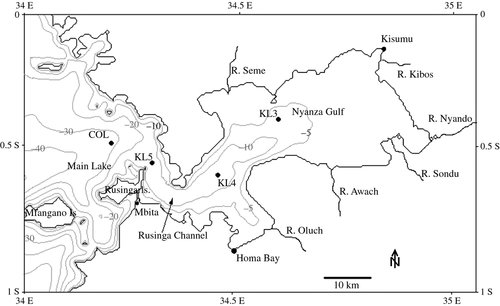
The study in north-eastern Lake Victoria compared the open lake basin in Kenya with the Nyanza Gulf (Fig. 1). Physical, chemical and biogeochemical measurements (Lung'ayia et al. 2001; Gikuma-Njuru & Hecky, 2005; Gikuma-Njuru, Hecky & Guildford, 2010) have demonstrated that the interconnecting Rusinga Channel effectively reduces interchange between the open lake basin of Lake Victoria (total surface area: 68 800 km2, mean depth: 40 m, catchment area: 193 000 km2) and the large and shallow Nyanza Gulf (surface area: 1333 km2, mean depth: 4.6 m, catchment area: 11 994 km2; Fig. 1, Table 1). The catchment-area-to-lake-area ratio of the gulf is more than three times that of lake (9 and 2.8, respectively). Nyanza Gulf receives substantial river inflows (Fig. 1) from several tributaries (total: 2.2 km3 year−1; over 10% of all tributary inflows to Lake Victoria), that, together with industrial and municipal effluent from Kisumu City, contribute sediment and nutrient loading into the gulf, resulting in marked water quality differences between the gulf and the open lake (Gikuma-Njuru et al., 2013). Compared to the lake, which has a water turnover time of over 100 years (Bootsma & Hecky, 2003) based on riverine inflows, the turnover time of Nyanza Gulf is much shorter, approximately 3 years, because of the strong river inputs and its small volume. Compared to the open lake, the gulf has relatively high turbidity, high concentrations of dissolved reactive silica (DRSi), total nitrogen (TN) and particulate organic carbon, nitrogen and phosphorus (PC, PN and PP). Remarkably, the gulf and the open lake have been found to have similar concentrations of TP, while the lake actually contributes soluble reactive phosphorus (SRP) to the gulf by exchange through Rusinga Channel (Gikuma-Njuru & Hecky, 2005; Gikuma-Njuru et al., 2013). The shallow gulf mixes to its bottom daily, whereas the much deeper open lake experiences prolonged seasonal stratification, but has a greater mixing depth (>15 m) than the gulf, even when stratified (Table 1).
 based on measurements taken between March 2005 and March 2006; Zmax, maximum depth; Zmix, mixing depth (effectively bottom depth except at COL which seasonally stratifies); Zeu, euphotic depth as 1% surface PAR; and
based on measurements taken between March 2005 and March 2006; Zmax, maximum depth; Zmix, mixing depth (effectively bottom depth except at COL which seasonally stratifies); Zeu, euphotic depth as 1% surface PAR; and  , mean irradiance over 24-h period
, mean irradiance over 24-h period| KL3 | KL4 | KL5 | COL | |
|---|---|---|---|---|
| Zmax (m) | 5.3 | 12.1 | 21.8 | 34.1 |
| Zmix (m) | 5.0–5.5 | 11.8–12.4 | 20.7–22.9 | 15–34 |
| Zeu (m) | 2.08 | 2.72 | 5.73 | 8.20 |
| Zeu/Zmix | 0.43 | 0.25 | 0.25 | 0.28 |
| Secchi depth (m) | 0.6 | 1.0 | 1.5 | 1.9 |
| Kd (m−1) | 1.2–3.4 | 1.1–2.7 | 0.5–1.2 | 0.4–0.8 |
 (μmol m−2 s−1) (μmol m−2 s−1) |
52.2 | 33.8 | 36.9 | 39.1 |
Field sampling and analysis
Sampling for nutrient status indicators, phytoplankton community structure, biomass and photosynthesis and stable isotopes analysis was carried out monthly between March 2005 and March 2006 (except May) at two stations each in the Nyanza Gulf (KL3 and KL4) and the open lake (KL5 and COL) (Fig. 1). Microscopic analysis of phytoplankton community structure and phytoplankton photosynthesis as well as metabolic nutrient status assays was only carried out between November 2005 and March 2006. Sampling was performed between 09:00 hours and 14:00 hours. Water samples were collected from the surface mixed layer (at approximately the Secchi depth) using a 5-L Van Dorn sampler and immediately filtered through pre-ignited GFF filters for the eventual analysis of chlorophyll a, particulate phosphorus, nitrogen and carbon (PP, PN and PC) and TP. Additionally, a subsample was passed through pre-ignited quartz fibre filters for the analysis of 13C and 15N isotope fractions (δ13C and δ15N) in particulate matter; the filtrate was used for the analysis of soluble fractions, namely SRP, nitrate (NO3-N), ammonium (NH4-N), nitrite (NO2-N) and DRSi. The filters and filtrate samples were kept under ice during transportation to the laboratory, where chlorophyll a and dissolved nutrients were analysed immediately. Chlorophyll a was extracted in the dark for 16 h using 90% ethanol and measured using a spectrophotometer (Wetzel & Likens, 1991). The PP, TP and dissolved nutrients were analysed according to methods described in APHA (1995). Suspended sediment (SS) concentration was estimated by filtering a known sample volume through pre-weighed GFF filters and then weighing after oven-drying (105 °C) for 16 h. Particulate carbon and nitrogen (PC and PN) were analysed using a Carbon–Nitrogen Analyzer (Model CE 440 Elemental Analyzer; Exeter Analytical, Inc.) at the University of Waterloo (Canada). The stable isotope samples were analysed at the Environmental Isotope Laboratory at the University of Waterloo using a Micromass VG-Isochrom Continuous Flow Isotope Ratio Mass Spectrometer (CF-IRMS) and PeeDee belemnite and ambient air nitrogen gas as standards for δ13C and δ15N, respectively.
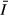 ) was calculated as follows:
) was calculated as follows:

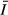 is the daily (24 h) mean irradiance that a freely circulating algal cell would receive within the mixed layer (Guildford et al., 2000).
is the daily (24 h) mean irradiance that a freely circulating algal cell would receive within the mixed layer (Guildford et al., 2000).Samples for the analysis of phytoplankton biomass and composition were preserved by adding 1% Lugol's iodine solution with further addition of few drops of formaldehyde (1%). Subsamples were analysed using a standard inverted microscope (Leitz Diavert), and biomass estimates were made as described for other areas of Lake Victoria in Kling et al. (2001). Phytoplankton productivity was measured using light–dark oxygen change after incubating lake water in a series of 150-mL bottles along a light gradient in an insulated shipboard incubator. Dissolved oxygen concentration in the initial and incubated samples was measured using modified Winkler titration (Wetzel & Likens, 1991). PAR at positions of exposure in the incubator was measured using a Li COR spherical probe, and the values were used to calculate photosynthetic parameters αB (the initial slope of the photosynthesis–light response curve expressed per unit chlorophyll), PBM (the maximum rate of photosynthesis per unit chlorophyll) and EK (the irradiance at the onset of light-saturated photosynthesis). Areal daily phytoplankton productivity was estimated from photosynthetic parameters (αB and PBM), phytoplankton biomass (chlorophyll a) and light attenuation coefficient (kd) using a modified Fee Model (Silsbe, 2004), which also calculates the photosynthetic parameters from the light response measurements in the incubator.
Metabolic nutrient status assays [P-debt, N-debt and alkaline phosphatase activity (APA)] were carried out in the field immediately after sampling. N-debt and P-debt were calculated as the amount of N and P, respectively, removed per unit of chlorophyll a after incubating a nutrient-supplemented sample for 24 h (Healey, 1977, 1978; Guildford et al., 2003) and is based on the principal that an algal population growing under a nutrient (N, P)-deficient environment takes up more nutrient than when the nutrient is sufficiently available to allow maximum growth rates (Healey, 1978; Healey & Hendzel, 1980). The P-debt assay was carried out in triplicate by adding potassium dihydrogen phosphate (KH2PO4) solution into a 100-mL water sample to achieve a final concentration of about 4.8 μM P and then incubating in the dark for 24 h. Orthophosphate concentration, before and after incubation, was determined and P-debt was taken as the amount of P uptake per unit chlorophyll a during the period of incubation. N-debt was determined following the same procedure as P-debt except that ammonium chloride (NH4Cl) solution was added (final concentration after the supplement of about 8.6 μm N).
Alkaline phosphatase is an inducible enzyme normally produced when many algal species are P deficient and acts to hydrolyse P bound to soluble organic compounds, thereby making it available to the phytoplankton; therefore, APA is higher in P-deficient cells (Healey, 1978; Rose & Axler, 1998). APA was measured fluorometrically using o-methyl fluorescein phosphate as the experimental substrate, following the method of Healey & Hendzel (1979). Total APA in the unfiltered sample and soluble APA in the filtered sampled (0.2-μm filter) were determined separately, and the difference between them was taken as the particulate APA. In addition to metabolic nutrient status indicators, seston atomic ratios (C : P, N : P and C : N) and carbon-to-chlorophyll a ratio (C : Chl) were sampled monthly between March 2005 and March 2006 and used as indicators of nutrient status (Healey & Hendzel, 1979). C : P and N : P can be indicative of P deficiency, C : N of N deficiency, while C : Chl can be a general indicator of nutrient deficiency (Healey & Hendzel, 1979, 1980).
Results
Physical and water quality conditions
The depths of the sampling stations increased from the gulf to the open lake, from 5.3 m at KL3 to more than 34 m at COL (Table 1). The shallower gulf water column mixes completely to the station depth daily, and therefore, the mixing depth was effectively the station depth. Station KL5 also mixed completely most days but it often exhibited diurnal stratification (data not shown) due to its more protected location compared to COL (Fig. 1). In the open lake at our deepest station COL, seasonal stratification resulted in varied mixing depths, from 15 m, the shallowest thermocline observed, to 34 m, the maximum depth at the site. Euphotic depth was lowest in the gulf (mean: 2.1 m) and increased along the study transect to 8.2 m in the open lake. The ratio of the euphotic depth to mixing depth (Zeu/Zmix) was always <1, but the shallower gulf station had the highest ratio because of its restricted mixing depth (Fig. 1 and Table 1).
Mean values for water quality parameters at the stations are given in Table 2. The concentration of suspended solids (SS) and water turbidity were highest in the gulf, whereas the open lake stations had lower SS values and higher transparency (Kd). The vertical extinction coefficient for PAR, Kd, was higher in the gulf (1.1–3.4 m−1) but was substantially lower (0.4–0.8 m−1) in the open lake (Fig. 2). Despite the higher transparency, however, mean irradiance (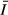 ) was lower in the open lake (Table 1) because of its greater mixing depth.
) was lower in the open lake (Table 1) because of its greater mixing depth.
| KL3 | KL4 | KL5 | COL | |
|---|---|---|---|---|
| SS (mg L−1) | 12.6 (0.6) | 10.7 (0.4) | 5.1 (0.6) | 3.5 (0.4) |
| Turbidity (NTU) | 17.6 (0.2) | 11.7 (1.2) | 4.4 (0.3) | 3.2 (0.4) |
| Temperature (°C) | 26.13 (0.1) | 26.79 (0.2) | 26.55 (0.1) | 26.00 (0.1) |
| pH | 8.4 (0.1) | 8.7 (0.1) | 8.8 (0.1) | 8.7 (0.1) |
| Alkalinity (meq L−1) | 1.3 | 1.4 | 1.0 | 0.8 |
| DO (mg L−1) | 7.9 (0.1) | 8.0 (0.2) | 7.8 (0.1) | 7.2 (0.1) |
| DRSi (μm) | 160 (5) | 157 (4) | 37 (2) | 27 (2) |
| TP (μm) | 3.6 (0.2) | 3.3 (0.1) | 3.5 (0.1) | 3.1 (0.1) |
| SRP (μm) | 0.9 (0.1) | 0.8 (0.1) | 0.9 (0.0) | 1.5 (0.1) |
| PP (μm) | 1.2 (0.0) | 1.1 (0.1) | 1.2 (0.1) | 0.8 (0.1) |
| TN (μm) | 83 (3.6) | 74 (2.7) | 45 (1.9) | 40 (2.9) |
| TN : TP | 20.7 (1.6) | 21.2 (1.0) | 16.6 (0.6) | 14.7 (1.0) |
| DIN (μm) | 6.8 (0.3) | 12.3 (0.7) | 7.0 (0.3) | 5.5 (0.2) |
| PN (μm) | 31.8 (1.2) | 21.5 (2.1) | 18.5 (1.5) | 13.8 (1.3) |
| PC (μm) | 218 (17) | 145 (14) | 118 (10) | 88 (7) |
| Chlorophyll (μg L−1) | 21.2 (0.9) | 18.5 (0.7) | 21.7 (1.1) | 11.1 (0.6) |
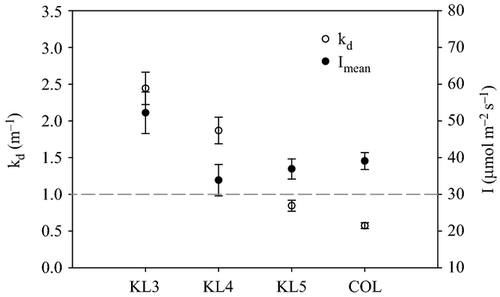
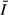 along the study transect from measurements taken monthly between March 2005 and March 2006. The dashed line is the light level below which Hecky & Guildford (1984) found phytoplankton to be strongly light limited.
along the study transect from measurements taken monthly between March 2005 and March 2006. The dashed line is the light level below which Hecky & Guildford (1984) found phytoplankton to be strongly light limited.Water temperatures were within a limited range at all stations (mean: 24.0–28.0 °C) and with little difference among stations in average values (Table 2), except the relatively protected station KL5, which was significantly warmer (anova; P < 0.05) than other stations. The pH ranged from 8.4 to 8.8, and the alkalinity ranged from 0.8 to 1.4 meq L−1. Alkalinity was highest (1.3 meq L−1) and pH lowest at KL3, while the inverse was true at the lake stations. Daily mixing of the water column maintained high oxygen concentrations in the gulf, but hypolimnetic hypoxia in the open lake occurred during seasonal stratification and substantially lowered the water column mean DO concentrations (Table 2). DRSi concentrations were high in the gulf (e.g. KL3, mean: 160 μm), but much lower in the lake stations (e.g. mean value of 27 μm at COL). SRP ranged from 0.8 to 1.5 μm, and DIN ranged from 5.5 to 18.9 μm. Statistical comparison (anova; P = 0.05) between the gulf and the lake showed marked biogeochemical differences between these morphologically distinct areas. The gulf had significantly higher concentrations of DRSi, DIN and seston fractions (PN, PC and PP) compared to the open lake, while the open lake at COL had significantly higher SRP compared to the gulf. Unlike SRP, which varied significantly between the gulf and the open lake, TP concentration showed a minimal variation between the gulf and open lake (Table 2) and was not significantly different between the sampled sites. However, TN was higher in the gulf and the TN : TP ratio was nearly twice as high in the gulf as in the open lake.
Phytoplankton biomass, composition and photosynthesis
Chlorophyll a mean concentrations exhibited well-defined trends, with highest values recorded at KL3 and KL5 (22 μg L−1) and lowest values (11 μg L−1) in the more offshore lake station (Fig. 3a). Seasonally, chlorophyll a varied widely with a 3- to 10-fold variation over all months at both lake and gulf stations (Fig. 3a). In the gulf (KL3), chlorophyll a followed a general increasing trend to a maximum in November 2005 and then decreased monotonically towards March 2006, whereas in the open lake at COL an increasing and decreasing trend over our period of observation was observed with maximum values in September and January and minimum value in April (Fig. 3b). Over this range of variability, KL3 had consistently higher chlorophyll a concentrations than COL. Between-year variation was also substantial in some stations. The gulf station KL3 had a 2-fold higher chlorophyll concentration in March 2006 compared to the same time in March 2005, but in the open lake the 2 years had similar chlorophyll a concentration.
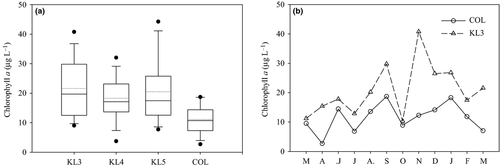
Phytoplankton biomass (wet weight), based on samples taken between December 2005 and March 2006, showed a similar spatial trend as chlorophyll a with the gulf having highest biomass (Fig. 4a). Diatoms (Bacillariophyceae) accounted for 43% of biomass at the offshore lake station COL. Cyanobacteria accounted for 72% of biomass in the gulf, but only 54% biomass in the open lake. The greater proportion of Cyanobacteria in the gulf also accounted for the nearly 50% higher biomass in the gulf compared to the open lake.
Diatoms in both open lake stations were dominated numerically by the long thin pennate species belonging to the genus Nitzschia spp., mainly N. lacustris, N. cf graciloides and N. cf acicularis morphotypes (taxonomy of the gulf Nitzschia is currently under investigation by others, e.g. L. Sitoki, pers. comm.), accompanied by Synedra (S. cunningtonii), but in the gulf the centric diatoms Cyclostephanos sp. as well as Aulacoseira (mainly A. granulata, A. agassizii, A. cf distens) were more common on the sampled dates. Several morphological forms (morphospecies) of the chroococcalean genus Aphanocapsa (including A. delicatissima, A. holsatica) and the non-nitrogen-fixing filamentous species in the genus Planktolyngbya (P. controta, P. tallingii, P. circumcreta, P. limnetica) and Pseudanabaena (P. limnetica, P. cf acutissima) were the dominant cyanobacteria in the open lake.
In the gulf Cyanodictyon (C. planktonica, C. reticulatum and C. imperfectum) and other small chroococcales, mainly of the genus Aphanocapsa and unidentified freely distributed picocyanobacterial cells contributed to the highest biomass. Anabaena (mainly A. cf discoidea) was the primary heterocystous cyanobacterial species both in the gulf and in the lake although Cylindrospermopsis africana, C. raciborskii, C. cuspis and Aphanizomenon sp also occurred at open lake stations (COL). The proportion of heterocysts to non-heterocystous cells in the primary nitrogen fixers Anabaena (mainly A. cf discoide, A. cf flos aquae; please note that all planktonic Anabaena have recently been shifted to genus Dolichospermum, Wacklin, Hoffmann & Komárek, 2009) was higher in the open lake (7.8%) compared to the gulf (1.8%), indicating differences in the importance of atmospheric nitrogen fixation as a nitrogen source between the gulf and open lake. Interestingly, the primary low-light N fixers in the genus Cylindrospermopsis, which were reported as dominant in the open lake and in the inshore Ugandan regions of the lake in the 1990s (Kling et al., 2001), were rare in the gulf and at KL5 during this study and their greatest contribution to biomass occurred at the open lake station COL in December 2005.
The means and standard errors of phytoplankton photosynthetic parameters (PBM, αB and integral primary productivity) in the gulf and open lake stations (KL3 and COL) are presented in Fig. 4b. PBM (the chlorophyll a-normalised maximum rate of photosynthesis) was similar in the gulf and the open lake, while the initial slope of the light response curve, αB, was higher in the open lake. Consequently, the Ek (irradiance at onset of light saturation) was somewhat lower in the open lake (mean: 148) compared to the gulf (mean: 167). The mean integral photosynthesis was twice as high in the open lake (COL) compared to the gulf (KL3), 11.7 and 5.4 g O2 m−2 day−1, as a result of the more efficient use of lower light intensities and the deeper depth of the euphotic zone.
Phytoplankton nutrient status
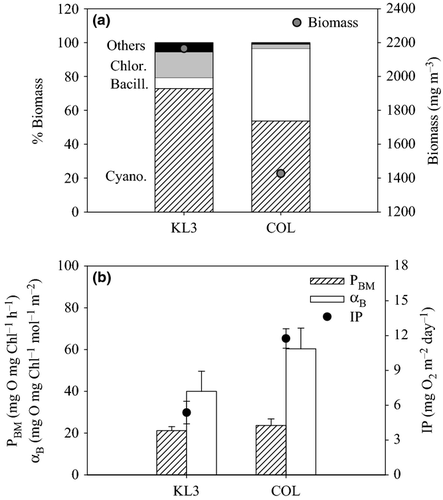
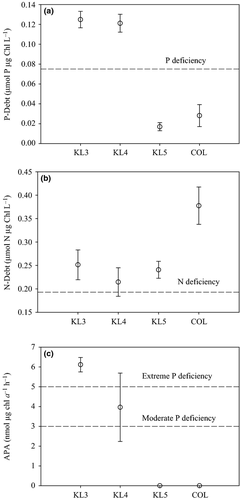
The indicator values for nutrient deficiency for phytoplankton growth by phosphorus and/or nitrogen (indicated by the dotted horizontal lines and the embedded text in Figs 5 and 6) are based on Healey & Hendzel (1979). The ratio of particulate nutrients to phosphorus (C : P, N : P; Fig. 5) as well as the P-debt and APA assays (Fig. 6) showed P deficiency in both gulf stations (KL3 and KL4). The C : N of particulate matter showed no indication of nitrogen deficiency of phytoplankton growth in any of the stations, but the N-debt assay showed nitrogen deficiency in phytoplankton in all the stations. The ratio of particulate carbon to chlorophyll (C : Chl) is a general indicator of nutrient deficiency (Healey & Hendzel, 1979, 1980) and did indicate nutrient deficiency in the gulf (at KL3 and KL4) and occasional deficiency in the open lake station, COL (Fig. 5c): a caveat for use of the C : Chl as nutrient deficiency indicator is that the ratio is also influenced by other environmental factors such as light and temperature (Wang et al., 2009). Because there are differences in the light environment between the gulf and open lake, this indicator may be responding more to the light conditions than to nutrients. C : Chl values indicating nutrient deficiency in the open lake were observed in October 2005 when strong thermal stratification occurred (Gikuma-Njuru, 2008) and higher light exposure in the shallower mixed layer may have increased the ratio at that time.
The particulate matter in the gulf was isotopically distinct from the open lake. The mean δ13C was more negative in the gulf by more than 2‰, while the δ15N was more positive in the gulf and fell to lower values in the open lake where δ15N was on the order of 3‰ lower than in the gulf (Fig. 7). These differences were maintained throughout our period of observation from June 2005 through until January 2006, which included one rainy season, and can be considered persistent feature of the particulate matter in these two lake regions.

Discussion
Nyanza Gulf and the open Lake Victoria provide remarkably different nutrient and light conditions that select for quite different phytoplankton communities and result in different primary productivities. Although Nyanza Gulf is connected to the lake at a common mean water level, its extensive area of shallow water and the restricted interchange through the Rusinga Channel allow it to maintain a distinct physicochemical environment. Although the gulf has not received as much attention in the published literature as the open lake (especially in Uganda), the intensive land use in its catchment and its significant contribution to the whole lake water budget have supported speculation that it may have played a disproportionate role in the historical changes observed in Lake Victoria since the 1960s. Our study allows quantitative evaluation of these different environments, how differences in nutrient sources and cycling may contribute to the differences and how management action might improve their conditions.
Nutrient status
Nyanza Gulf
Nyanza Gulf is unique among the other inshore areas and coastal embayments of Lake Victoria because of its expansive area of shallow waters and its hydrology, which is dominated by river inflows and a constricted connection to the open lake. The inflowing rivers bring substantial loads of suspended sediments from surrounding highlands, and the shallow depth and expansive area of the gulf facilitates resuspension. Consequently, the gulf has high mineral turbidity that reduces light penetration. However, the effect of the high light extinction on algal growth is moderated by the shallow mixing depths so that the mean PAR in the mixed layer falls within the range of lake stations and was, on average in the year of our observation, above PAR values that might limit algal photosynthesis. Sediment loading and resuspension may also contribute to the higher concentrations of particulate C, N and P in the gulf compared to the lake. The higher particulate P concentrations in the gulf, however, do not result in higher TP concentrations than those in the open lake. In contrast, the SRP was a smaller fraction (c. 25%) of the TP in the gulf stations compared to more offshore lake station COL, where SRP was 50% of TP (Fig. 8).
Hecky (1993) and Lehman & Branstrator (1993) have previously reported the declining proportion of SRP to TP in the inshore Ugandan waters of Lake Victoria compared to SRP of >50% of TP in offshore waters. Our trend between COL and KL5 and into the gulf is consistent with the declining availability of SRP in shallower coastal waters, as reported by them. Hecky (1993) and Hecky et al. (2010) attributed this pattern to reduced mixing depths in inshore waters resulting in higher irradiance and SRP uptake resulting in higher algal biomass in shallower inshore waters. In the gulf, the well-oxygenated water column and the higher suspended mineral load may also contribute to the lower SRP/TP ratio by ensuring efficient binding of phosphate to the oxidised sediment surfaces (Gikuma-Njuru et al., 2010). The oxygenated water column would also inhibit microbial denitrification and may account for the maintenance of higher DIN concentrations in the gulf compared to the open lake. The greater extent of hypoxic waters in the open lake (Hecky et al., 1994) should favour denitrification as well as the release of SRP from sediments compared to the well-oxygenated gulf (Hecky et al., 1996). Gulf waters also have high concentrations of DRSi as a consequence of high riverine loading of DRSi and low demand for Si by diatoms, which were a relatively minor component of the phytoplankton in the gulf. The higher DRSi in the gulf contrasts with the low DRSi found in inshore coastal waters in Uganda (Hecky et al., 2010) where diatoms have remained a significant proportion of the algal community (Kling et al., 2001).
Chlorophyll a concentrations and algal biomass in the gulf were generally higher than in the open lake. Cyanobacteria accounted for most of the difference in algal biomass with diatoms only a very minor contributor in the gulf. The phytoplankton in the gulf was on average quite P deficient based on both seston composition measures (over the year of observation) and metabolic indicators (from November 2005 to March 2006). Seston C : P ratios consistently indicated extreme P deficiency, while N : P ratios indicated moderate to severe deficiency. Similarly, P-debt indicated severe P deficiency in the gulf, while alkaline phosphatase was consistent with moderate to severe P deficiency on average. Although the stoichiometric measure of N deficiency did not show any indication of N-debt in the gulf, with C : N consistently <8, the N-debt assay did suggest N deficiency. DIN and TN concentrations in the gulf were significantly higher than in the open lake, and so lower N stress might be expected compared to the open lake. Sitoki et al. (2012) also report nitrate concentrations of 2–3 μm in the gulf that were more than three times higher than in the open lake. We infer that nutrient status of phytoplankton in the gulf most likely tends to strong P deficiency, while N deficiency is less likely to strongly limit algal biomass.
Lake Victoria proper
In strong contrast to the gulf, our two stations in Lake Victoria did not show any evidence of P deficiency; especially striking was the complete elimination of APA, which is usually considered the most sensitive indicator of P deficiency. The absence of P deficiency is consistent with the higher SRP concentrations especially at COL. The lack of P stress is also consistent with observations from offshore and coastal waters in the Ugandan portion of Lake Victoria that indicated SRP is available in excess supply relative to demand, and P stress is generally minimal (Lehman & Branstrator, 1993; Guildford et al., 2003). The lack of strong P demand occurs because light availability, due to self-shading by increasing algal biomass, limits N fixation by heterocystous cyanobacteria (Mugidde et al., 2003), which would otherwise drive further SRP uptake. Although N fixation was not measured in our study, we do have indirect evidence that N fixation is important in the open lake, especially compared to Nyanza Gulf. The stable isotope signature of seston in the gulf is much more enriched with δ15N greater than 6‰ compared to the lake where values of δ15N fall below 2‰ at COL. Atmospheric nitrogen has a δ15N of 0.0 by convention (Peterson & Fry, 1987), and so the lower values of δ15N in the open lake are consistent with higher rates of nitrogen fixation. Our δ15N values at lake stations are also similar to the average, δ15N = 2.8, observed in coastal waters in Uganda where N fixation has been measured as well as its effect on the δ15N of particulate matter (Hecky et al., 2010). The frequency and abundance of heterocysts is also correlated with N fixation rates (Gondwe, Guildford & Hecky, 2008), and our lake stations had higher frequencies of heterocysts. We conclude that the more positive δ15N in the gulf indicates a different source of DIN to the gulf, most likely tributary derived, compared to the lake proper where N fixation has been established as the main source of N supporting phytoplankton biomass (Hecky et al., 1996; Mugidde et al., 2003).
The N-debt results for the lake stations do not conflict with higher rates of N fixation in the open lake as even actively N-fixing cyanobacteria would energetically benefit by taking up ammonia when it is available. This would be a short-term response to the availability of ammonia in the assay that may not be readily available in situ. Consequently, short-term enrichment experiments based on the addition of DIN can lead to positive uptake of the added substrate and apparent N limitation even when N-fixing taxa are present (Lehman & Branstrator, 1993; Guildford et al., 2003). Perhaps most convincing is the lack of any indication of N deficiency in our C : N data. Our C : N values for seston integrate N availability over the whole growth cycle of the phytoplankton, and they did not indicate any strong N deficiency at the lake stations. This suggests that N fixation together with recycled DIN must be able to meet the N demand of phytoplankton and maintain C : N ratios, indicating that N availability is sufficient for algal growth at our open lake stations, as in other coastal waters in Uganda (Mugidde et al., 2003).
Phytoplankton community composition and productivity
The results of nutrient status measurements indicate two very different nutrient regimes. Nyanza Gulf showed persistent P deficiency, while the lake was P sufficient and likely dependent on N fixation by heterocystous cyanobacteria and N recycling to meet its N requirements. Despite these differences, cyanobacterial taxa dominated the phytoplankton communities in both the gulf and the lake, although diatoms composed a significant portion of the community in the lake. The advantage of the cyanobacteria at the lake station is understandable if N fixation was critical to meeting N demand by phytoplankton as only the cyanobacteria can do this in the plankton. It is noteworthy that diatoms were relatively abundant in the lake in response to higher SRP concentrations despite the lake having much lower DRSi concentrations than the gulf. The higher αB and lower Ek measured at COL are probably a consequence of the significant contribution of diatoms at COL. Silsbe et al. (2006) demonstrated that the historic shift from a diatom-dominated phytoplankton to cyanobacteria in Lake Victoria resulted in a lowering of αB in the phytoplankton. The more efficient use of low light by diatoms combined with the deeper euphotic zone at COL resulted in higher integral primary productivity measured in the open lake despite the lower algal biomass and chlorophyll concentrations in the lake.
The more positive δ13C in seston at the lake stations was consistent with the lake stations being more productive and having a higher C demand relative to CO2 availability (Peterson & Fry, 1987; Hecky & Hesslein, 1995). The observed δ13C increase from the gulf stations to the lake stations is in agreement with our primary productivity results that indicate an approximate doubling in integral primary productivity between the gulf and the open lake. Phytoplankton preferentially assimilate the lighter CO2diss during photosynthesis as it requires no energy expenditure for uptake or charge balance (Hecky & Hesslein, 1995), but under low CO2diss conditions, as happens during high rates of phytoplankton production, DIC uptake becomes less discriminating as CO2diss diminishes and results in heavier δ13C in phytoplankton. Our most positive δ13C values are comparable to inshore, coastal values in Ugandan and Tanzanian waters of Lake Victoria, as observed by Hecky et al. (2010) who concluded that sestonic δ13C was responsive to primary productivity patterns in Lake Victoria with more positive δ13C occurring in regions of higher algal biomass and primary productivity in the modern lake. Also, δ13C increased towards the sediment surface in three sediment cores from Lake Victoria, recording the increased primary productivity of the lake over the last several decades of the 20th century (Hecky et al., 2010). Within Nyanza Gulf, poor light penetration due to mineral turbidity limits the depth of the euphotic zone, while P stress may limit phytoplankton growth rates. The δ13C in organic matter serves as a tracer for higher rates of photosynthesis in the lake as the euphotic zone deepens and P deficiency is relieved. In the open lake, the phytoplankton community is more efficient at low irradiances and achieves higher integral rates of productivity. From the standpoint of primary productivity, the open lake is more productive and in that sense more eutrophic than Nyanza Gulf, even though chlorophyll concentrations are higher in the gulf.
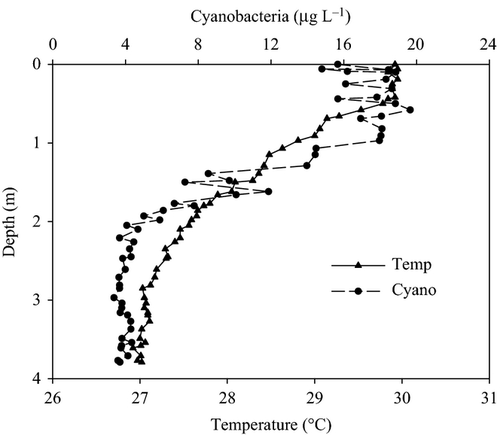
The low abundance of diatoms in the gulf and the overwhelming dominance of cyanobacteria are somewhat unexpected. The shallower mixing depths should favour resuspension of diatoms, and the higher DRSi should allow a broad range of diatom species to compete for N and P. The phytoplankton in the gulf is strongly P deficient, but diatoms are generally thought to be efficient competitors for P, yet they are nearly excluded from the gulf phytoplankton. The high mineral turbidity in the gulf reduces light penetration and may induce light limitation for many phytoplankton taxa, but high light extinction may favour the growth of cyanobacterial species (often in the genera Microcystis or Anabaena, depending on N availability), which have the capacity to control buoyancy during quiescent periods to maximise acquisition of light and nutrients (Ganf, 1974). The ability of the cyanobacteria to rise in the water column to overcome temporary low light conditions (Ibelings et al., 1991a) and attain higher growth rates than would be accomplished under the mean light conditions in a well-mixed water column may give them a competitive advantage in the gulf. This opportunity to alter vertical position in the water column to maximise growth rates is further enhanced by diurnal thermal stratification (Ganf, 1974; Ibelings, Mur & Walsby, 1991b) allowing the cyanobacteria to aggregate near the surface with the reduction in mixing depth during daytime (Fig. 8). Thus, buoyancy control allows these taxa to increase their access to light, while other taxa such as the negatively buoyant diatoms sink below the diurnal thermocline into darkness. Therefore, despite KL3 and KL4 having mean water column irradiance ( ), based on a well-mixed water column, even exceeding the critical PAR on average, diurnal thermal stratification and the shallow water column over which vertical migration brings an advantage probably allow the buoyancy-controlling cyanobacteria to dominate in the gulf and develop high biomasses. The shallow water column and particularly the steep light extinction gradient will result in substantial improvement in light exposure for even a small range of vertical migration, 1 or 2 m in the case of Nyanza Gulf. In the absence of diurnal stratification, diatoms would have better light conditions during the day and would probably be more prominent in the gulf.
), based on a well-mixed water column, even exceeding the critical PAR on average, diurnal thermal stratification and the shallow water column over which vertical migration brings an advantage probably allow the buoyancy-controlling cyanobacteria to dominate in the gulf and develop high biomasses. The shallow water column and particularly the steep light extinction gradient will result in substantial improvement in light exposure for even a small range of vertical migration, 1 or 2 m in the case of Nyanza Gulf. In the absence of diurnal stratification, diatoms would have better light conditions during the day and would probably be more prominent in the gulf.
P and N in Lake Victoria
The clear differentiation of P deficiency in the gulf and N deficiency in the open lake is a result of the constricted circulation, imposed by the Rusinga Channel, between the two hydrological regimes. Although there is a significant exchange between the lake and the gulf through the connecting channel (Gikuma-Njuru et al., 2013), there is much less exchange of offshore water with coastal water than in other inshore embayments of Lake Victoria which have an active interchange driven by gradients in surface energy exchange between the open lake and shallow, more open embayments (MacIntyre, Romero & Kling, 2002). Because of the constricted interchange with the lake, the shallow gulf is more heavily influenced by terrestrial run-off from its highly populated catchment. The gulf is also shallow and well mixed diurnally, which maintains an oxygenated water column and sediment–water interface (Gikuma-Njuru et al., 2010). These conditions result not only in high terrestrial nutrient loading to the gulf, but they also favour P binding and sedimentation while N is more efficiently recycled under an oxygenated regime. In contrast, the sediments of the deeper open lake stations are exposed at least seasonally to hypoxic conditions that will favour the release of mineral-bound P and denitrification (Hecky et al., 1996). These contrasting nutrient cycling regimes result in the gulf having a TN : TP ratio nearly double the open lake and strong P deficiency that we observed in the phytoplankton. The low TN : TP regime in the open lake requires N fixation to meet the N demand of the phytoplankton (Mugidde et al., 2003). Although the differentiation of these two nutrient cycling regimes is especially clear between the gulf and the lake, they are probably characteristic of a continuum between these different regimes that exist between the deep waters of the lake and the shallower, nearshore coastal regime throughout Lake Victoria.
Hecky (1993) reported evidence of declining SRP concentrations and increasing stoichiometric evidence of P deficiency along a transect from offshore to nearshore Lake Victoria. Other studies in Lake Victoria have also shown this pattern of higher inshore algal biomass, lower SRP (but little change in TP) and higher TN : TP along transects from offshore to nearshore (Lehman & Branstrator, 1994; Kling et al. 2000; Hecky et al., 2010). The clear differentiation, so evident between the gulf and lake in our study, may be exemplary of a more general pattern in Lake Victoria in which shallow, well-oxygenated coastal waters yield higher algal biomass and P drawdown. Mugidde (1993) and Mugidde et al. (2003) have previously shown that light availability limits photosynthesis, nitrogen fixation and algal biomass offshore in Lake Victoria, due to the deep mixed layer, and that shoaling depths further inshore allow higher N fixation and photosynthetic rates that result in higher algal biomasses. Consequently, increases in P availability over the past century have led to increases in algal biomass in general in the lake and in particular to algal blooms in coastal waters of Lake Victoria (Hecky et al., 2010). These blooms acquire much of their nitrogen from nitrogen fixation, which accounts for nearly 80% of the annual input of N input into Lake Victoria (Mugidde et al., 2003).
Temporal variability in Nyanza Gulf
Water quality results in our study (2005–2006) are, ingeneral, comparable to those observed by Gikuma-Njuru & Hecky (2005) at the same stations between 2000 and 2002, suggesting stability over time in the gulf. But other observations on water quality and phytoplankton composition indicate that the gulf may experience a significant year-to-year variability because of its relative sensitivity to riverine discharges. Thus, nano- (Cyanodictyon and Aphanocapsa spp.) and picocyanobacteria were dominant in the gulf in our year of study, but in other years colonial Microcystis spp. have produced spectacular blooms; heavy algal blooms have been observed in the gulf (Sitoki et al., 2012; P. Gikuma-Njuru, pers. obser.), especially after intense seasonal rains and the associated influx of mineral turbidity and nutrients (P) from the river inflows. For example, after heavy rains in March 2007, high concentrations of SRP (7 μm), DIN (55 μm) and high turbidity (74 NTU; Secchi depth: 0.2 m) were measured at KL3 and resulted in the development of dense algal blooms (>180 μg L−1 chlorophyll) by early April (P. Gikuma-Njuru unpubl. data). The blooms were dominated by Microcystis species (M. panniformis, M. protocystis and M. cf aeruginosa), which have been found to produce the algal toxin microcystin in Lake Victoria (Sitoki et al., 2012) as well as in other tropical parts of the world (Kotut, Ballot & Krienitz, 2006). In addition to this extreme event in 2007, Sitoki et al. (2012) observed Microcystis dominance over a complete annual cycle in 2008–2009 during which they also observed lower mean Secchi transparencies (0.4 m) and much higher mean TP (210 μg L−1) than we did at two stations near our two gulf stations. The phytoplankton composition and water quality of the gulf are clearly very responsive to long-term and interannual variability in suspended sediment concentrations and accompanying nutrient loading because of its shallow depth and several substantial inflowing rivers. Dramatic turbidity events and resultant algal blooms appear to be becoming more frequent and are a challenge to water quality management in the gulf.
Historic trends
Initial concern about the possible eutrophication of Nyanza Gulf (e.g. Calamari et al., 1995) began with the observations of algal blooms in Kenya waters by Ochumba & Kibaara (1989). But, in fact, the blooms they observed were more prominent in coastal waters outside Nyanza Gulf than within the gulf where they reported a mean chlorophyll a concentration of 17 μg L−1. Their reported mean concentration in the gulf is remarkably similar to what we observed in the gulf proper nearly 20 years later. Although chlorophyll measurements are sparse prior to 1986, Ochumba & Kibaara (1989) point out that the mean chlorophyll concentration in the gulf that they reported was remarkably similar to a single measurement of 20 μg L−1 made in 1960 by Talling (1965) and another single measurement, 17 μg L−1, made by Melack (1976) in 1971. Lung'ayia et al. (2000) reported chlorophyll values in the range of 10–20 μg L−1 and Sitoki, Kurmayer & Rott (2012) reported only one chlorophyll concentration >20 μg L−1 for three stations in the gulf sampled quarterly in 2009, further evidence that algal biomass (as chlorophyll) in the gulf has not changed over the past five decades. Similarly, comparison of available historical primary productivity measurements with our measurements (Table 3) shows no evidence for an increase in primary productivity within Nyanza Gulf from at least 1973 through 2009.
| Kavirondo (Talling 1965) | Winam (Melack 1976) | Nyanza (this study; KL3, KL4) | |
|---|---|---|---|
| SD (cm) | na | 134 (120–150, 4) | 80 (24) |
| kd (540 or PAR) (m−1) | 1.67 (1) | 1.68 (3) | 2.15 (24) |
| EC (μS cm−1) | 144 (2) | 174 (170–179, 3) | 160 (24) |
| Alkalinity (meq L−1) | 1.39 (2) | 1.68 (1.57–1.86, 3) | 1.35 (12) |
| TP (μg L−1) | 67 (1) | na | 107 (24) |
| Dissolved SiO2 (mg L−1) | 5.1 (2) | 4.3 (2–7, 3) | 9.5 (24) |
| Chlorophyll (μg L−1) | 20 (1) | 17 (1) | 19.8 (12) |
| PBM (mg O2 mg Chl−1 h−1) | 25 (1) | 16 (1) | 20 (5) |
| Primary productivity (mgO2 m−2 day−1) | 10.7 (1) | 6.2 (4.3–9.8, 9) | 5.4 (5) |
- Means and number of measurements in parentheses; parenthetic data for Melack also include range of observations when available. Note that Kavirondo, Winam and Nyanza are the same gulf but are referred to by different names in the publications.
- na = not available.
In contrast to this apparent stability in chlorophyll concentrations and integral productivity over time in Nyanza Gulf, transparency has declined substantially, measured either by Secchi disc (reduced by 40% from 1973) or by light extinction coefficient (increase of 30%), while TP concentrations may have risen by as much as 60% over time as turbidity increased over the same period. The higher turbidity in the gulf may be selecting for algal taxa that can utilise buoyancy regulation to gain access to higher light in the water column and overcome the light limitation imposed on many species by the high mineral turbidity. The gulf may therefore be poised at a critical light condition and any deterioration below the Secchi values we observed may favour Microcystis, as observed in seasons with high turbidity (Lungayia et al. 2000; Sitoki et al., 2012), over the community that we observed in 2005–2006. The larger Microcystis colonies that have a buoyancy advantage will also be more resistant to zooplankton grazing and so reduce their loss rates compared to other phytoplankton. The shift towards dominance by the potentially toxic Microcystis (Sitoki et al., 2012) is a cause for concern not only for food-web integrity and productivity of consumers including fish but also for human consumption of water and fish (Poste, Hecky & Guildford, 2011).
Management implications
Both seston ratios and metabolic nutrient assays show the gulf to be P limited in our year of study, indicating that the maximum phytoplankton biomass and productivity in the gulf may be determined by phosphorus availability. This finding is important for the management of the gulf's water quality since an increase in P loading, and especially bioavailable P, may translate to even higher maximum algal biomass, mainly of bloom-forming and potentially toxic cyanobacteria (Kreinitz et al., 2001; Sitoki et al., 2012). However, such an ‘enrichment scenario’ necessarily leading to higher algal biomass may be modified if much of the increased P loading is in the form of mineral-bound P coming from soil erosion (Gikuma-Njuru et al., 2010). In such a case, the release of phosphate from mineral-bound complexes probably requires the exposure of the mineral grains to persistent anoxic conditions currently found only in the open lake. These conditions probably account for the higher SRP concentrations found there, while the gulf with similar TP concentrations remains strongly P deficient in regard to phytoplankton growth. In this case, the gulf may be enriched in total P over time but may not suffer a proportionate enrichment in available P to sustain phytoplankton growth.
Reduction in sediment as well as P loading into the gulf should be a management priority in order to protect the gulf environment from further deterioration. Both mineral turbidity and associated P concentrations have certainly increased in Nyanza Gulf over the last 50 years of rapidly growing populations and increasing agricultural land use. Mineral turbidity may be the greater threat to the gulf through its inducement of Microcystis blooms in years of high turbidity. Although the increased P concentrations have not resulted in increased chlorophyll on average in the gulf because the gulf is well oxygenated and the P is strongly bound to the oxidised mineral surfaces, TP bound in sediments exported from the gulf will eventually reach reducing environments in the open lake where SRP can be released and contribute to coastal blooms. Phosphorus control can be achieved through reduction in the use of phosphate-free detergents and more effective treatment of municipal effluents and solid wastes from Kisumu City and other urban areas in the gulf catchment. However, given the sensitivity of the gulf to Microcystis blooms induced by high mineral turbidity, the implementation of good land-use practices and catchment reforestation to reduce sediment loading and mineral-associated P loading to the gulf and lake may be an even greater priority.
Acknowledgments
We thank the Deputy Director Kenya Marine and Fisheries Research Institute (KMFRI), Kisumu, for availing a research vessel during lake sampling. This study was supported by a research grant (A/3869-1) from International Foundation for Science (IFS) to PGN, Government of Kenya through Lake Victoria Environmental Management Project (LVEMP) and NSERC grants to REH and SJG. The assistance of M. Ndungu, F. Onyango, M. Ouma and G. Agunda in field sampling and laboratory analysis is highly appreciated. We thank two anonymous reviewers and Professor Colin Townsend for their comments that significantly improved the manuscript.




